Abstract
Long interspersed nucleotide element-1 (LINE-1) retrotransposons are located throughout the human genome. Those retaining an intact 5′ promoter can copy and insert themselves into the DNA of neural progenitor cells that express tyrosine hydroxylase, which may influence differentiation and survival of these cells. Because LINE-1 promoter methylation is associated with decreased LINE-1 propagation, we compared LINE-1 methylation profiles in blood mononuclear cells between 292 newly diagnosed Parkinson’s disease (PD) cases and 401 unrelated, neurologically normal controls. Overall, PD was not associated with percent methylation of the LINE-1 promoter. However, the predictable inverse association between PD and ever smoking tobacco was strongest for men and women with the lowest LINE-1 promoter methylation, and less apparent as LINE-1 methylation increased. Underlying this possible interaction, ever regularly smoking tobacco was associated with decreased LINE-1 methylation in controls (age- and sex-adjusted linear regression β = −0.24, 95% confidence interval [CI] −0.43, −0.04), but not in cases (β = 0.06, 95% CI −0.17, 0.28, interaction p = 0.06). PD cases may have innate differences in their ability to respond to tobacco smoke.
Introduction
Parkinson’s disease (PD) is a neurodegenerative movement disorder due to selective loss of dopaminergic neurons in midbrain substantia nigra. The causes of PD remain largely unknown, although both genetic and environmental factors are etiologically relevant [1]. One factor not previously examined in relation to PD is methylation of long interspersed nucleotide element-1 (LINE-1 or L1). LINE-1 DNA sequences are repeated throughout the genome, and those with intact 5’ promoters can copy and insert themselves elsewhere in cellular DNA [2]. These insertions potentially affect gene expression [3], and both germline and somatic LINE-1 insertions may result in disease [4]. In the brain, most LINE-1 insertions are somatic [5]. Notably, they occur in the subtype of human neural progenitor cells that express tyrosine hydroxylase [6], a protein suggestive of differentiation into dopaminergic neurons. Integration of LINE-1 into neural progenitor cells might affect progenitor cell fate, including differentiation [3] or survival [7]. LINE-1 expression may be especially important during neurogenesis [8].
The number of LINE-1 insertions in brain cell DNA varies between persons [5]. One factor that may affect retrotransposition of LINE-1 within neurons is methylation of DNA in the LINE-1 promoter [6]. Methylation of individual CG dinucleotides (CpG sites) is mitotically heritable yet is alterable within each cell. Mean percent LINE-1 methylation, although somewhat stable [9], appears to be affected by a variety of environmental exposures [10]. Recently, a small study found that genome-wide methylation, which is correlated with LINE-1 methylation, was lower in PD than control brains [11]. However, this study was limited to frontal cortex samples from deceased patients, whose DNA methylation may have been altered by their advanced disease state, treatment [12–14] or physical inactivity [15–16] in the months preceding death. We investigated the relation between PD and methylation of the LINE-1 promoter using blood collected close in time to PD diagnosis in a relatively large case-control study. In addition, because oxidative stress increases LINE-1 retrotransposition in human neuroblastoma cells [17], we examined whether the known inverse association between PD and tobacco smoking [18–19] was modified by LINE-1 methylation. As we were unable to obtain dopaminergic neurons from study participants and assess de novo LINE-1 insertions, we measured percent LINE-1 methylation in blood mononuclear cells, as a proxy and as an initial step in directly exploring the relation between PD and LINE-1.
Materials and Methods
Participant identification and data collection
Idiopathic PD cases (N=490) were newly diagnosed during 1992–2008 at Group Health Cooperative (N=387) or the University of Washington Neurology Clinic (N=103) in western Washington State [19–20]. Briefly, all cases had at least two of four cardinal signs of PD (bradykinesia, resting tremor, cogwheel rigidity, postural reflex impairment). Diagnoses not made by neurologists were verified by chart reviews by study neurologists (W.T.L., G.M.F., P.D.S.) We excluded patients with an established cause of parkinsonism, such as stroke, recent brain trauma, brain tumor, or use of selected medications. Controls (N=644) were unrelated Group Health Cooperative enrollees who were free of PD and other neurodegenerative disorders. Controls were frequency matched to cases on age, sex, race/ethnicity, clinic and length of enrollment. We made no exclusions for cases or controls with regard to history of cancer or other non-neurological outcomes.
We determined history of tobacco (cigarette, cigar and pipe) smoking through a structured, in-person questionnaire. Most participants (96% of cases, 95% of controls) also provided blood or buccal cells at interview. Because LINE-1 methylation may differ by race/ethnicity [21–22] and cell type [23–24], we restricted analysis to non-Hispanic Caucasians with sufficient DNA from blood-derived mononuclear cells at the time of laboratory analysis, 292 (60%) of cases and 401 (62%) of controls. University of Washington and Group Health Cooperative Institutional Review Boards approved all study procedures, and all participants provided written informed consent.
Assessment of LINE-1 methylation
The Functional Genomics Laboratory at the Center for Ecogenetics and Environmental Health at the University of Washington (Seattle, Washington) isolated DNA using the DNeasy Blood and Tissue Kit (Qiagen) following centrifugation of whole blood to isolate the mononuclear cells and then treatment with RNase. Blind to case-control status, the Center for Environmental Health and Technology at Brown University (Providence, Rhode Island) conducted quantitative LINE-1 methylation assays by pyrosequencing. Bisulfite conversion of genomic DNA (250ng) was performed using the EZ DNA Methylation Direct Kit (Zymo Research) per the manufacturer’s instructions. Four CpG sites in the 5′ promoter of LINE-1 (TT[T/C]GTGGTG[T/C]GT[T/C]GTTTTTTAAGT[T/C]GGTTT) were analyzed in triplicate on a PyroMark MD system (Qiagen) using primers as described [25]. We retained replicates with complete data, that is, percent methylation for all four CpG sites. All three replicates were complete for 92% of cases and 94% of controls, and the remainder had at least one complete replicate. We took the mean across the four CpG sites for each replicate, and then the mean of these, hereafter LINE-1 methylation, expressed as a percentage.
Statistical analysis
We used Stata 11.1 (College Station, Texas) for all statistical analyses. LINE-1 methylation was normally distributed, and we compared cases to controls using a t-test, followed by multivariable linear regression to adjust for age, sex and tobacco smoking (ever/never regularly smoked tobacco, number of cigarettes per day, and number of years smoking cigarettes). These factors are associated with PD occurrence [18–19] or LINE-1 methylation [10]. We also adjusted for assay plate to account for any inter-plate effects.
We then examined whether the known inverse association between smoking and PD risk previously seen in this study [18–20] varied by LINE-1 methylation. We calculated age- and sex-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) as an estimate of the relative risk of PD in relation to tobacco smoking, overall and by LINE-1 methylation quartiles. We formally tested interactions in multivariate regression models; we used the p-value for the multiplicative interaction term between smoking and LINE-1 methylation as a continuous variable, while including main effects terms and the same covariates.
Results
Characteristics of participants
Other than the restriction to non-Hispanic Caucasians, cases and controls with LINE-1 methylation data were similar to those in the parent study [19–20], including greater tobacco smoking by controls than cases (Table 1). For most cases (91%) we had obtained DNA less than two years after diagnosis.
Table 1.
Characteristics of Parkinson’s disease cases and controls, Group Health Cooperative and University of Washington, 1992–2008
| All participants | Non-Hispanic Caucasians with LINE-1 data | |||
|---|---|---|---|---|
| Cases N=490 | Controls N=644 | Cases N=292 | Controls N=401 | |
| n (%) | n (%) | n (%) | n (%) | |
| Male | 310 (63) | 409 (64) | 196 (67) | 256 (64) |
| Non-Hispanic Caucasian | 456 (93) | 595 (92) | 292 (100) | 401 (100) |
| Family history of PDa | 43 (11) | 23 (5) | 27 (12) | 16 (5) |
| Age at diagnosis/reference, years | ||||
| ≥ 60 | 359 (73) | 526 (82) | 206 (71) | 325 (81) |
| Mean (standard deviation) | 65.6 (10.2) | 68.0 (8.7) | 64.9 (10.5) | 68.0 (8.7) |
| Time between diagnosis/reference and DNA collection, years | ||||
| <1 | 175 (36) | 230 (36) | 100 (34) | 146 (36) |
| 1 | 274 (56) | 362 (56) | 167 (57) | 221 (55) |
| 2–4 | 41 (8) | 52 (8) | 25 (9) | 34 (8) |
| Ever tobacco smokingb | 245 (50) | 398 (62) | 148 (51) | 244 (61) |
| Cigarette smokingc | ||||
| Never | 272 (56) | 276 (43) | 159 (54) | 179 (45) |
| Former | 196 (40) | 307 (48) | 116 (40) | 192 (48) |
| Current | 22 (4) | 61 (9) | 17 (6) | 30 (7) |
| Smoking among ever smokers, mean (standard deviation) | ||||
| Packs per day | 0.88 (0.57) | 0.92 (0.55) | 0.84 (0.53) | 0.92 (0.53) |
| Years | 22.5 (15.2) | 26.8 (16.4) | 22.5 (15.1) | 26.3 (16.4) |
| Pack-years | 21.7 (22.0) | 26.4 (23.2) | 20.5 (20.7) | 25.1 (22.0) |
| Years since smoked | 25.1 (14.9) | 22.7 (16.8) | 24.2 (14.5) | 23.1 (16.5) |
Any first degree relative with Parkinson’s disease (PD), based on 390 cases and 494 controls with complete data, including 231 cases and 316 controls who were non-Hispanic Caucasian and for whom LINE-1 methylation was assessed
Ever smoked >100 cigarettes, cigar regularly or pipe regularly
History of cigarette smoking (>100 cigarettes) up to diagnosis/reference
Abbreviations: PD Parkinson’s disease, LINE-1 long interspersed nucleotide element-1
PD and LINE-1 methylation
Overall, percent LINE-1 methylation was similar in cases and controls (Table 2; p = 0.40). We confirmed the well-established association between LINE-1 methylation and sex [10] among both cases and controls (Table 2; both p < 0.001); therefore, we repeated LINE-1 comparisons separately for men and women. Again no association between PD and LINE-1 methylation was evident, nor when we used multivariate linear regression to adjust for age, sex and smoking (all p > 0.40, data not shown).
Table 2.
Percent LINE-1 methylation in Parkinson’s disease cases and controls, overall and by sex
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | |
| N=292 | N=401 | N=196 | N=256 | N=96 | N=145 | |
| Minimum | 76.83 | 76.72 | 77.82 | 77.39 | 76.83 | 76.72 |
| Median | 80.33 | 80.23 | 80.52 | 80.41 | 80.02 | 79.98 |
| Maximum | 83.67 | 84.14 | 82.52 | 83.38 | 83.67 | 84.14 |
| Mean | 80.33 | 80.26 | 80.52 | 80.44 | 79.95 | 79.95 |
| Standard deviation | 1.03 | 1.06 | 0.94 | 0.99 | 1.10 | 1.11 |
Abbreviations: LINE-1 long interspersed nucleotide element-1
PD, smoking and LINE-1 methylation
In participants with LINE-1 methylation data, ever smoking tobacco was associated with reduced risk of PD (age- and sex-adjusted OR = 0.63, 95% CI 0.46–0.87, data not shown). Risk was 0.61, 95% CI 0.41–0.91 in men, and 0.67, 95% CI 0.39–1.14 in women. This inverse association between PD and ever smoking was strongest among men and women with the lowest LINE-1 methylation, and became less apparent with greater LINE-1 methylation (Fig. 1; interaction p = 0.14 for men and women combined). Underlying this potential interaction, ever smoking was associated with reduced LINE-1 methylation among controls (linear regression β = −0.24, 95% CI −0.43, −0.04), but ever smoking was not associated with LINE-1 methylation among cases (β = 0.06, 95% CI −0.17, 0.28, interaction p = 0.06).
Figure 1. Age-adjusted odds ratio between Parkinson’s disease and ever tobacco smoking, by sex and percent LINE-1 methylation quartile.
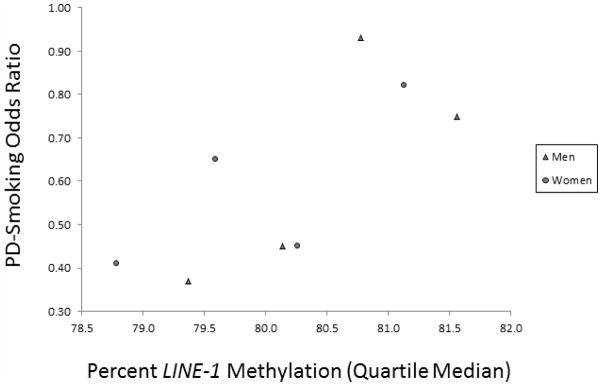
PD-ever tobacco smoking odds ratio adjusted for age (continuous) and assay plate
Symbols: ▲ Men ● Women
Abbreviations: LINE-1 long interspersed nucleotide element-1, PD Parkinson’s disease
Discussion
To our knowledge, this is the first study of PD and LINE-1 methylation. We observed no difference in LINE-1 methylation between cases and controls overall, despite studying a reasonably large number of newly diagnosed PD patients and highly comparable, unrelated controls. However, the known inverse association between smoking and PD risk was most evident in men and women with the lowest LINE-1 methylation, and it markedly diminished with greater LINE-1 methylation. Although this potential interaction was not statistically significant, it was probably attenuated by our use of non-neural cells. This predictable effect may have been substantial because even the correlation between LINE-1 methylation in serum vs. buffy coat cells is modest [24]. This conservative bias is a limitation with regard to the lack of overall case-control differences, but the use of cells obtained close in time to diagnosis likely minimized bias related to disease progression and survival. Thus, insofar as mononuclear cell LINE-1 DNA methylation reflects dopaminergic neural cell LINE-1 methylation – and hence retrotransposition frequency [6] – the potential PD-smoking-LINE1 interaction we report may have a biological basis related to neural cell differentiation or survival [3, 7–8]. Alternatively, lower LINE-1 methylation in blood may be a surrogate for glutathione depletion [26] or exposure to oxidative stress [27], persistent organic pollutants [28] or heavy metals [29–31]. It is plausible that smoking might be more protective with these exposures.
Underlying the interaction of smoking and LINE-1 methylation on PD risk was an inverse association between ever smoking and LINE-1 methylation among controls, which agrees with some but not all epidemiologic studies [9, 32–36]. Experimental studies indicate that cigarette smoke condensate [37] and benzo(a)pyrene specifically [38] are associated with reduced LINE-1 methylation. Heavy metals that are present in tobacco smoke are also associated with reduced LINE-1 [29–31]. The lack of association between smoking and LINE-1 methylation in cases probably was not due to lower statistical power, because the β estimate was close to null. Thus, insofar as our results are not simply due to chance, the most straightforward explanation is that PD cases respond to smoking differently than their counterparts. This possibly includes innate differences in cases’ ability to methylate or demethylate DNA. Because DNA methylation may play a role in smoking initiation [39], the inverse association between PD and ever smoking might even simply reflect poor (de)methylation by persons who later develop PD, rather than a true protective effect of smoking. A limitation of our study is that we did not have longitudinal samples to directly assess the effect of smoking on LINE-1 methylation. However, a recent study with longitudinal blood samples, which examined post-traumatic stress disorder among military personnel deployed in Afghanistan and Iraq, reported findings that parallel ours: No difference in LINE-1 methylation between cases and controls prior to deployment, but a deployment-related change in LINE-1 methylation among controls but not cases [22]. In summary, persons with less ability to respond to a changing environment may be at increased risk of developing a variety of neurological disorders.
Additional similar studies of LINE-1 methylation and PD among newly diagnosed cases and a carefully selected comparison group will be required to confirm our results. Studies that include blood samples collected longitudinally may prove particularly useful, although feasibility for such studies is limited. Targeted examination of PD and methylation of smoking-related genes also may help elucidate potential biological mechanisms pertinent to PD pathogenesis and susceptibility.
Acknowledgments
This work was supported by the University of Washington Superfund Research Program, grant P42ES004696 from the National Institute of Environmental Health Sciences (NIEHS); and byf NIEHS grants R01ES10750 and P30ES007033.
References
- 1.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 2.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–910. doi: 10.1038/nature03663. [DOI] [PubMed] [Google Scholar]
- 4.Hancks DC, Kazazian HH., Jr Active human retrotransposons: variation and disease. Curr Opin Genet Dev. 2012;22:191–203. doi: 10.1016/j.gde.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baillie JK, Barnett MW, Upton KR, Gerhardt DJ, Richmond TA, De Sapio F, Brennan PM, Rizzu P, Smith S, Fell M, Talbot RT, Gustincich S, Freeman TC, Mattick JS, Hume DA, Heutink P, Carninci P, Jeddeloh JA, Faulkner GJ. Somatic retrotransposition alters the genetic landscape of the human brain. Nature. 2011;479:534–537. doi: 10.1038/nature10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, Morell M, O’Shea KS, Moran JV, Gage FH. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460:1127–1131. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer T, McConnell MJ, Marchetto MC, Coufal NG, Gage FH. LINE-1 retrotransposons: mediators of somatic variation in neuronal genomes? Trends Neurosci. 2010;33:345–354. doi: 10.1016/j.tins.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas CA, Muotri AR. LINE-1: creators of neuronal diversity. Front Biosci (Elite Ed) 2012;4:1663–1668. doi: 10.2741/e488. [DOI] [PubMed] [Google Scholar]
- 9.Wu HC, Wang Q, Delgado-Cruzata L, Santella RM, Terry MB. Genomic methylation changes over time in peripheral blood mononuclear cell DNA: differences by assay type and baseline values. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0300. Advance online publication on June 6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson HH, Marsit CJ, Kelsey KT. Global methylation in exposure biology and translational medical science. Environ Health Perspect. 2011;119:1528–1533. doi: 10.1289/ehp.1103423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desplats P, Spencer B, Coffee E, Patel P, Michael S, Patrick C, Adame A, Rockenstein E, Masliah E. Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem. 2011;286:9031–9037. doi: 10.1074/jbc.C110.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottiglieri T, Arning E, Wasek B, Nunbhakdi-Craig V, Sontag JM, Sontag E. Acute administration of L-Dopa induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of tau protein in mouse brain. J Neurosci. 2012;32:9173–9181. doi: 10.1523/JNEUROSCI.0125-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isobe C, Abe T, Terayama Y. L-Dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson’s disease. Journal of Clinical Neuroscience. 2010;17:717–721. doi: 10.1016/j.jocn.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Simma N, Kaut O, Schmitt I, Waha A, Wüllner U. Neurowoche: Arbeitsgemeinschaft Klinische Neurowissenschaften. Mannheim, Germany: 2010. [Accessed August 6, 2012]. DNA methylation in lymphocytes and brain samples of Parkinson’s disease patients. http://registration.akm.ch/2010neuro_einsicht.php?XNABSTRACT_ID=113351&XNSPRACHE_ID=1&XNKONGRESS_ID=122&XNMASKEN_ID=900. [Google Scholar]
- 15.Muotri AR, Zhao C, Marchetto MC, Gage FH. Environmental influence on L1 retrotransposons in the adult hippocampus. Hippocampus. 2009;19:1002–1007. doi: 10.1002/hipo.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang FF, Cardarelli R, Carroll J, Zhang S, Fulda KG, Gonzalez K, Vishwanatha JK, Morabia A, Santella RM. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics-Us. 2011;6:293–299. doi: 10.4161/epi.6.3.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgi G, Marcantonio P, Del Re B. LINE-1 retrotransposition in human neuroblastoma cells is affected by oxidative stress. Cell Tissue Res. 2011;346:383–391. doi: 10.1007/s00441-011-1289-0. [DOI] [PubMed] [Google Scholar]
- 18.Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 19.Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 20.Searles Nielsen S, Gallagher LG, Lundin JI, Longstreth WT, Jr, Smith-Weller T, Franklin GM, Swanson PD, Checkoway H. Environmental tobacco smoke and Parkinson’s disease. Mov Disord. 2012;27:293–297. doi: 10.1002/mds.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 22.Rusiecki JA, Chen L, Srikantan V, Zhang L, Yan L, Polin ML, Baccarelli A. DNA methylation in repetitive elements and post-traumatic stress disorder: a case-control study of US military service members. Epigenomics. 2012;4:29–40. doi: 10.2217/epi.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu HC, Delgado-Cruzata L, Flom JD, Kappil M, Ferris JS, Liao Y, Santella RM, Terry MB. Global methylation profiles in DNA from different blood cell types. Epigenetics-Us. 2011;6:76–85. doi: 10.4161/epi.6.1.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Bemmel D, Lenz P, Liao LM, Baris D, Sternberg LR, Warner AC, Johnson AT, Jones MA, Kida M, Schwenn M, Schned A, Silverman DT, Rothman N, Moore LE. Correlation of LINE-1 methylation levels in patient matched buffy coat, serum, buccal cell and bladder tumor tissue DNA samples. Cancer Epidemiol Biomarkers. 2012 doi: 10.1158/1055-9965.EPI-11-1030. Advance online publication on April 28 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, Byun HM, Jiang J, Marinelli B, Pesatori AC, Bertazzi PA, Yang AS. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Jacobs DR, Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environ Health Persp. 2009;117:1799–1802. doi: 10.1289/ehp.0900741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patchsung M, Boonla C, Amnattrakul P, Dissayabutra T, Mutirangura A, Tosukhowong P. Long interspersed nuclear element-1 hypomethylation and oxidative stress: correlation and bladder cancer diagnostic potential. PLoS One. 2012;7:e37009. doi: 10.1371/journal.pone.0037009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright RO, Schwartz J, Wright RJ, Bollati V, Tarantini L, Park SK, Hu H, Sparrow D, Vokonas P, Baccarelli A. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect. 2010;118:790–795. doi: 10.1289/ehp.0901429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hossain MB, Vahter M, Concha G, Broberg K. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ Health Perspect. 2012;120:879–884. doi: 10.1289/ehp.1104600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambrou A, Baccarelli A, Wright RO, Weisskopf M, Bollati V, Amarasiriwardena C, Vokonas P, Schwartz J. Arsenic exposure and DNA methylation among elderly men. Epidemiology. 2012 doi: 10.1097/EDE.0b013e31825afb0b. Advance online publication on July 24 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cash HL, Tao L, Yuan JM, Marsit CJ, Houseman EA, Xiang YB, Gao YT, Nelson HH, Kelsey KT. LINE-1 hypomethylation is associated with bladder cancer risk among nonsmoking Chinese. Int J Cancer. 2012;130:1151–1159. doi: 10.1002/ijc.26098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langevin SM, Houseman EA, Christensen BC, Wiencke JK, Nelson HH, Karagas MR, Marsit CJ, Kelsey KT. The influence of aging, environmental exposures and local sequence features on the variation of DNA methylation in blood. Epigenetics-Us. 2011;6:908–919. doi: 10.4161/epi.6.7.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelm CS, Kelsey KT, Butler R, Plaza S, Gagne L, Zens MS, Andrew AS, Morris S, Nelson HH, Schned AR, Karagas MR, Marsit CJ. Implications of LINE1 methylation for bladder cancer risk in women. Clin Cancer Res. 2010;16:1682–1689. doi: 10.1158/1078-0432.CCR-09-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu ZZ, Hou L, Bollati V, Tarantini L, Marinelli B, Cantone L, Yang AS, Vokonas P, Lissowska J, Fustinoni S, Pesatori AC, Bonzini M, Apostoli P, Costa G, Bertazzi PA, Chow WH, Schwartz J, Baccarelli A. Predictors of global methylation levels in blood DNA of healthy subjects: a combined analysis. Int J Epidemiol. 2012;41:126–139. doi: 10.1093/ije/dyq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, Rao M, Meltzer PA, Schrump DS. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene. 2010;29:3650–3664. doi: 10.1038/onc.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teneng I, Montoya-Durango DE, Quertermous JL, Lacy ME, Ramos KS. Reactivation of L1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation. Epigenetics-Us. 2011;6:355–367. doi: 10.4161/epi.6.3.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q, Ma JZ, Payne TJ, Li MD. Determination of methylated CpG sites in the promoter region of catechol-O-methyltransferase (COMT) and their involvement in the etiology of tobacco smoking. Front Psychiatry. 2010;1:16. doi: 10.3389/fpsyt.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]


