Abstract
Background
In the United States, expenditures related to spine care are estimated to account for $86 billion annually. Policy makers have set a cost-effectiveness benchmark of less than $100,000/quality adjusted life year (QALY), forcing surgeons to defend their choices economically. This study projects the cost/QALY for surgical treatment of adult spinal deformity at 5-year follow-up based on 2-year cost- and health-related quality-of-life (HRQOL) data.
Methods
In a review of 541 patients with adult spinal deformity, the patients who underwent revision or were likely to undergo revision were identified and cost of surgery was doubled to account for the second procedure; all other patients maintained the cost of the initial surgery. Oswestry Disability Index (ODI) was modeled by revision status based on literature findings. Total surgical cost was based on Medicare reimbursement. Chi square and student t tests were utilized to compare cost-effective and non–cost-effective patients.
Results
The average cost/QALY at 5-year follow-up was $120,311.73. A total of 40.7% of patients fell under the threshold of a cost/QALY <$100,000. Cost-effective patients had higher baseline ODI scores (45% vs 34% [P=0.001]), lower baseline total Scoliosis Research Society scores (2.89 vs 3.00 [P=0.04]), and shorter fusions (8.23 vs 9.87 [P=0.0001]).
Conclusion
We found 40.7% of patients to be below the threshold of cost effectiveness. Factors associated with reaching the threshold <$100,000/QALY were greater preoperative disability, diagnosis of idiopathic scoliosis, poor preoperative HRQOL scores, and fewer fusion levels.
Keywords: Cost-benefit analysis, quality adjusted life years, quality of life, scoliosis, spine, surgical procedures–operative
INTRODUCTION
The United States spent $2.5 trillion, 17.6% of gross domestic product, on healthcare in 2009—the highest per capita spending for any industrialized nation.1-3 Spine care–related expenditures in the United States are estimated to account for $86 billion annually.4 Compared to other well-studied pathologies—arthritis, with estimated annual expenditures of $80.3 billion, and cancer, with estimated annual expenditures of $89 billion—the price tag for spine care is relatively high.5 Consequently, the spine community is under increasing pressure to demonstrate both the clinical effectiveness and the cost effectiveness of the treatments provided.
Establishing cost-effectiveness profiles for surgical treatment of even the most common spinal pathologies has proven difficult given the inherent heterogeneity of these patient populations.6 Recent studies have used strict inclusion criteria and rigid application of published guidelines to determine cost-effectiveness values for several of the more common pathologies.7-10 These studies continue to evolve with regards to data collection and defining the direct and indirect costs that are necessary to consider when establishing a truly societal perspective.6,10,11
Cost-effectiveness research is conducted using the outcomes measure cost/quality adjusted life years (QALYs): the cost of the health intervention divided by the QALYs. A QALY measures the health state of an individual on a scale with zero the equivalent of death and 1 equal to the optimal state for 1 year of life. A change in QALY induced by a health intervention can represent substantial clinical benefit.12,13 Woven into the mathematical description of cost effectiveness are 2 facts: (1) if you assume a durable treatment benefit, then long-term follow-up will increase the perceived value of a given intervention, and (2) complex interventions with high upfront direct care costs (DCCs) need to demonstrate durable treatment over the long term to fall within acceptable thresholds of cost effectiveness. Cost effectiveness has been demonstrated most recently with the study of lumbar fusion in the setting of spondylolisthesis.10 Traditionally, $100,000/QALY is accepted as the threshold for cost effectiveness.14-17
The surgical management of adult spinal deformity (ASD) carries extraordinary DCCs in relation to less complex and more common counterparts such as discectomy and decompressive laminectomy. These costs are reflected in the cost-effectiveness profile for ASD surgery at 2 years that is outside the target range in terms of cost/QALY.18,19 However, the surgical benefit for ASD patients is known to be durable over the long term despite the surgery being associated with high complication and revision rates that can increase cost and affect outcome.20-23
The purpose of this study was to create a patient cohort model based on a comprehensive review of the literature to estimate cost effectiveness of ASD surgery at 5-year follow-up utilizing baseline and 2-year data. A secondary aim was to initiate the development of a predictive patient risk profile so patients can be stratified and preoperative planning and counseling can be adjusted appropriately.
METHODS
Institutional review board approval for this study was obtained by each participating institution.
Patient Population
Included in this retrospective review of patients enrolled in a prospective consecutive database were adults (>18 years) who underwent an operative intervention for a spinal deformity (adolescent idiopathic scoliosis of adulthood [AISA], de novo degenerative scoliosis [DDS], global sagittal malalignment, or other spinal conditions). Patients had a minimum of 5 levels fused and had follow-up data for 2 years.
Data Collection
Demographic parameters included age, sex, prior spine surgery, and medical history. Surgical parameters included operative approach, number of levels fused, fusion to the pelvis, osteotomy details, operative time, estimated blood loss (EBL), and type of intraoperative monitoring. This information was used to apply International Classification of Diseases (ICD)-9 Current Procedural Terminology (CPT) codes. We evaluated the hospital length of stay (LOS), intraoperative and immediate postoperative complications, and comorbidities as components of the diagnosis-related group (DRG). We retrieved postoperative complications (including pseudarthrosis, curve progression, infection, painful/prominent implants, adjacent segment degeneration with back pain, implant failure, and neurologic deficit) and reoperation rates at 2-year follow-up from the database.
Reoperation
We conducted a literature review to determine an established revision rate for ASD surgery over the 5-year follow-up period,20,22 as well as known ASD risk factors and the most common reasons for reoperation. We individually reviewed patient-related parameters to identify patients presenting with those risk factors who were therefore at risk of requiring a reoperation. Any patient who was known to have revision by 2-year follow-up or had greater than 4 risk factors for revision surgery was included in the modeled reoperation population. All other patients were included in the nonreoperation population.
Costs
Direct costs included both hospital and physician reimbursements, calculated based on Medicare reimbursement rates. These data were estimates because they represented an average, large urban hospital and were not modified for any socioeconomic factors.
Hospital reimbursements were DRG based; each patient was assigned a DRG based on his/her spine-related diagnosis with consideration for coexisting and comorbid conditions. Physician reimbursements included surgeon, anesthesiologist, and neuromonitoring fees. These costs were based on ICD-9 CPT codes and because these were multicenter data, the costs were not modified by a geographic practice cost index multiplier particular to a specific region.
For the modeled reoperation population, we doubled the cost of the primary surgery to establish a conservative estimate of the cost of revision surgery. The majority of revision procedures would cost less than the initial corrective measure; however, we chose to use the highest reasonable cost for the modeling protocol. Patients in the nonreoperation group retained a cost equal to their initial procedure.
Clinical Outcomes
Clinical outcomes included 2 measures of disease-specific health-related quality of life (HRQOL): (1) Scoliosis Research Society (SRS) scores, which assess pain, appearance, activity, and mental domains on a scale of 0 to 5 with higher scores reflecting better health status, and (2) Oswestry Disability Index (ODI) scores, which range from 0% to 100% with higher percentages reflecting greater disability. Baseline and 1- and 2-year postoperative ODI and SRS scores were captured. Bridwell et al20 determined that patients with revision by 5 years had an average increase of 7 ODI percentage points between the 2- and 5-year time points, while those who did not have revision had an average decrease of 2 ODI percentage points during the same time period. Therefore, the ODI scores at 5 years for patients in the modeled reoperation group were calculated by adding 7 percentage points to their 2-year ODI scores, while ODI scores for patients in the nonreoperation group were decreased by 2 percentage points. Patients were assumed to maintain 2-year ODI scores at the 3- and 4-year time points.20,24
We based the calculation of QALY on the conversion of ODI scores to Short Form Health Survey scores using a published regression model.14 The QALY utility value varies only when an HRQOL is available for that time point. For this study, data were available for baseline and the 1-year and 2-year follow-ups. Because we used the literature to predict 5-year ODI scores, we modeled that no change would occur over the 2-, 3-, and 4-year intervals to ODI and ultimately to the utility values. Utility at 5 years is based upon the modeled 5-year ODI score, accounting for the increase in utility at 5-year follow-up.
Statistical Analysis
Data were analyzed using SPSS Statistics, version 17.0 (IBM). Descriptive statistics were used to summarize collected data (means, standard deviation, etc). Changes in HRQOL scores were evaluated using a paired t test. Differences between groups were measured using unpaired t tests and chi square analysis with a level of significance set at 0.05.
RESULTS
Dataset
The database query identified 541 patients who underwent surgery for ASD with the required preoperative and postoperative data points available for review. The mean age of the patients was 53.9 years and the majority were female (468, 86.5%). Among the identified patients, 162 (29.9%) were known to have had spinal surgery prior to database baseline surgery, and 379 (70.1%) were primary surgery cases. The breakdown by pathology was 274 patients (50.6%) with AISA, 104 patients (19.2%) with DDS, 92 patients (17.0%) with sagittal plane deformity, and 71 patients (13.1%) with other conditions.
Reoperations
Of the 541 patients we reviewed, 108 (19.9%) had undergone reoperation by 2-year follow-up. Upon reviewing the relevant literature, we determined that approximately 24 (4.4%) additional patients would be expected to require reoperation between 2- and 5-year follow-up. In our analysis of the literature,25-28 we identified 8 possible risk factors for developing these specific complications. We then identified 26 additional patients having at least 4 risk factors and included them in the modeled reoperation group. The most common reasons for these reoperations include pseudarthrosis, curve progression, infection, and painful or prominent implant.22
HRQOL Scores by Time Point
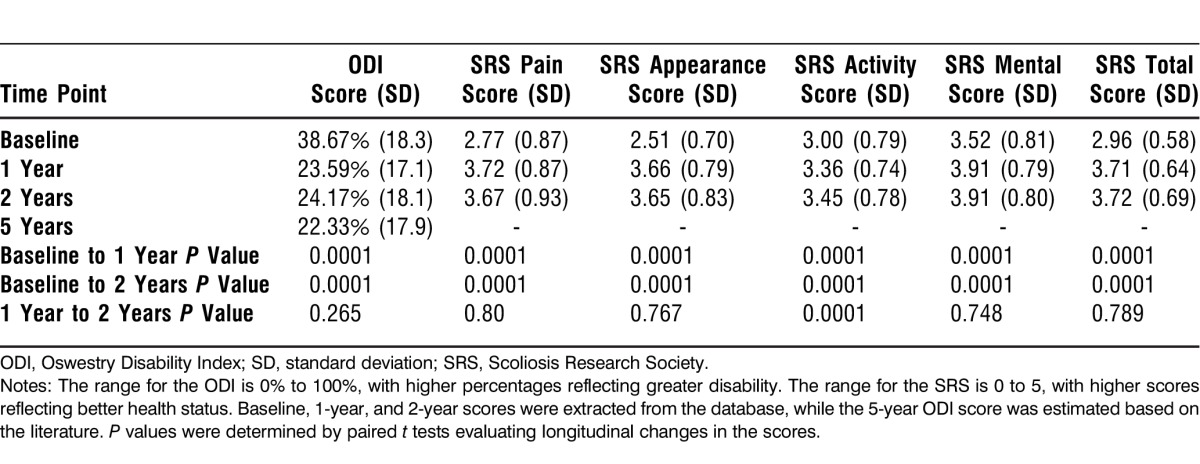
HRQOL scores at baseline and at 1 year and 2 years postoperatively are provided in Table 1. All HRQOL scores significantly improved between baseline and 1 year as well as between baseline and 2 years. The only significant improvement between the 1- and 2-year time points occurred in the SRS activity score.
Table 1.
Health-Related Quality of Life Scores at Baseline and 1 and 2 Years
Cost and Cost-Utility Analysis
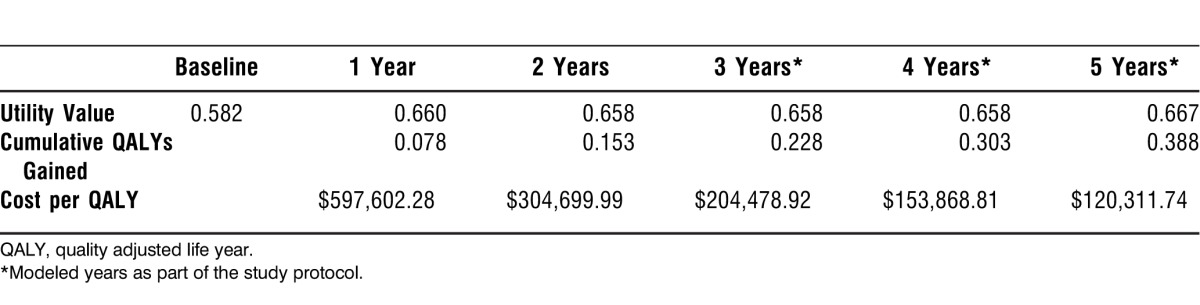
Cost of surgery was calculated by using Medicare reimbursement rates for surgeon, anesthesia, and neuromonitoring fees, as well as hospital reimbursement, based on DRG and ICD-9 CPT coding. The average reimbursement for the entire population was $46,690.63. The mean total reimbursement of the entire set of patients was $37,050.90 without taking into account the reoperations and $46,599.18 with the reoperations. Calculation of health state utility values24,29 revealed that on average there was a cumulative gain in HRQOL. As a result, the projected cost per QALY decreased from $597,602.28 at year 1 to $120,311.74 at year 5 (Table 2).
Table 2.
Utility Values, Associated Cumulative QALYs Gained, and Cost per QALY
Cost-Effectiveness Analysis
Taking $100,000/QALY as the established cost-effective threshold, 220 patients (40.7%) were cost effective at 5-year follow-up.
Demographic Parameters
Table 3 shows that cost-effective patients were significantly older than non–cost-effective patients—55.44 years old compared to 52.9 years old—even though, as Table 4 shows, the 2 groups had nearly equal percentages of patients greater than 55 years old (58.6% in the cost-effective group vs 53.3% in the non–cost-effective group). Sex and history of spine surgery distributions were similar between the 2 groups. Overall, the majority of patients were non–cost-effective as reflected in all diagnosis groups. Among the 4 diagnosis groups, the highest percentage of cost-effective patients occurred in the AISA group.
Table 3.
Demographic Data for Cost-Effective vs Non–Cost-Effective Patients
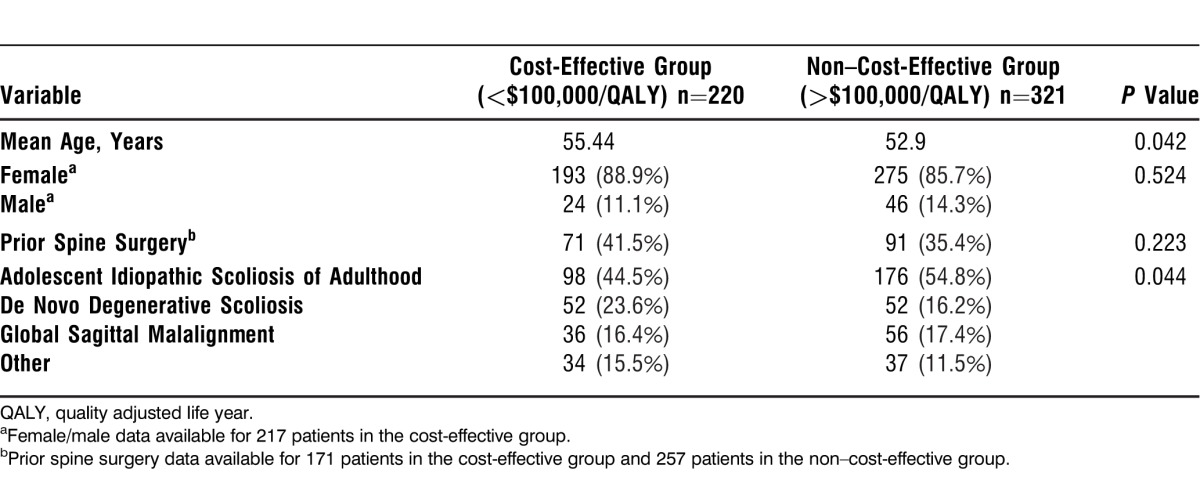
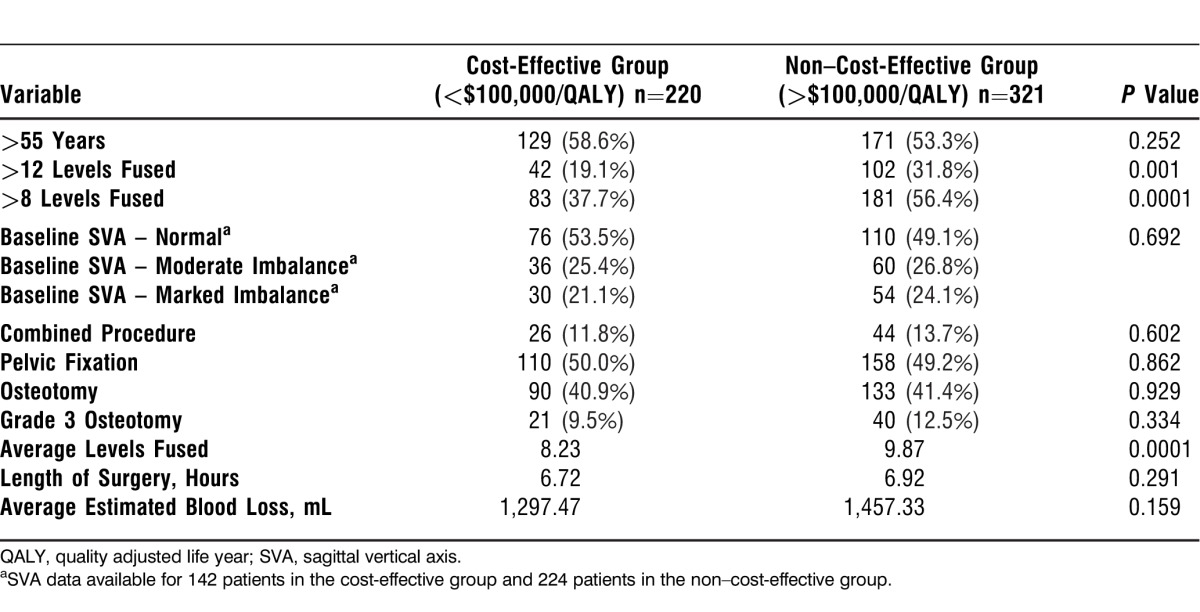
Table 4.
Intraoperative Data for Cost-Effective vs Non–Cost-Effective Patients
Intraoperative Parameters
As shown in Table 4, cost-effective patients had significantly fewer levels fused than non–cost-effective patients, 8.23 vs 9.87 (P=0.0001). More than 8 levels were fused in 37.7% of cost-effective patients compared to 56.4% of non–cost-effective patients (P=0.0001). No significant differences were detected between groups for length of surgery, EBL, or surgical procedure.
HRQOL Scores by Group
Table 5 shows significant differences in HRQOL measures at baseline between cost-effective and non–cost-effective patients. Cost-effective patients had significantly worse scores for SRS pain, activity, and total domains (P<0.05). ODI scores for cost-effective patients were worse at baseline compared to non–cost-effective patients, 44.87% vs 34.42% (P<0.05). The baseline SRS appearance and mental domains do not seem to be related to cost effectiveness; no significant difference between the 2 groups occurred for these measures.
Table 5.
Average Health-Related Quality-of-Life Scores for Cost-Effective vs Non–Cost-Effective Patients at Baseline
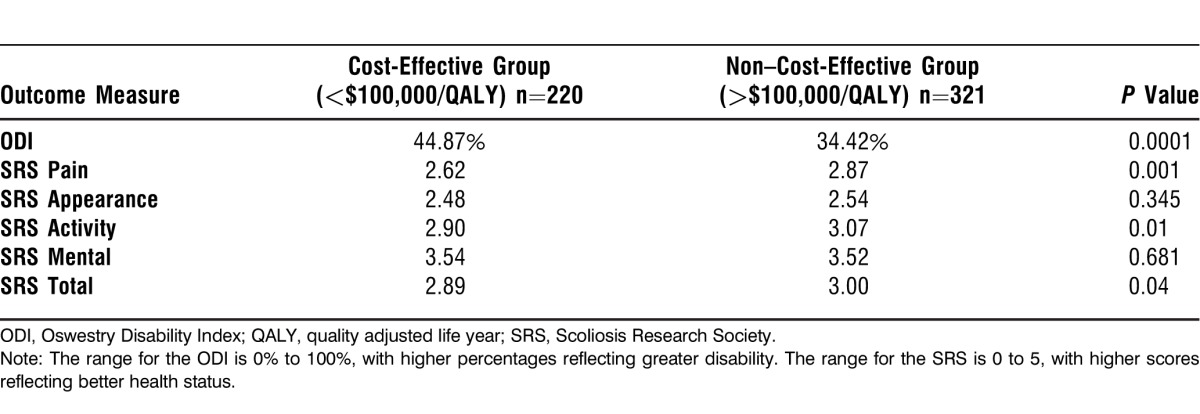
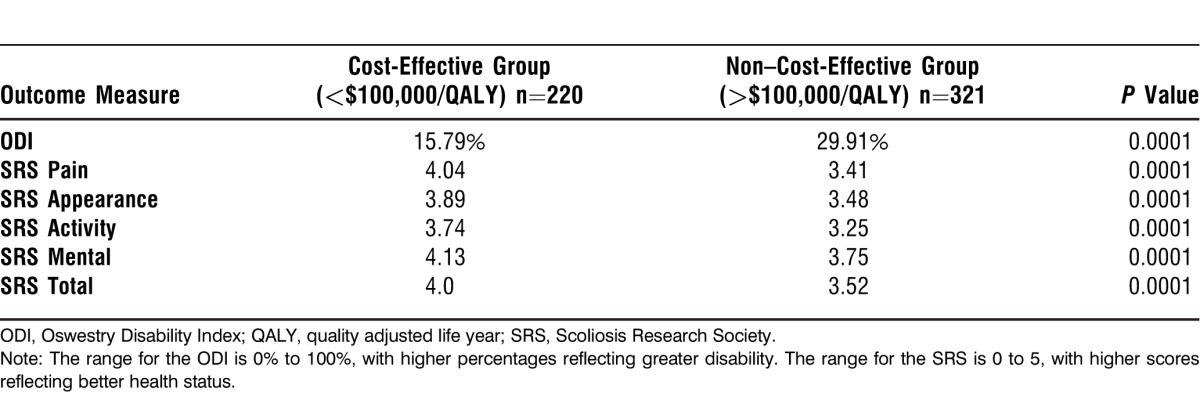
Table 6 shows differences in HRQOL at 2-year follow-up between cost-effective and non–cost-effective patients. Cost-effective patients had significantly better HRQOL scores in all SRS domains and in the ODI at 2-year follow-up (P<0.05).
Table 6.
Average Health-Related Quality-of-Life Scores for Cost-Effective vs Non–Cost-Effective Patients at 2-Year Follow-up
DISCUSSION
We analyzed 541 patients at 2 years and subsequently modeled them to a 5-year endpoint. Overall mean projected direct reimbursement (a measure of cost) at 5 years was $46,690.63, with a mean cumulative QALY gained of 0.388 at 5 years. Cost was calculated utilizing ICD-9 CPT and DRG coding to determine Medicare reimbursement rates for surgeon, anesthesia, neuromonitoring, and hospital costs. This value was used as a measure of projected direct cost of the surgical interventions provided to the patient. Despite employing a highly conservative estimate for the cost of revision surgeries (double the initial cost) and an elevated reoperation rate (24.8%), the cost-effectiveness profile at 5-year follow-up was improved compared with 2-year follow-up ($120k/QALY vs $304k/QALY). Additionally, a much higher percentage of the patient cohort approached cost effectiveness at 5 years when compared to 2-year follow-up (40.7% vs 9%).20,30
Patients with AISA made up a significant portion of the database population. Consequently, patients with this diagnosis accounted for the largest blocks of patients in both the cost-effective (44.5%) and non–cost-effective groups (54.8%). Additionally, most patients did not reach the cost-effectiveness threshold, likely accounting for some of the statistical variation by diagnosis. In the cost-effective group, 23.6% of patients were diagnosed with DDS, 16.4% had global sagittal malalignment, and 15.5% had other diagnoses. In the non–cost-effective group, 11.5% of patients had other diagnoses.
Patients with greater preoperative disability were more likely to reach cost effectiveness as were patients with fusions of less than 8 vertebral levels. The average number of levels fused in the cost-effective group was 8.23 compared to 9.87 in the non–cost-effective group. A review of the population according to fusion level groups (>8 levels, >12 levels) indicates that patients with shorter fusions (<8 levels, <12 levels) were more cost effective compared to those with >8 or >12 levels fused. Approximately 60% of patients in the cost-effective group had <8 levels fused. More than 55% of the non-cost-effective group had >8 levels fused. Patients with greater disability are more likely to improve significantly and would subsequently have a larger number of QALYs gained. Assuming similar direct costs, this degree of improvement would have the effect of lowering the cost/QALY. Similarly, patients requiring shorter fusions require less direct costs and assuming a similar gain in QALYs would increase the trend to cost effectiveness.
Based upon the model for 5-year follow-up,20 patients who did not require reoperation had continued improvement from 2 to 5 years in their QALYs gained (2 ODI percentage points were subtracted from their 2-year scores), whereas patients requiring reoperation had a decline in their health state utility value from 2 to 5 years (7 ODI percentage points were added to their 2-year score), lowering their overall QALYs gained at 5-year follow-up. This decline in effectiveness, coupled with the increase in cost (double the primary cost), greatly affected the projected cost effectiveness of this group.
This study does not suggest modification of current surgical indications. Future studies will further delineate the preoperative characteristics of patients likely to reach cost-effectiveness thresholds and potentially have an impact on surgical indications; however, current measures of determining cost effectiveness do not satisfactorily identify all patients receiving significant clinical benefit. Societal factors such as return to work must be acknowledged as equally important variables in determining the economic value of a health intervention. Surgical indications will always include a balance of disability and radiographic and clinical findings. Economic findings are not likely to definitively alter surgical indications.
At a cost of $120,000 per QALY gained based on this model, surgery for ASD appears close to the accepted threshold (<$100,000) of cost effectiveness when considered over the long term. Our findings regarding cost over an extended time horizon are not surprising. Complex surgical interventions that carry high upfront DCCs by definition take longer to achieve cost effectiveness compared with less complex surgeries with lower DCCs (assuming equivalent outcomes). This result was demonstrated recently in the Spine Patient Outcomes Research Trial (SPORT) data, in which decompressive laminectomy for spinal stenosis and discectomy for disc herniation (2 procedures with relatively low DCCs) showed cost effectiveness at 2 years ($77,600/QALY and $34,355/QALY, respectively), whereas lumbar fusion for spondylolisthesis (a more complex procedure with higher DCCs) was outside the acceptable range ($115,600/QALY).10 However, when reevaluated over 4 years, not only was fusion surgery considered cost effective ($64,300/QALY), but cost effectiveness had improved to a greater extent than surgery for spinal stenosis.10
The marked improvement in the number of patients reaching cost-effectiveness thresholds of lumbar fusion surgery from 2-4 years can, in part, be attributed to the offsetting increase in costs of nonoperative care over that time period—mostly due to productivity losses.13 The societal ramifications that are not adequately captured in cost-effectiveness calculations are particularly relevant when evaluating ASD surgery that is performed to prevent curve progression rather than to treat debilitating symptoms. These patients have a lower QALYs-gained value; however, they avoid the significant and escalating costs that would have been associated with nonoperative management of a rapidly progressive spinal deformity.
This point is perhaps best demonstrated with an incremental cost-effectiveness ratio; however, withholding surgery from patients with rapidly progressing spinal deformity is unethical. We utilize a single cohort model in which baseline QALYs represent the maximum gain from nonoperative management.6,31,32 Unfortunately, this methodology does not capture the value inherent in staving off progressive deformity and its associated disability with preventive surgical treatment—one of the limitations of assessing cost effectiveness using QALYs gained.
A recent study by Glassman et al suggests nonoperative costs for ASD in “high symptom patients” of $14,022 at 2 years.32 If we look to the SPORT data on nonoperative costs in fusion patients as an example, we would expect the nonoperative costs in ASD patients to continue to rise over the longer term, further unmasking the true value of ASD surgery.10
A separate challenge in establishing the cost effectiveness of ASD surgery stems from the significant heterogeneity among the patient population. The same problem was encountered in the cost-effectiveness studies for lumbar fusion, with a range of reported QALYs gained at 2 years (0.26 to 0.86) and a range of surgical techniques (instrumented and noninstrumented) along with the corresponding variation in cost effectiveness.6,9,10,31 Indeed, with both a more homogenous patient cohort, excluding patients who have predominant low back pain symptoms, and a primary focus on instrumented fusions in the setting of spondylolisthesis, cost effectiveness will be demonstrated for lumbar fusion at 2 years.6,31 This issue is magnified in the setting of ASD surgery, where a greater degree of variability with regard to patient population and surgical treatment22,27 is reflected in both the variety of diagnostic groups (AISA, DDS, sagittal imbalance, etc) within ASD and the range of reported outcomes (eg, pseudarthrosis rates range from 0%-35%).27 This added complexity highlights the need to develop a universal classification system that can be used to define appropriately rigid inclusion criteria and operative treatment guidelines. Additionally, such data can aid in identifying factors that are associated with cost effectiveness.
This study is not without significant limitations. As a modeling study based on prospectively collected data analyzed in a retrospective fashion, certain assumptions had to be made and such limitations warrant consideration. Similar techniques have been used in the past to estimate the relative value of surgical interventions.33 The limitations of data collection and the pressing need to establish the cost effectiveness of this treatment make studies such as this one pivotal in that they provide a rational projection of value based on what is currently known regarding the relative costs and clinical effectiveness of ASD surgery over the long term.
We explicitly made efforts to be as conservative as possible when making assumptions with the stated goal of estimating the real value (ie, overestimating the cost/QALY). The majority of revision surgeries are unlikely to entail the same DCCs as the initial surgery; however, such an assumption will err on the side of underestimating value. Likewise, the published revision rates for ASD surgery range from 6%-20% over 5 years,20,22 but we observed an estimated 20% reoperation rate and modeled an additional 4.8% of patients chosen based on literature findings of revision risk factors (minimum 4 risk factors) for a total reoperation rate of 24.8% at 2 years in our data. Published revision rates from 2-5 years range from 2.6%-4.4%, and the higher value was used in conjunction with risk factor stratification.20,22 Modeling the lower revision rate of approximately 7% at 5 years would have greatly reduced the average direct cost per patient.
Additionally, our cost estimations are based on Medicare fee scheduling and do not capture hospital LOS data that can be significant in these complicated patients. Our analysis only uses direct costs and excludes consideration of indirect costs in the calculations because indirect costs were unavailable. Indirect cost is an area of future study in ASD surgery cost effectiveness.
Finally, we included patients with a variety of diagnoses to obtain a sufficient sample size. However, the heterogeneity of such a group presents specific limitations as discussed above.
Future studies must include an analysis of indirect costs alongside direct costs of ASD surgery, ideally including true billed values rather than Medicare reimbursements rates. Analyses should include a direct comparison of operative and nonoperative cost effectiveness. These studies could assist physicians in establishing surgical indications and identifying patients who would benefit the most from operative intervention.
Despite the study limitations, however, we have demonstrated that ASD surgery approaches cost effectiveness at 5-year follow-up.
CONCLUSION
The cost-effective threshold has been set at $100,000/QALY. Based upon a conservatively modeled population with a revision rate close to 25% and including only the direct costs of surgical management, 40.7% of patients reach this cost-effectiveness threshold, indicating a clear trend towards cost effectiveness as early as 5 years after surgery. Continued review of cost effectiveness in operative spinal deformity populations will further elucidate a patient profile likely to reach the $100,000/QALY threshold.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Systems-Based Practice.
REFERENCES
- 1.Anderson GF, Hussey PS, Frogner BK, Waters HR. Health spending in the United States and the rest of the industrialized world. Health Aff (Millwood) 2005 Jul-Aug;24(4):903–914. doi: 10.1377/hlthaff.24.4.903. [DOI] [PubMed] [Google Scholar]
- 2.Chapman JE, Jr, Sinicrope RA, Clark DM. Angio and peritoneal access for endstage renal disease in the community hospital: a cost analysis. Am Surg. 1986 Jun;52(6):315–319. [PubMed] [Google Scholar]
- 3.Kindlmann GL, Weinstein DM, Jones GM, Johnson CR, Capecchi MR, Keller C. Practical vessel imaging by computed tomography in live transgenic mouse models for human tumors. Mol Imaging. 2005 Oct-Dec;4(4):417–424. doi: 10.2310/7290.2005.05166. [DOI] [PubMed] [Google Scholar]
- 4.Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997-2006. Spine (Phila Pa 1976) 2009 Sep 1;34(19):2077–2084. doi: 10.1097/BRS.0b013e3181b1fad1. [DOI] [PubMed] [Google Scholar]
- 5.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008 Feb 13;299(6):656–664. doi: 10.1001/jama.299.6.656. Erratum in: JAMA. 2008 Jun 11;299(22):2630. [DOI] [PubMed] [Google Scholar]
- 6.Adogwa O, Parker SL, Davis BJ, et al. Cost-effectiveness of transforaminal lumbar interbody fusion for Grade I degenerative spondylolisthesis. J Neurosurg Spine. 2011 Aug;15(2):138–143. doi: 10.3171/2011.3.SPINE10562. Epub 2011 May 6. Erratum in: J Neurosurg Spine. 2011 Aug;15(2):211. [DOI] [PubMed] [Google Scholar]
- 7.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976) 2000 May 1;25(9):1132–1139. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Tosteson AN, Lurie JD, Tosteson TD, et al. SPORT Investigators. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008 Dec 16;149(12):845–853. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tosteson AN, Skinner JS, Tosteson TD, et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008 Sep 1;33(19):2108–2115. doi: 10.1097/brs.0b013e318182e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976) 2011 Nov 15;36(24):2061–2068. doi: 10.1097/BRS.0b013e318235457b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009 Mar;12(Suppl 1):S5–S9. doi: 10.1111/j.1524-4733.2009.00515.x. Erratum in: Value Health. 2010 Dec;13(8):1065. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto JM. Quality-Adjusted Life Years (QALY) Utility Models under Expected Utility and Rank Dependent Utility Assumptions. J Math Psychol. 1999 Jun;43(2):201–237. doi: 10.1006/jmps.1999.1256. [DOI] [PubMed] [Google Scholar]
- 13.Sloan FA. Valuing Health Care: Costs, Benefits, and Effectiveness of Pharmaceuticals and Other Medical Technologies. New York: Cambridge University Press;; 1995. [Google Scholar]
- 14.Kaplan RM, Bush JW. Health-related quality of life measurement for evaluation research and policy analysis. Health Psychol. 1982;1(1):61–80. [Google Scholar]
- 15.Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ. When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. 2004 May;13(5):429–436. doi: 10.1002/hec.853. [DOI] [PubMed] [Google Scholar]
- 16.Grosse SD, Teutsch SM, Haddix AC. Lessons from cost-effectiveness research for United States public health policy. Annu Rev Public Health. 2007;28:365–391. doi: 10.1146/annurev.publhealth.28.021406.144046. [DOI] [PubMed] [Google Scholar]
- 17.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000 Jul-Sep;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 18.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008 Apr;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 19.Whitmore RG, Schwartz JS, Simmons S, Stein SC, Ghogawala Z. Performing a cost analysis in spine outcomes research: comparing ventral and dorsal approaches for cervical spondylotic myelopathy. Neurosurgery. 2012 Apr;70(4):860–867. doi: 10.1227/NEU.0b013e3182367272. discussion 867. [DOI] [PubMed] [Google Scholar]
- 20.Bridwell KH, Baldus C, Berven S, et al. Changes in radiographic and clinical outcomes with primary treatment adult spinal deformity surgeries from two years to three- to five-years follow-up. Spine (Phila Pa 1976) 2010 Sep 15;35(20):1849–1854. doi: 10.1097/BRS.0b013e3181efa06a. [DOI] [PubMed] [Google Scholar]
- 21.Glassman SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007 Nov 15;32(24):2764–2770. doi: 10.1097/BRS.0b013e31815a7644. [DOI] [PubMed] [Google Scholar]
- 22.Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O'Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976) 2010 Jan 15;35(2):219–226. doi: 10.1097/BRS.0b013e3181c91180. [DOI] [PubMed] [Google Scholar]
- 23.Rinella A, Bridwell K, Kim Y, et al. Late complications of adult idiopathic scoliosis primary fusions to L4 and above: the effect of age and distal fusion level. Spine (Phila Pa 1976) 2004 Feb 1;29(3):318–325. doi: 10.1097/01.brs.0000111838.98892.01. [DOI] [PubMed] [Google Scholar]
- 24.Carreon LY, Glassman SD, McDonough CM, Rampersaud R, Berven S, Shainline M. Predicting SF-6D utility scores from the Oswestry disability index and numeric rating scales for back and leg pain. Spine (Phila Pa 1976) 2009 Sep 1;34(19):2085–2089. doi: 10.1097/BRS.0b013e3181a93ea6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C. 2nd. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005 Jul 15;30(14):1643–1649. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Bridwell KH, Lenke LG, Cho KJ, Edwards CC. 2nd, Rinella AS. Pseudarthrosis in adult spinal deformity following multisegmental instrumentation and arthrodesis. J Bone Joint Surg Am. 2006 Apr;88(4):721–728. doi: 10.2106/JBJS.E.00550. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006 Sep 15;31(20):2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 28.Yagi M, Akilah KB, Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine (Phila Pa 1976) 2011 Jan 1;36(1):E60–E68. doi: 10.1097/BRS.0b013e3181eeaee2. [DOI] [PubMed] [Google Scholar]
- 29.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002 Mar;21(2):271–292. doi: 10.1016/s0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 30.Fischer CR, Lonner BS, Terran JS, et al. Scoliosis Research Society 47th Annual Meeting. Chicago, IL: 2012. Factors Predicting Cost-Effectiveness of Adult Spinal Deformity Surgery at Two Years Follow-Up. September 5-8. [Google Scholar]
- 31.Glassman SD, Polly DW, Dimar JR, Carreon LY. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 years after surgery. Spine (Phila Pa 1976) 2012 Apr 20;37(9):769–774. doi: 10.1097/BRS.0b013e3181e03099. [DOI] [PubMed] [Google Scholar]
- 32.Glassman SD, Carreon LY, Shaffrey CI, et al. The costs and benefits of nonoperative management for adult scoliosis. Spine (Phila Pa 1976) 2010 Mar 1;35(5):578–582. doi: 10.1097/BRS.0b013e3181b0f2f8. [DOI] [PubMed] [Google Scholar]
- 33.Polly DW, Jr, Glassman SD, Schwender JD, et al. Lumbar Spine Study Group. SF-36 PCS benefit-cost ratio of lumbar fusion comparison to other surgical interventions: a thought experiment. Spine (Phila Pa 1976) 2007 May 15;32((11 Suppl)):S20–S26. doi: 10.1097/BRS.0b013e318053d4e5. [DOI] [PubMed] [Google Scholar]


