Abstract
Colostrum-replacement products are an alternative to provide passive immunity to neonatal calves; however, their ability to provide adequate levels of antibodies recognizing respiratory viruses has not been described. The objective of this study was to compare the serum levels of IgG at 2 d of age and the duration of detection of antibodies to bovine viral diarrhea virus 1 (BVDV-1), bovine viral diarrhea virus 2 (BVDV-2), bovine respiratory syncytial virus (BRSV), bovine herpesvirus 1 (BHV-1), and bovine parainfluenza virus 3 (BPIV-3) in calves fed maternal colostrum (MC) or a colostrum replacement (CR) at birth. Forty newborn male Holstein calves were assigned to the CR or the MC group. Group CR (n = 20) received 2 packets of colostrum replacement (100 g of IgG per 470-g packet), while group MC (n = 20) received 3.8 L of maternal colostrum. Blood samples for detection of IgG and virus antibodies were collected from each calf at birth, at 2 and 7 d, and monthly until the calves became seronegative. Calves in the MC group had greater IgG concentrations at 2 d of age. The apparent efficiency of absorption of IgG was greater in the MC group than in the CR group, although the difference was not significant. Calves in the CR group had greater concentrations of BVDV neutralizing antibodies during the first 4 mo of life. The levels of antibodies to BRSV, BHV-1, and BPIV-3 were similar in the 2 groups. The mean time to seronegativity was similar for each virus in the 2 groups; however, greater variation was observed in the antibody levels and in the duration of detection of immunity in the MC group than in the CR group. Thus, the CR product provided calves with more uniform levels and duration of antibodies to common bovine respiratory viruses.
Résumé
Les produits de remplacement du colostrum sont une alternative pour fournir une immunité passive aux veaux nouveau-nés; toutefois, leur capacité à fournir des niveaux adéquats d’anticorps reconnaissant les virus respiratoires n’a pas été décrite. L’objectif de la présente étude était de comparer les niveaux d’IgG sériques à 2 jours d’âge et la durée de détection des anticorps contre le virus de la diarrhée virale bovine de type 1 (BVDV-1), le virus de la diarrhée virale bovine de type 2 (BVDV-2), le virus respiratoire syncitial bovin (BRSV), l’herpesvirus bovin de type 1 (BHV-1), et le virus parainfluenza bovin de type 3 (BPIV-3) chez des veaux nourris avec du colostrum maternel (MC) ou du colostrum de remplacement (CR) à la naissance. Quarante veaux nouveau-nés mâles de race Holstein ont été assignés soit au groupe CR ou MC. Les animaux du groupe CR (n = 20) ont reçu deux paquets de substitut de colostrum (100 g d’IgG par paquet de 470 g), alors que les animaux du groupe MC (n = 20) ont reçu 3,8 L de colostrum maternel. Des échantillons sanguins pour la détection d’IgG et d’anticorps contre les virus ont été prélevés de chaque veau à la naissance, à 2 et 7 j d’âge, et à chaque mois jusqu’à ce que les veaux deviennent séronégatifs. Les veaux dans le groupe MC avaient des concentrations d’IgG plus élevées à 2 j d’âge. L’efficacité d’absorption apparente d’IgG était plus grande dans le groupe MC que dans le groupe CR, bien que la différence ne fût pas significative. Les veaux dans le groupe CR avaient des concentrations plus élevées d’anticorps neutralisants envers BVDV durant les 4 premiers mois de vie. Les niveaux d’anticorps contre BRSV, BHV-1, et BPIV-3 étaient similaires dans les deux groupes. Le temps moyen pour atteindre la séronégativité était similaire pour chaque virus dans les deux groupes; toutefois, de plus grandes variations étaient observées dans les niveaux d’anticorps et la durée de détection de l’immunité dans le groupe MC comparativement au groupe CR. Ainsi, le produit CR a fourni des veaux avec des niveaux d’anticorps contre les virus respiratoires bovins communs plus uniformes et de plus longue durée.
(Traduit par Docteur Serge Messier)
Introduction
Bovine respiratory disease (BRD) is one of the most common diseases affecting cattle in the United States and is an important cause of economic losses in cattle operations worldwide (1,2). Viral respiratory pathogens such as bovine viral diarrhea virus 1 (BVDV-1), bovine viral diarrhea virus 2 (BVDV-2), bovine respiratory syncytial virus (BRSV), bovine herpesvirus 1 (BHV-1), and bovine parainfluenza virus 3 (BPIV-3) play an important role in the pathogenesis of BRD because of their ability to impair the integrity of the upper respiratory tract, cause immunosuppression, promote secondary bacterial infection, and cause acute clinical disease (3–6). Antibodies against respiratory viruses transmitted through maternal colostrum protect calves against acute BRD (4,7,8), and calves with high serum titers of antibodies against bovine viral diarrhea viruses, BRSV, BHV-1, and BPIV-3 on arrival at the feedlot have a lower risk of BRD as evidenced by lower morbidity rates and reduced frequency of treatment (8,9). As maternally derived immunity decays, calves become susceptible to acute bovine viral diarrhea (10). The duration of maternally derived immunity to respiratory viruses in the calf is directly proportional to the serum level of antibodies to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 ingested and absorbed from colostrum (11,12): the higher the levels, the longer the duration (11). However, previous research has shown a large range of initial serum antibody titers in calves that receive maternal colostrum (13,14) and, as a consequence, variability in the age at which calves become susceptible to acute BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 infections (11,13).
Provision of high-quality colostrum to neonatal calves in a timely manner is a key management practice in preventing the failure of passive transfer of immunoglobulins (FPT); however, maternal colostrum may not be consistently available. As an alternative, commercial colostrum-replacement products may provide adequate passive immunity while reducing the risk of infection with colostrum-transmitted pathogens (15–17). When such a product is used, a minimum of 100 g of IgG is recommended to achieve acceptable levels of serum immunoglobulins, and a greater mass of IgG (150 to 200 g) increases passive immunity and more reliably prevents FPT in calves (15,16). The ability of colostrum-replacement products to provide calves with adequate levels of antibodies against BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 and the duration of the replacement-derived immunity have not been described. The objective of this study was to compare the serum levels and duration of detection of antibodies to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 in neonatal calves that received maternal colostrum or a colostrum-replacement product at birth.
Materials and methods
Calves and experimental treatment
Forty newborn male Holstein calves from 3 dairy farms local to Auburn, Alabama, USA were used in this study. Pertinent management practices at 2 of the dairies included prebreeding vaccination of heifers with a modified live-virus vaccine that included BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 antigens and vaccination of adult cows with a killed-virus vaccine that included the same antigens. One dairy farm did not vaccinate cattle against these viruses. The calves were removed from their dams immediately after birth and strategically assigned to a colostrum-replacement (CR) group or a maternal-colostrum (MC) group. All 16 calves born on the farm that did not vaccinate against BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 were assigned to the CR group. Strategic assignment was necessary to ensure the presence of virus antibody in the serum of calves from the nonvaccinated herd after colostrum intake. Four calves from the vaccinated herds were randomly assigned to the CR group and 20 were assigned to the MC group.
Group CR received 2 packets of a colostrum-replacement product (Land O’Lakes Animal Milk Products, Shoreview, Minnesota, USA) providing 100 g of IgG per packet. Group MC received 3.8 L of fresh or frozen maternal colostrum. The colostrum-replacement product was not reconstituted according to the label directions; instead, a larger water volume, 1.5 L, was used to reconstitute each packet so that the volumes of colostrum given to all the calves were the same. The maternal colostrum was evaluated with a colostrometer (Biogenics, Mapleton, Oregon, USA). If the specific gravity was 1.055 or greater the calf received the maternal colostrum from the dam. If the specific gravity was less than 1.055 the calf received stored frozen colostrum collected from cows of the same herd that had a higher specific gravity. Nine calves in the MC group received stored frozen colostrum. Maternal colostrum or colostrum replacement was administered in a single feeding by bottle within the first 2 h of life. If the calf did not take the bottle during the first 10 min of feeding, an esophageal feeder was used. Seven calves in the CR group and 11 calves in the MC group required an esophageal feeder.
After the colostrum feeding, all the calves were moved to individual pens in a calf isolation barn at the Animal Health Research facilities at Auburn University, Auburn, Alabama, USA. This barn provided biosecurity to prevent exposure to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 during the trial. After 12 h of life the calves were examined and fed 1.8 L of a commercial milk replacer (Land O’Lakes Animal Milk Products) twice daily until weaned, between 6 and 8 wk of age. A calf starter, hay, and free-choice water were offered to all the calves from 1 wk of age until weaning. Once weaned, all the calves were moved to a biosecure paddock at the Animal Health Research facilities, where they had access to free-choice hay, water, and a calf-grower grain mix. During the study period the calves were evaluated once daily and were cared for under the guidelines of Auburn University’s Institutional Animal Care and Use Committee (PRN 2009-1647).
Sample testing
Blood samples were collected from each calf before the colostrum feeding for isolation of BVDV-1 and BVDV-2 and testing for antibodies to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3. Serum titers of antibodies against BVDV-1 and BVDV-2 were measured by virus neutralization (VN), whereas titers of antibodies against BRSV, BHV-1, and BPIV-3 were measured by indirect enzyme-linked immunosorbent assay (ELISA). Any calf with positive results was to be removed from the study. Additionally, the total serum IgG concentration was measured by single radial immunodiffusion in the serum from these samples and from those obtained when the calves were 2 d old. Failure of passive transfer of immunoglobulins was defined as a total serum IgG concentration of less than 10 g/L at 2 d of age (18). Samples of the maternal colostrum and the colostrum-replacement product were evaluated for IgG concentration, and the apparent efficiency of absorption (AEA) of IgG was calculated with the following formula: serum IgG concentration at 2 d of age (g/L) × plasma volume (L) ÷ total IgG intake (g) (19,20). The plasma volume was estimated as 9.9% of the body weight. Blood samples were collected from all the calves for virus antibody testing at 2 and 7 d of age and monthly until the calves became seronegative for each virus or until 8 mo of age.
Virus neutralization testing
The standard VN microtiter assay was used to detect serum antibodies against BVDV-1 and BVDV-2 (21). The BVDV-1 cytopathic strain NADL and the BVDV-2 cytopathic strain 125c were used (21). Briefly, after heat inactivation at 56°C for 30 min, serial 2-fold dilutions (1:2 to 1:4096) of serum were made in 50 μL of culture medium. For each dilution, 3 wells of a 96-well polystyrene microtiter plate (Immulon 4HBX; Thermo Electron Corporation, Milford, Massachusetts, USA) were inoculated with an equal volume (50 μL) of culture medium containing a median tissue culture infective dose (TCID50) of the test strain of 100 to 500 per milliliter. After inoculation, the plate was incubated at 38.5°C in a humidified atmosphere of 5% CO2 and air for 1 h. Then 2.5 × 103 Madin–Darby bovine kidney (MDBK) cells in 50 μL of culture medium were added to each well. The plate was incubated for 72 h and evaluated visually for a cytopathic effect (21,22). Mean log2 antibody titers were calculated from the endpoint titers for the animals in each group. Seronegativity to BVDV-1 and BVDV-2 was defined as a serum antibody titer less than 2, which equates to a log2 antibody titer of 0. The mean time to reach seronegative status with respect to the 2 viruses and the proportion of calves with a serum titer ≤ 1:16 or a mean log2 antibody titer ≤ 4 per time point were calculated and compared between the groups. These levels have been associated with an increased susceptibility to acute BVDV infection (23).
Indirect ELISA
This assay for antibodies to BRSV, BHV-1, and BPIV-3 was done as previously described (24,25). Briefly, 96-well Immulon 4HBX microtiter plates were coated with BRSV, BHV-1, and BPIV-3 antigen diluted in carbonate buffer (pH 9.6) in alternating rows with uninfected cell lysate as a control and incubated overnight at 4°C, then the plates were washed and blocked with 0.2% gelatin (Sigma Chemical Company, St. Louis, Missouri, USA) in carbonate buffer. Serum was added to the wells at a dilution of 1:50 (BRSV) or 1:200 (BHV-1 and BPIV-3), and the plates were incubated at 37°C for 1 h. Positive and negative control serum was included in each plate. The plates were washed, and then a 1:5000 dilution of protein G conjugated with horseradish peroxidase (Zymed, San Francisco, California, USA) was added. Single-component 2,2′-azino-di(3-ethyl-benzthiazoline-6-sulfonate (Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA) was used as the enzyme substrate. Sample and antibody dilutions were made in ELISA working buffer (0.01 M phosphate buffer, pH 7.2, with 0.75 M NaCl and 0.05% Tween 20 with 0.2% gelatin). Standard positive-control serum samples were obtained from cattle with high titers of neutralizing antibody against BHV-1 and BPIV-3. Convalescent serum from an unvaccinated calf with naturally occurring BRSV infection was used as a positive control for BRSV. Fetal bovine serum (FBS) was used as a negative control for all the serum samples. All ELISA results were calibrated against the results for their respective standard positive controls and the negative control to give uniformity to the results over the time that the tests were done. Optical density (OD) values were measured with a Benchmark microplate reader (Bio-Rad Laboratories, Mississauga, Ontario) at 492 nm and converted to ELISA units (EU) with a software program (Microplate Manager, version 5.0.1; Bio-Rad Laboratories). The net OD value of the sample was calculated by subtracting the mean sample OD values of the wells coated with the cell control from the mean sample OD values of the wells coated with BRSV, BHV-1, and BPIV-3 antigen. The net OD values were similarly calculated for the positive-control and negative-control serum samples (24). Final serum results were expressed in EU as follows: (mean net sample OD — mean net negative-control OD) ÷ (mean net positive-control OD — mean net negative-control OD) × 100. The results for each virus were classified as positive or negative according to whether the ELISA reactivity was greater or less than 10 EU, which is equivalent to a serum neutralizing-antibody titer of 1:3 for the 3 viruses (26). The mean time to reach seronegative status with respect to the 3 viruses and the proportion of seronegative calves per time period were calculated and compared between the groups.
Single radial immunodiffusion
Undiluted serum samples obtained before colostrum feeding, serum samples diluted 1:4 obtained after colostrum feeding, maternal colostrum diluted 1:15, and colostrum-replacement samples diluted 1:15 were assayed for total IgG concentration by single radial immunodiffusion, essentially as previously described (20). Antiserum against bovine IgG (heavy and light chains; Jackson Laboratories, West Grove, Pennsylvania, USA) was used. Immunodiffusion plates were prepared from 2% agarose containing 2.5% antiserum in phosphate-buffered saline, pH 7.25. Standard curves (1.06 to 8.5 g/L) were produced by means of duplicate samples of a bovine IgG serum calibrator (Midland BioProducts Corporation, Boone, Iowa, USA). Validity was assessed with a reference serum from the Center for Veterinary Biologics, Animal and Plant Health Inspection Service, United States Department of Agriculture, Ames, Iowa. All samples were tested in triplicate and incubated in a humid atmosphere at 25°C for 18 to 24 h. Ring diameters were measured with a computer-assisted plate reader (The Binding Site Group, Birmingham, England) and the values for the samples calculated with a program for linear analysis.
Statistical analysis
All statistics were calculated with SAS software (SAS Institute, Cary, North Carolina, USA) with an α level of 0.05. To detect the decay in serum antibody titer over time after the intake of maternal colostrum or colostrum replacement, the mean titer of antibody against each virus measured at 2 d of life was compared with the mean titer at 7 d and at 1, 2, 3, 4, 5, 6, 7, and 8 mo of life by repeated-measures analysis in a mixed generalized linear model. Analysis of variance (ANOVA) was done to compare the mean time to reach seronegative status among treatment groups with the use of a generalized linear model. Since the antibody data did not have a normal distribution and the variances were not homogeneous between the groups, a nonparametric 1-way ANOVA (Kruskal–Wallis test) was done to compare the mean serum antibody levels for each virus at each time point and to compare the serum and colostrum or replacement IgG concentrations among the treatment groups. To compare variation in antibody values between the groups, extremes of antibody titers were determined and coefficients of variation (CV) calculated at 2 d, 2 mo, and 5 mo of age. The Levene test was used to statistically compare the extent of variation of antibody levels within the MC and CR groups. Logistic regression (PROC LOGISTIC) was used to compare the proportion of calves reaching seronegative status with respect to each virus at each time point between the treatment groups, and significant differences were determined by the chi-squared test.
Results
Before the calves were fed maternal colostrum or the colostrum-replacement product, IgG was not detectable in the serum. The mean total mass of IgG administered by feeding was higher with maternal colostrum than with colostrum replacement, at 277.4 versus 208.5 g (P = 0.0089). The calves in the MC group had higher mean concentrations of serum IgG at 2 d of age than the calves in the CR group: 20.65 versus 12.41 g/L (P = 0.021). There were no significant differences in serum IgG concentration at 2 d of age between the calves fed with a bottle and those fed with an esophageal feeder within the 2 groups (P = 0.098). The AEA at 2 d of age was higher, but not significantly so (P = 0.2238), in the MC group than in the CR group, at 27.21% versus 23.40%.
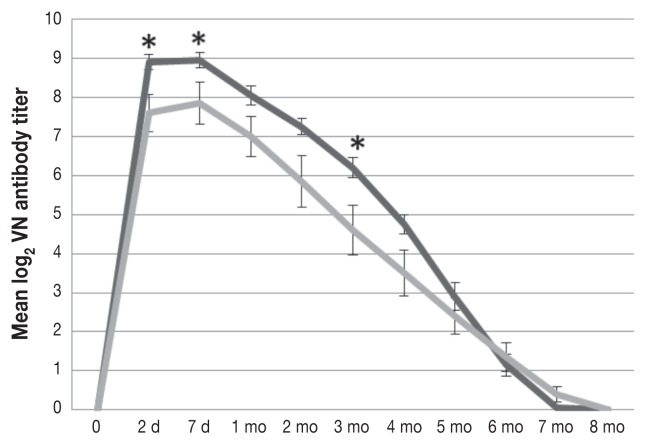
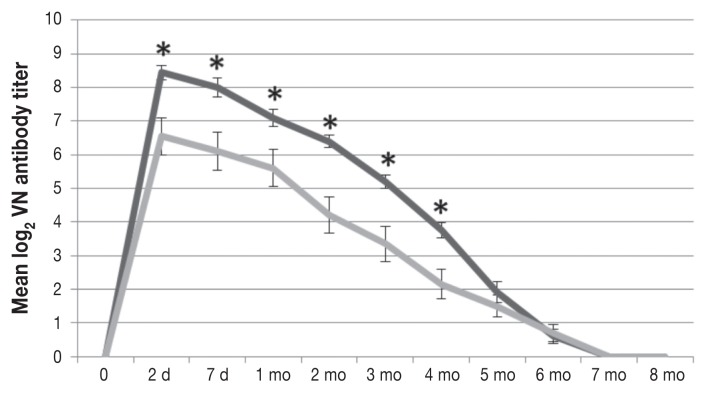
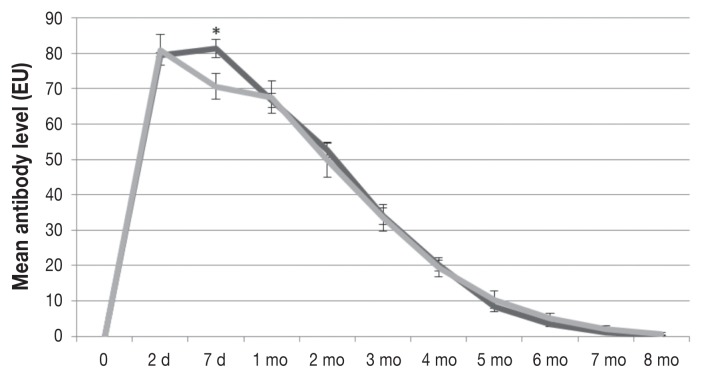
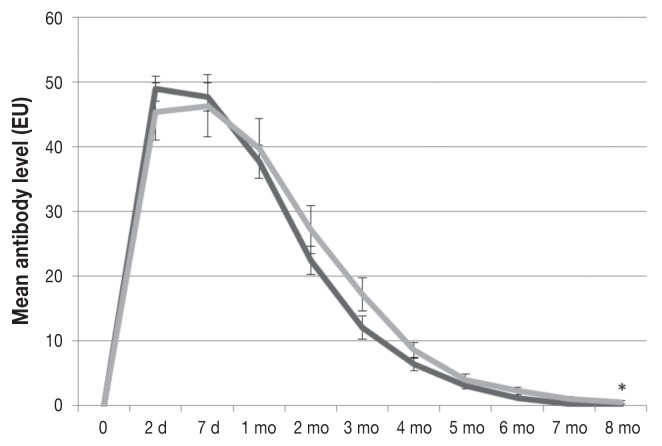
All the calves were seronegative for all the test viruses before colostrum intake. The CR group had mean BVDV-1 and BVDV-2 antibody titers that were significantly greater than those in the MC group at 2 d, 7 d, and 3 mo of age (Figure 1) and at 2 d, 7 d, 1 mo, 2 mo, 3 mo, and 4 mo of age (Figure 2), respectively. For BRSV the mean antibody level was significantly higher at 7 d of age in the CR group than in the MC group (81.3 versus 70.65 EU; P = 0.0384); however, significant differences were not observed at the other time points (Figure 3). The mean BHV-1 antibody level was significantly higher at 8 mo of age in the MC group than in the CR group (0.47 versus 0.0 EU; P = 0.0328); however, additional significant differences were not observed (Figure 4). For BPIV-3, statistically significant differences were not observed between the groups at any time point.
Figure 1.
Titers of virus-neutralizing (VN) antibody [mean ± standard error (SEM)] against bovine viral diarrhea virus 1 (BVDV-1) in calves fed a colostrum replacement (CR; darker grey) or maternal colostrum (MC) within the first 2 h of life. The mean log2 titers were significantly higher in the CR group than in the MC group at 2 d (P = 0.0194), 7 d (P = 0.05), and 3 mo of age (P = 0.0427). The decay rate was similar in the 2 groups.
Figure 2.
Titers of VN antibody against bovine viral diarrhea virus 2 (BVDV-2) in the same groups of calves. The mean log2 titers were significantly higher in the CR group than in the MC group at 2 d (P = 0.0119), 7 d (P = 0.0074), 1 mo (P = 0.05), 2 mo (P = 0.0024), 3 mo (P = 0.0098), and 4 mo of age (P = 0.0107). The decay rate was similar in the 2 groups.
Figure 3.
Levels of antibody against bovine respiratory syncytial virus in the same groups of calves, as measured by enzyme-linked immunosorbent assay (ELISA). The mean level, in ELISA units (EU), ± SEM was significantly higher in the CR group than in the MC group at 7 days of age (P = 0.0384). The decay rate was similar in the 2 groups.
Figure 4.
Levels of antibody against bovine herpesvirus 1 in the same groups of calves, as measured by ELISA. The mean level was significantly higher in the MC group than in the CR group at 8 mo of age (P = 0.032). The decay rate was similar in the 2 groups.
Table I shows the variation in virus-antibody levels between the groups at 2 d, 2 mo, and 5 mo of age. For BVDV-1, BVDV-2, BRSV, and BHV-1 the variation was significantly greater (P < 0.05) in the MC group than in the CR group at 2 d and 2 mo of age, whereas for BPIV-3 the variation was significantly greater (P = 0.0178) in the MC group than in the CR group only at 2 mo of age. At 5 mo of age there were no significant differences in variation between the groups.
Table I.
Variation in levels of antibody against respiratory viruses and their respective coefficients of variation in groups of calves fed a colostrum-replacement product or maternal colostrum within the first 2 h of life
| Treatment; measures of variation | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Colostrum replacement | Maternal colostrum | |||||
|
|
|
|||||
| Virus | Time pointa | Extremesb | CVc (%) | Extremesb | CVc (%) | P-valued |
| BVDV-1 | 2 d | 8, 10 | 9.55 | 5, 11 | 28.03 | 0.0084 |
| 2 mo | 6, 9 | 12.55 | 1, 11 | 51.11 | 0.0003 | |
| 5 mo | 0, 5 | 55.52 | 0, 5 | 87.92 | 0.0611 | |
| BVDV-2 | 2 d | 7, 10 | 11.12 | 2, 11 | 37.40 | 0.0028 |
| 2 mo | 5, 8 | 13.75 | 0, 7 | 57.14 | 0.0003 | |
| 5 mo | 0, 4 | 74.21 | 0, 4 | > 100 | 0.6535 | |
| BRSV | 2 d | 74, 84 | 3.50 | 26, 119 | 24.98 | 0.0269 |
| 2 mo | 45, 70 | 17.59 | 3, 97 | 45.32 | 0.0118 | |
| 5 mo | 0, 21 | 78.46 | 0, 44 | > 100 | 0.1802 | |
| BHV-1 | 2 d | 33, 71 | 17.59 | 11, 85 | 43.49 | 0.0002 |
| 2 mo | 8, 41 | 44.42 | 0, 52 | 61.48 | 0.0069 | |
| 5 mo | 0, 3 | > 100 | 0, 9 | > 100 | 0.8752 | |
| BPIV-3 | 2 d | 83, 187 | 20.06 | 22, 168 | 34.19 | 0.3416 |
| 2 mo | 28, 134 | 35.72 | 4, 128 | 55.88 | 0.0178 | |
| 5 mo | 0, 23 | > 100 | 0, 37 | > 100 | 0.2659 | |
The 2-d time point represents the time the first sample was obtained after administration of the colostrum-replacement product or maternal colostrum.
Extremes of log2 antibody titer for bovine viral diarrhea virus 1 (BVDV-1) and bovine viral diarrhea virus 2 (BVDV-2) and of enzyme-linked immunosorbent assay units for bovine respiratory syncytial virus (BRSV), bovine herpesvirus 1 (BHV-1), and bovine parainfluenza virus 3 (BPIV-3).
Coefficient of variation (CV = σ/μ × 100) of the mean levels of antibody against each virus at each time point.
Based on the Levene test for homogeneity of variances.
The duration of passively acquired immunity, expressed as the mean time to reach seronegative status with respect to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3, did not differ significantly between the CR and MC groups (Table II). Significant decay of antibody titers was observed after 1 mo of age for each virus in both groups, and the decay rate was similar in the 2 groups. The calves in the MC group reached serum titers of antibody against BVDV-1 and BVDV-2 of ≤ 1:16 (log2 ≤ 4) in greater proportions at each time point compared with the calves in the CR group (Figures 5 and 6, respectively). However, significant differences between the groups were observed only at 2 and 3 mo of age for BVDV-1 and at 7 d, 1 mo, 2 mo, 3 mo, 4 mo, and 5 mo of age for BVDV-2 (P < 0.05). Similarly, for BRSV and BHV-1 the calves in the MC group started to become seronegative in greater proportions at each time point compared with the CR calves; however, significant differences were observed only at 4 mo of age (P < 0.05). For BPIV-3 the proportion of calves becoming seronegative at each time point did not differ significantly between the groups.
Table II.
Mean time for the 2 groups of calves to reach seronegative status with respect to BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3
| Treatment; mean time ± standard deviation (mo) | ||
|---|---|---|
|
|
||
| Virus | Colostrum replacement | Maternal colostrum |
| BVDV-1 | 6.1 ± 0.7 | 5.5 ± 1.7 |
| BVDV-2 | 6.5 ± 0.6 | 6.1 ± 1.6 |
| BRSV | 5.5 ± 0.6 | 5.2 ± 1.5 |
| BHV-1 | 3.75 ± 0.8 | 3.8 ± 1.6 |
| BPIV-3 | 5.1 ± 0.8 | 4.9 ± 1.66 |
Figure 5.
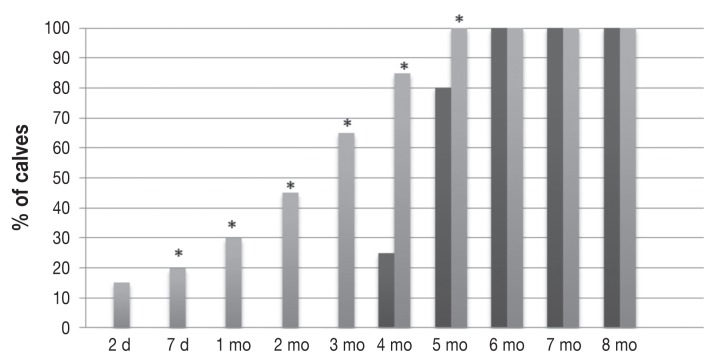
Proportions of calves with a serum titer ≤ 1:16 or a mean log2 titer ≤ 4 of antibody against BVDV-1 in the same groups of calves. These antibody levels started to be reached earlier and in greater proportions of the calves per time period in the MC group (lighter grey bars) compared with the CR group; the differences between the groups were significant only at 2 and 3 mo of age.
Figure 6.
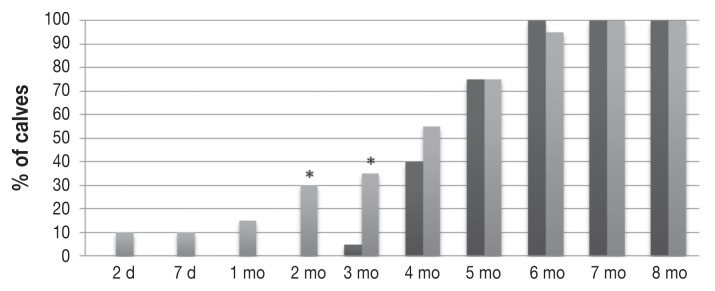
Proportions of calves with a serum titer ≤ 1:16 or a mean log2 titer ≤ 4 of antibody against BVDV-2 in the same groups of calves. These antibody levels started to be reached earlier and in greater proportions of the calves per time period in the MC group (lighter grey bars) compared with the CR group; the differences between the groups were significant at 7 d, 1 mo, 2 mo, 3 mo, 4 mo, and 5 mo of age.
Discussion
This is the first report demonstrating the efficacy of a colostrum-replacement product in providing neonatal calves with adequate serum levels of antibodies against common respiratory viruses, including BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3. Two doses of the product used in this study provided serum levels of virusspecific antibodies that were similar to those in calves that received 3.8 L of good-quality maternal colostrum (11,13). In addition, the calves that received the replacement product had greater mean serum titers of antibody against BVDV-1 and BVDV-2 during the first 4 mo of life compared with the calves that receive maternal colostrum. Higher mean concentrations of BVDV-specific antibodies in the colostrum-replacement product and low BVDV-specific antibody concentrations in the maternal colostrum could have contributed to the greater titers of antibody against BVDV-1 and BVDV-2 observed in the CR group. The concentrations of BVDV-specific antibodies in maternal colostrum could have been influenced by factors such as the degree of exposure of cattle within the herd to field strains of BVDV, the herd vaccination program, the frequency of vaccination, the response to vaccination, and the ability to transfer specific antibodies to colostrum (11,14,27). The levels of antibodies in the replacement product, in contrast, would be expected to be much more uniform given that the product is created from pools of large numbers of individual collections of colostrum that are selected according to IgG concentration. However, the CR group received twice the amount of colostrum-replacement product recommended by the manufacturer, which may suggest that a single dose of the product could potentially be inadequate to provide calves with antibody levels similar to those found in this study.
There was greater variability in the levels of antibody against BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 among the calves in the MC group compared with the calves in the CR group during the first 2 mo of life. A wide range of levels of antibodies to respiratory viruses has been reported in calves after maternal colostrum intake (12,13). These levels have a direct effect on the duration of colostrum-derived immunity, as calves with low initial levels become seronegative earlier in life (11,12). Factors such as diversity of maternal colostrum sources, differences in concentration of virus-specific antibodies in maternal colostrum, and prevalence of FPT influence the levels of colostrum-derived antibodies in calves fed maternal colostrum. However, the mean serum IgG concentrations at 2 d of life in both groups in this study were above the reported concentration associated with FPT in calves (< 10 g/L) (18); therefore, FPT was unlikely as a factor contributing to the variability in antibody levels observed in the MC group. The lower AEA observed in the CR group compared with the MC group and compared with results of the use of this product in previous studies (15,16) could have been due to the use of more water when reconstituting the product than the manufacturer recommended.
Several studies have reported the duration of detection of maternal colostrum-derived antibodies against BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 as the mean time to reach seronegativity for each virus (11,13). The longevity of passively acquired immunity in calves that receive maternal colostrum at birth is highly variable (12,14,26,27). One study estimated the mean time to reach seronegativity for respiratory viruses in a group of calves that had received maternal colostrum as being 117.7 ± 37.7 d for BVDV-1, 93.9 ± 61.9 d for BVDV-2, 200.2 ± 116.7 d for BRSV, 65.1 ± 37.8 d for BHV-1, and 183.8 ± 100.0 d for BPIV-3 (14). Another study found the mean duration of colostrum-derived antibodies in beef calves to be 185.6 ± 59.8 d for BVDV-1, 157.8 ± 56.1 d for BVDV-2, 183.7 ± 33 d for BRSV, 122.9 ± 46.6 d for BHV-1, and 190.6 ± 58.3 d for BPIV-3 (12). In the present study the mean time to reach seronegativity for BVDV-1, BVDV-2, BRSV, BHV-1, and BPIV-3 varied with the virus but was similar for each virus between the groups. However, the variability in duration was greater in the MC group than in the CR group: although the standard deviation for time to seronegativity ranged from 1.5 mo (45 d) to 1.7 mo (53 d) in the MC group, it ranged from only 0.6 mo (18 d) to 0.8 mo (24 d) in the CR group. When the proportion of calves becoming seronegative for the various viruses per time period was evaluated, greater proportions of calves in the MC group than in the CR group were found to have reached serum titers of antibodies against BVDV-1 and BVDV-2 of ≤ 1:16 (log2 ≤ 4), a level associated with an increased susceptibility to acute BVDV infection (23), at several points during the first months of life. Additionally, at 4 mo of age a greater proportion of calves in the MC group than in the CR group had become seronegative to BRSV and BHV-1. Variable duration of colostrum-derived immunity and differences in the proportion of animals becoming seronegative for viral respiratory pathogens could result in poor calf-herd immunity and increase the risk of introduction of BVDV-1, BVDV-2, BRSV, and BHV-1 into the calf herd. Variability in the duration of colostrum-derived immunity against various respiratory viruses has been related to multiple factors, including differences in the rate of decay of colostrum-derived antibodies (11,14,26,27), which is usually influenced by active viral infections or vaccination. The decay rate of passively derived neutralizing antibodies in this study was similar in the 2 groups. The calves were not vaccinated at any point, and seroconversion was not observed to any of the viruses, so an active viral infection was unlikely. The variation in the levels of colostrum-derived antibodies observed during the first 2 mo of life in the calves in the MC group was likely responsible for the variation in the times to reach seronegative status within this group.
The results of this study indicate that calves that receive timely and adequate amounts of high-quality maternal colostrum at birth absorb amounts of IgG into the blood that are well above the threshold defined for FPT but still demonstrate highly variable levels of immunity to individual respiratory viruses, including BVDV-1, BVDV-2, BRSV, and BHV-1. This results in variable times at which calves become seronegative for the viral pathogens, which could increase the risk of acute disease. In contrast to 3.8 L of maternal colostrum, 2 doses of the colostrum-replacement product provided calves with adequate passive transfer and less variable levels of passive immunity to BVDV-1, BVDV-2, BRSV, and BHV-1, which may have resulted in the greater uniformity in the time at which the calves became seronegative for the viral pathogens. The present study did not attempt to evaluate the ability of passive immunity derived from maternal colostrum or a colostrum-replacement product to protect calves against challenge with virulent strains of respiratory viruses or to determine the antibody levels associated with protection against each virus. Nevertheless, from the results it is reasonable to suggest that, overall, calves fed this product will have longer immunity compared with calves fed the more variable maternal colostrum, and therefore programs of vaccination against respiratory viruses should start earlier in calves that receive maternal colostrum, as the calves could become seronegative for those pathogens earlier in life. The more uniform time to seronegativity suggests that it should be possible to better predict the optimum time to vaccinate calves fed the replacement product used in this study compared with maternal colostrum, and this should be the subject of additional study.
References
- 1.Griffin D. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract. 1992;13:367–377. doi: 10.1016/s0749-0720(15)30302-9. [DOI] [PubMed] [Google Scholar]
- 2.Stokka GL. Prevention of respiratory disease in cow/calf operations. Vet Clin North Am Food Anim Pract. 2010;26:229–241. doi: 10.1016/j.cvfa.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis JA, Gow SP, Goji N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J Am Vet Med Assoc. 2010;236:991–999. doi: 10.2460/javma.236.9.991. [DOI] [PubMed] [Google Scholar]
- 4.Martin SW, Nagy E, Armstrong D, Rosendal S. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can Vet J. 1999;40:560–567. 570. [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor A, Martin SW, Nagy E, Menzies P, Harland R. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can J Vet Res. 2001;65:137–142. [PMC free article] [PubMed] [Google Scholar]
- 6.Walz PH, Grooms DL, Passler T, et al. Control of bovine viral diarrhea virus in ruminants. J Vet Intern Med. 2010;24:476–486. doi: 10.1111/j.1939-1676.2010.0502.x. [DOI] [PubMed] [Google Scholar]
- 7.Moerman A, Straver PJ, de Jong MC, Quak J, Baanvinger T, van Oirschot JT. Clinical consequences of a bovine virus diarrhoea virus infection in a dairy herd: A longitudinal study. Vet Q. 1994;16:115–119. doi: 10.1080/01652176.1994.9694430. [DOI] [PubMed] [Google Scholar]
- 8.Fulton RW, Cook BJ, Step DL, et al. Evaluation of health status of calves and the impact on feedlot performance: Assessment of a retained ownership program for postweaning calves. Can J Vet Res. 2002;66:173–180. [PMC free article] [PubMed] [Google Scholar]
- 9.Fulton RW, Cook BJ, Blood KS, et al. Immune response to bovine respiratory disease vaccine immunogens in calves at entry to feedlot and impact on feedlot performance. Bov Practitioner. 2011;45:1–12. [Google Scholar]
- 10.Ridpath J, Neill J, Endsley J, et al. Effect of passive immunity on the development of a protective immune response against bovine viral diarrhea virus in calves. Am J Vet Res. 2003;64:65–69. doi: 10.2460/ajvr.2003.64.65. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Zanzi C, Thurmond M, Johnson W, et al. Predicted ages of dairy calves when colostrum derived bovine viral diarrhea virus antibodies would no longer offer protection against disease or interfere with vaccination. J Vet Med Assoc. 2002;221:678–685. doi: 10.2460/javma.2002.221.678. [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick JG, Step DL, Payton ME, et al. Effect of age at the time of vaccination on antibody titers and feedlot performance in beef calves. J Am Vet Med Assoc. 2008;233:136–142. doi: 10.2460/javma.233.1.136. [DOI] [PubMed] [Google Scholar]
- 13.Fulton RW, Briggs RE, Payton ME, et al. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus, bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine. 2004;22:643–649. doi: 10.1016/j.vaccine.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick J, Fulton RW, Burge LJ, Dubois WR, Payton M. Passively transferred immunity in newborn calves, rate of antibody decay, and effect on subsequent vaccination with modified live virus vaccine. Bov Practitioner. 2001;35:47–54. [Google Scholar]
- 15.Godden SM, Haines DM, Hagman D. Improving passive transfer of immunoglobulins in calves. I: Dose effect of feeding a commercial colostrum replacer. J Dairy Sci. 2009;92:1750–1757. doi: 10.3168/jds.2008-1846. [DOI] [PubMed] [Google Scholar]
- 16.Godden SM, Haines DM, Konkol K, Peterson J. Improving passive transfer of immunoglobulins in calves. II: Interaction between feeding method and volume of colostrum fed. J Dairy Sci. 2009;92:1758–1764. doi: 10.3168/jds.2008-1847. [DOI] [PubMed] [Google Scholar]
- 17.Pithua P, Godden SM, Wells SJ, Oakes MJ. Efficacy of feeding plasma-derived commercial colostrum replacer for the prevention of transmission of Mycobacterium avium subsp. paratuberculosis in Holstein calves. J Am Vet Med Assoc. 2009;234:1167–1176. doi: 10.2460/javma.234.9.1167. [DOI] [PubMed] [Google Scholar]
- 18.Weaver DM, Tyler JW, VanMetre DC, Hostetler DE, Barrington GM. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med. 2000;14:569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Quigley JD, 3rd, Kost CJ, Wolfe TM. Absorption of protein and IgG in calves fed a colostrum supplement or replacer. J Dairy Sci. 2002;85:1243–1248. doi: 10.3168/jds.S0022-0302(02)74188-X. [DOI] [PubMed] [Google Scholar]
- 20.Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993;34:407–412. [PMC free article] [PubMed] [Google Scholar]
- 21.Walz PH, Givens MD, Cochran A, Navarre CB. Effect of dexamethasone administration on bulls with a localized testicular infection with bovine viral diarrhea virus. Can J Vet Res. 2008;72:56–62. [PMC free article] [PubMed] [Google Scholar]
- 22.Passler T, Walz PH, Ditchkoff SS, Walz HL, Givens MD, Brock KV. Evaluation of hunter-harvested white-tailed deer for evidence of bovine viral diarrhea virus infection in Alabama. J Vet Diagn Invest. 2008;20:79–82. doi: 10.1177/104063870802000116. [DOI] [PubMed] [Google Scholar]
- 23.Bolin SR, Ridpath JF. Differences in virulence between two non-cytopathic bovine viral diarrhea viruses in calves. Am J Vet Res. 1992;53:2157–2163. [PubMed] [Google Scholar]
- 24.Durham PJ, Sillars HM. Evaluation of an enzyme-linked immunosorbent assay (ELISA) for serodiagnosis of infectious bovine rhinotracheitis infection, with results of a preliminary survey. N Z Vet J. 1986;34:27–30. doi: 10.1080/00480169.1986.35266. [DOI] [PubMed] [Google Scholar]
- 25.Durham PJ, Hassard LE. Prevalence of antibodies to infectious bovine rhinotracheitis, parainfluenza 3, bovine respiratory syncytial, and bovine viral diarrhea viruses in cattle in Saskatchewan and Alberta. Can Vet J. 1990;31:815–820. [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Poel WHM, Midel WGJ, Schukken YH. Antibody titers against bovine respiratory syncytial virus in colostrum-fed dairy calves born in various seasons. Am J Vet Res. 1999;60:1098–1101. [PubMed] [Google Scholar]
- 27.Menateau-Horta AM, Ames TR, Johnson DW, Meiske JC. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine viral diarrhea vaccines. Can Comp Med. 1985;49:10–14. [PMC free article] [PubMed] [Google Scholar]