Abstract
Background. Rotarix (GlaxoSmithKline), a newly licensed rotavirus vaccine requiring 2 doses, may have the potential to save hundreds of thousands of lives in Africa. Nations such as Malawi, where Rotarix is currently under phase III investigation, may nevertheless face difficult economic choices in considering vaccine adoption.
Methods. The cost-effectiveness of implementing a Rotarix vaccine program in Malawi was estimated using published estimates of rotavirus burden, vaccine efficacy, and health care utilization and costs.
Results. With 49.5% vaccine efficacy, a Rotarix program could avert 2582 deaths annually. With GAVI Alliance cofinancing, adoption of Rotarix would be associated with a cost of $5.07 per disability-adjusted life-year averted. With market pricing, Rotarix would be associated with a base case cost of $74.73 per disability-adjusted life-year averted. Key variables influencing results were vaccine efficacy, under-2 rotavirus mortality, and program cost of administering each dose.
Conclusions. Adopting Rotarix would likely be highly cost-effective for Malawi, particularly with GAVI support. This finding holds true across uncertainty ranges for key variables, including efficacy, for which data are becoming available.
Rotavirus is one of the top causes of childhood mortality worldwide, leading to >600,000 annual deaths, the majority of which are in Africa and Asia [1]. The virus causes an acute, self-limited febrile gastroenteritis typically lasting 3–7 days [2]. Since 2006, 2 new vaccines, each with at least 80% efficacy against severe rotavirus-associated disease, have been developed and licensed by the US Food and Drug Administration [2]. Rotarix (GlaxoSmithKline), the more recent of these vaccines, is currently being evaluated in a phase III study in Malawi and South Africa. Preliminary results show that efficacy in Malawi may be ∼50% [3]. Unlike Rotateq (Merck), Rotarix requires 2 rather than 3 doses for a full course. This may be particularly important to developing nations that face difficulty in paying the program costs of administering vaccines.
This study determined the cost-effectiveness of adding Rotarix to the existing vaccination infrastructure in Malawi. Malawi is eligible for the highest degree of support from the GAVI Alliance for cofinancing the introduction of new vaccines. The GAVI Alliance price of $0.15 per dose (2008 US dollars) fundamentally changes the cost-effectiveness profile, compared with the projected market price of $5.50 (potential range, $1.00–$10.00) [4]. The goal of the GAVI Alliance is to provide several years of financial support, with vaccine costs then gradually transferred to the recipient nation. This analysis, therefore, examines the cost-effectiveness of rotavirus vaccination in Malawi with use of both the subsidized GAVI Alliance price and the market price.
Methods
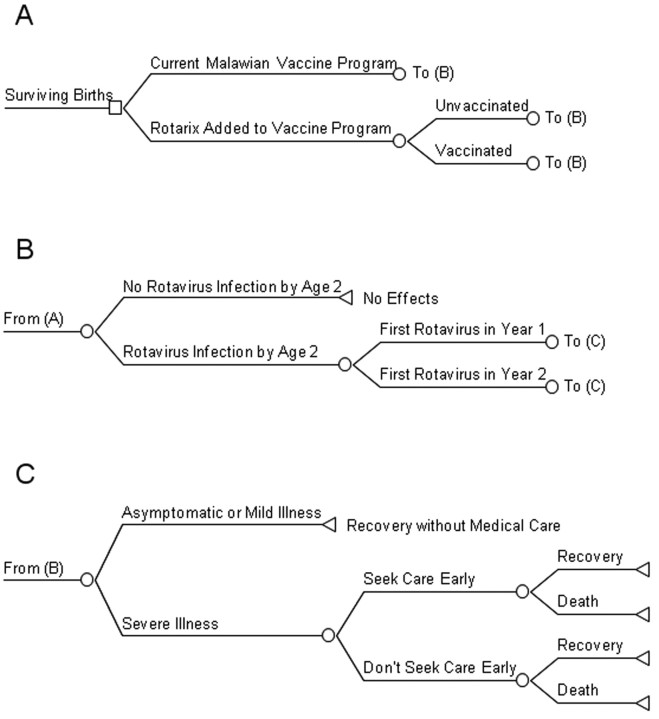
A 2-year time frame was chosen for examining the disease burden, because a study by Cunliffe et al [5] that comprised >1000 Malawian children with hospitalization or outpatient clinic visits for diarrhea found that 99% of rotavirus cases occurred within the first 2 years of life. Figure 1 illustrates the decision tree used in analysis. A birth cohort enters the tree and stays with the current Malawian vaccine program (ie, no rotavirus vaccination) or has a Rotarix vaccine program added. Infants will all be subjected to rotavirus infection or not, with some children having their first infection in their second year of life. Vaccination asserts its effect by changing the proportion of first rotavirus infection resulting in severe illness. Parents are assumed not to present their children for care for asymptomatic or mild illness, and some parents will seek care for severe illnesses. A portion of severe illnesses will result in death, and all others will result in full recovery. Although repeat infections occur, natural immunity acquired from the first infection tends to limit these to asymptomatic or mild cases only [6]. Costs and effects were modeled using TreeAge Pro 2009 and Excel 2003 software.
Figure 1.
Decision analysis tree for a rotavirus vaccination program.
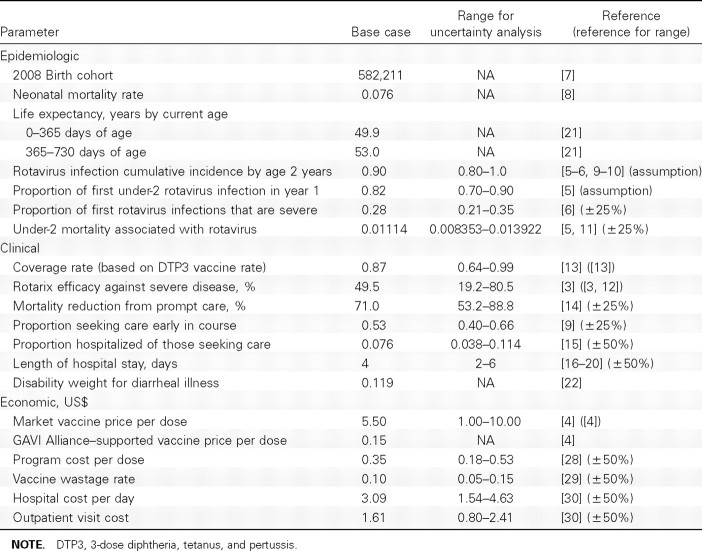
Epidemiologic variables considered in the analysis are listed in Table 1. All aggregate costs and effects were based on an estimated 2008 birth cohort of 582,211 [7], adjusted downward for neonatal mortality [8]. Rotavirus is very common in Malawi, with ∼90% of Malawian children infected at least once by their second birthday [5–6, 9–10]. In a rotavirus natural history study in Mexico by Velazquez et al [6], 28% of first rotavirus infections involved severe diarrhea. Cumulative under-5 rotavirus mortality was estimated by multiplying 0.00225, the World Health Organization (WHO) estimate of annual risk of death due to rotavirus among children <5 years if age in Malawi, by 5 years [11]. Cumulative under-2 mortality associated with rotavirus infection was extrapolated by multiplying cumulative under-5 rotavirus-associated mortality by 0.99 [5]. The range for uncertainty was estimated by multiplying the base case by ±25%.
Table 1.
Epidemiologic, Clinical, and Economic Variable Estimates
The efficacy of a full course of Rotarix against severe rotavirus was estimated to be 49.5% (95% confidence interval, 19.2%–68.3%) on the basis of 1-year preliminary results of an ongoing phase III study in Malawi [3]. The efficacy range for sensitivity analyses was estimated to be 19.2%–80.5%, with the upper bound coming from a completed 2-year phase III Rotarix trial in Latin America [12]. The vaccine coverage rate was estimated to be 87%, on the basis of mean reported Malawian 3-dose diphtheria, tetanus, and pertussis (DTP3) vaccine coverage rates during 1996–2007, with uncertainty bounds (64%-99%) defined by the range across these years [13].
For medical care to be successful in severe cases, it should be initiated early before significant hypovolemia or electrolyte deficiencies have occurred. A study of 760 Malawian infants assessed monthly through 1 year of age revealed that Western medical care was sought for 53% of diarrheal illnesses [9]. The effect of prompt medical care in reducing mortality was estimated from an analysis of 14 studies from developing nations on the reduction in diarrheal mortality after the institution of aggressive oral rehydration therapy [14]. All children with severe diarrhea who were brought to care were assumed to receive outpatient treatment and an evaluation of the need for hospitalization. The portion of children subsequently hospitalized was estimated from a systematic review of the burden of rotavirus in developing nations in which the ratio of rotavirus hospitalizations to rotavirus outpatient visits was 1.9:23 [15]. Mean length of hospital stay was estimated to be 4 days on the basis of 5 reports from developed nations where this parameter ranged from 3.2 to 4.8 days [16–20]. Because of the lack of data from sub-Saharan Africa, this parameter was subjected to a wide potential range (±50%) in sensitivity analyses.
Disability-adjusted life-years (DALYs) were estimated using 2006 WHO life expectancy data [21] with use of age weighting. The disability weight for children <5 years of age from “The Global Burden of Disease” was used to calculate years lost because of disability, with the mean duration of diarrhea of 5 days [2, 22]. Survivors of both severe and mild infections are not known to have any long-term disability resulting from infection [2]. DALYs were calculated in the base case, with 3% annual discounting of future costs and effects, in accordance with WHO convention [23]. Discounting future costs reflects the opportunity to invest present capital. Discounting future health effects reflects a time preference for immediate rather than postponed benefits. Because discounting is controversial [24, 25], a common approach (used in our study) is to generate comparative results without it [26].
All costs were estimated in 2008 US dollars with use of International Monetary Fund inflation rates [27]. Vaccination costs per child are composed of the vaccine price and program costs to administer the vaccine. Program costs (Table 1) are the incremental costs of adding 2 Rotarix doses to the existing vaccine delivery infrastructure for DTP3 vaccine in Malawi. Because the vaccine would be delivered with the existing DTP vaccine schedule, no additional visits would be required by children, resulting in no additional costs to households. Packed vaccines need to be refrigerated, transported, and stored at local sites before reconstitution and oral administration. Cold chain, transportation, personnel time, and stationery cost data were available for delivery of measles vaccines in Zambia [28]. The cold chain and transportation costs were multiplied by 3.83 (ie, the number of times a single dose of Rotarix is greater in packed volume compared with a single dose of measles vaccine) [29]. Because of the oral method of delivery, no costs were included for needles, safe boxes, or staff training. A vaccine wastage rate of 10% was estimated because of single-dose packaging with oral delivery [29]. Program cost and wastage rate uncertainty bounds were assumed to be ±50% of base case.
The estimated cost of an outpatient visit was $1.61. This was derived from the 2005 WHO estimated local currency cost of a 20-min visit to a health care center with 50% population coverage, inflated to the 2008 local value and then converted to US dollars [27, 30]. Cost per bed-day for hospitalization was derived from the mean cost of primary, secondary, and tertiary hospital stays and was similarly converted to 2008 US dollars [30]. Neither health care center visit nor hospitalization cost estimates included diagnostics and treatments and are thus likely to be underestimates. The degree of underestimation may be small, however, because specific diagnostics are not likely to be frequently available for diarrhea in Malawi, and fluid resuscitation, the mainstay of treatment, is relatively inexpensive. Because of the inherent uncertainty, ranges for hospital and outpatient costs were estimated to be ±50% of the base case. On the basis of the authors' experience in Malawi, many children with severe diarrhea who are dying and have not previously been brought to medical attention will be brought to medical facilities at or just before the time of death. The cost of a 1-day hospital stay was therefore added to each death that occurred among children whose parents did not seek care early in the disease course.
A 1-way sensitivity analysis is the range of incremental costeffectiveness ratio (ICER) results created by varying a single input parameter throughout its range of possible values while keeping all other parameters at their base case. Tornado diagrams display several 1-way sensitivity analyses on the same axis, starting with the most influential parameter at the top and moving down in order of importance [31]. These were calculated separately for GAVI Alliance and market price ICERs. Multiway sensitivity analyses consist of a set of ICER results created by varying >1 parameter simultaneously in the model.
ICER results from the base case and sensitivity analyses were compared with a willingness-to-pay threshold of 1 times the per capita Malawi gross domestic product (GDP) per DALY averted. The WHO generally considers interventions that cost 1–3 times the per capita GDP per DALY averted to represent good value for money and labels them cost-effective [23, 32]. Interventions that cost <1 times the per capita GDP are considered to be very cost-effective.
Results
With use of GAVI Alliance pricing, a Rotarix program would cost $482,069 per year (Table 2). With use of market pricing, the cost would be nearly $6 million per year. Currently, the Malawi health care system spends an estimated $187,211 on rotavirus treatment in the form of outpatient and inpatient clinical care. A Rotarix program would be expected to avert an estimated 58,380 severe cases, which would normally result in 28,590 outpatient visits and 11,354 hospital bed-days. Therefore, annual treatment costs would be expected to decrease by $80,748 (Table 2).
Table 2.
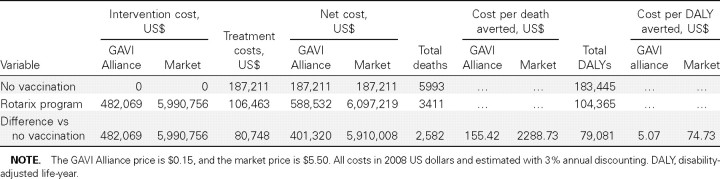
Costs and Outcomes from a Rotarix Vaccination Program under GAVI Alliance and Market Vaccine Pricing
An estimated 5993 deaths due to rotavirus infection currently occur each year among children <2 years of age in Malawi (Table 2). A Rotarix program would be expected to avert 2582 deaths annually. As anticipated, with no long-term complications from illness, years of life lost because of death contribute the vast majority (99.9%) of DALYs attributable to rotavirus infection. A Rotarix program would be expected to avert 79,081 DALYs.
With use of GAVI Alliance pricing, a Rotarix program would be associated with an ICER of $5.07 per DALY averted. With use of market pricing, the estimated ICER would be $74.73 per DALY averted. Without 3% annual discounting, costs were negligibly different, but each case of rotavirus infection generated almost twice the number of DALYs. The market price ICER with no annual discounting would be $36.23 per DALY averted.
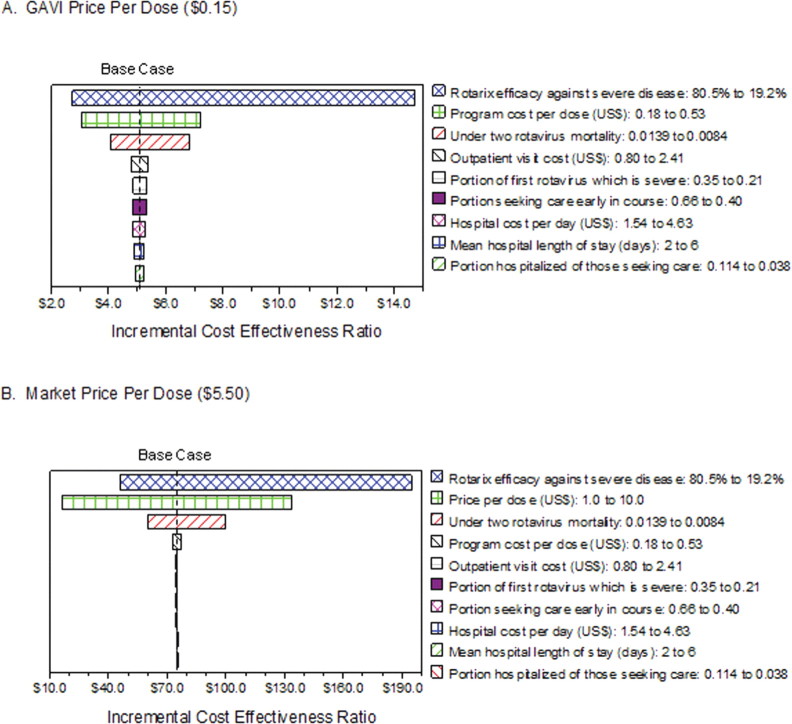
Figure 2 shows 1-way sensitivity analyses for the variables that had the most potential effect on the Rotarix program ICERs. With use of GAVI Alliance pricing, the estimated Rotarix program ICER was most sensitive to variation in vaccine efficacy, the program cost of administering the vaccine, and under-2 rotavirus-associated mortality (Figure 2A). When vaccine efficacy was varied from 80.5% to 19.2%, the resulting ICERs ranged from $2.70 to $14.70 per DALY averted. None of the ranges of uncertainty included the Rotarix program becoming cost-saving.
Figure 2.
Tornado diagrams of 1-way sensitivity analyses. End points of each bar represent incremental cost-effectiveness ratios at the low and high ends of the range for each parameter shown to the right. The base case incremental cost-effectiveness ratio is indicated.
The ICER for implementing Rotarix based on market pricing was most sensitive to variation in vaccine efficacy, the expected market price, and under-2 rotavirus-associated mortality (Figure 2B). When efficacy was varied from 80.5% to 19.2%, the resulting ICERs ranged from $45.70 to $194.80. To examine whether the ICER could cross the potential willingness-to-pay threshold of per capita Malawi GDP ($312) [27], a 3-way sensitivity analysis was performed on efficacy, price per dose, and under-2 mortality. Figure 3 shows the ranges of efficacy and price per dose under the scenario of the lowest possible value for under-2 mortality (0.0084). The estimated ICER would cross the willingness-to-pay threshold for the higher range of vaccine prices only when efficacy was <29%. Even when efficacy was varied as low as 19.2%, any vaccine price per dose <$6.50 still resulted in the ICER remaining below the willingness-topay threshold. The ICER in the worst case scenario of lowest efficacy, highest market price, and lowest under-2 mortality would be $461.90 per DALY averted.
Figure 3.
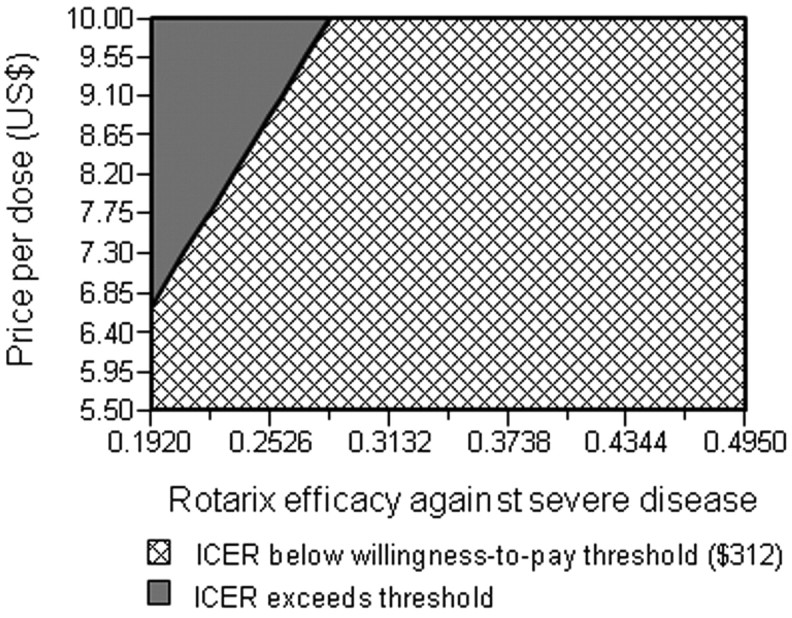
Two-way sensitivity analysis of the Rotarix program incremental cost-effectiveness ratio (ICER) on vaccine efficacy and market price per dose. The ICER estimates are determined under the assumption that under-2 rotavirus-associated mortality is at its lowest potential value of 0.0084. Combinations of price and efficacy highlighted in the hatched area would result in an ICER <$312 per disability-adjusted life-year averted.
Discussion
This analysis shows that adding Rotarix vaccination to the existing vaccine infrastructure in Malawi would likely be a highly cost-effective intervention. With GAVI Alliance cofinancing, the full-course program would be associated with an ICER of $5.07 per DALY averted. Even under the worst potential vaccine efficacy of 19.2%, the ICER would be only $14.70 per DALY averted. With market pricing, a vaccine program would cost $74.73 per DALY averted. This number compares favorably with the WHO's conservative willingness-to-pay threshold of per capita annual GDP ($312). The ICER crosses this threshold only when the most extreme potential values of efficacy, market vaccine price, and under-2 rotavirus-associated mortality are combined in a worst case scenario.
A recent analysis of the cost-effectiveness of a Rotarix program in Vietnam found an ICER of $550 per DALY averted [33]. The investigators used a vaccine efficacy value of 77%, and an under-5 rotavirus-associated mortality in Vietnam of ∼0.001, which is just 9% of the under-2 rotavirus-associated mortality that we estimated for Malawi. Multiplying 9% times $550 produces an ICER of $49.50 per DALY averted, which is similar to the ICER of $74.73 found in this study.
Although the annual per capita GDP is often used as a rule of thumb for willingness-to-pay in developing nations, Malawi's total vaccine budget in 2008 was projected to be only $18.5 million [34]. With GAVI Alliance pricing, the introduction of a Rotarix program would amount to 2.6% of the annual vaccine budget. With market pricing, a Rotarix program would amount to nearly one-third of $18.5 million. Malawian health authorities may thus have reservations about introducing Rotarix because of the expectation that, over time, the country will pay for an increasing proportion of the vaccine costs and, particularly, because of the projected upper bounds on ICERs resulting from uncertainty in key parameters, including vaccine efficacy, market price, and under-2 rotavirus-associated mortality. New empirical data that allow better estimates of these parameters would thus be useful.
As more data from the phase III Rotarix trial in Malawi and South Africa become available, more precise region-specific vaccine efficacy estimates for low-and middle-income countries in sub-Saharan Africa should become known. This trial's preliminarily efficacy estimate of 49.5% in Malawi is much lower than the 80.5% found in Latin American countries [12] and 90.4% in Europe [35]. Although preliminary, the finding underscores the need to perform efficacy studies in sub-Saharan Africa. A theoretical possibility for the efficacy difference is rotavirus serotype diversity [10]. Serotype analysis of rotavirus isolates from the vaccine trial would help in evaluating this. Nevertheless, our analysis indicates that, even at 49.5% efficacy, the vaccine would still represent good value for money. The phase III trial may also make available additional data on rotavirus-associated mortality that are specific to Malawi. The cost of outpatient visits and hospitalizations due to severe rotavirus diarrhea could be more precisely determined through economic studies at Malawian health care centers.
This analysis does not consider alternatives to a Rotarix program, such as a Rotateq program or an expansion of efforts to encourage medical care for diarrhea. The decision to focus on Rotarix was made because of available efficacy data for Malawi. By assuming that RotaTeq would have similar efficacy against severe rotavirus illness, it is possible to use the model in this analysis to estimate the cost-effectiveness of RotaTeq in Malawi. The GAVI Alliance may provide additional price support for Rotateq, so that the price of a full course will equal that of a full course of Rotarix ($0.30) [4]. Because of competition, market pricing for a full course may also be assumed to be similar ($11). Therefore, the only parameter to be changed in the model would be the program cost of vaccine delivery. After adjusting this parameter for 3 doses rather than 2 and factoring that the packed volume of RotaTeq is >4 times that of Rotarix [4], the base case estimated RotaTeq ICERs under GAVI Alliance and market pricing would be $21.35 and $91.21 per DALY averted, respectively. These projected results are highly speculative because of the assumption about vaccine efficacy; however, it is clear that difference in program cost is potentially an issue favoring Rotarix over RotaTeq.
Expanding efforts to seek medical care for diarrhea may be less attractive than adopting a new vaccine. The cost of an outpatient clinic visit is comparable to the market price and program costs of delivering Rotarix. Diarrheal illnesses are extremely common, and there are no reliable signs or symptoms to distinguish rotavirus from less-aggressive causes of infectious diarrhea near the onset of illness. Many rural parents may continue to seek traditional healers or home remedies for several days before traveling to Western medical clinics.
Adding Rotarix to the existing vaccine program in Malawi is likely to be advantageous from the perspective of the Malawian health sector. With GAVI Alliance-supported vaccine pricing, introduction of Rotarix will result in thousands of deaths averted annually with very good value for the cost to the Health Ministry. At such a time when GAVI Alliance price support expires, the currently projected market price of Rotarix still represents potentially good value. Further data on vaccine efficacy against severe disease and on the under-2 rotavirusassociated mortality rate in Malawi may allow a more accurate cost-effectiveness estimate. Even so, the cost per DALY averted is likely to remain less than the WHO recommended willingness-to-pay threshold for very cost-effective interventions.
Acknowledgments
We thank Drs Nigel Cunliffe and Duncan Steele for data and discussion related to vaccine efficacy in Malawi.
Footnotes
Financial support: National Center for Research Resources (1KL2RR025006-01).
Potential conflicts of interest: none reported.
Supplement sponsorship: This article is part of a supplement entitled “Rotavirus Infection in Africa: Epidemiology, Burden of Disease, and Strain Diversity,” which was prepared as a project of the Rotavirus Vaccine Program, a partnership among PATH, the World Health Organization, and the US Centers for Disease Control and Prevention, and was funded in full or in part by the GAVI Alliance.
References
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein DI. Rotavirus overview. Pediatr Infect Dis J. 2009;28(3 Suppl):S50–S53. doi: 10.1097/INF.0b013e3181967bee. [DOI] [PubMed] [Google Scholar]
- 3.Meeting of the Immunization Strategic Advisory Group of Experts April 2009: conclusions and recommendations. Wkly Epidemiol Rec. 2009;84(23):220. [PubMed] [Google Scholar]
- 4.Introduction of rotavirus vaccine with support from the GAVI Alliance: information to assist the national decision-making and application process. http://www.gavialliance.org/resources/RVP_GAVI_InfoPack_17Jan08.pdf. [13 March 2009].
- 5.Cunliffe NA, Gondwe JS, Kirkwood CD, et al. Effect of concomitant HIV infection on presentation and outcome of rotavirus gastroenteritis in Malawian children. Lancet. 2001;358(9281):550–555. doi: 10.1016/s0140-6736(01)05706-3. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335(14):1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 7.CIA World Factbook: Malawi. https://www.cia.gov/library/publications/the-world-factbook/print/mi.html 13 March 2009.
- 8.The Partnership for Maternal Newborn and Child Health-MNCH in Malawi. http://www.who.int/pmnch/countries/malawi/en/index.html 13 March 2009.
- 9.Vaahtera M, Kulmala T, Maleta K, Cullinan T, Salin ML, Ashorn P. Epidemiology and predictors of infant morbidity in rural Malawi. Paediatr Perinat Epidemiol. 2000;14(4):363–371. doi: 10.1046/j.1365-3016.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe NA, Ngwira BM, Dove W, et al. Serotype g12 rotaviruses, Lilongwe, Malawi. Emerg Infect Dis. 2009;15(1):87–90. doi: 10.3201/eid1501.080427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Immunization surveillance, assessment, and monitoring. http://www.who.int/immunization_monitoring/burden/rotavirus_estimates/en/index.html 27 May 2009.
- 12.Linhares AC, Velazquez FR, Perez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Immunization surveillance, assessment and monitoring. http://www.who.int/immunization_monitoring/diseases/en 13 March 2009.
- 14.Claeson M, Merson MH. Global progress in the control of diarrheal diseases. Pediatr Infect Dis J. 1990;9(5):345–355. doi: 10.1097/00006454-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5)(5):565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsella M, Raimondi L, Bergamini M, et al. Epidemiology of rotavirus-associated hospital admissions in the province of Ferrara, Italy. Eur J Pediatr. 2009;168:1423–1427. doi: 10.1007/s00431-009-0942-z. [DOI] [PubMed] [Google Scholar]
- 17.abutti G, Lazzara C, Marsella M, Bergamini M, Malaventura C, Borgna-Pignatti C. Burden of hospitalizations due to rotavirus infection in Emilia Romagna, Italy. Acta Biomed. 2007;78(3):176–181. [PubMed] [Google Scholar]
- 18.Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J. 2006;25(6):489–493. doi: 10.1097/01.inf.0000215234.91997.21. [DOI] [PubMed] [Google Scholar]
- 19.de Wit MA, Koopmans MP, van der Blij JF, van Duynhoven YT. Hospital admissions for rotavirus infection in the Netherlands. Clin Infect Dis. 2000;31(3):698–704. doi: 10.1086/314025. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-de-ndres A, Jimenez-Garcia R, Carrasco-Garrido P, lvaro-Meca A, Galarza PG, de Miguel AG. Hospitalizations associated with rotavirus gastroenteritis in Spain, 2001–2005. BMC Public Health. 2008;8:109. doi: 10.1186/1471-2458-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Life Tables for WHO Member States. http://www.who.int/whosis/database/life_tables/life_tables.cfm 21 April 2009.
- 22.Murray C, Lopez A, editors. The global burden of disease. Boston: Harvard University Press; 1996. [Google Scholar]
- 23.The World Health Report 2002. Reducing risks, promoting healthy life. Geneva: World Health Organization; [DOI] [PubMed] [Google Scholar]
- 24.Gravelle H, Smith D. Discounting for health effects in cost-benefit and cost-effectiveness analysis. Health Econ. 2001;10(7):587–599. doi: 10.1002/hec.618. [DOI] [PubMed] [Google Scholar]
- 25.Tasset A, Nguyen VH, Wood S, Amazian K. Discounting: technical issues in economic evaluations of vaccination. Vaccine. 1999;17(Suppl 3):S75–S80. doi: 10.1016/s0264-410x(99)00298-4. [DOI] [PubMed] [Google Scholar]
- 26.Bilcke J, Beutels P. Reviewing the cost effectiveness of rotavirus vaccination: the importance of uncertainty in the choice of data sources. Pharmacoeconomics. 2009;27(4):281–97. doi: 10.2165/00019053-200927040-00002. [DOI] [PubMed] [Google Scholar]
- 27.International Monetary Fund World Economic Outlook Database. http://www.imf.org/external/pubs/ft/weo/2009/01/weodata/index.aspx 31 May 2009.
- 28.Dayan GH, Cairns L, Sangrujee N, Mtonga A, Nguyen V, Strebel P. Cost-effectiveness of three different vaccination strategies against measles in Zambian children. Vaccine. 2004;22:475–484. doi: 10.1016/j.vaccine.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Wolfson LJ, Gasse F, Lee-Martin SP, et al. Estimating the costs of achieving the WHO-UNICEF global immunization vision and strategy, 2006–2015. Bull World Health Organ. 2008;86(1):27–39. doi: 10.2471/BLT.07.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Choosing Interventions that are Cost Effective (WHO-CHOICE): Malawi. http://www.who.int/choice/country/mwi/cost/en/index.html 13 March 2009. [Google Scholar]
- 31.Muennig P. Designing and conducting cost-effectiveness analyses in medicine and health care. 1st ed. San Francisco: Jossey-Bass; 2002. [Google Scholar]
- 32.Macroeconomics and health: investing in health for economic development. http://whqlibdoc.who.int/publications/2001/924154550x.pdf 24 June 2009.
- 33.Kim SY, Goldie SJ, Salomon JA. Cost-effectiveness of Rotavirus vaccination in Vietnam. BMC Public Health. 2009;9:29. doi: 10.1186/1471-2458-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comprehensive multi-year plan 2006–2010: Malawi. http://www.gavialliance.org/performanc/country_results 7 May 2009.
- 35.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]