Abstract
The objective of the present work was to determine the effect of Lactobacillus murinus strain LbP2 on canine fecal immunoglobulin A (IgA) levels. Seven dogs were orally treated with a 3-mL suspension of L. murinus LbP2 containing 5 × 109 colony-forming units on alternate days for 2 wk. Six dogs were treated with 3 mL of phosphate-buffered saline as placebo. Fecal samples were taken from the rectal ampulla on days 0 and 16, and the total canine fecal IgA concentration was determined with an immunoperoxidase assay kit. The IgA levels of individual dogs were compared with the nonparametric Wilcoxon test. Differences were considered significant when the P-value was less than 0.05. An increase in the total fecal IgA concentration was observed in the 7 dogs after treatment with L. murinus LbP2 (P = 0.01796). No differences were detected between the initial total fecal IgA values and those obtained at the end of placebo treatment. Thus, after oral administration L. murinus LbP2 showed potential immunomodulatory effects, an important property to assess in a microorganism being considered for use as a probiotic.
Résumé
L’objectif de la présente étude était de déterminer l’effet de la souche Lactobacillus murinus LbP2 sur les niveaux d’immunoglobulines A (IgA) fécales chez le chien. Sept chiens ont reçu oralement 3 mL d’une suspension de L. murinus LbP2 contenant 5 × 109 unités formatrices de colonies à chaque 2 jours pendant 2 semaines. Six chiens ont reçu 3 mL de saline tamponnée comme placebo. Des échantillons de fèces ont été prélevés au niveau de l’ampoule rectale aux jours 0 et 16, et la concentration d’IgA canine totale déterminée au moyen d’une épreuve d’immunoperoxydase. Les niveaux d’IgA des chiens individuels ont été comparés avec le test non-paramétrique de Wilcoxon. Les différences étaient considérées significatives lorsque la valeur de P était inférieure à 0,05. Une augmentation dans la concentration d’IgA fécale totale a été observée dans les sept chiens après traitement avec L. murinus LbP2 (P = 0,01796). Aucune différence ne fut détectée entre les valeurs initiales des IgA fécales totales et celles à la fin du traitement avec le placebo. Ainsi, l’administration orale de L. murinus LbP2 montre des effets immunomodulateurs potentiels, une propriété intéressante à évaluer chez un microorganisme considéré pour usage comme probiotique.
(Traduit par Docteur Serge Messier)
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (1). The beneficial effects and the use of probiotics in the management of specific diseases are well-accepted (2). Commonly used bacterial probiotics include Lactobacillus spp. and Bifidobacterium spp.; Lactococcus lactis and some Enterococcus species have also been used (3).
The mechanisms of action of probiotics differ and are not unique. One potential mechanism is based on the ability to modulate the mucosal immune system and the local production of IgA. In some cases probiotics have been associated with total and pathogen-specific IgA levels upon infection while typically not inducing the production of specific antiprobiotic IgA. Treatment with L. casei induced a significant increase in the numbers of IgA-producing cells in the small-bowel lamina propria of mice (4). However, not all probiotic strains are equal in terms of their effects on IgA production (5).
The domestic dog (Canis lupus familiaris) plays an important role in our society and has been used as a model to study various diseases. One of the dominant lactobacilli in the dog’s intestinal microbiota and milk, L. murinus, plays an important role in the immunity of puppies (6,7). Different L. murinus strains have, in vitro, shown interesting properties for probiotic use, exhibiting acid and bile salt resistance, adhesion to canine intestinal mucus, and antimicrobial activity against specific bacterial pathogens (8,9). An in-vivo assay in dogs proved the enteric persistence and safety of L. murinus (10).
The aim of the present work was to evaluate the immunomodulation potential of L. murinus strain LbP2 through the effect on total fecal IgA production in orally treated dogs.
One group of 7 and another of 6 healthy dogs between 3 and 7 y old from the Veterinary Faculty of the University of Uruguay, Montevideo, were used; all animal procedures were approved by the Bioethics Committee of the Veterinary Faculty. The probiotic-treated group included 5 females (3 that were 7 y old and 1 each that were 4 and 5 y old) and 2 males (7 and 3 y old). The group treated with phosphate-buffered saline (PBS) included 4 females (3 that were 7 y old and 1 that was 5 y old) and 2 males (3 and 5 y old). The animals were fed once a day with 270 g of dry commercial food (Royal Canin, Buenos Aires, Argentina) from 10 d before the start of the study. All the dogs were kept at the same place and isolated. At the beginning and at the end of the study (days 0 and 16) their body condition, body weight, and fecal consistency were determined.
The probiotic-treated group received orally 3 mL of a suspension of L. murinus LbP2 containing 5 × 109 colony-forming units on alternate days for 2 wk, for a total of 8 doses. The bacteria had been grown in MRS broth for 48 h, washed twice, and resuspended in PBS at an adequate concentration. The control group received orally 3 mL of PBS on the same days as the probiotic-treated group.
Fecal samples were taken from the rectal ampulla of each dog on study days 0 and 16 (2 d after the last dose) and suspended in PBS (w/v ratio 1:4) supplemented with ethylene diamine tetraacetic acid and phenylmethylsulfonyl fluoride, 0.5 mM each, to inhibit protease activity (11). The samples were homogenized by means of a vortex blender at maximum speed for 30 min at 4°C and the homogenates centrifuged twice at 10 000 × g for 20 min at 4°C. The supernatants were collected and stored at −80°C for subsequent analysis.
An immunoperoxidase assay kit (Immunology Consultants Laboratory, Portland, Oregon, USA) was used to determine the total canine fecal IgA concentration. The standard was prepared in 7 serial dilutions of 0 to 1000 ng/mL. Successively, 100-μL aliquots of the fecal samples diluted 1:500 were added to predesignated wells coated with antibody against dog IgA provided by the manufacturer. The microplates were incubated for 30 min and then aspirated and washed 3 times. Antibody conjugated with horseradish peroxidase was added, and the plates were incubated for another 30 min and then washed and blotted again. Next, 100 μL of cromogen-substrate solution (3,3′,5,5′-tetramethybenzidine and hydrogen peroxide in citric acid buffer, pH 3.3) was added to each well, and the plates were incubated for 10 min. Finally, stop solution (0.3 M sulfuric acid) was added to end the reaction, and the absorbance was determined (at 450 nm) with a Varioskan Flash plate reader (Thermo Scientific, Asheville, North Carolina, USA). For standards and samples, duplicate measures were obtained. The results were expressed as milligrams of IgA per gram of feces.
A 4-parameter logistics curve was constructed from the results observed for the standards to interpolate the values for the test samples. The IgA levels of individual dogs were compared with the nonparametric Wilcoxon test. Differences were considered significant when the P-value was less than 0.05.
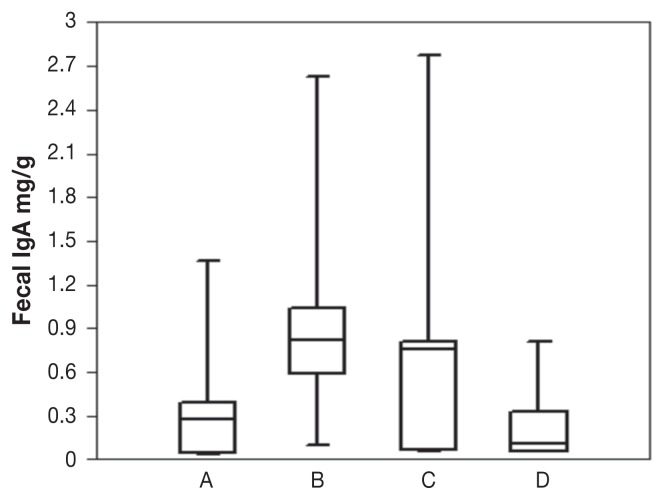
No significant changes in body condition, body weight, or fecal consistency were observed in the dog populations before and after treatment (data not shown). The standard curve constructed with the use of the standard absorbance values showed an r2 value of 0.989. The total fecal IgA concentration increased significantly (P = 0.01796) in the 7 probiotic-treated dogs: immediately before treatment the values ranged from 0.037 to 1.36 (median 0.269) mg/g, and at the end of treatment they ranged from 0.096 to 2.621 (median 0.821) mg/g (Figure 1, Table I). The total fecal IgA concentration did not change significantly (P > 0.05) in the 6 PBS-treated dogs: immediately before treatment the values ranged from 0.05 to 2.77 (median 1.53) mg/g, and at the end of treatment they ranged from 0.05 to 0.81 (median 0.2367) mg/g. When the total fecal IgA levels of the 2 groups of dogs were compared, no significant differences were detected at the beginning of the study (P > 0.05), whereas there were significant differences at the end of the study (P = 0.02681).
Figure 1.
Total fecal IgA concentration in dogs before and after treatment with Lactobacillus murinus strain LbP2 (A, B) or phosphate-buffered saline (C, D). Each plot shows the median (line within box), 25th and 75th percentiles (box) and extremes. The difference in median concentration after treatment with L. murinus was significant (P = 0.01796), whereas the difference after placebo treatment was not (P > 0.05).
Table I.
Changes in total fecal immunoglobulin A (IgA) concentration in 7 dogs treated with Lactobacillus murinus strain LbP2 and 6 dogs treated with phosphate-buffered saline as placebo for 2 wk
| Fecal IgA level, mg per gram of feces; treatment | |||
|---|---|---|---|
|
| |||
| Lactobacillus murinus strain LbP2 | Phosphate-buffered saline | ||
|
|
|
||
| Day 0 | Day 16 | Day 0 | Day 16 |
| 0.366 | 0.581 | 0.06 | 0.101 |
| 0.127 | 0.584 | 0.75 | 0.32 |
| 0.038 | 0.096 | 0.49 | 0.08 |
| 0.385 | 0.821 | 2.77 | 0.05 |
| 0.269 | 1.04 | 0.05 | 0.055 |
| 0.037 | 1.01 | 0.81 | 0.81 |
| 1.36 | 2.621 | ||
| P = 0.01796 | P > 0.05 | ||
From the results of the present study it can be concluded that oral administration of L. murinus LbP2 significantly influences the production of total canine fecal IgA. Not all probiotics are equal in terms of their effects on IgA production. In a study comparing different probiotic properties, including intestinal IgA production, Saccharomyces boulardii induced greater production of IgA than did Escherichia coli, B. animalis, and L. casei, whereas B. animalis and L. casei were better as pathogen inhibitors than S. boulardii (11). Thus, a particular property such as immunomodulation through local IgA production is not necessarily associated with other probiotic-related attributes. No effect on fecal IgA concentration was observed after administration of the probiotic Enterococcus faecium SF68 to treat giardiasis in dogs (13). Others have reported that the use of prebiotics such as inulin in association with probiotics could have a synergistic effect on the modulation of gut immunity, including an increase in IgA production (5). All these findings suggest that there is a remarkable diversity of stimuli that can lead to an enhanced enteric immune response. In addition, canine fecal IgA levels are highly variable, and the influence of diverse factors such as age and breed has been reported (14,15).
As in the previous in-vivo assay in which L. murinus LbP2 was administered to dogs (10), the microorganism did not affect body condition or fecal consistency. This could indicate that no inflammatory disorders were induced by the probiotic. It is important to take into account that L. murinus LbP2 treatment did not significantly affect the dogs fecal microbiota as observed in a previous study (10) or the current one.
Considering these encouraging results, further studies are being conducted to assess other potential probiotic effects of L. murinus LbP2 in dogs.
References
- 1.Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. [Last accessed January 4, 2014]. Available from: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
- 2.Narayan SS, Jalgaonkar S, Shahani S, Kulkarnin VN. Probiotics: Current trends in the treatment of diarrhea. Hong Kong Med J. 2010;16:213–218. [PubMed] [Google Scholar]
- 3.De Vrese M, Schrezenmeir J. Probiotics, prebiotics, symbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 4.Galdeano CM, Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis modulates intestinal immune functions in rats. J Nutr. 2004;134:153–156. doi: 10.1093/jn/134.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Rinkinen ML, Koort JM, Ouwehand AC, Westermarck E, Björkroth KJ. Streptococcus alactolyticus is the dominating culturable lactic acid bacterium species in canine jejunum and feces of four fistulated dogs. FEMS Microbiol Lett. 2004;230:35–39. doi: 10.1016/S0378-1097(03)00851-6. [DOI] [PubMed] [Google Scholar]
- 7.Martin R, Olivares M, Pérez M, et al. Identification and evaluation of the probiotic potential of lactobacilli isolated from canine milk. Vet J. 2010;185:193–198. doi: 10.1016/j.tvjl.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Nardi R, Santoro M, Oliveira J, et al. Purification and molecular characterization of antibacterial compounds produced by Lactobacillus murinus strain L1. J Appl Microbiol. 2005;99:649–656. doi: 10.1111/j.1365-2672.2005.02632.x. [DOI] [PubMed] [Google Scholar]
- 9.Perelmuter K, Fraga M, Zunino P. In vitro activity of potential probiotic Lactobacillus murinus isolated from the dog. J Appl Microbiol. 2008;104:1718–1725. doi: 10.1111/j.1365-2672.2007.03702.x. [DOI] [PubMed] [Google Scholar]
- 10.Perelmuter K, Fraga M, Delucchi L, Zunino P. Safety assessment and enteric colonization ability of a native canine Lactobacillus murinus strain. World J Microbiol Biotechnol. 2011;27:1725–1730. [Google Scholar]
- 11.Sainz T, Perez J, Fresan MC, et al. Histological alterations and immune response induced by pet toxin during colonization with enteroaggregative Escherichia coli (EAEC) in a mouse model infection. J Microbiol. 2002;40:91–97. [Google Scholar]
- 12.Martins FS, Silva AA, Vieira AT, et al. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191:623–630. doi: 10.1007/s00203-009-0491-x. [DOI] [PubMed] [Google Scholar]
- 13.Simpson KW, Rishniw M, Bellosa M, et al. Influence of Enterococcus faecium SF68 probiotic on giardiasis in dogs. J Vet Intern Med. 2009;23:476–481. doi: 10.1111/j.1939-1676.2009.0283.x. [DOI] [PubMed] [Google Scholar]
- 14.Zaine L, Ferreira C, de Gomes MO, et al. Faecal IgA concentration is influenced by age in dog. Br J Nutr. 2011;106(S1):S183–S186. doi: 10.1017/S0007114511000559. [DOI] [PubMed] [Google Scholar]
- 15.Littler RM, Batt RM, Lloyd DH. Total and relative deficiency of gut mucosal IgA in German shepherd dogs demonstrated by faecal analysis. Vet Rec. 2006;158:334–341. doi: 10.1136/vr.158.10.334. [DOI] [PubMed] [Google Scholar]