Abstract
Smell identification deficits (SIDs) are relatively specific to schizophrenia and its negative symptoms, and may predict transition to psychosis in clinical high-risk (CHR) individuals. Moreover, eventrelated potentials (ERPs) to odors are reduced in schizophrenia. This study examined whether CHR patients show SIDs and abnormal olfactory N1 and P2 potentials. ERPs (49 channels) were recorded from 21 CHR and 20 healthy participants (13 males/group; ages 13–27 years) during an odor detection task using three concentrations of hydrogen sulfide (H2S) or blank air presented unilaterally by a constant-flow olfactometer. Neuronal generator patterns underlying olfactory ERPs were identified and measured by principal components analysis (unrestricted Varimax) of reference-free current source densities (CSD). Replicating previous findings, CSD waveforms to H2S stimuli were characterized by an early N1 sink (345 ms, lateral-temporal) and a late P2 source (600 ms, mid-frontocentroparietal). N1 and P2 varied monotonically with odor intensity (strong > medium > weak) and did not differ across groups. Patients and controls also showed comparable odor detection and had normal odor identification and thresholds (Sniffin’ Sticks). However, olfactory ERPs strongly reflected differences in odor intensity and detection in controls, but these associations were substantially weaker in patients. Moreover, severity of negative symptoms in patients was associated with reduced olfactory ERPs and poorer odor detection, identification and thresholds. Three patients who developed psychosis had poorer odor detection and thresholds, and marked reductions of N1 and P2. Thus, despite the lack of overall group differences, olfactory measures may be of utility in predicting transition to psychosis among CHR patients.
Keywords: Event-Related Potentials (ERP), Current Source Density (CSD), Principal Components Analysis (PCA), Olfaction, Prodrome, Schizophrenia, Clinical High-Risk (CHR), Negative Symptoms
1. Introduction
Schizophrenia is a chronic illness with an onset of symptoms typically occurring early in life (i.e., during young adulthood). Before a first onset of psychosis, a prodromal period occurs in over 70% of schizophrenia cases (Häfner et al., 2003), which is characterized by attenuated psychotic symptoms, anxiety, social and role dysfunction, and affective symptoms. In the hope of reducing morbidity and preventing or delaying onset through early intervention, current efforts aim at identifying young people at risk during this prodromal stage (e.g., Corcoran et al., 2010; Fusar-Poli et al., 2012b). Little is known, however, about the underlying pathophysiology of emerging psychosis. A large multisite study (Cannon et al., 2008) that followed individuals at clinical high risk (CHR) for psychosis for 2.5 years reported that certain clinical characteristics assessed at baseline predicted psychosis, including genetic risk with recent functional decline, positive symptom severity, social impairment and substance abuse; however, no psychophysiological measures were included. These findings are consistent with previous studies which identified as predictors poor role function, earlier onset, and longer duration and greater severity of prodromal symptoms (Amminger et al., 2006; Haroun et al., 2006; Yung et al., 2004). Although less impaired than schizophrenia, CHR patients have generalized neuropsychological deficits (Brewer et al., 2006; Hawkins et al 2004; Woodberry et al., 2010), and verbal memory deficits may be a predictor of psychosis (Brewer et al., 2005; Lencz et al., 2006; Woodberry et al., 2010). A promising line of research has recently implicated various electrophysiologic measures obtained during active and passive auditory paradigms as helpful tools in predicting transition to psychosis (Atkinson et al., 2012; Bodatsch et al., 2011; Frommann et al., 2008; Koh et al., 2011; Shaikh et al., 2012; van der Stelt et al., 2005; van Tricht et al., 2010). However, only smell identification deficits have been shown to discern whom among high-risk cases will specifically develop schizophrenia and its spectrum disorders (Brewer et al., 2003), which is agreement with evidence showing that impairments in odor identification are present before individuals develop psychotic symptoms (Woodberry et al., 2010). Moreover, a cross-sectional study found that CHR individuals were impaired not only in odor identification but also in odor discrimination, with both deficits being comparable to schizophrenia patients (Kamath et al., 2011).
1.1. Olfactory deficits in schizophrenia
Deficits of olfactory function are common in schizophrenia, affecting threshold sensitivity, discrimination and identification of odors (e.g., reviewed by Moberg et al., 1999; Moberg & Turetsky, 2003). Although olfactory abnormalities have also been found in several neurological diseases (Barresi et al., 2012) and other psychiatric disorders (Buron & Bulnena, 2013; Schecklmann et al., 2013), most research in this area has been performed in schizophrenia (Atanasova et al., 2008). Studies using psychophysical measures of odor identification have consistently demonstrated that schizophrenia patients, when compared to healthy controls, have a robust impairment in correctly naming or identifying different odors (Cohen et al., 2012a; Kamath et al., 2011). This deficit in odor identification is not due to increased odor detection threshold (Moberg et al., 2006), the findings for which have been less consistent (Martzke et al., 1997; Moberg et al., 1999; Purdon & Flor-Henry, 2000). Moreover, it has been suggested that smell identification deficits are relatively specific to schizophrenia (Hurwitz et al., 1988) and its negative symptoms (Malaspina & Coleman, 2003), including in young people with psychotic disorders (Corcoran et al., 2005), which can not be accounted for by cognitive impairment (Seidman et al., 1991, 1997), socioeconomic status, smoking or medication (Coleman et al., 2002; Malaspina & Coleman, 2003; Turetsky et al., 2003b). Interestingly, unaffected relatives of schizophrenia patients also showed poorer smell identification (Kopala et al., 2001; Turetsky et al., 2008) and elevated odor thresholds, which were intermediate between patients and controls (Roalf et al., 2006). Although these data suggest a genetic component, there has been some controversy about the extent to which olfactory identification deficits may constitute a meaningful, broader vulnerability marker of schizophrenia pathology (Cohen et al., 2012a,b; Turetsky et al., 2012).
Given the functional anatomy of human olfactory pathways (e.g., Martzke et al., 1997; Seubert et al., 2013), olfactory deficits likely originate from brain structures in medial temporal lobe regions and orbitofrontal and dorsolateral prefrontal cortex linked to olfactory as well as cognitive and emotional disturbances in schizophrenia (e.g., Atanasova et al., 2008), and may help elucidate limbic system dysfunctions (Moberg et al., 2003). Thus, decreased olfactory threshold sensitivity in schizophrenia patients was associated with reduced volume in the perirhinal, but not entorhinal, region of the anterior ventromedial temporal lobe (Turetsky et al., 2003b), and both patients and their healthy relatives had reduced olfactory bulb volumes compared to healthy controls (Turetsky et al., 2003c). Also, Rupp et al. (2005) reported that poorer olfactory discrimination in schizophrenia patients was related to smaller hippocampal volumes, but not volumes in the orbitofrontal region. However, given that olfactory deficits have been observed across several neuropsychiatric and neurodegenerative disorders, including Parkinson’s and Alzheimer’s disease, it has been proposed that some aspects of impaired odor processing may share a common dopaminergic pathology, which may affect neurotransmission in the olfactory bulbs (Schecklmann et al., 2013). This is of particular interest given the refined dopamine hypothesis of schizophrenia (e.g., Howes & Kapur, 2009) and evidence that dopaminergic abnormalities precede psychosis onset (Egerton et al., 2013; Howes et al., 2011).
Nonetheless, reports of behavioral deficits in olfactory function and structural abnormalities in the olfactory system in schizophrenia offer limited insights into the relevant brain activity. Recent functional magnetic resonance imaging (fMRI) evidence in healthy adults suggests that odor identification, as opposed to smelling of nonidentified odors, is specifically associated with activity of entorhinal cortex and hippocampus (Kjelvik et al., 2012), but it remains to be seen whether these structures can be linked to smell identification deficits in schizophrenia. While functional imaging methods (e.g., PET, SPECT, fMRI) have shown decreased activation in schizophrenia in limbic as well as frontal and temporal regions in response to olfactory cues (e.g., Crespo-Facarro et al., 2001; Malaspina et al., 1998; Schneider et al., 2007), only electrophysiological correlates of information processing, with far greater temporal resolution, can provide direct, ‘real-time’ measures of olfactory function in schizophrenia and its risk states. Because event-related potentials (ERPs) trace the sequence of information processing by indexing neuronal activity, ERP components (e.g., N1, P2, P3), time-locked to the onset of sensory events, reflect brain activity representative of the underlying neurophysiologic processes associated with successive stages of stimulus information processing. These characteristics, in combination with their cost-effectiveness and development of advanced data analytic techniques, have been recognized as offering unique opportunities to identify and study translational biomarkers in schizophrenia (e.g., Javitt et al., 2008).
1.2. Neurophysiologic abnormalities in the psychosis prodrome
There is ample evidence of neurophysiologic abnormalities in schizophrenia and unaffected relatives for processing auditory or visual stimuli, although prominent ERP reductions, such as the decrease in P3 amplitude, are not specific to schizophrenia (e.g., Ford, 1999; Javitt et al., 2008; Winterer et al., 2003). Of relevance for high risk studies, P3 amplitude reduction has elements of being both a state and trait marker of schizophrenia (e.g., Mathalon et al., 2000). In CHR patients, several studies have reported abnormalities of auditory P3 amplitude (e.g., Frommann et al., 2008; van Tricht et al., 2010; van der Stelt et al., 2005) and duration mismatch negativity (e.g., Atkinson et al., 2012; Bodatsch et al., 2011; Shaikh et al., 2012), as well as for visual ERPs during recognition of facial affect (e.g., Wölwer et al., 2012), which has been linked to odor identification in schizophrenia (Kohler et al., 2007).
In contrast to electrophysiologic studies probing the auditory and visual modality, olfactory ERPs have rarely been used due to methodological challenges linked to the precise timing of odor stimulation (e.g., Lorig, 2000), but the limited evidence suggests that abnormal olfactory ERPs may be a vulnerability marker for schizophrenia. Compared to healthy controls, schizophrenia patients showed reduced N1 and P2 amplitudes to three different concentrations of hydrogen sulfide (H2S) despite similar ratings of odor intensity (Turetsky et al., 2003a), and similar reductions of N1 (left nostril only) and P2 (bilaterally) were observed in first degree relatives of patients with schizophrenia (Turetsky et al., 2008). Moreover, family members had increased odor detection thresholds for the left nostril, and showed poorer odor identification for both nostrils as measured by the University of Pennsylvania Smell Identification Test (UPSIT; Doty et al., 1984), thereby supporting smell identification deficit as a candidate endophenotype for schizophrenia (Brewer et al., 2003). Using an odor detection task with two concentrations of H2S, we replicated and extended olfactory ERP findings for schizophrenia patients (Kayser et al., 2010). The patients (n = 32) showed regional amplitude reductions of N1 over inferior frontotemporal sites and of P2 over medial parietal sites, despite patients having similar odor detection performance as healthy controls (n = 35).
Olfactory ERPs have not yet been evaluated in CHR patients, namely help-seeking young people with attenuated psychotic symptoms and/or functional decline in the context of genetic risk. For “persons at risk” identified within a sample of 948 young adults who scored in the uppermost deciles on German scales for physical anhedonia and/or perceptual aberration, Becker et al. (1993) reported a reduction in P1/N1 peak-to-peak amplitude at vertex after left nostril stimulation with H2S. However, although this early study provided some evidence for an abnormality in processing of odor stimuli possibly related to risk for psychosis in young adults, several methodological weaknesses considerably limit the value of this report. The present study sought to improve on these shortcomings, including ascertainment of the clinical high-risk status for psychosis, the use of a complete EEG montage, and the application of unbiased, data-driven statistics.
1.3. Methodological issues in olfactory ERP research
Limitations in ERP methodology with regard to the study of olfactory function have been discussed previously (Kayser et al., 2010). Briefly, most olfactory ERP studies have used peak and latency measurements of “prominent” deflections in individual ERP waveforms at midline or central scalp locations (i.e., Cz, Pz, C3/4) referenced to linked ears (cf. recommendations by Evans et al., 1993). However, the choice of the EEG recording reference for surface potentials is arbitrary, with linked ears, linked mastoids, nose, or common average reference schemes likely rendering a different ERP morphology (i.e., sequence and location of “prominent” deflections), and thereby potentially masking effects of interest (e.g., Kayser & Tenke, 2010). A related problem is the operational definition of an ERP component by means of identifying the “obvious” ERP waveform peaks (or approximations thereof by determining appropriate time integrals), and the selection of scalp regions or sites for statistical analysis, all of which is affected by the reference choice. However, these problems can be efficiently addressed by combining temporal principal components analysis (PCA) and current source density (CSD) methods (e.g., Kayser & Tenke, 2003, 2005, 2006a, 2006b).
CSD, also known as the scalp surface Laplacian, provides a representation of current generators underlying an ERP topography, which reflects the magnitude of radial current flow entering (source) and leaving (sink) the scalp (e.g., Nunez & Srinivasan, 2006). CSD analysis is a reference-free technique because any EEG recording reference scheme will yield the same, unique CSD transform for a given EEG montage. A CSD transform yields sharper topographies compared to those of scalp potentials, and also reduces redundant contributions due to volume conduction (e.g., see review by Tenke & Kayser, 2012), which also enhances the temporal resolution of the component structure. CSD waveform topographies faithfully summarize and simplify the putative generators of a scalp potential topography, and therefore represent a common bridge between scalp-recorded EEG and the underlying neuronal generators (Tenke & Kayser, 2012). The entire set of CSD waveforms can then be submitted to temporal PCA to identify relevant, data-driven components in the form of unique, orthogonal variance factors associated with generator patterns underlying stimulus processing, and thereby provide a concise and unbiased summary of the observed ERP/CSD activity (e.g., Kayser & Tenke, 2003, 2005, 2006a, Kayser et al., 2007).
For H2S stimuli, this CSD-PCA strategy has supported the importance of N1 and P2 as two distinct ERP components reflecting distinct, sequential stages of odor processing (Lorig, 2000). Our previous study (Kayser et al., 2010) revealed prominent bilateral N1 sinks over lateral frontotemporal sites, along with a corresponding mid-frontopolar source, presumably reflecting an early (about 300 ms), modality-specific processing stage during odor perception, with putative generators within the medial temporal lobe and/or basal cortical regions (i.e., piriform cortex and orbital frontal cortex; cf. Martzke et al., 1997; Seubert et al., 2013). Importantly, this prominent negative deflection is substantially attenuated at lateral temporal recording sites in surface potentials when a linked-mastoid, linked-ears or nose reference is used because the generator underlying an olfactory N1 evidently creates an isopotential line involving these common reference locations and lateral-inferior sites (i.e., T7/8, FT9/10, P9/10). These common reference schemes yield a smaller, volume-conducted N1 at midline sites, which is nevertheless considered an integral part of the basic olfactory ERP morphology (e.g., Turetsky et al., 2003a). In contrast, the ensuing olfactory P2 is not compromised by a linked-ear or -mastoid reference. It corresponds to a robust P2 source spanning mid-centroparietal regions, is accompanied by current sinks over lateral frontotemporal sites, and appears to reflect odor evaluation, as its topography resembles the P3 source associated with a classical P3b (e.g. Lorig, 2000; Olofsson et al., 2008). Taken together, this N1/P2 complex may be the olfactory equivalent of an N2/P3 complex in the auditory or visual modality, perhaps with similar functional properties (Kayser et al., 2010).
1.4. The present study
Despite evidence of olfactory dysfunction in schizophrenia and suggestions that it may be an endophenotypic marker of this disorder, there have been few studies of olfaction in CHR individuals (e.g., Brewer et al., 2003; Kamath et al., 2011; Woodberry et al., 2010). None of these studies employed direct electrophysiologic measures of olfactory cortical function. The present study sought to fill this gap by measuring olfactory ERPs in CHR patients and age- and gender-matched healthy participants. Among several procedural improvements to our previous study in schizophrenia (Kayser et al., 2010), we: (a) employed an odor detection task with three different concentrations of H2S (strong, medium, weak) and blank air as a control condition; (b) increased the spatial resolution from a 31- to a denser 49-channel EEG montage to further refine the characterization of current generators underlying distinct olfactory ERP components (N1, P2); and (c) used randomization tests of component topographies (cf. Kayser et al., 2007) as a tool to identify regions associated with odorspecific stimulus processing (H2S vs. blank air). Following our previous developments in ERP analysis (e.g., Kayser & Tenke, 2003, 2006a, 2006b), we relied on a combined CSD-PCA approach to obtain meaningful and unique olfactory ERP component measures that are independent of the EEG recording reference and therefore have an unambiguous polarity and topography (Tenke & Kayser, 2012). Given our prior olfactory ERP findings (Kayser et al., 2010), we hypothesized that both N1 sink and P2 source would show monotonic increases in amplitude paralleling increases in odor intensity, with each component characterized by an activation topography that is significantly different from non-odor (blank air) stimulation. It was further predicted that CHR patients as a group would exhibit reductions of N1 sink and P2 source and their monotonic increases, although possibly to a lesser degree than seen for schizophrenia patients (Kayser et al., 2010).
In addition to obtaining standardized measures of odor detection thresholds and odor identification (Kobal et al., 2000), odor detection sensitivity was measured during the olfactory ERP paradigm to estimate the extent to which the parametric manipulation of odor intensity reflects a co-variation of the ability to detect odors and their electrophysiologic correlates. Given that the abnormal olfactory ERPs in family members were associated with odor identification, a predictor for development of schizophrenia in CHR patients (Brewer et al., 2003), a secondary focus was whether electrophysiologic, behavioral and nasal chemosensory performance measures of olfactory function in CHR patients may improve prediction of transition to schizophrenia. Finally, it was hypothesized that deficits in olfactory function would be associated with severity of negative symptoms (Brewer et al., 2001; Corcoran et al., 2005; Good et al., 2006; Malaspina & Coleman, 2003; Moberg et al., 2006).
2. Material and Methods
2.1. Participants
Twenty-one CHR patients (13 male, 8 female) were ascertained from the Center of Prevention & Evaluation (COPE) at New York State Psychiatric Institute at Columbia University, a clinical research program that evaluates and treats adolescents and young adults (ages 12–30) who are considered at heightened clinical risk for psychosis on the basis of attenuated psychotic symptoms and/or genetic risk in the context of functional decline. Patients are followed for up to four years to determine transition to psychotic disorder, typically schizophrenia. Patients were compared in cross-section to 20 healthy volunteers (13 male, 7 female), who were ascertained from the same source population in the New York metropolitan area using flyers, brochures, and the internet. All participants received US$10/hr plus an extra US$10 travel compensation for each research appointment. Although the initial sample consisted of 24 patients and 21 controls, the data of 3 patients and 1 control had to be excluded due to technical issues during the olfactory EEG recordings. Demographic and clinical characteristics of the final sample are summarized in Table 1. Participants, who had no history of neurological illness or substance abuse, were between 13 and 27 years of age (median 22 years), had between 9 and 19 years of education (median 14 years), and were primarily right-handed (mean laterality quotient 71.9 ± 7.4; Oldfield, 1971); there were no significant differences between patients and controls in these demographic variables. Likewise, the small number of smokers did not differ between patients (n = 3) and controls (n = 2), χ2(1) = 0.18, n.s. = .41. Participants were instructed to refrain from smoking or applying any cosmetic fragrance on the day of testing. Olfactory ERP (OERP) recording sessions, which lasted about 1.5 h, were scheduled between 2 and 6 pm to control for putative circadian influences on chemosensory ERP amplitudes (Nordin et al., 2003). Time of testing did not differ between groups, F(1, 37) < 1.0, n.s. Following the OERP recordings, participants also performed a novelty oddball ERP paradigm (Bruder et al., 2009; Tenke et al., 2010), and these results will be reported elsewhere (Kayser et al., submitted).
Table 1.
Means, standard deviations (SD), and ranges for demographic and clinical variables
| Prodromal Patients (n = 21; 13 male; 3 smokers) |
Healthy Controls (n = 20; 13 male; 2 smokers) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Range | Mean | SD | Range | F | p |
| Age (years) | 21.4 | 3.8 | 13 – 27 | 21.7 | 3.3 | 16 – 27 | ||
| Education (years) | 13.7 | 2.3 | 9 – 18 | 14.4 | 1.8 | 12 – 18 | ||
| Handedness (LQ) a | 65.7 b | 36.9 | −40 – 100 | 78.8 c | 49.1 | −100 – 100 | ||
| SOPS positive d | 11.0 | 4.2 | 4 – 20 | 0.7 | 1.1 | 0 – 3 | 98.9 | <.0001 |
| SOPS negative d | 12.3 | 6.1 | 3 – 27 | 1.1 | 1.7 | 0 – 6 | 53.5 | <.0001 |
| SOPS disorganization d | 7.0 | 3.3 | 1 – 14 | 0.4 | 0.8 | 0 – 2 | 65.2 | <.0001 |
| SOPS general d | 8.6 | 4.2 | 0 – 14 | 0.5 | 1.1 | 0 – 4 | 72.9 | <.0001 |
| SOPS modified GAF d | 46.8 | 6.5 | 38 – 60 | 83.6 | 7.1 | 68 – 95 | 273.4 | <.0001 |
Note. Only F ratios with p < .10 are detailed (df = 1, 37).
Laterality quotient (Oldfield, 1971) can vary between −100.0 (completely left-handed) and +100.0 (completely right-handed).
n = 16.
n = 18.
Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS; Miller et al., 2003) subscales (possible range): positive symptoms (0 #x02013; 30); negative symptoms (0 – 36); disorganization symptoms (0 – 24); general symptoms (0 – 24); modified global assessment of function score (0 – 100).
All participants were screened with the Structured Interview for Prodromal Syndromes and Scale of Prodromal Symptoms (SIPS/SOPS; Miller et al., 2003). The inclusion/exclusion criteria were largely identical to those described by Piskulic et al. (2012) and included: 1) meeting criteria for at least one of three prodromal syndromes using the SIPS/SOPS; 2) no current or lifetime Axis I psychotic disorder; 3) IQ greater than 70 (Wechsler Adult Intelligence Scale [WAIS] or Wechsler Intelligence Scale for Children [WISC] only administered if low IQ suspected); and 4) no current or past CNS disorder (medical or psychiatric) which may account for prodromal symptoms. CHR patients differed highly significantly from healthy controls in all SOPS subscales in the expected direction (cf. Table 1). There were no group × gender interactions or gender main effects for any of the SOPS subscales. Notably, the SOPS negative symptoms were highly comparable to what has been reported for a larger cohort (Piskulic et al., 2012), as deterioration in role function was most prominent (with 81% of the current CHR patients reporting a score of 3 or higher), followed by social isolation and withdrawal (71%), avolition (57%), and decreased experience of emotion (48%), with decreased expression of emotion (14%) and decreased ideational richness (14%) being the least frequently reported negative symptoms. The current mean (±SD) total SIPS negative symptom score (12.2 ±6.1) was intermediate between that previously reported for males (13.6 ±7.3) and females (8.9 ±6.6; cf. Piskulic et al., 2012). All CHR patients in the current study met criteria for the attenuated positive symptom syndrome of the SIPS/SOPS.
The ethnic composition in both groups was representative for the New York region, including 15 Caucasian, 10 African-American, 3 Asian, 9 individuals of more than one race, and 4 participants with race unknown. The experimental protocol had been approved by the institutional review board and was undertaken with the understanding and written consent of each participant.
Since the time of the OERP sessions (from November 2009 to May 2011), the prospective followup identified three CHR patients (1 male, 2 female) who developed threshold psychosis as determined by the “Presence of Psychosis” criteria in the SIPS/SOPS (i.e., a score of 6 on one of the five positive symptom domains; cf. Miller et al., 1999, 2003). These ‘converters’ were 16, 23 and 27 years old at the time of testing, with 10, 14 and 17 years of education, respectively. Their SIPS/SOPS scores were largely comparable to the overall patient sample, although converters tended to show more negative symptoms (M ± SD, positive, 9.3 ± 5.0; negative, 19.0 ± 6.9; disorganization, 9.3 ± 4.0; general, 12.0 ± 1.7; global assessment of function, 40.3 ± 2.1). Given that investigating whether olfactory deficits have predictive value for transition to psychosis in CHR individuals is the underlying purpose of the current study, descriptive summaries of the core dependent measures are also separately reported for these three converters.
2.2. Stimuli and procedure
The study builds on the procedure outlined in our previous OERP report (Kayser et al., 2010). Participants were seated in an IAC (Industrial Acoustics Company) sound-attenuated booth using a chin and forehead rest, with a video camera monitoring participants’ compliance and behavior. They were instructed to focus on a monitor that presented visual cues signaling the interval when an odor may be present and when to respond, and to keep breathing normally through the nose but not the mouth (velopharyngeal closure as an active breathing technique was not incorporated; cf. footnote 2 in Kayser et al., 2010; Seubert et al., 2013). H2S stimuli (10 ppm, Scott Speciality Gases, Plumsteadville, PA) at strong (undiluted), medium (diluted to 70%) or weak (diluted to 40%) concentrations or blank air were delivered to the left or right nostril by a constant-flow olfactometer (OM2s, Heinrich Burghart GmbH, Wedel, Germany) through a Teflon™ tube inserted approximately 1 cm into the naris. Stimulus duration was 200 ms (not more than 50 ms rise time according to manufacturer specification). For any given session, the air stream at the exit of the olfactometer had a constant flow rate (about l/min), temperature (the measured range was 40° –44 °C at the olfactometer’s head to approximately 37 °C body temperature when entering the nasal cavity), and relative humidity (above 80%). White noise of approximately 75 dB SPL was presented binaurally via Telephonics TDH-49P earphones to preclude hearing the switching valves.
Odor and blank air stimuli were presented in 8 blocks of 20 trials each (160 total trials), with a variable stimulus onset asynchrony (SOA 14.5–20.5 s). A trial was initiated with the foveal presentation of digits counting down in 1-s intervals from 3 to 1. This was followed by the display of a smell icon which remained on the screen for 5.5 s, with an odor or blank air presented within 2 and 4 s. The smell icon was replaced by the question ‘Did you smell anything?’ above a picture of two foot pedals labeled ‘Yes’ and ‘No,’ prompting participants for 2.5 s to indicate whether or not they had detected an odor by pressing the corresponding foot pedal with the left or right foot. A variable delay interval warranted an average SOA of 17.5 s. Each of the three H2S concentrations and blank air were presented 40 times, and for any given block, stimuli were delivered to either the right or left nostril (4 blocks each in a counterbalanced order). Stimuli were presented in a pseudorandomized sequence based on Latin squares, with each of the four conditions occurring once within four consecutive trials. Before beginning the OERP test, participants were given four practice trials to ensure that they understood the task.
Using a standardized screening test of nasal chemosensory performance (Sniffin’ Sticks; Hummel et al., 1997; Kobal et al., 2000), odor identification and thresholds were assessed for each participant immediately before the OERP test. For testing odor thresholds, 2-phenylethanol (rose-like smell) was used as a pure olfactory alternative to n-butanol because of concerns of trigeminal co-activation with higher odor concentrations (Doty et al., 1978; Jacquot et al., 2004), as both odorants have produced reliable threshold results and were equally good to distinguish between patients and normosmic subjects (Croy et al., 2009).
2.3. Data acquisition, recording, and artifact procedures
Continuous EEG, stimulus onset, response and all other trigger codes were recorded at 200 samples/s with a gain of 10k within .01–30 Hz (−6dB/octave) using a 48-channel Grass Neurodata acquisition system and NeuroScan software (NeuroScan, 1993). A Lycra stretch electrode cap with tin electrodes was used for an expanded 10–20 scalp montage (Pivik et al., 1993; Jurcak et al., 2007) consisting of 10 midline (Nz, Fpz, AFz, Fz, FCz, Cz, Pz, POz, Oz, Iz) and 19 homologous pairs of scalp placements over each hemisphere (Fp1/2, AF7/8, F9/10, F7/8, F3/4, FT9/10, FC5/6, FC1/2, T7/8, C3/4, TP9/10, TP7/8, CP5/6, CP1/2, P9/10, P7/8, P3/4, PO7/8, O1/2). All electrode impedances were maintained at or below 5 kΩ. EEG was recorded with a nose tip reference, and the implicit nose reference channel was added to the montage offline. Cap placement was optimized by precise measurements of electrode locations with respect to landmarks of the 10–20 system (nasion, inion, auditory meatus, vertex).
Bipolar eye activity (left and right outer canthi; above and below right eye) was estimated from the raw data by spherical spline interpolation (Perrin et al., 1989) to monitor lateral eye movements and blinks. However, volume-conducted blink artifacts were removed from the raw EEG by spatial PCA generated from identified blinks and artifact-free EEG periods (NeuroScan, 2003). Recording epochs of 2,000 ms (250 ms prestimulus baseline) were extracted off-line, tagged for A/D saturation, and lowpass filtered at 50 Hz (−24 dB/octave). A reference-free approach identified residual artifacts on a channel-by-channel and trial-by-trial basis (Kayser & Tenke, 2006d). Artifactual surface potentials were replaced by spherical spline interpolation (Perrin et al., 1989) using the data from artifact-free channels if possible (i.e., when less than 25% of all EEG channels contained an artifact); otherwise, a trial was rejected.
Separate OERPs for strong, medium and weak odor intensity and blank air were averaged from artifact-free trials using the entire 2-s epoch. To obtain more stable waveforms, ERPs were pooled across nostrils because of their blocked presentation order (cf. Kayser et al., 2010) and because the side of odor stimulation is ranked as less important when measuring OERPs (e.g., Olofsson et al., 2006; Stuck et al., 2006). The means for the number of trials (±SD) used to compute these OERP averages were 27.8 ±5.0, 28.9 ±5.5, 30.1 ±4.1, and 32.8 ±5.5 (strong, medium, weak intensity and blank air, respectively) for CHR patients, and 31.0 ±4.2, 30.7 ±4.1, 32.1 ±5.8, and 33.5 ±4.5 for healthy controls (no fewer than 16 trials per OERP average), and there were no significant differences between patients and controls, F(1, 37) = 1.32, p = .26. ERP waveforms were screened for electrolyte bridges (Tenke & Kayser, 2001), low-pass filtered at 12.5 Hz (−12 dB/octave), and baseline-corrected using the 100 ms preceding stimulus onset.
As an additional preprocessing step, temporal PCA was employed as an effective filter to reduce or eliminate persistent drifts. All OERP waveforms (41 participants, 49 sites, 4 conditions: 8,036 cases; −250 to 1,750 ms: 401 variables) were submitted to a covariance-based PCA, followed by Varimax rotation of all covariance loadings (Kayser & Tenke, 2003). The time course of the first extracted factor (55% explained variance) was characterized by a monotonic, virtually linear, increase from the baseline to the end of the recording epoch, which accounted for unsystematic drifts across conditions, sites, and participants. By virtue of the linear decomposition, this unsystematic variance was removed from the data by reconstructing the surface potentials from all but the first factor loadings and corresponding factor scores and the grand mean (i.e., summed factor loadings multiplied by corresponding factor scores plus grand mean waveform; cf. Sinai & Pratt, 2002; Tenke et al., 2011). The drift-corrected ERPs were re-referenced to linked mastoids (TP9/10) for comparison to prior OERP studies using linked ear lobes or mastoids as reference.
2.4. Current Source Density (CSD) and Principal Components Analysis (PCA)
As in our previous study (Kayser et al., 2010), OERP waveforms were transformed into CSD estimates (µV/cm2 units; 10 cm head radius; 50 iterations; m = 4; smoothing constant λ = 10−5) using a spherical spline surface Laplacian (Perrin et al., 1989; Kayser & Tenke, 2006a, 2006b; Kayser, 2009). To determine their common sources of variance, CSD waveforms were submitted to temporal PCA derived from the covariance matrix, followed by unrestricted Varimax rotation of the covariance loadings (Kayser & Tenke, 2003, 2006c). The input data matrix consisted of 301 variables (time interval −100 to 1,400 ms) and 8,036 observations stemming from 41 participants, 4 odor conditions, and 49 electrode sites. By virtue of the reference-independent Laplacian transform (see Tenke & Kayser, 2012, for a review), CSD factors have an unambiguous component polarity and topography.
As is common when using temporal PCA as a multivariate, linear data decomposition approach for ERP analysis, there is no need to back-project the extracted factors into the original data space (in this case, µV/cm2) because the associated factor scores already provide optimal quantifications of the factors (e.g., Chapman & McCrary, 1995; Donchin & Heffley, 1978; Kayser & Tenke, 2003; van Boxtel, 1998). In case of a covariance-based temporal PCA, the factor scores can be considered as weighted time window integrals (i.e., amplitudes) for each factor, with the additional statistical benefit of having a mean of zero (across all cases) and a standard deviation of one (Kayser & Tenke, 2003). Thus, it may be not surprising that these factors describe the variance contributions of temporally and spatially overlapping ERP or CSD components more efficiently than conventional measures, such as baseline-to-peak or integrated time windows, yielding larger effect sizes and higher reliabilities (e.g., Beauducel et al., 2000; Beauducel & Debener, 2003; Kayser et al., 1997, 1998).
2.5. Statistical analysis
Factor scores of two targeted CSD-PCA factors corresponding to N1 and P2 were submitted to repeated measures ANOVAs with group (patients, controls) and gender (male, female) as betweensubjects factors, and odor intensity (weak, medium, strong) as a within-subjects factor. As there were no specific hypotheses regarding sex differences for olfactory function in the CHR patients, and because the sample included almost twice as many male than female participants in each group, gender was only considered as a control factor in all statistical analyses. The selection of recording sites for comparing experimental effects in these ANOVAs was guided by our previous findings using a 31-channel EEG montage (Kayser et al., 2010) and by means of randomization tests (cf. Maris, 2004; Mewhort et al., 2010) evaluating the topographic differences between H2S stimuli pooled across intensities and blank air. For a given CSD factor, randomization distributions (10,000 repetitions) were estimated from the observed data of the entire sample (N = 41) to compute univariate (channel-specific) T2 statistics for paired samples (see Kayser et al., 2007, for computational details), which allowed determination of regional sink and source activity in response to H2S stimulation as compared to blank air. Significant differences were used to identify individual sites or subsets of sites to be included in the conventional repeated measures ANOVA, which consisted of either midline sites or lateral, homologous recording sites over both hemispheres, and thereby adding either site, or site and hemisphere as within-subjects factors to the design. However, because recording sites were selected on the premise that they collectively represent sink or source activity associated with odor detection, site effects were not further pursued in these analyses.
For analyses of the behavioral data, percentages of correct responses (‘yes’ to odors, ‘no’ to blank air) were submitted to repeated measures ANOVA with condition (weak, medium, strong, blank air) as within-subjects factor, and group and gender as between-subjects factors. A d’-like sensitivity measure dL (logistic distribution; Snodgrass & Corwin, 1988) was calculated from the hit rates for each odor intensity and the false alarm rates for blank air and submitted to a similar ANOVA using a three-level within-subjects factor condition (weak, medium, strong).
For nasal chemosensory performance (Sniffin’ Sticks), odor thresholds were analyzed via a repeated measures ANOVA with nostril (left, right) as a within-subjects factor, and group and gender as between-subjects factors, and odor identification was analyzed via an ANOVA with group and gender.
Simple effects (BMDP-4V; Dixon, 1992) provided means to systematically examine interaction sources, or to further explore group effects even in the absence of superordinate interactions. When appropriate, Greenhouse-Geisser epsilon (ε) correction was used to compensate for violations of sphericity (e.g., Keselman, 1998). A conventional significance level (p < .05) was applied for all effects.
Pearson’s correlations were used to evaluate associations between nasal chemosensory performance, behavioral and electrophysiological measures of olfactory function separately for each group, and also with the clinical variables for patients only. In addition, the parametric manipulation of odor intensity was exploited to compute within-subjects Pearson’s correlations between odor concentrations (i.e., assuming fixed H2S dilutions of 40%, 70%, or 100%), behavioral (dL) and CSD measures (i.e., using pairwise observations for the three levels of odor intensity), which were then Fisher z-transformed, averaged, back-transformed to correlation coefficients for interpretability, and assessed using conventional inference statistics (df = n − 2). Given our a priori hypotheses about the direction of these associations (e.g., better performance coupled with greater CSD amplitudes, or poorer odor identification linked to more negative symptoms), one-tailed significance levels are reported.
3. Results
3.1. Odor thresholds and odor identification
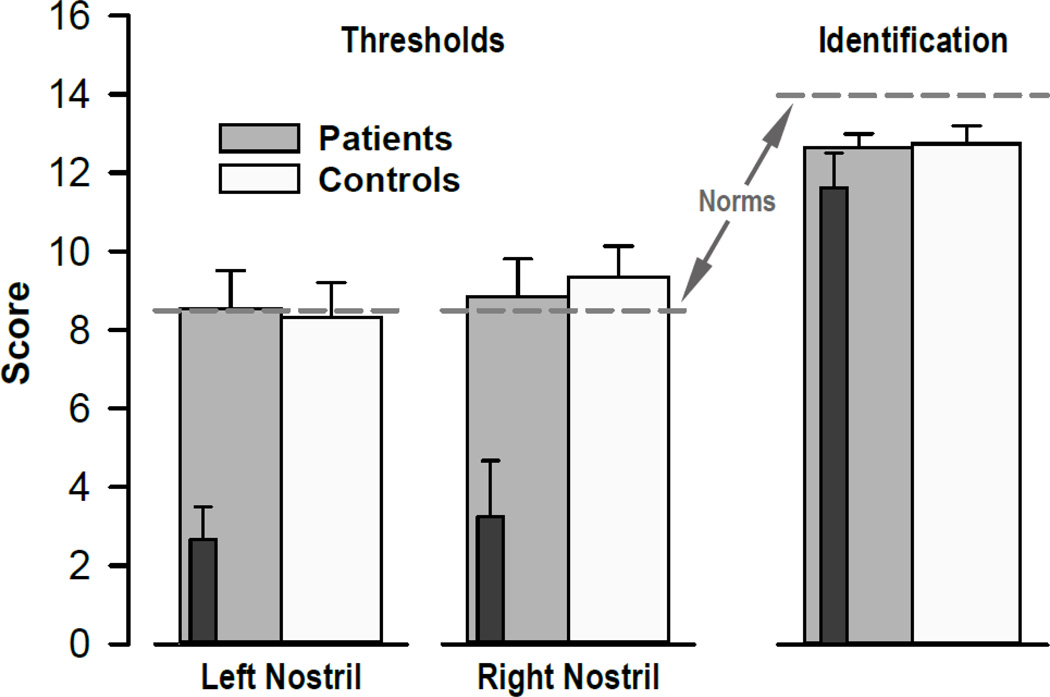
Nasal chemosensory performance (Sniffin’ Sticks) was well within the reported normal range (Kobal et al., 2000) for healthy controls and CHR patients (Fig. 1). There were no significant group main effects or group × nostril interaction effects for odor thresholds or odor identification (all F < 1.0), indicating preserved olfactory function in CHR patients. However, the three converters, while showing normal odor identification, had substantially poorer odor thresholds (cf. inserts in Fig. 1).
Figure 1.
Mean scores (±SEM) of odor thresholds and odor identification (Sniffin’ Sticks) for HR patients and healthy controls. Dark insert bars reflect nasal chemosensory performance for three converters. Dashed lines indicate published normative values (medians) for healthy controls aged 16 to 35 years (Kobal et al., 2000).
3.2. Behavioral data
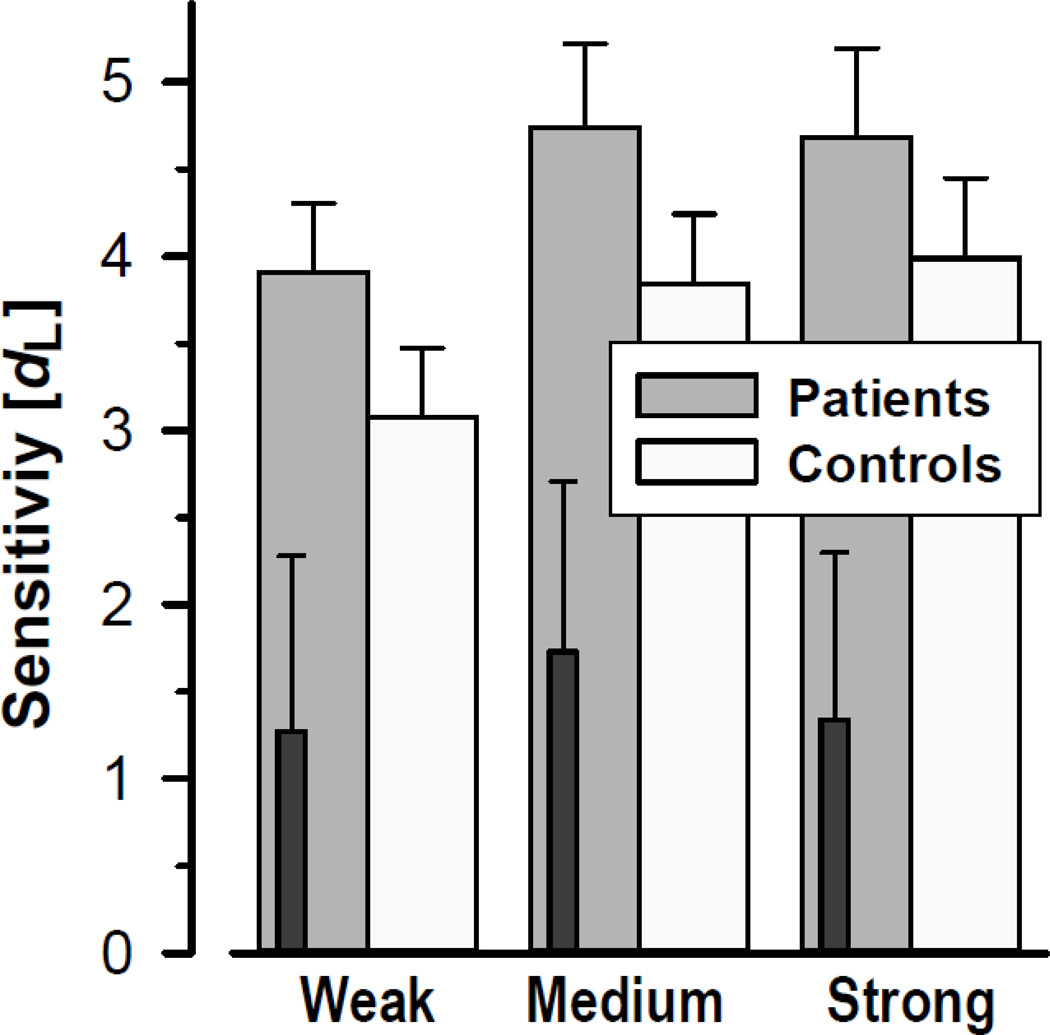
Table 2 summarizes the behavioral performance during the OERP paradigm. CHR patients and healthy controls correctly rejected blank air at a rate of almost 90% and detected the presentation of H2S stimuli, with detection accuracy improving with greater odor intensity. Independent of odor intensity, patients had greater performance accuracy than controls (Tab. 2, left column). Although these effects were essentially also observed in the sensitivity measure dL (Fig. 2), only the increase in odor detection performance was preserved in the corresponding repeated measures ANOVA (Tab. 2, right column), suggesting that a difference in response bias between patients and controls contributed to the significant group difference in odor detection accuracy.
Table 2.
Behavioral data summary: Grand means (±SD) and ANOVA F ratios
| Correct Responses [%] | Sensitivity [dL] | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Weak | Medium | Strong | Air | Weak | Medium | Strong | |
| Patients | 69.4 | 81.0 | 80.5 | 89.2 | 3.90 | 4.74 | 4.68 | |
| ±19.3 | ±15.8 | ±17.5 | ±15.8 | ±1.83 | ±2.19 | ±2.32 | ||
| Controls | 56.8 | 71.8 | 73.6 | 89.4 | 3.07 | 3.84 | 3.99 | |
| ±15.6 | ±14.3 | ±15.2 | ±12.8 | ±1.79 | ±1.80 | ±2.06 | ||
| Effect a | df | F | p | ε | df | F | p | ε |
| Group | 1, 37 | 4.15 | .049 | 1, 37 | 2.38 | |||
| Condition | 3, 111 | 31.1 | <.0001 | 0.5184 | 2, 74 | 30.0 | < .0001 | 0.9959 |
| Condition × Group | 3, 111 | 2.38 | 2, 74 | 0.24 | ||||
Note
Only F ratios with p < .10 are detailed.
Figure 2.
Mean (±SEM) sensitivity (dL) of odor detection as a function of odor intensity, revealing a monotonic increase for both CHR patients and healthy controls. Dark insert bars reflect behavioral performance for three converters.
As with odor thresholds, the three converters showed markedly poorer odor detection for all H2S intensities, which also failed to reflect the increase in odor concentration (cf. inserts in Fig. 2).
3.3 Electrophysiologic data
3.3.1. Grand mean ERP and CSD waveforms
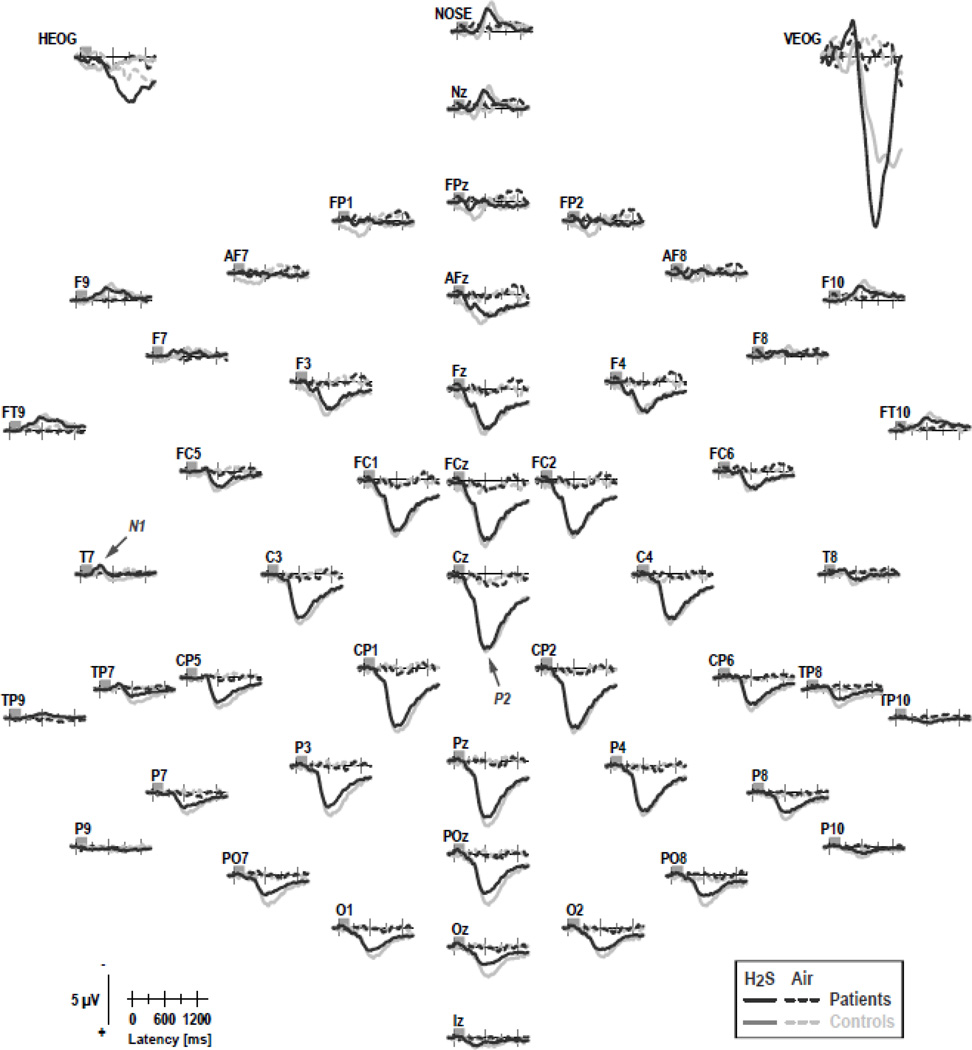
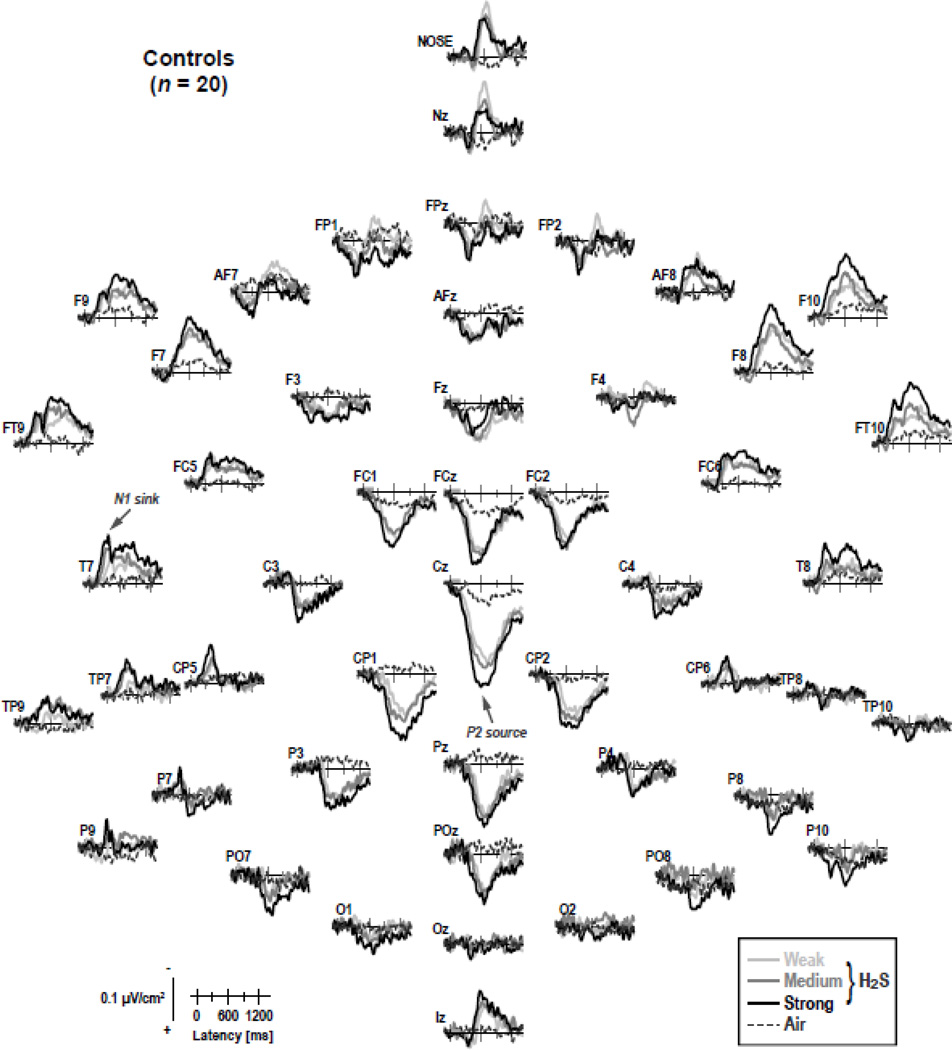
Figure 3 shows the grand mean ERP waveforms (referenced to linked mastoids) for patients and controls for H2S stimuli (pooled across intensity) and blank air at all 49 recording sites. As in previous OERP studies using H2S stimuli (e.g., Kayser et al., 2010; Turetsky et al., 2003b, 2008), a prominent P2 at about 600 ms, which was broadly distributed over centroparietal sites, and an earlier N1, peaking at 350 ms at lateral-temporal sites, were present in both groups. In contrast, ERPs to blank air were virtually flat at all recording sites, indicating that any procedural requirements specific to H2S detection (e.g., those related to stimulus delivery, visual cuing, odor expectation, or foot pedal response) were not causing these OERP components. Similarly, although considerable eye movements, particularly blinks, were associated with H2S stimuli, eye artifacts, which occurred mostly beyond 600 ms, were effectively eliminated from the EEG traces.
Figure 3.
Grand mean olfactory ERP [µV] waveforms (−100 to 1400 ms, 100 ms pre-stimulus baseline) referenced to linked mastoids of CHR patients and healthy controls for H2S stimuli (pooled across intensity) and blank air at all 49 recording sites. Horizontal and vertical electrooculograms (EOG) are shown before blink correction. Two prominent ERP components identified in previous studies are labeled at sites T7 (N1) and Cz (P2).
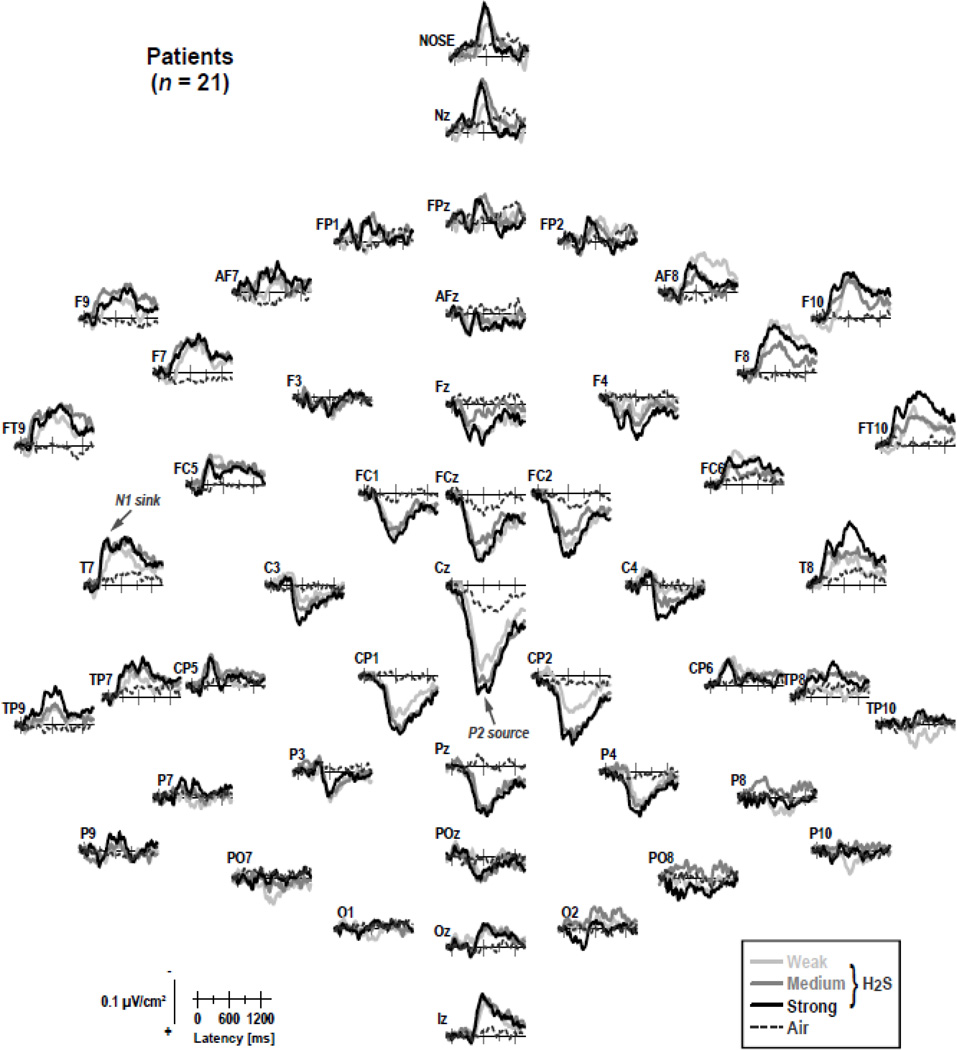
Figures 4 and 5 show the CSD transformations of the ERP waveforms separately for patients and controls, comparing differences for H2S intensities and blank air (the corresponding ERPs referenced to the nose or to the average of all recording sites are shown in supplementary Figures S1–S4).1 A robust centroparietal P2 source, peaking between 500 and 800 ms, was accompanied by lateral-frontotemporal (F9/10, FT9/10, F7/8), mid-anterior (Nz, Nose), and inferior-occipital (Iz) sinks. However, these sinks differed from lateral-temporal (T7/8) and frontocentral (FC5/6) N1 sinks, which peaked between 250 and 450 ms over each hemisphere. Both N1 sink and P2 source, which directly corresponded to the N1 and P2 potentials in the OERP waveforms, and were present in both patients and controls, revealed a monotonic increase in amplitude with an increase in odor intensity, closely corresponding to our previous findings for low and high H2S concentrations (Kayser et al., 2010).
Figure 4.
Reference-free current source density ( SD) [µV/cm²] waveforms for 21 HR patients comparing weak, medium and strong H2S stimuli and blank air at all 49 recording sites. Two prominent CSD components are labeled at sites T7 (N1 sink) and Pz (P2 source), where they closely corresponded to their ERP counterparts.
Figure 5.
CSD waveforms as in Figure 4 for 20 healthy controls.
3.3.2. PCA component waveforms and topographies
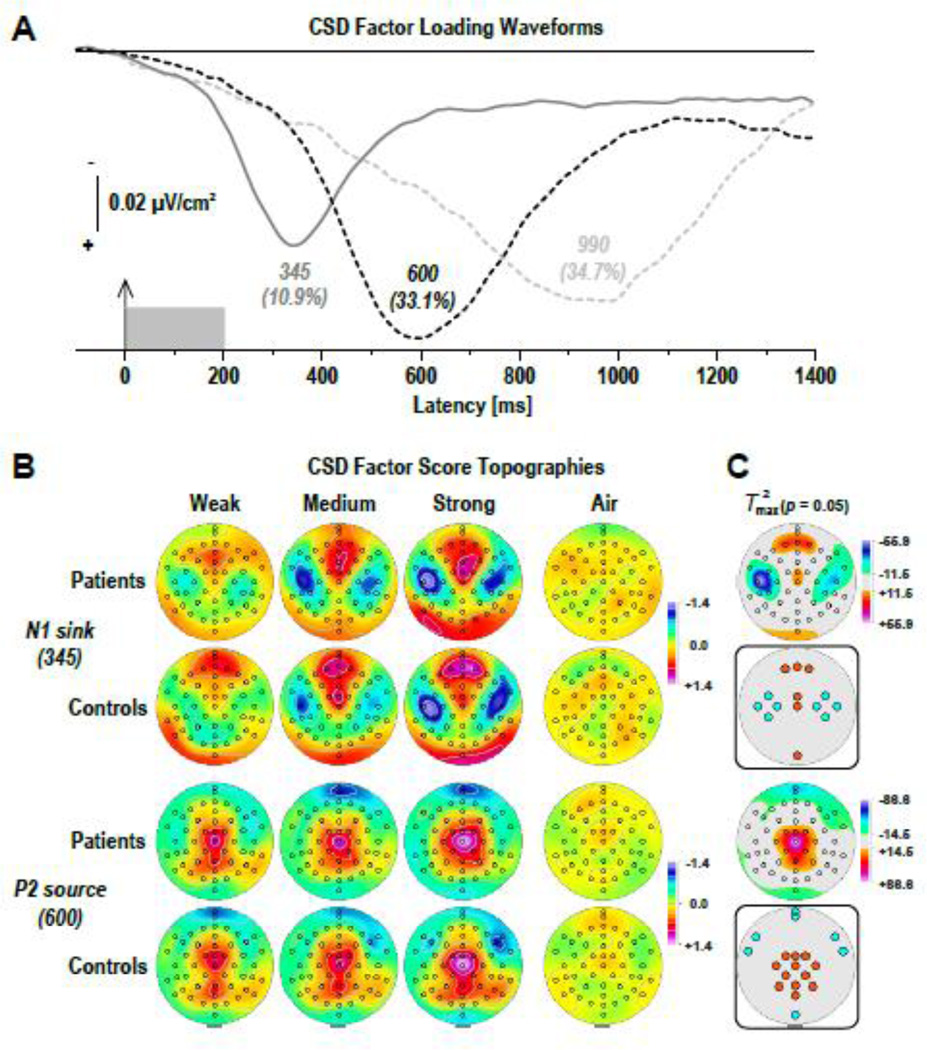
Figure 6 shows the time courses of factor loadings for the first three CSD factors extracted (78.6% explained variance after rotation) and the corresponding factor score topographies of the two targeted CSD components, separately plotted for odor conditions and groups. Labels reflect the peak latency of the factor loadings relative to stimulus onset.
Figure 6.
(A) Factor loadings of the first three PCA factors (with explained variance) extracted from olfactory CSD waveforms (N = 41). (B) CSD factor score topographies corresponding to N1 sink (top) and P2 source (bottom) for 21 CHR patients and 20 healthy controls comparing H2S stimuli of weak, medium, and strong intensity and blank air. (C) Squared univariate (channel-specific) paired samples T statistics thresholded at the 95th quantile (p = 0.05) of the corresponding randomization distribution (maximum of all 49-channel squared univariate paired samples T statistics) of difference between H2S stimuli (pooled across intensity) and blank air (N = 41). To facilitate comparisons of the max(T2) topographies with the underlying sink-source difference topographies, the sign of the difference at each site was applied to the respective T2 value, which is otherwise always positive. Inset topographies show the sites selected for repeated measures ANOVA models performed on CSD factors 345 (N1 sink) and 600 (P2 source) to probe region-specific sink (cyan) or source (orange) activations associated with odor detection, as indicated by colored locations. All topographies are two-dimensional representations of spherical spline interpolations (m = 2; λ = 0) derived from the mean factors scores or T2 statistics available for each recording site.
CSD factors corresponding to N1 sink (peak latency 345 ms; lateral-temporal maximum accompanied by a mid-frontopolar source; 10.9% explained variance) and P2 source (600 ms; midcentroparietal maximum with mid-anterior and lateral-frontal sinks; 33.1%) distinctly reflected the parametric manipulation of odor intensity (Fig. 6B), whereas a later factor (990 ms; parietal maximum; 34.7%) did not. Furthermore, N1 sink and P2 source factors revealed several region-specific sink and source activations to H2S stimuli that differed significantly from blank air (Fig. 6C), whereas factor 990 failed to show similar odor-specific effects. The statistical analysis focused therefore on the previously identified and targeted factors representing N1 sink and P2 source.
3.3.3. Repeated measures ANOVA of PCA factor scores
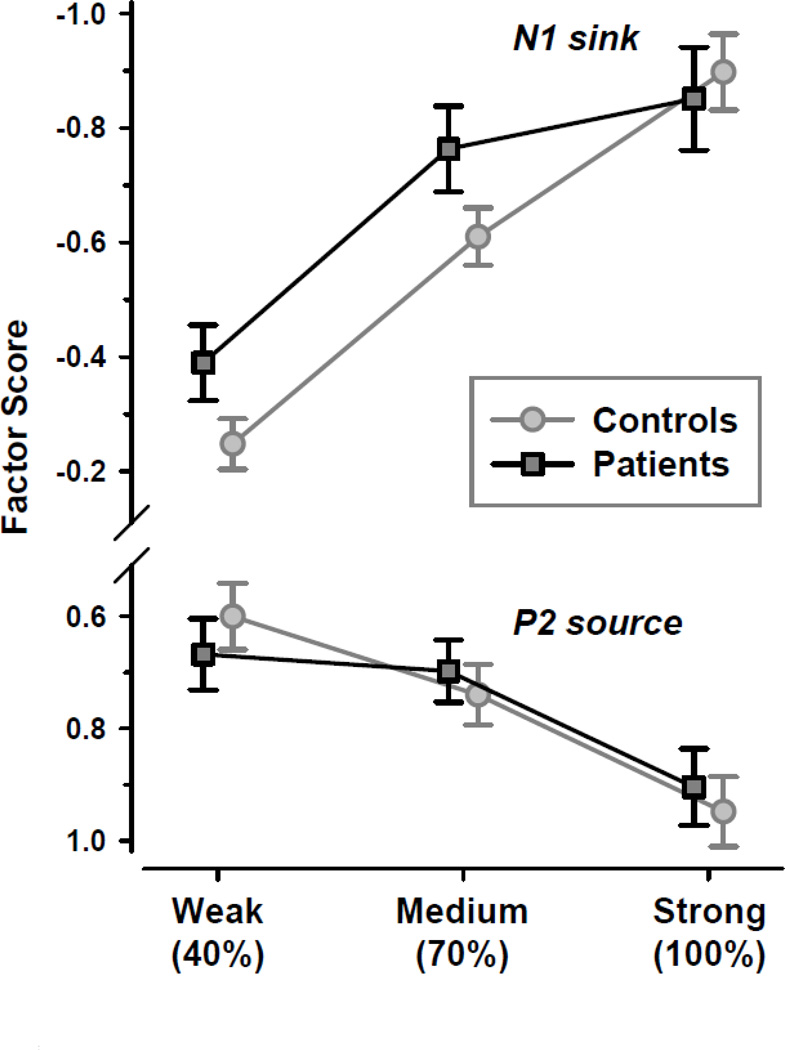
3.3.3.1. N1 sink
At lateral-temporal and frontocentral sites (T7/8, C3/4, FC5/6, CP5/6) for factor 345 (cf. Fig. 6C, row 2; for detailed ANOVA means, see supplementary Table S5), there was a highly significant main effect of intensity, F(2, 74) = 23.8, p < .0001, ε = 0.9062, confirming the monotonic increase of N1 sink from weak to strong odor intensities (Fig. 7, top). There was also a significant main effect of hemisphere, F(1, 37) = 7.12, p = .01, which stemmed from a greater N1 sink over the left than right hemisphere (M ±SD, −0.68 ±0.92 vs. −0.58 ±0.86). However, no other effect attained a conventional level of significance, including effects involving group (all F < 1.0).
Figure 7.
Mean (±SEM) factor scores of N1 sink at lateral-temporal and medial-frontocentroparietal sites (T7/8, FC5/6, C3/4, CP5/6) and P2 source at mid-frontocentral and mid-parietal sites (FCz, FC1/2, Cz, C3/4, CP1/2, Pz, P3/4, POz) as a function of odor intensity, revealing a monotonic increase for both CSD components in prodromal patients and healthy controls. Please note the inverted ordinate (negativity up), showing increases in N1 sink amplitude upwards and increases in P2 source amplitude downwards.
Additional analyses were performed to probe regional source activities associated with factor 345 (cf. Fig. 6C, row 2). However, there were no significant effects at frontopolar sites (Fpz, Fp1/2), and only a significant main effect of intensity at mid-frontocentral (FCz, Cz; F[2, 74] = 10.0, p = .0002, ε = 0.9857) and inferior-occipital sites (Iz; F[2, 74] = 3.81, p = .03, ε = 0.9435), both resulting from a source increase with an increase in odor intensity (Fig. 6B, rows 1 and 2).
Marked reductions of N1 sink were observed for the three converters, who also showed no monotonic increase with odor intensity (M ± SD, weak, −0.08 ± 0.07; medium, 0.05 ± 0.11; strong, −0.14 ± 0.88).
3.3.3.2. P2 source
At mid-frontocentral and mid-parietal sites (FCz, FC1/2, Cz, C3/4, CP1/2, Pz, P3/4, POz) for factor 600 (cf. Fig. 6C, row 4; for detailed ANOVA means, see supplementary Table S6), a highly significan main effect of intensity, F(2, 74) = 9.76, p = .0002, ε = 0.9637, resulted from the monotonic increase fo P2 source with increased odor intensity (Fig. 7, bottom). Again, there were no significant effects involving group (all F < 1.0).
Additional analyses probing sink activities associated with factor 600 (cf. Fig. 6C, row 4) at inferior sites revealed several significant effects. A marginal group × intensity interaction at mid-anterior sites (Nz, Nose; F[2, 74] = 3.02, p = .06, ε = 0.9245) originated from a reduced sink to weak (−0.72 ±1.07) compared to medium (−1.29 ±0.95) and strong (−1.25 ±1.26) odor intensities in patients (simple intensity main effect, F[2, 74] = 3.19, p = .05, ε = 0.9245), which was not observed for controls (F[2, 74] < 1.0; cf. Fig. 6B, rows 3 and 4). In contrast, a significant group × intensity interaction at lateral frontotemporal sites (F9/10, FT9/10; F[2, 74] = 4.06, p = .03, ε = 0.8863) stemmed from a monotonic sink increase with odor intensity in controls (F[2, 74] = 3.28, p = .05, ε = 0.8863) but not patients (F[2 74] = 1.46, p > .24, ε = 0.8863; cf. Fig. 6B, rows 3 and 4). This analysis also revealed a significant main effect of hemisphere, F(1, 37) = 10.5, p = .01, stemming from a right-greater-than left sink asymmetry, but this did not interact with group or intensity. Finally, a highly significant main effect of intensity, F(2, 74) = 6.50, p = .004 , ε = 0.8852, emerged at Iz, but the underlying sink increase from weak to strong odor intensity did not interact with group nor did the groups differ overall (all F < 1.0; cf. Fig. 6B, rows 3 and 4).
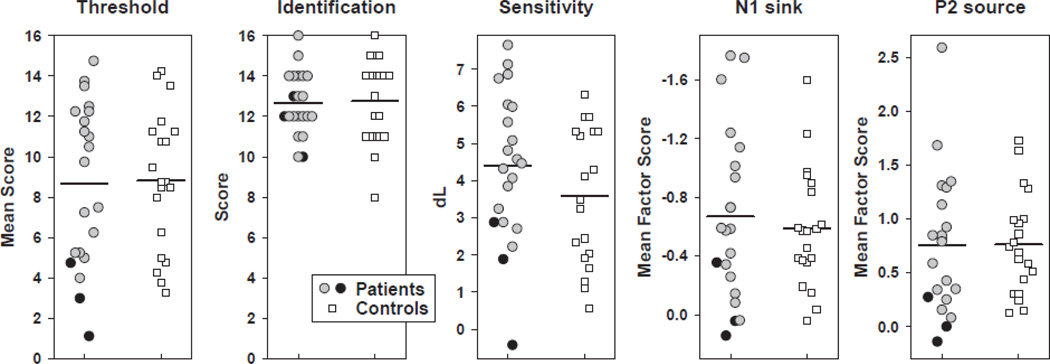
Again, the three converters showed marked reductions of P2 source and no monotonic increase with odor intensity (M ± SD, weak, −0.07 ± 0.48; medium, 0.21 ± 0.05; strong, 0.00 ± 0.45). To better appreciate the marked reductions of the three converters across the different olfactory function measures, and to better represent the inter-subject variability within the group of CHR patients and healthy controls, Figure 8 shows summary scatter plots for nasal chemosensory (Sniffin’ Sticks) and odor detection performance, and the two electrophysiologic measures. Except for odor identification, the three converters scored at or close to the bottom of the data range for each measure of olfactory function.
Figure 8.
Scatter plots for odor thresholds (pooled across nostril), odor identification, odor detection sensitivity, N1 sink and P2 source (sites as in Figure 8) comparing CHR patients (three converters marked by dark circles) and healthy controls. Detection sensitivity, N1 sink and P2 source are pooled across odor intensity. Group means are indicated by black horizontal lines.
3.3.4. Correlational findings
Strong associations were found between the three-way complex of odor concentrations, the individual ability to detect stimuli at different concentrations, and N1 and P2 measures obtained for different concentrations (Tab. 3). For controls, N1 sink pooled across lateral-temporal and frontocentral sites (cf. Fig. 6C, row 2) showed robust correlations over both hemispheres with odor concentration and odor detection sensitivity (dL), confirming that increases in odor intensity were accompanied by increases in behavioral performance and N1 sink amplitude. These associations were also significant for patients, but nevertheless weaker when compared to controls, as indicated by significant differences between correlations for each group over each hemisphere (odor concentration: left, z = −2.78, p = 0.003; right, z = −1.87, p = 0.03; odor detection: left, z = −3.01, p = 0.001; right, z = −2.42, p = 0.008). Likewise, P2 source pooled across mid-frontocentral and mid-parietal sites (cf. Fig. 6C, row 4) showed positive correlations with odor concentration and odor detection, indicating that greater P2 was linked to greater odor intensity and better performance, although the association between odor detection and P2 source amplitude was insignificant in patients (r = 0.25, p = 0.13); however, these correlations did not differ between groups (both z ≤ 1.0 , both p ≥ 0.15). inally, both groups showed a strong association between odor concentration and odor detection, with increases in odor intensity yielding better performance, but this link was nevertheless marginally weaker in patients compared to controls (z = 1.48, p = 0.07).
Table 3.
Intraindividual correlations between olfactory ERPs, odor concentrations, and odor detection
| Patients (n = 21) | Controls (n = 20) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Detection [dL] | Concentration | Detection [dL] | ||||||
| R | p | r | p | r | p | r | p | ||
| N1 sink | LH | −0.6550 | 0.0006 | −0.3986 | 0.0368 | −0.9382 | <0.0001 | −0.8936 | <0.0001 |
| RH | −0.6548 | 0.0006 | −0.3935 | 0.0388 | −0.8890 | <0.0001 | −0.8441 | <0.0001 | |
| P2 source | 0.7842 | <0.0001 | 0.2519 | 0.1353 | 0.8235 | <0.0001 | 0.5432 | 0.0067 | |
| Detection [dL] | 0.8075 | <0.0001 | 0.9245 | <0.0001 | |||||
Note. Olfactory ERP measures are pooled factor scores of N1 sink (LH: left hemisphere [T7, C3, FC5, CP5]; RH: right hemisphere [T8, C4, FC6, CP6]) and P2 source (FCz, FC1/2, Cz, C3/4, CP1/2, Pz, P3/4, POz). Fixed odor concentrations were 40%, 70%, and 100% dilutions. Odor detection reflects performance sensitivity. Reported are Pearson product-moment correlation coefficients r with corresponding one-tailed significance levels (p; df = n − 2) based on mean individual (Fisher z-transformed) correlations.
Interestingly, the conventional Pearson’s correlations computed among participants between OERP measures and odor detection, each pooled across odor intensity, revealed significant associations for patients (dL with N1 sink, left vs. right hemisphere, r = −0.45 vs. −0.47, both p = 0.02; with P2 source, r = 0.70, p < 0.001), but not for controls (r = −0.23, −0.22, and −0.13, respectively; all p > 0.16). Thus, patients having larger N1 and P2 had higher odor detection scores.
In patients, odor detection sensitivity (pooled across odor intensity) showed significant positive correlations with odor identification (r = 0.57, p = 0.04) and odor thresholds (right, r = 0.38, p = 0.05; left, r = 0.30, p = 0.09), and P2 source was likewise significantly correlated with odor identification (r = 0.69, p = 0.0003) and right (r = 0.58, p = 0.004) but not left (r = 0.23, p = 0.16) odor thresholds, indicating that poorer odor identification and odor thresholds in patients were associated with reduced odor detection and P2 source. There were no other significant correlations between behavioral, electrophysiologic and nasal chemosensory measures in patients or controls.
Among the clinical variables considered for patients, severity of negative symptoms was correlated with reduced P2 source (r = −0.52, p = 0.007) and reduced N1 sink, but this was significant only for the right (r = 0.41, p = 0.03) but not left hemisphere (r = 0.23, p = 0.16). Severity of negative symptoms was also correlated with poorer odor identification (r = −0.37, p = 0.05), and right (r = −0.44, p = 0.02) but not left (r = −0.14, p = 0.27) odor thresholds. However, neither of the correlations between negative symptoms and N1 sink or odor thresholds differed between the left and right hemisphere or nostril (both z < 0.98, both p > 0.16). Finally, lower global assessment of function score on SIPS was associated with poorer odor detection ( r = 0.44, p = 0.02) and reduced right (r = −0.64, p < 0.001) but not left (r = −0.29, p = 0.10) N1 sink, which attained a marginal significant difference between hemispheres (z = 1.37, p = 0.08).
3.3.5. Supplementary analyses for peak-based ERP measures
At the request of a reviewer, conventional peak amplitudes and latencies were extracted from ERPs referenced to linked mastoids (cf. supplementary Figures S1 and S2) or to the average of all recording sites (cf. supplementary Figures S3 and S4). These reference-dependent ERP component measures were analyzed for N1 at T7 and T8 (minimum between 100 and 600 ms) and P2 at Cz and Pz (maximum between 550 and 1400 ms), using the time intervals specified by Turetsky et al. (2008). The repeated measures ANOVAs included group (patients, controls), gender (male, female), intensity (weak, medium, strong), and hemisphere (T7, T8) or site (Cz, Pz) as independent variables.
For linked-mastoid ERPs, no significant main effects (all F[1, 37] ≤ 1.09, all p ≥ .30) or interactions (all p ≥ .11) involving group were observed in any of the analyses. or N1 amplitude, a significant main effect of hemisphere, F(1, 37) = 5.66, p = .02, confirmed a left-greater-than-right asymmetry, but the monotonic increase of N1 (M ±SD, weak to strong, −1.09 ± 1.16, −1.31 ±1.15, −1.47 ± 1. µV) was insignificant, F(2, 74) = 1.98, p = .15, ε = 0.9301. There were no significant effects for N1 latency (patients vs. controls, 316 ±132 vs. 326 ±110 ms; weak to strong, 322 ±134, 331 ±119, 309 ±112 ms). For P2 amplitude, highly significant main effects of intensity, F(2, 74) = 15.1, p < .0001, ε = 0.9885, and site, F(1, 37) = 9.72, p = .003, confirmed a monotonic increase (weak to strong, 8.71 ±4.69, 9.10 ±4.26, 11.31 ± .69 µV) and greater P2 at z than Pz (10.29 ±5.12 vs. 9.12 ± .11 µV), but there was no difference between patients and controls (9.75 ±5.36 vs. 9.66 ±3. 5 µV). This paralleled highly significant main effects of intensity, F(2, 74) = 6.14, p = .004, ε = 0.9631, and site, F(1, 37) = 12.4, p = .001, for P2 latency, stemming from a monotonic decrease in latency (weak to strong, 827 ±257, 760 ±225, 721 ±213 ms) and shorter latency at Cz than Pz (741 ±213 vs. 798 ±254 ms), but there was no significant difference between patients and controls (793 ±245 vs. 745 ±223 ms).
Similarly, for average-referenced ERPs, there were no significant main effects (all F[1, 37] ≤ 1.7 , all p ≥ .20) or interactions (all p ≥ .22) involving group. However, in contrast to linked mastoids, N1 amplitude showed a highly significant monotonic increase with intensity, F(2, 74) = 7.26, p = .001, ε = 0.9920, with means more than doubled (weak to strong, −2.22 ± 1.54, −2.46 ±1.40, −2.9 ± 1. 3 µV). There was also a significant left-greater-than-right N1 asymmetry, F(1, 37) = 6.00, p = .02. While there were no significant effects for N1 latency, N1 peaks were over 120 ms delayed compared to linked-mastoid ERPs (patients vs. controls, 463 ±134 vs. 445 ±136 ms; weak to strong, 470 ±139, 450 ±132, 444 ±133 ms). For P2 amplitude, highly significant main effects of intensity, F(2, 74) = 15.7, p < .0001, ε = 0.9022, and site, F(1, 37) = 5.58, p = .02, were comparable to those found for linked mastoids, but amplitudes were substantially smaller (weak to strong, 5.77 ±3.38, 6.06 ±2.92, 7.33 ±3.01 µV; z vs. Pz, 6. 3 ±3. 2 vs. 5.95 ±2. µV; patients vs. controls, 6.15 ±3. 7 vs. 6.6 ±2. 1 µV). Again, this paralleled the findings for P2 latency, revealing significant main effects of intensity, F(2, 74) = 3.48, p = .05, ε = 0.7982, and site, F(1, 37) = 14.9, p = .0004, but in this case P2 peaked earlier compared to the linked-mastoid data (weak to strong, 781 ±222, 730 ±189, 719 ±192 ms; Cz vs. Pz, 710 ±175 vs. 776 ±222 ms; patients vs. controls, 760 ±220 vs. 726 ±182 ms).
Thus, the peak-based findings are consistent with the CSD-PCA findings, although weaker for N1. Importantly, peak-based measures, being subject to the EEG reference, differed regarding both presence and size of statistical effects, as well as their overall amplitudes and latencies. These differences conflict with the implicit assumption that they are valid estimates of the ERP component construct.
4. Discussion
CHR patients and healthy controls as a group showed highly comparable levels of odor identification and odor thresholds, as well as odor detection performance and olfactory ERPs. While the current findings for odor identification are in disagreement with prior reports (Brewer et al., 2003; Kamath et al., 2011; Woodberry et al., 2010), there are no previous studies in CHR patients measuring odor thresholds, odor detection or olfactory ERPs. However, there was considerable variability in these measures of olfactory function among patients, and three CHR patients who later developed psychosis had marked reductions of odor thresholds, odor detection performance and olfactory N1 and P2, which further underscores the potential value of olfactory measures for predicting transition to psychosis in high-risk individuals (Corcoran et al., 2010; Turetsky et al., 2012). In agreement with previous studies in patients with psychosis (Brewer et al., 2001; Corcoran et al., 2005; Good et al., 2006; Malaspina & Coleman, 2003; Moberg et al., 2006), negative symptoms in CHR patients were associated not only with poorer odor identification and right odor thresholds, but also showed a strong association with olfactory ERPs, with more negative symptoms linked to reduced N1 and P2 amplitudes. Moreover, a new finding is that healthy controls showed intraindividually a strong association between olfactory ERP amplitudes and odor detection, in that both N1 and P2 amplitudes increased monotonically on an individual basis with increases in odor intensity. These associations, however, were substantially weaker in CHR patients, which is a further indicator that certain characteristics of normal olfactory processing are disturbed in some but not all CHR individuals.
4.1. Monotonic increases of N1 sink and P2 source as a function of odor intensity
Replicating our prior findings using CSD-PCA methodology, the morphology of olfactory ERPs in response to H2S stimuli, which consists of the two major components named N1 and P2 (e.g., Lorig, 2000; Pause & Krauel, 2000), is efficiently represented by two distinct neuronal generator patterns at scalp, which we have termed N1 sink and P2 source (Kayser et al., 2010). However, the distinct sequence of N1 sink, peaking around 350 ms, and P2 source, peaking approximately between 500 and 800 ms, appears to be rather generic, as a highly comparable olfactory CSD component complex has been also observed for citronalva, a pleasant odorant with a lemony smell (Kayser et al., 2012). That study also found that N1 sink was closely related to individual ratings of arousal, whereas P2 source was associated with individual ratings of odor valence, suggesting a functional dissociation between these two olfactory components. This agrees with the proposition that early aspects of olfactory processing, such as odor categorization, are reflected by N1 sink, whereas P2 source reflects later aspects of olfactory processing, such as odor evaluation (Kayser et al., 2010).
The parametric manipulation of odor intensity in the current study, combined with a non-odor (blank air) control condition, and the use of a denser EEG montage and unbiased randomization tests allowed an improved characterization of the regional activation patterns of odor perception. Hence, N1 sink was most prominent over lateral frontotemporal sites, particularly the left hemisphere, and had corresponding mid-frontopolar, mid-frontocentral and inferior-occipital sources. The increase in odor intensity was strongly paralleled by increases in N1 sink, and to a lesser degree by increases in the corresponding mid-frontocentral and inferior-occipital sources, but not in the mid-frontopolar source. P2 source was broadly distributed over mid-frontocentral and mid-parietal sites, and had corresponding sinks at inferior frontotemporal, frontopolar, and occipital sites. P2 source also showed a monotonic increase in amplitude with increases in odor intensity, and so did the corresponding sinks at inferior sites, but the strength of this association was weaker compared to N1 sink. Both of these scalp CSD patterns are entirely consistent with assumed generator activity within primary and secondary olfactory cortices (i.e., piriform cortex and orbitofrontal cortex), and possibly additional contributions from insular cortex, amygdala, hippocampus and anterior cingulate gyrus (cf. Seubert et al., 2013).
The monotonic increase of N1 sink and P2 source with increased concentrations of H2S is in close agreement with previous findings (Huart et al., 2012; Stuck et al., 2006; Turetsky et al., 2003a; Wang et al., 2002). Furthermore, the present findings demonstrate that the intensity-dependent amplitudes of N1 sink and P2 source were closely related to the individual ability to correctly detect H2S stimuli, revealing almost 80% common variance between increases in odor detection sensitivity and N1 sink in healthy controls. Notably, this robust relationship may be weakened or entirely obscured in betweensubjects correlations because of a lack of interindividual variability. Although prior studies reported between-subjects correlations between nasal chemosensory performance and olfactory P2 amplitude (Stuck et al., 2006) or an olfactory time-frequency theta component overlapping a late P2 time interval (Huart et al., 2012), the present findings reveal robust within-subjects (i.e., intraindividual) correlations with odor intensity not only for P2 source but also for N1 sink. Given that similar within-subjects correlations could not be obtained for odor thresholds and odor identification, it is plausible that an absence of significant between-subjects correlations for these measures with odor detection and CSD amplitudes in healthy controls is due to the same methodological limitation (i.e., lack of variability). In contrast, for CHR patients, who exhibited a greater variability in these measures, between-subjects correlations of odor identification and odor thresholds with odor detection and also P2 source were found in the expected direction (i.e., better nasal chemosensory performance, better odor detection, greater P2 source), which agrees with the correlational findings for P2 amplitude in a substantially larger (N = 95) and more heterogenous sample of healthy adults (Stuck et al., 2006). For these reasons, it seems prudent to interpret the intensity-dependent variations of N1 sink and P2 source amplitudes as direct, electrophysiologic correlates of odor perception, categorization and evaluation.
4.2. Clinical, electrophysiological and behavioral correlates of odor detection in CHR patients
In agreement with prior findings in patients with psychosis (Corcoran et al., 2005; Malaspina & Coleman, 2003), negative, but not positive, symptoms were associated with poorer nasal chemosensory performance, for both odor identification and odor thresholds. Moreover, the present findings show that negative symptoms inversely impacted on odor detection and olfactory ERP measures, with reduced amplitudes of N1 sink and P2 source both linked to more negative symptoms. However, there was no overall difference between CHR patients and healthy controls in the morphology of olfactory ERP/CSD waveforms, N1 and P2 component topographies, and responsivity to changes in odor intensity. The latter finding in particular makes it unlikely that the lack of group differences is merely due to poor data quality, yielding a low signal-to-noise ratio and therefore obscuring true effects – quite the contrary. Given evidence of markedly reduced olfactory ERPs in schizophrenia (Kayser et al., 2010; Turetsky et al., 2003a), the preserved olfactory ERPs in CHR patients may indicate that olfactory ERP abnormalities do not emerge before disease onset, thereby implicating a state rather than a trait measures. However, such an interpretation would be at odds with evidence of reduced olfactory ERPs in first-degree relatives of schizophrenia patients (Turetsky et al., 2008), and also with the present observations that olfactory ERPs were markedly reduced in the three patients who later developed psychosis. Rather, compared to healthy controls, CHR patients had individually a less robust association between odor intensity, odor detection, and olfactory ERPs, suggesting either a less coherent interplay of different elements and functions within the olfactory system, or a greater variability in olfactory processing among CHR patients, or both. An intriguing consideration is whether the sequence of olfactory ERPs reflects different stages of conscious odor processing, thereby providing insight into odor perception preceding smell awareness and its correct detection, but this is admittedly speculative. Thus, despite the lack of overall group differences in olfactory function, the present findings provide strong evidence for the hypothesis that certain aspects of olfactory function are impaired in at least a subgroup of young individuals at risk for psychosis, and also linked to the presence of negative symptoms, which has been found to be a predictor for conversion to psychosis (e.g., Cannon et al., 2008; Piskulic et al., 2012; Velthorst et al., 2009; but see also Corcoran et al., 2011).
In contrast to the main analyses of N1 sink and P2 source, some subtle group effects were observed for regional sink activities associated with P2 source. These differences originated from more variable increases in amplitude with increases in odor intensity for CHR patients at lateral frontotemporal sites, for which healthy controls showed a monotonic sink increase. While the exact meaning of these effects are not clear, these findings nevertheless underscore the greater variability in CHR patients in how these electrophysiologic measures directly reflect intensity-dependent odor detection. Thus, although these group findings provide no or little evidence of abnormal olfactory function in individuals at risk for psychosis, olfactory deficits may nonetheless prove to be a marker of risk of transition to psychosis (Brewer et al., 2003).
Recent literature reviews indicate that on average about 1/4 to 1/3 of cases included in high-risk studies show a conversion to psychosis within 2–3 years, but there is also a considerable range in transition rates between studies (Gee & Cannon, 2011; Fusar-Poli et al., 2012a; Simon et al., 2011). The incidence rate for transition to psychosis for the current sample falls within these reported ranges (i.e., 3/21 = 14%). Most importantly, the three converters differed substantially from the observed group means in odor thresholds, odor detection, and olfactory ERPs, all indicative of markedly reduced olfactory function, which strongly implies that measures of olfactory function may be a promising endophenotype for schizophrenia and its risk states (Turetsky et al., 2012). However, in contrast to prior studies (i.e., Brewer et al., 2003; Woodberry et al., 2010), the three converters did not show any abnormalities in odor identification, which warrants a more careful review of these results. Relying on the 40-item version of the UPSIT (Doty et al., 1984), Brewer et al. (2003) failed to find SIDs between 59 CHR nonconverters (M = 32.2 ±0.9) or 22 CHR converters (31.2 ±1.6) compared to 31 healthy controls (33.4 ±1.4), but a subgroup of 12 CHR patients who later developed schizophrenia (28.8 ±2.2) differed significantly from all other groups. Whereas Woodberry et al. (2010), using the abbreviated 12-item Brief Smell Identification Test (BSIT) version of the UPSIT, found SIDs for 55 CHR patients (9.9 ±1.5) compared to 34 healthy controls (11.0 ±0.7), apparently the 7 CHR converters (9.7 ±2.2) did not differ from the 44 CHR nonconverters (10.0 ±1.3).2Kamath et al. (2011), a cross-sectional study that also used the Sniffin’ Sticks (Kobal et al., 2000) and therefore addressed the question of pre-conversion differences in at-risk individuals, reported SIDs for 10 CHR (11.40 ±1.07) and 14 genetically at-risk participants (11.93 ±1.49) compared to 17 healthy controls (13.12 ±1.50); however, group differences were not based on raw scores but instead examined via Generalized Linear Latent and Mixed Models (GLLAMM) algorithm. Furthermore, the CHR participants were neither help- seeking nor patients, merely young people who reported psychotic-like experiences, thereby rendering this a different sample compared to our and other studies in CHR patients (e.g., Brewer et al., 2003; Piskulic et al., 2012).
Apart from recognizing that SIDs in CHR patients may underlie a more complex pattern, with critical methodological details necessarily lost in a broader review of the literature (Schecklmann et al., 2013), it should also be noted that all reported group differences for smell identification were small in absolute terms (i.e., about 1–3 test items), and our own data indicated less variance for odor identification compared with all other olfactory function measures (cf. Fig. 8). Moreover, the use of different olfaction tests (Sniffin’ Sticks, UPSIT/BSIT) may impede cross-study comparisons. Although both standardized tests have been found to be reliable instruments for evaluating smell identification deficits in Parkinson’s disease, overall test scores had only 58% common variance (Silveira-Moriyama et al., 2008). The specific Sniffin’ Sticks and UPSIT test versions used in the present and the prior studies differ in a variety of methodological aspects, including the delivery procedure (felt tip pens vs. scratch and sniff booklets) and the number of odorants (16 vs. 40 vs. 12), which may be of critical importance for revealing and understanding smell identification deficits in schizophrenia and individuals at clinical high risk for psychosis.
Another concern is confounding group characteristics that will affect olfactory performance, such as those described by Brewer et al. (2003), who reported significant differences between groups for smoking and premorbid intelligence (i.e., mean IQ = 96.8 ±9.6 for those who developed schizophrenia vs. 108.5 ±9.7 in healthy controls). Of particular interest regarding the puzzling observation that the three converters showed marked threshold deficits but normal odor identification performance, a similar pattern of reduced odor thresholds but preserved odor identification as measured by the Sniffin’ Sticks was found in children with autism (Dudova et al., 2011). For the present study, there was no indication that the patient cohort differed from those of other studies (e.g., Piskulic et al., 2012). However, recruitment of healthy controls was likely different given our emphasis on ascertaining individuals having the same sociocultural background as CHR patients. Thus, one could argue that the recruitment of more closely-matched healthy controls may have accounted for the difference in SID findings between the current and prior studies. In any case, more research is warranted regarding specific aspects of olfactory processing and its electrophysiologic correlates in schizophrenia and how it relates to an early prodromal phase of the disease. The available preliminary data from three converters is extremely encouraging as this suggests that behavioral and neurophysiological deficits in olfactory processing may have predictive value for transition to psychosis.
4.3. Limitations and conclusions
A major limitation of the current study is its small sample size of 21, which included only 3 CHR patients who later developed psychosis. Although these three patients showed striking abnormalities in electrophysiologic, behavioral and standardized test measures of olfactory function, no meaningful statistical comparisons were possible. Thus, our observations should be considered preliminary, at best, and treated with great caution. However, the findings underscore the importance of further research with larger samples as to whether and how electrophysiological and behavioral correlates of odor detection may be helpful in predicting transition to psychosis in adolescents and young adults with subclinical positive symptoms.
The lack of smell identification deficits warrants further study, particularly about the role of specific standardized test or testing procedures for olfactory function (i.e., UPSIT vs. Sniffin’ Sticks; cf. also Atanasova et al., 2008). It would also be desirable to obtain a more complete screening of basic olfactory function, as the current study evaluated only odor thresholds and odor identification but not odor discrimination (cf. Rupp, 2010), particularly given evidence of odor discrimination deficits in CHR individuals (Kamath et al., 2011).
The inclusion of only a single odorant, in this case, stimuli associated with strong negative affective valence, is also a limiting factor. For example, recent evidence suggests odor-specific reductions in thresholds in CHR individuals, which may provide a key for uncovering the mechanisms underlying impaired olfactory signal transduction in psychosis (Kamath et al., 2012). At the same time, H2S stimuli have frequently been employed in ERP studies of olfactory function, the associated ERP morphology is well documented, and alternating odor intensity is comparably easy. In fact, the parametric manipulation of odor intensity (e.g., Turetsky et al., 2003a) combined with a no-odor control condition warranted conclusions that would otherwise not have been possible. Systematic variations of odor quality and intensity could provide additional insights into the arousal and valence dimensions of olfactory processing (e.g., Anderson et al., 2003; Kayser et al., 2012), and how these relate to deficits in olfactory function in schizophrenia and its prodromal state.
Although patient and control group were closely matched for gender, the unbalanced cell size in combination with the limited sample size prevented the investigation of sex differences. Olfactory function appears to be enhanced in women compared to men, as indicated by nasal chemosensory and OERP measures, and different aspects of olfactory processing in males and females may be differently affected by age (e.g., Olofsson & Nordin, 2004; Stuck et al., 2006). Thus, only with substantially larger samples may it be possible to address the impact of gender (and age).
The present study soundly replicates our previous olfactory ERP findings using a combined CSD-PCA approach (Kayser et al., 2010), and underscores the value of reference-free techniques for a comprehensive description of surface potentials (Kayser & Tenke, 2010; Tenke & Kayser, 2012). Despite the widely held concern that CSDs may preferentially highlight superficial cortical sources at the expense of deep contributions (Nunez & Srinivasan, 2006, p. 333), such as those stemming from putative generators in basal cortical regions, N1 sink and P2 source clearly reflected olfactory stimulation. This parallels prior findings demonstrating adequate, or even superior condition-sensitivity for other ‘deep-generator’ components, such as P3b (Kayser & Tenke, 2006a, 2006b; Tenke et al., 2008, 2010). The neuronal generator patterns underlying olfactory N1 and P2 are not only reliable and stable but are also closely related to odor intensity and odor detection, thereby rendering these unbiased measures suitable tools to study interindividual differences in olfactory processing. This is a necessary prerequisite for ongoing efforts for identifying endophenotypes for schizophrenia, which may aid an early intervention before the onset of psychosis (e.g., Corcoran et al., 2010; Fusar-Poli et al., 2012b). Although one may invoke the often suspected limitations of the surface Laplacian (see Tenke & Kayser, 2012, p. 2342) as a cause of attenuated group differences, there is no empirical basis for this argument for the present data (see supplementary Figures S1–S4). Despite the absence of overall group differences in nasal chemosensory performance or olfactory N1 and P2 amplitudes, the present results imply a greater variability of odor processing among high-risk individuals and thereby contribute to the growing evidence showing pre-psychosis abnormalities of olfactory function in schizophrenia, which merits further research in a larger sample employing either a cross-sectional (e.g., including CHR, first-episode, and chronic schizophrenia patients) or a longitudinal design with more comprehensive measures of olfactory processing.
Supplementary Material
Supplementary Material: Mastoid- and average-referenced ERP waveforms and CSD-PCA component means for N1 sink and P2 source
Highlights.
We studied olfactory processing in clinical high-risk patients and healthy controls.
We recorded olfactory ERPs during odor detection of hydrogen sulfide and blank air.
ERPs reflected odor intensity and detection more strongly in controls than patients.
Negative symptoms correlated with reduced ERPs, detection, and Sniffin’ Sticks score.
Three patients who developed psychosis had markedly reduced N1, P2, and thresholds.
Acknowledgments
This research was supported by grant MH086125 from the National Institute of Mental Health (NIMH). We are grateful to Bruce Turetsky at the University of Pennsylvania and Holly Moore at New York State Psychiatric Institute for their thoughtful advice on odor concentrations, and Charles L. Brown, III, for providing waveform plotting software. We also thank Dolores Malaspina at New York University for initiating our studies of olfactory ERPs in schizophrenia. We are grateful for several helpful comments received during the review process by Dr. Tyler Lorig and one anonymous reviewer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Animated ERP (linked-mastoid reference) and CSD topographies comparing groups and intensities can be obtained at URL http://psychophysiology.cpmc.columbia.edu/oerp2013.html.
Although explicit statistics were not reported for this comparison, smell identification did not differ between converters and nonconverters (K. A. Woodberry, personal communication, April 18, 2013).
Preliminary analyses of these data were presented at the 67th Annual Meeting of the Society of Biological Psychiatry (SoBP), Philadelphia, PA, May 3–5, 2012.
References
- Amminger GP, Leicester S, Yung AR, Phillips LJ, Berger GE, Francey SM, Yuen HP, McGorry PD. Early-onset of symptoms predicts conversion to non-affective psychosis in ultra-high risk individuals. Schizophr. Res. 2006;84(1):67–76. doi: 10.1016/j.schres.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci. Biobehav. Rev. 2008;32(7):1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatry. 2012;71(2):98–104. doi: 10.1016/j.biopsych.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012;323(1–2):16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Beauducel A, Debener S. Misallocation of variance in event-related potentials: simulation studies on the effects of test power, topography, and baseline-to-peak versus principal component quantifications. J. Neurosci. Methods. 2003;124(1):103–112. doi: 10.1016/s0165-0270(02)00381-3. [DOI] [PubMed] [Google Scholar]
- Beauducel A, Debener S, Brocke B, Kayser J. On the reliability of augmenting/reducing: peak amplitudes and principal components analysis of auditory evoked potentials. J. Psychophysiol. 2000;14(4):226–240. [Google Scholar]
- Becker E, Hummel T, Piel E, Pauli E, Kobal G, Hautzinger M. Olfactory event-related potentials in psychosis-prone subjects. Int. J. Psychophysiol. 1993;15(1):51–58. doi: 10.1016/0167-8760(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Müller R, Schultze Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkötter J, Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biol. Psychiatry. 2011;69(10):959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am. J. Psychiatry. 2001;158(1):107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D, Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am. J. Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, Yung AR, Anderson VA, McGorry PD. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry. 2005;162(1):71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, Cornblatt B, McGorry PD. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr. Bull. 2006;32(3):538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kroppmann CJ, Kayser J, Stewart JW, McGrath PJ, Tenke CE. Reduced brain responses to novel sounds in depression: P3 findings in a novelty oddball task. Psychiatry Res. 2009;170(2–3):218–223. doi: 10.1016/j.psychres.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buron E, Bulbena A. Olfaction in affective and anxiety disorders: a review of the literature. Psychopathology. 2013;46(2):63–74. doi: 10.1159/000338717. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RM, McCrary JW. EP component identification and measurement by principal components analysis. Brain Cogn. 1995;27(3):288–310. doi: 10.1006/brcg.1995.1024. [DOI] [PubMed] [Google Scholar]
- ohen AS, Brown LA, Auster TL. Olfaction, “olfiction,” and the schizophrenia-spectrum: an updated meta-analysis on identification and acuity. Schizophr. Res. 2012a;135(1–3):152–157. doi: 10.1016/j.schres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Brown LA, Auster TL. Clarifying the nature of olfaction deficits in the schizophrenia-prone: “clinical high-risk state” versus “vulnerability”. Schizophr. Res. 2012b;139(1–3):262–263. [Google Scholar]
- Coleman E, Goetz RR, Leitman D, Yale S, Stanford A, Gorman JM, Malaspina D. Odor identification impairments in schizophrenia: relationship with demographic measures, clinical variables, and diagnostic subtypes. CNS Spectr. 2002;7(1):43–48. doi: 10.1017/s1092852900022252. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, Malaspina D. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr. Res. 2005;80(2–3):283–293. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, First MB, Cornblatt B. The psychosis risk syndrome and its proposed inclusion in the DSM-V: a risk-benefit analysis. Schizophr. Res. 2010;120(1–3):16–22. doi: 10.1016/j.schres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Kimhy D, Parrilla Escobar MA, Cressman VL, Stanford AD, Thompson J, David SB, Crumbley A, Schobel S, Moore H, Malaspina D. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol. Med. 2011;41(2):251–261. doi: 10.1017/S0033291710000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. J.A.M.A. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- Croy I, Lange K, Krone F, Negoias S, Seo HS, Hummel T. Comparison between odor thresholds for phenyl ethyl alcohol and butanol. Chem. Senses. 2009;34(6):523–527. doi: 10.1093/chemse/bjp029. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, editor. BMDP Statistical Software Manual: To Accompany the 7.0 Software Release. Berkeley, CA: University of California Press; 1992. [Google Scholar]
- Donchin E, Heffley EF. Multivariate analysis of event-related potential data: a tutorial review. In: Otto DA, editor. Multidisciplinary Perspectives in Event-Related Brain Potential Research., Proceedings of the Fourth International Congress on Event-Related Slow Potentials of the Brain (EPIC IV) Washington, DC: The Office; 1978. pp. 555–572. Hendersonville, NC, April 4–10, 1976. [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol. Behav. 1978;20(2):175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Dudova I, Vodicka J, Havlovicova M, Sedlacek Z, Urbanek T, Hrdlicka M. Odor detection threshold, but not odor identification, is impaired in children with autism. Eur. Child Adolesc. Psychiatry. 2011;20(7):333–340. doi: 10.1007/s00787-011-0177-1. [DOI] [PubMed] [Google Scholar]
- Egerton A, Chaddock CA, Winton Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, McGuire PK, Howes OD. Presynaptic striatal dopamine dysfunction in people at ultrahigh risk for psychosis: findings in a second cohort. Biol. Psychiatry. 2013 Jan 8; doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Kobal G, Lorig TS, Prah JD. Suggestions for collection and reporting of chemosensory (olfactory) event-related potentials. Chem. Senses. 1993;18(6):751–756. [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry. 2012a;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, McGuire P, Borgwardt S. Mapping prodromal psychosis: a critical review of neuroimaging studies. Eur. Psychiatry. 2012b;27(3):181–191. doi: 10.1016/j.eurpsy.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Frommann I, Brinkmeyer J, Ruhrmann S, Hack E, Brockhaus-Dumke A, Bechdolf A, Wölwer W, Klosterkötter J, Maier W, Wagner M. Auditory P300 in individuals clinically at risk for psychosis. Int. J. Psychophysiol. 2008;70(3):192–205. doi: 10.1016/j.ijpsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Gee DG, Cannon TD. Prediction of conversion to psychosis: review and future directions. Rev. Bras. Psiquiatr. 2011;332:129–142. doi: 10.1590/s1516-44462011000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good KP, Whitehorn D, Rui Q, Milliken H, Kopala LC. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am. J. Psychiatry. 2006;163(5):932–933. doi: 10.1176/ajp.2006.163.5.932. [DOI] [PubMed] [Google Scholar]
- Haroun N, Dunn L, Haroun A, Cadenhead KS. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophr. Bull. 2006;32(1):166–178. doi: 10.1093/schbul/sbj007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner H, Maurer K, Löffler W, ander Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr. Bull. 2003;29(2):325–340. doi: 10.1093/oxfordjournals.schbul.a007008. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R, Woods SW, Miller TJ, Marquez E, Breier A, McGlashan TH. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr. Res. 2004;67(2–3):115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III – the final common pathway. Schizophr. Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]- DOPA PET imaging study. Am. J. Psychiatry. 2011;168(12):1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huart C, Legrain V, Hummel T, Rombaux P, Mouraux A. Time-frequency analysis of chemosensory event-related potentials to characterize the cortical representation of odors in humans. PLoS One. 2012;7(3):33221. doi: 10.1371/journal.pone.0033221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol. Psychiatry. 1988;23(2):123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- Jacquot L, Monnin J, Brand G. Unconscious odor detection could not be due to odor itself. Brain Res. 2004;(1–2):51–54. doi: 10.1016/j.brainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34(4):1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kamath V, Turetsky BI, Calkins ME, Kohler CG, Conroy CG, Borgmann Winter K, Gatto DE, Gur RE, Moberg PJ. Olfactory processing in schizophrenia, non-ill first-degreefamily members, and young people at-risk for psychosis. World J. Biol. Psychiatry. 2011 Nov 10; doi: 10.3109/15622975.2011.615862. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath V, Moberg PJ, Calkins ME, Borgmann-Winter K, Conroy CG, Gur RE, Kohler CG, Turetsky BI. An odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosis. Schizophr. Res. 2012;138(2–3):280–284. doi: 10.1016/j.schres.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J. Current source density (CSD) interpolation using spherical splines - CSD Toolbox (Version 1.1) Division of Cognitive Neuroscience: New York State Psychiatric Institute; 2009. [ http://psychophysiology.cpmc.columbia.edu/Software/CSDtoolbox] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin. Neurophysiol. 2003;114(12):2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Trusting in or breaking with convention: towards a renaissance of principal components analysis in electrophysiology. Clin. Neurophysiol. 2005;116(8):1747–1753. doi: 10.1016/j.clinph.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 2006a;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin. Neurophysiol. 2006b;117(2):369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Consensus on PCA for ERP data, and sensibility of unrestricted solutions. Clin. Neurophysiol. 2006c;117(3):703–707. [Google Scholar]
- Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006d;43:S51. (available at http://psychophysiology.cpmc.columbia.edu/mmedia/SPR2006/ElecDistArti.pdf) [Google Scholar]
- Kayser J, Tenke CE. In search of the Rosetta Stone for scalp EEG: converging on reference- free techniques. Clin. Neurophysiol. 2010;121(12):1973–1975. doi: 10.1016/j.clinph.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke C, Nordby H, Hammerborg D, Hugdahl K, Erdmann G. Event-related potential (ERP) asymmetries to emotional stimuli in a visual half-field paradigm. Psychophysiology. 1997;34(4):414–426. doi: 10.1111/j.1469-8986.1997.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Bruder GE. Dissociation of brain ERP topographies for tonal and phonetic oddball tasks. Psychophysiology. 1998;35(5):576–590. doi: 10.1017/s0048577298970214. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Gates NA, Bruder GE. Reference-independent ERP old/new effects of auditory and visual word recognition memory: joint extraction of stimulus- and response-locked neuronal generator patterns. Psychophysiology. 2007;44(6):949–967. doi: 10.1111/j.1469-8986.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Malaspina D, Kroppmann CJ, Schaller JD, Deptula A, Gates NA, Harkavy-Friedman JM, Gil R, Bruder GE. Neuronal generator patterns of olfactory event-related brain potentials in schizophrenia. Psychophysiology. 2010;47(6):1075–1086. doi: 10.1111/j.1469-8986.2010.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Fekri S, Stewart JW, McGrath PJ, Turetsky BI, Bruder GE. Effects of odor valence and arousal on event-related potentials (ERPs) in healthy adults and depressed patients. Biol. Psychiatry. 2012;71(8):78S–79S. (available at http://psychophysiology.cpmc.columbia.edu/mmedia/SoBP2012/oERPemot.pdf) [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Fekri S, Ben-David S, Corcoran CM, Bruder GE. Auditory event-related potentials and alpha oscillations in the psychosis prodrome: Neuronal generator patterns during a novelty oddball task. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2013.12.003. http://dx.doi.org/10.1016/j.ijpsycho.2013.12.003 (Dec 13, Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman HJ. Testing treatment effects in repeated measures designs: an update for psychophysiological researchers. Psychophysiology. 1998;35(4):470–478. [PubMed] [Google Scholar]
- Kjelvik G, Evensmoen HR, Brezova V, Haberg AK. The human brain representation of odor identification. J. Neurophysiol. 2012;108(2):645–657. doi: 10.1152/jn.01036.2010. [DOI] [PubMed] [Google Scholar]
- Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur. Arch. Otorhinolaryngol. 2000;257(4):205–211. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- Koh Y, Shin KS, Kim JS, Choi JS, Kang DH, Jang JH, Cho KH, O’Donnell BF, Chung CK, Kwon JS. An MEG study of alpha modulation in patients with schizophrenia and in subjects at high risk of developing psychosis. Schizophr. Res. 2011;126(1–3):36–42. doi: 10.1016/j.schres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Barrett FS, Gur RC, Turetsky BI, Moberg PJ. Association between facial emotion recognition and odor identification in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2007;19(2):128–131. doi: 10.1176/jnp.2007.19.2.128. [DOI] [PubMed] [Google Scholar]
- Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am. J. Psychiatry. 2001;158(8):1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol. Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The application of electroencephalographic techniques to the study of human olfaction: a review and tutorial. Int. J. Psychophysiol. 2000;36(2):91–104. doi: 10.1016/s0167-8760(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch. Gen. Psychiatry. 2003;60(6):578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Perera GM, Lignelli A, Marshall RS, Esser PD, Storer S, Furman V, Wray AD, Coleman E, Gorman JM, Van Heertum RL. SPECT imaging of odor identification in schizophrenia. Psychiatry Res. 1998;82(1):53–61. doi: 10.1016/s0925-4927(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Maris E. Randomization tests for ERP topographies and whole spatiotemporal data matrices. Psychophysiology. 2004;41(1):142–151. doi: 10.1111/j.1469-8986.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- Martzke JS, Kopala LC, Good KP. Olfactory dysfunction in neuropsychiatric disorders: review and methodological considerations. Biol. Psychiatry. 1997;42(8):721–732. doi: 10.1016/s0006-3223(96)00442-8. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol. Psychiatry. 2000;47(5):434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Mewhort DJK, Johns BT, Kelly M. Applying the permutation test to factorial designs. Behav. Res. Methods. 2010;42(2):366–372. doi: 10.3758/BRM.42.2.366. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr. Bull. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21(3):325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, Gur RE, Turetsky BI. Impairment of odor hedonics in men with schizophrenia. Am. J. Psychiatry. 2003;160(10):1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ, Turetsky BI. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J. Clin. Exp. Neuropsychol. 2006;28(8):1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Turetsky BI. Scent of a disorder: olfactory functioning in schizophrenia. Curr. Psychiatry Rep. 2003;5(4):311–319. doi: 10.1007/s11920-003-0061-x. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Flor-Henry P. Asymmetrical olfactory acuity and neuroleptic treatment in schizophrenia. Schizophr. Res. 2000;44(3):221–232. doi: 10.1016/s0920-9964(99)00212-1. [DOI] [PubMed] [Google Scholar]
- NeuroScan, Inc. SCAN Manual II Version 3.0. Herndon, VA: Author; 1993. [Google Scholar]
- NeuroScan Inc. SCAN 4.3 - Vol. II. EDIT 4.3 - Offline analysis of acquired data (Document number 2203, Revision D) El Paso, TX: Compumedics Neuroscan; 2003. [Google Scholar]
- Nordin S, Lötsch J, Murphy C, Hummel T, Kobal G. Circadian rhythm and desensitization in chemosensory event-related potentials in response to odorous and painful stimuli. Psychophysiology. 2003;40(4):612–619. doi: 10.1111/1469-8986.00062. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. New York: Oxford University Press; 2006. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S. Gender differences in chemosensory perception and event-related potentials. Chem. Senses. 2004;29(7):629–637. doi: 10.1093/chemse/bjh066. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Broman DA, Gilbert PE, Dean P, Nordin S, Murphy C. Laterality of the olfactory event-related potential response. Chem. Senses. 2006;31(7):699–704. doi: 10.1093/chemse/bjl011. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Ericsson E, Nordin S. Comparison of chemosensory, auditory and visual event-related potential amplitudes. Scand. J. Psychol. 2008;49(3):231–237. doi: 10.1111/j.1467-9450.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- Pause BM, Krauel K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int. J. Psychophysiol. 2000;36(2):105–122. doi: 10.1016/s0167-8760(99)00105-1. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping [Corrigenda EEG 02274, EEG Clin. Neurophysiol, 1990, 76, 565] Electroenceph. Clin. Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, McGlashan TH. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196(2–3):220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Turetsky BI, Owzar K, Balderston CC, Johnson SC, Brensinger CM, Gur RE, Siegel SJ, Moberg PJ. Unirhinal olfactory function in schizophrenia patients and firstdegree relatives. J. Neuropsychiatry Clin. Neurosci. 2006;18(3):389–396. doi: 10.1176/jnp.2006.18.3.389. [DOI] [PubMed] [Google Scholar]
- Rupp CI. Olfactory function and schizophrenia: an update. Curr. Opin. Psychiatry. 2010;23(2):97–102. doi: 10.1097/YCO.0b013e328336643f. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, Kremser C, Bilder RM, Mechtcheriakov S, Szeszko PR, Walch T, Scholtz AW, Klimbacher M, Maier C, Albrecht G, Lechner Schoner T, Felber S, Hinterhuber H. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr. Res. 2005;74(2–3):149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Schwenck C, Taurines R, Freitag C, Warnke A, Gerlach M, Romanos M. A systematic review on olfaction in child and adolescent psychiatric disorders. J. Neural. Transm. 2013;120(1):121–130. doi: 10.1007/s00702-012-0855-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. Neuroimaging. 2007;155(2):103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, Pepple JR, Tsuang MT. Neuropsychological probes of fronto-limbic system dysfunction in schizophrenia. Olfactory identification and Wisconsin Card Sorting performance. Schizophr. Res. 1991;6(1):55–65. doi: 10.1016/0920-9964(91)90021-i. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner WM, Faraone SV, Tsuang MT. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol. Psychiatry. 1997;42(2):104–115. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- Seubert J, Freiherr J, Djordjevic J, Lundström JN. Statistical localization of human olfactory cortex. Neuroimage. 2013;66:333–342. doi: 10.1016/j.neuroimage.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, Woolley J, Tabraham P, Walshe M, Johns L, Fusar-Poli P, Howes O, Murray RM, McGuire P, Bramon E. Reduced mismatch negativity predates the onset of psychosis. pSchizophr. Res. 2012;134(1):42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Silveira-Moriyama L, de Carvalho Jesus M, Katzenschlager R, Petrie A, Ranvaud R, Barbosa ER, Lees AJ. The use of smell identification tests in the diagnosis of Parkinson’s disease in Brazil. Mov. Disord. 2008;23(16):2328–2334. doi: 10.1002/mds.22241. [DOI] [PubMed] [Google Scholar]
- Simon AE, Velthorst E, Nieman DH, Linszen D, Umbricht D, de Haan L. Ultra highrisk state for psychosis and non-transition: a systematic review. Schizophr. Res. 2011;132(1):8–17. doi: 10.1016/j.schres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Sinai A, Pratt H. Electrophysiological evidence for priming in response to words and pseudowords in first and second language. Brain Lang. 2002;80(2):240–252. doi: 10.1006/brln.2001.2597. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J. Exp. Psychol. Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stuck BA, Frey S, Freiburg C, Hormann K, Zahnert T, Hummel T. Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin. Neurophysiol. 2006;117(6):1367–1375. doi: 10.1016/j.clinph.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin. Neurophysiol. 2001;112(3):545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol. 2012;123(12):2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Shankman SA, Griggs CB, Leite P, Stewart JW, Bruder GE. Hemispatial PCA dissociates temporal from parietal ERP generator patterns: CSD components in healthy adults and depressed patients during a dichotic oddball task. Int. J. Psychophysiol. 2008;67(1):1–16. doi: 10.1016/j.ijpsycho.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Alschuler DM, Stewart JW, McGrath PJ, Bruder GE. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biol. Psychiatry. 2011;70(4):388–394. doi: 10.1016/j.biopsych.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Stewart JW, Bruder GE. Novelty P3 reductions in depression: characterization using principal components analysis (PCA) of current source density (CSD) waveforms. Psychophysiology. 2010;47(1):133–146. doi: 10.1111/j.1469-8986.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol. Psychiatry. 2003a;53(5):403–411. doi: 10.1016/s0006-3223(02)01865-6. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch. Gen. Psychiatry. 2003b;60(12):1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Arnold SE, Doty RL, Gur RE. Low olfactory bulb volume in first-degree relatives of patients with schizophrenia. Am. J. Psychiatry. 2003c;160(4):703–708. doi: 10.1176/appi.ajp.160.4.703. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Gur RE, Moberg PJ. Olfactory physiological impairment in first-degree relatives of schizophrenia patients. Schizophr. Res. 2008;102(1–3):220–229. doi: 10.1016/j.schres.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kamath V, Calkins ME, Brewer WJ, Wood SJ, Pantelis C, Seidman LJ, Malaspina D, Good KP, Kopala LC, Moberg PJ. Olfaction and schizophrenia clinical risk status: just the facts. Schizophr. Res. 2012;139(1–3):2–3. doi: 10.1016/j.schres.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel GJM. Computational and statistical methods for analyzing event-related potential data. Behav. Res. Methods Instrum. Comput. 1998;30(1):87–102. [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr. Res. 2005;77(2–3):309–320. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, vander Meer JN, Bour LJ, de Haan L, Linszen DH. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol. Psychiatry. 2010;68(7):642–648. doi: 10.1016/j.biopsych.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Velthorst E, Nieman DH, Becker HE, vande Fliert R, Dingemans PM, Klaassen R, de Haan L, van Amelsvoort T, Linszen DH. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr. Res. 2009;109(1–3):60–65. doi: 10.1016/j.schres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Walker VE, Sardi H, Fraser C, Jacob TJ. The correlation between physiological and psychological responses to odour stimulation in human subjects. Clin. Neurophysiol. 2002;113(4):542–551. doi: 10.1016/s1388-2457(02)00029-9. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Egan MF, Goldberg TE, Weinberger DR. Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biol. Psychiatry. 2003;54(11):1181–1192. doi: 10.1016/s0006-3223(03)00532-8. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr. Res. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr. Res. 2010;123(2–3):188–198. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W, Brinkmeyer J, Stroth S, Streit M, Bechdolf A, Ruhrmann S, Wagner M, Gaebel W. Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophr. Bull. 2012;38(5):1021–1029. doi: 10.1093/schbul/sbr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material: Mastoid- and average-referenced ERP waveforms and CSD-PCA component means for N1 sink and P2 source