SUMMARY
Protein acetylation has emerged as a major mechanism in regulating cellular metabolism. Whereas most glycolytic steps are reversible, the reaction catalyzed by pyruvate kinase is irreversible and the reverse reaction requires phosphoenolpyruvate carboxykinase (PEPCK1) to commit for gluconeogenesis. Here we show that acetylation regulates the stability of the gluconeogenic rate limiting enzyme PEPCK1, thereby modulating cellular response to glucose. High glucose destabilizes PEPCK1 by stimulating its acetylation. PEPCK1 is acetylated by the P300 acetyltransferase and this acetylation stimulates the interaction between PEPCK1 and UBR5, a HECT domain containing E3 ubiquitin ligase, therefore promoting PEPCK1 ubiquitinylation and degradation. Conversely, SIRT2 deacetylates and stabilizes PEPCK1. These observations represent an example that acetylation targets a metabolic enzyme to a specific E3 ligase in response to metabolic condition changes. Given that increased levels of PEPCK is linked with type II diabetes, this study also identifies potential therapeutic targets for diabetes.
INTRODUCTION
Metabolic enzymes are regulated by various mechanisms such as transcription, post translational modifications (PTM), and allosteric regulation. The role of PTM in metabolism regulation has received close attention not only because its ability of acutely responding to changes in cellular metabolic status but also its regulation by upstream signaling pathways. Phosphorylation was first discovered in the study of metabolic enzymes and plays a very broad role in metabolic control through mostly regulating the conformation and activity of metabolic enzymes. Phosphorylation of glycogen phosphorylase and glycogen synthase exemplifies a typical mechanism that PTM directly regulate catalytic activity of metabolic enzymes (Browner and Fletterick, 1992; Johnson, 1992; Soderling et al., 1979). Very few examples are known whether a metabolic enzyme is regulated by a PTM that affects the proteins stability and links to nutrient condition.
Acetylation has been identified as an evolutionarily conserved modification in metabolic enzymes and has emerged to play major roles in metabolic regulation (Wang et al., 2010; Zhao et al., 2010). In bacteria, acetylation has been found not only to control activities of key metabolic enzymes such as acetyl CoA synthetase and glyceraldehyde dehydrogenase but also to play critical roles in coordinating activities of metabolic pathways according to different carbon source availability (Starai et al., 2002; Wang et al., 2010). In eukaryotic cells, a number of recent studies have rapidly revealed that acetylation regulates key metabolic enzymes in urea and TCA cycles, gluconeogenesis, fatty acids metabolism and reactive oxygen species scavenge system (Hirschey et al.; Kim et al.; Nakagawa et al., 2009; Qiu et al.; Someya et al.; Zhao et al., 2010). The consequence of acetylation on these enzymes is that they directly affect catalytic activity of these enzymes via different mechanisms (Lin et al., 2009; Someya et al.; Wang et al., 2010; Zhao et al., 2010).
PEPCK1 is a cataplerotic enzyme that plays important functions in gluconeogenesis, glyceroneogenesis, serine synthesis and amino acid metabolism (Hanson and Patel, 1994; Nye et al., 2008; Tannen, 1978). It catalyzes the first committed and rate limiting step of gluconeogenesis, which plays critical functions, mainly in liver and to a lesser extent in kidney and small intestine, to maintain glucose homeostasis (Chakravarty et al., 2005). It is well established that changes in the rate of transcription of PEPCK1, an event regulated by transcription factors such as PGC-1α and HNF-1 in response to hormones and diets (Granner and O'Brien, 1992; Hanson and Reshef, 1997; Yoon et al., 2001), is of critical importance in maintaining the overall PEPCK1 activity. Since elevated gluconeogenesis is an important marker in the evaluation of type II diabetes (Granner and O'Brien, 1992), mechanisms that are involved in PEPCK1 regulation have been extensively studied.
Yeast PEPCK1 has been reported to be acetylated at Lys19 and Lys514 and acetylation inactivates its catalytic activity (Lin et al., 2009). Human PEPCK1 was identified to be acetylated at Lys70, Lys71 and Lys594 by cell wide protein acetylation profiling (Zhao et al., 2010), and acetylation of PEPCK1 leads to decreased protein stability (Zhao et al., 2010). This study is directed toward to understand the underlining mechanism of acetylation-induced PEPCK1 reduction.
RESULTS
Acetylation promotes PEPCK1 degradation via ubiquitin-proteosome pathway
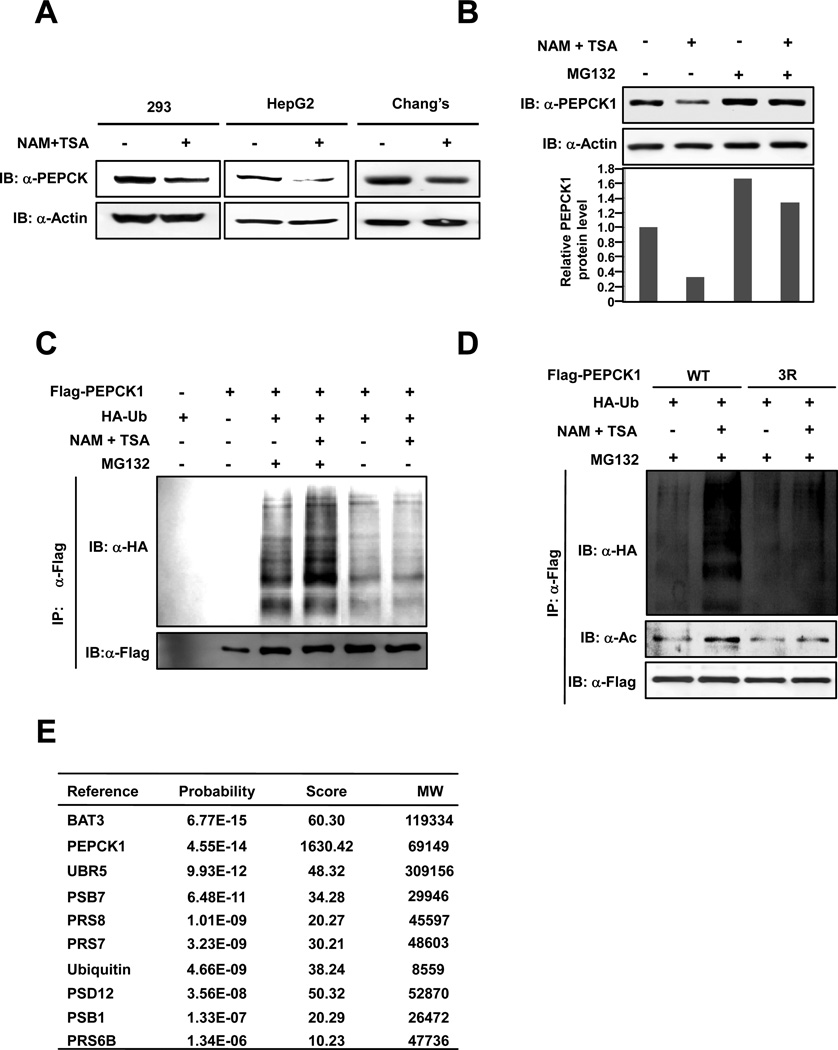
In order to elucidate the nature of acetylation in human PEPCK1 stability regulation, HEK293, HepG2, Chang’s and HEK293T cells were each treated with a deacetylase inhibitor cocktail that contains nicotinamide (NAM) and trichostatin A (TSA), a chemical combination that supposedly inhibits all four classes of known deacetylases (Xu et al., 2007). Confirming our previous finding (Zhao et al., 2010), steady state levels of endogenous PEPCK1 were decreased 50–70% by inhibition of deacetylases (Figures 1A, 1B). When MG132 was included in cell culture medium to inhibit proteosomal degradation, steady state PEPCK1 levels increased by more than 60% in HEK293T cells and, notably, this treatment cancelled the destabilization effect of NAM+TSA treatment on PEPCK1 (Figure 1B). These results suggested that acetylation-promoted decrease of PEPCK1 is likely mediated by the ubiquitin-proteosome pathway. Consistent with this notion, when Flag-tagged PEPCK1 was co-expressed with HA-tagged ubiquitin, active PEPCK1 ubiquitinylation was detected and inhibition of deacetylases significantly increased PEPCK1 ubiquitinylation (Figures 1C, 1D). Moreover, inhibition of deacetylases further increased PEPCK1 ubiquitination in the presence of MG132. The promotional role of inhibition of deacetylases on PEPCK1 ubiquitination, however, was not observed when all three putative acetylation lysine residues of PEPCK1 were changed to non-acetylable arginine residues (PEPCK13K/R). When PEPCK13K/R was expressed in HEK293T cells, purified PEPCK13K/R protein had a low basal level of ubiquitination, and more importantly, its ubiquitination level did not respond to inhibition of deacetylases (Figure 1D), indicating that PEPCK1 ubiquitination depends on acetylation.
Figure 1. Acetylation promotes ubiquitin-proteosome degradation of PEPCK1.
(A) Acetylation promotes cellular PEPCK1 degradation. Endogenous PEPCK1 levels of HEK293, HepG2 and Chang’s liver cells were determined under both with and without deacetylase inhibitor treatment. (B) Acetylation-promoted PEPCK1 degradation is inhibited by proteosome inhibitor. 293T cells were treated or not treated by TSA and NAM in the absence or presence of MG132. Endogenous PEPCK1 was probed by anti-PEPCK1 antibody. (C) Deacetylase inhibitors increase the ubiquitination of PEPCK1. Ubiquitination levels of affinity purified Flag-PEPCK1 proteins expressed under treated or not treated by NAM+TSA in the absence or presence of MG132 were detected. (D) Deacetylase inhibitors do not affect PEPCK13K/R acetylation level and protein stability. The acetylation levels and ubiquitination levels of affinity purified PEPCK13K/R protein expressed under with or without NAM+TSA treatment were determined. (E) Identification of PEPCK1 interacting proteins. PEPCK1 interacting proteins involved in acetylation and ubiquitination are shown. See also Table S1.
PEPCK1 interacts with proteins involved in proteosome degradation
To elucidate the regulatory mechanism of PEPCK1 degradation, we established a HEK293T derivative cell line that stably expressed PEPCK1 with both Flag and streptavidin-binding peptide (SBP) tagged to the N-terminus. Tandem affinity purification with Flag beads and streptavidin- agarose beads followed by mass spectrometry analysis allowed us to identify proteins that specifically interact with PEPCK1. Among the consistently identified proteins were BAT3, UBR5, ubiquitin, and various proteosome associated proteins (Figure 1E, Table S1). That PEPCK1 interacts with many proteins associated with the ubiquitin-proteosome pathway further strengthens the case for PEPCK1 degradation through ubiquitin-proteosome pathway. Interestingly, BAT3 is a known enhancer of the p300 acetyltransferase as well as a co-chaperone that regulates protein degradation via the ubiquitin-proteosome pathway (Sasaki et al., 2007; Takayama and Reed, 2001); UBR5 (ubiquitin protein ligase E3 component n-recognin 5, also known as EDD1, DD5 and HYD) is a HECT domain E3 ubiquitin ligase (Callaghan et al., 1998; Honda et al., 2002). These observations indicate that BAT3 and p300 may be involved in PEPCK1 acetylation, while UBR5 could serve as a potential E3 ubiquitin ligase to regulate PEPCK1 stability.
UBR5 is involved in glucose dependent PEPCK1 degradation
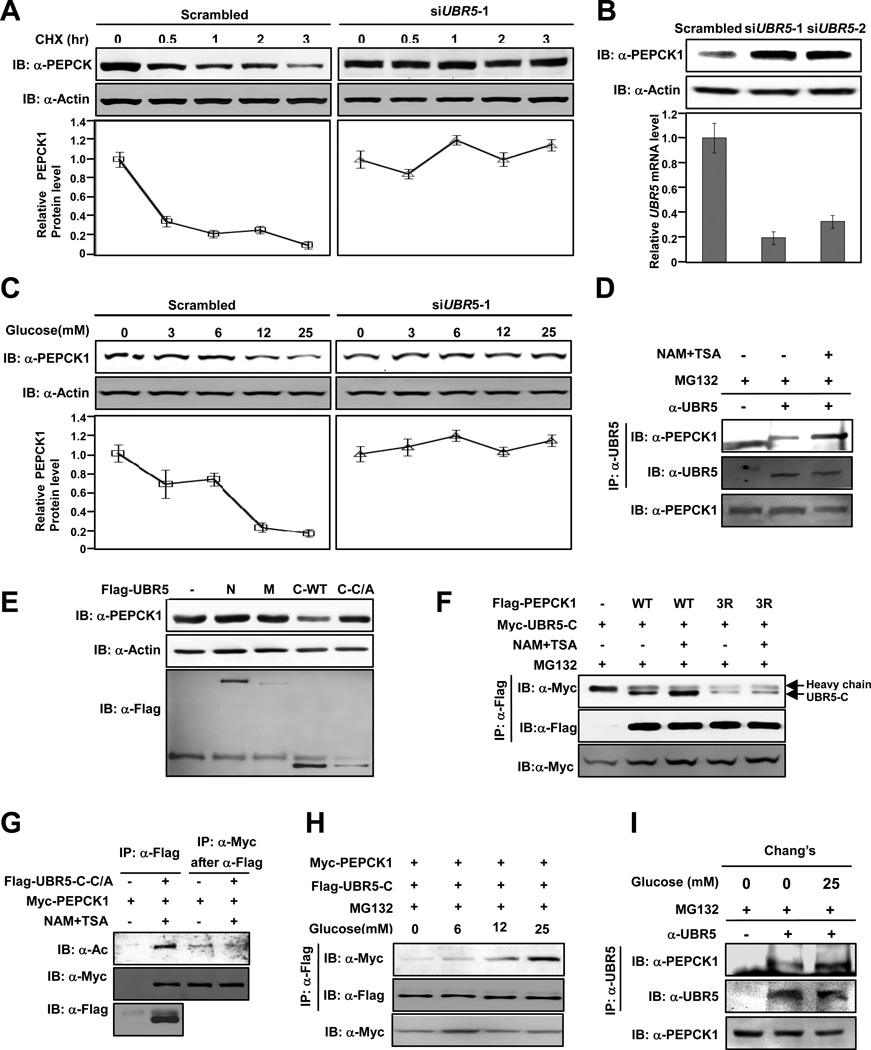
We examined the role of UBR5 in regulation of PEPCK1 protein stability. PEPCK1 had a rather short half-life of approximately 30 minutes under high glucose conditions. However, PEPCK1 protein stability was dramatically increased when UBR5 was knocked down by siRNA (Figure 2A, Figure S1A). Consistently, UBR5 knockdown resulted in a significant increase in steady state level of PEPCK1 (Figure 2B). These results suggest that UBR5 plays a role in PEPCK1 degradation.
Figure 2. UBR5 is an E3 ligase of PEPCK1.
(A) UBR5 knockdown stabilizes PEPCK1. PEPCK1 levels of 293T cells with or without UBR5 knocked down were determined at different time points after CHX was added. Shown are representative western blot results of three replicates. Average quantified relative protein abundance from all three repeats is shown with SD. (B) UBR5 knockdown increases steady state PEPCK1 level. UBR5 in 293T cells was knocked down by siRNA and steady state PEPCK1 level was determined. Results of two different siRNA oligos are shown. (C) UBR5 knockdown abolishes glucose induced PEPCK destabilization. Steady state PEPCK1 levels of HEK293T cells and HEK293T cells with UBR5 knocked down were determined under different glucose concentrations. (D) Inhibition of deacetylases promotes endogenous PEPCK1-UBR5 interaction. Endogenous PEPCK1 levels co-precipitated with endogenous UBR5 under with and without MG132 treatment were detected. (E) Overexpressed UBR5-C decreases steady state PEPCK1 level. Steady state PEPCK1 levels of 293T cells were measured when UBR5 domains were overexpressed. (F) Deacetylase inhibitors affect PEPCK1-UBR5 interaction. PEPCK1-UBR5-C and PEPCK13K/R–UBR5-C interactions were determined with or without TSA and NAM treatment. (G) UBR5 binds to acetylated PEPCK1. UBR5-C-C/A and PEPCK1 were co-expressed in 293T cells. Acetylation levels of PEPCK1 associated with UBR5-C-C/A. (H) PEPCK1-UBR5 interaction is enhanced by high glucose. Myc-PEPCK1 and Flag-UBR5-C were co-expressed in 293T cells maintained at different glucose concentrations and PEPCK-UBR5-C interaction was by IP-western. (I) Glucose increase PEPCK1-UBR5 interaction. Endogenous UBR5 proteins in Chang’s cells cultured in 25mM glucose and glucose free media were precipitated and PEPCK-UBR5-C interaction was determined. See also Figure S1.
As the key enzyme in balancing cellular glucose homeostasis, PEPCK1 activity is inversely regulated by glucose concentrations [(Panin et al., 1979; Scott et al., 1998), Figure 2C]. However, in cells with UBR5 knocked down, high glucose failed to reduce PEPCK1 levels (Figure 2C, right panel). Furthermore, inhibition of deacetylases decreased the steady state PEPCK1 levels (Figure S1B, upper panel); whereas UBR5 knock down prevented the reduction of endogenous PEPCK1 by inhibition of deacetylases (Figure S1B, lower panel). Collectively, these data demonstrate that UBR5 is required for PEPCK1 regulation by glucose and it promotes PEPCK1 degradation possibly by serving as the E3 ubiquitin ligase for PEPCK1.
UBR5 interacts with and degrades PEPCK1 through its C-terminal domain
In an attempt to analyze UBR5-PEPCK1 interaction, we found endogenous UBR5 co-precipitated with overexpressed PEPCK1 (Figure S1C), conversely, endogenous PEPCK1 co-precipitated with overexpressed UBR5 (Figure S1D). Moreover, the interaction between endogenous PEPCK1 and endogenous UBR5 was enhanced by deacetylase inhibitor treatment (Figure 2D), suggesting acetylation promotes PEPCK1-UBR5 interaction. UBR5 is a large protein containing 2,799 amino acid residues. To map the specific domain of UBR5 that is responsible for PEPCK1-UBR5 interaction, we generated N-terminal zinc finger containing fragment (1-1245, UBR5-N), central fragment (1246-2375, UBR5-M), and C-terminal HECT domain containing fragment (2375-2799, UBR5-C) of UBR5 (Figure S1E) and tested their interaction with PEPCK1 and found that PEPCK interacted with UBR5-C but not with either UBR5-N or UBR5-M (Figure S1F). Since UBR5-C contains catalytic HECT domain, it could act as an E3 ligase towards PEPCK1 and thus promote PEPCK1 degradation. We tested this possibility by ectopically expressing UBR5-C and a catalytic inactive mutant of UBR5-C (UBR5-C C2768A, referred thereafter as UBR5-C-C/A) that still keep the ability to bind to PEPCK1 (see Figure 2G) and determined steady state endogenous PEPCK1 protein levels. Only in cells overexpressing UBR5-C the endogenous PEPCK1 level was markedly decreased and ectopically expression of UBR5-C-C/A could not reduce PEPCK1 protein level (Figure 2E). Together, these results indicate that the E3 ligase activity of UBR5-C is required for UBR5-C to decrease PEPCK1 protein level.
Acetylation of PEPCK1 promotes UBR5-PEPCK1 interaction
A key mechanism in regulation of protein ubiquitination is the interaction between a target protein and its specific E3 ligase (Skowyra et al., 1997). We investigated the role of acetylation in UBR5 dependent PEPCK1 degradation. Since inhibition of deacetylases enhances the interaction between PEPCK1 and UBR5 in vivo (Figure 2D), we investigated the role of PEPCK1 acetylation in PEPCK1-UBR5 interaction by comparing the binding of PEPCK1 and the non-acetylable PEPCK1 mutants with UBR5-C in response to inhibition of deacetylases. The interaction between Flag-PEPCK1 and Myc-UBR5-C was readily detectable and was enhanced by more than two-fold upon deacetylase inhibitor treatment; the interaction between PEPCK13K/R and UBR5-C was barely detectable and was not responsive to inhibition of deacetylases (Figure 2F). Moreover, acetylation mimetic PEPCK13K/Q mutant had stronger interaction with UBR5-C than wild type PEPCK1 (Figure S1G). These results support the notion that acetylation of PEPCK1 facilitates binding to UBR5. To further confirm this notion, we tested whether the UBR5-associated PEPCK1 is highly acetylated compared with the free PEPCK1. UBR5-C-C/A and PEPCK1 was co-expressed in HEK293T cells. PEPCK1 was co-immunoprecipitated by UBR5-C-C/A and the relative acetylation level of the co-precipitated PEPCK1 was compared with the free PEPCK1 that was not co-precipitated by UBR5-C-C/A. We observed that PEPCK1 protein co-purified with UBR5-C-C/A had a much higher relative acetylation level than PEPCK1 protein purified from lysates post UBR5 immunoprecipitation (Figure 2G). This result clearly demonstrates that acetylated PEPCK1 is preferentially associated with UBR5 and suggests an underlining molecular mechanism of PEPCK1 acetylation in promoting its degradation by increasing interaction with the UBR5.
The physiological correlation between PEPCK1 acetylation and its UBR5 binding was investigated by determining PEPCK1-UBR5 binding under increasing glucose concentrations. High glucose increases PEPCK1, but not PEPCK13K/R acetylation [(Zhao et al., 2010), Figure S1H]. When UBR5-C and PEPCK1 was coexpressed in HEK293T cells maintained under different glucose concentrations and in the presence of MG132, we observed that high glucose promoted UBR5-C-PEPCK1 interaction in a dosage dependent manner (Figure 2H). Furthermore, the amount of UBR5-associated PEPCK1 in cells cultured in 25 mM glucose increased approximately one-fold compared with cells cultured in glucose-free medium (Figure 2I). In contrast, the non-acetylable PEPCK13K/R mutant displayed little interaction with UBR5, and more importantly, glucose could not increase the interaction between UBR5 and PEPCK13K/R (Figure S1I). These results show that the UBR5-PEPCK1 interaction is under control by acetylation, which is regulated by glucose concentrations.
P300 acetylates and destabilizes PEPCK1
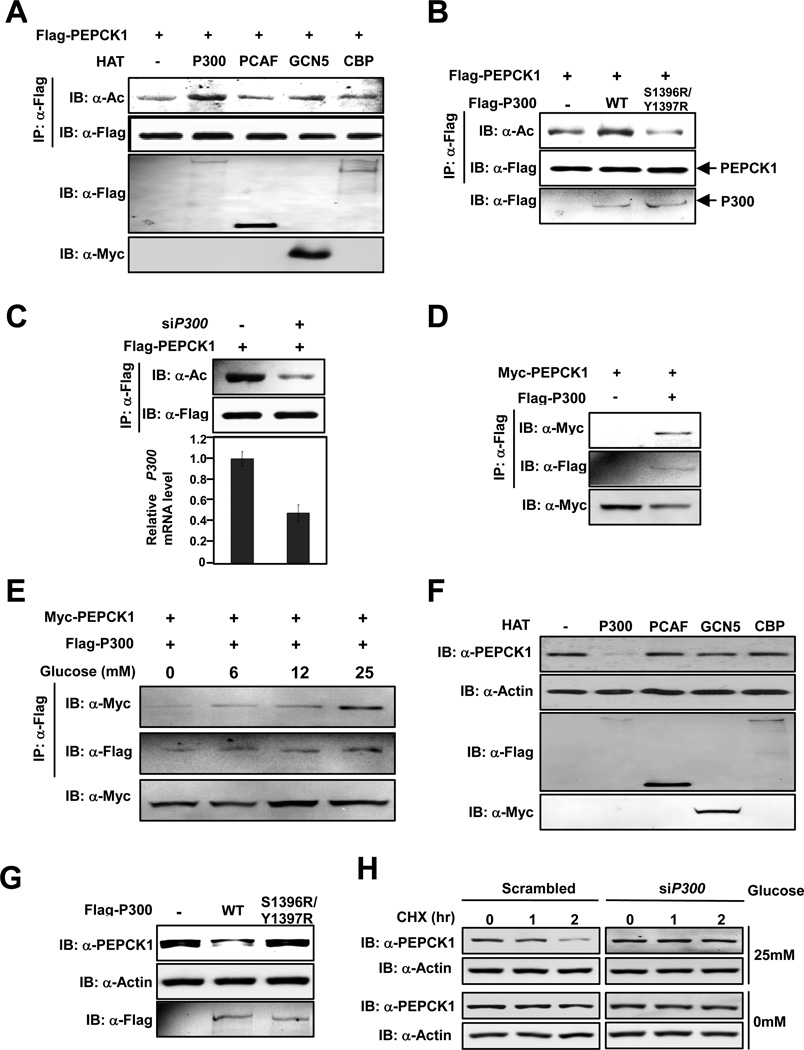
Our affinity purification identified BAT3, a known enhancer of P300 (Mantelingu et al., 2007), as an interacting protein of PEPCK1 led us to investigate a possible involvement of P300 in PEPCK1 acetylation. Notably, the sequence flanking acetylated Lys70 and Lys71 of PEPCK1 matches the consensus sequence of P300 substrates [(Liu et al., 2008), Figure S2A]. To experimentally demonstrate PEPCK1 is a substrate of P300, P300 and other acetyltransferases, including PCAF, CBP and GCN5, were co-transfected with PEPCK1 and the acetylation status of PEPCK1 was determined. Our data showed that only P300, but not the other acetyltransferases, increased PEPCK1 acetylation (Figure 3A). Moreover, co-expression of the catalytic inactive P300S1396R/Y1397R mutant did not increase PEPCK1 acetylation (Figure 3B), indicating that P300 acetyltransferase activity is required for PEPCK1 acetylation. Furthermore, co-expression of P300 increased acetylation levels of wild type PEPCK1, but not PEPCK13K/R, indicating that P300 acts on these lysine residues of PEPCK1 (Figure S2B). Contrary to overexpressing P300 increased PEPCK1 acetylation (Figure 3A), knockdown of P300 significantly decreased PEPCK1 acetylation level (Figure 3C). In addition, PEPCK1 could be co-immunoprecipitated by P300 when both were co-expressed in 293T cells (Figure 3D) and P300-PEPCK1 interaction increased with glucose concentration in a dose dependent manner (Figure 3E). These results support the notion that P300 is an acetyltransferase for PEPCK1. Consistent with this notion is a finding that although P300 mainly localized in nucleus, when PEPCK1 was overexpressed in Chang’s Liver cells, increased amount of P300 was detected localized in cytoplasm, the compartment in which PEPCK1 is located (Figure S2C).
Figure 3. P300 acetylates PEPCK1 and promotes its degradation.
(A) P300 acetylates PEPCK1. Flag-PEPCK1 was co-expressed with different acetyltransferases and purified by Flag beads. Acetylation levels of purified Flag-PEPCK1 proteins were determined. (B) Catalytic activity of P300 is required to acetylate PEPCK1. Acetylation levels of PEPCK1 co-expressed with P300 or its catalytic mutant were determined. (C) P300 knockdown decreases PEPCK1 acetylation level. Acetylation levels of Flag-PEPCK1 expressed and purified from 293T cells with or without P300 knocked down by siRNA were detected. (D) P300 interacts with PEPCK1. Interaction between co-expressed Flag-P300 and Myc-PEPCK1 was detected. (E) P300-PEPCK1 interaction is enhanced by glucose. Interaction between Flag-P300 and Myc-PEPCK1 co-expressed under different concentrations of glucose was determined. (F) P300 overexpression decreases steady state PEPCK1 level. Steady state PEPCK1 levels of 293T cells overexpressing different acetyltransferases were determined. (G) Catalytic activity of P300 is required for decreasing steady state PEPCK1 level. PEPCK1 levels of 293T cells and 293T cells expressing P300 and P300S1396R/Y1397R were determined. (H) P300 is required for PEPCK1 degradation. PEPCK1 degradation rates of Chang’s cells with and without P300 knocked down were measured under both low (0mM) and high (25mM) glucose concentrations. See also Figure S2.
The effect of P300 on PEPCK1 stability was also studied. Steady state PEPCK1 levels were determined when P300 and other related acetyltransferases were each ectopically expressed in HEK293T cells. Expression of P300 decreased the steady state PEPCK1 protein level by 70%, while expression of other acetyltransferases caused no discernable change of PEPCK1 protein levels (Figure 3F), suggesting that increased P300 activity in cells destabilized PEPCK1. The destabilizing ability of P300 on PEPCK1 was again found to be dependent on the catalytic activity of P300 because ectopically expressing of catalytic inactive P300S1396R/Y1397R mutant in HEK293T cells did not cause any change in steady state PEPCK1 protein level (Figure 3G), consistent with the finding that knockdown of P300 in Chang’s cells by siRNA reduced endogenous PEPCK1 decreasing rate with increased glucose concentrations (Figures 3H, S2D), supporting P300 as the acetyltransferase of PEPCK1.
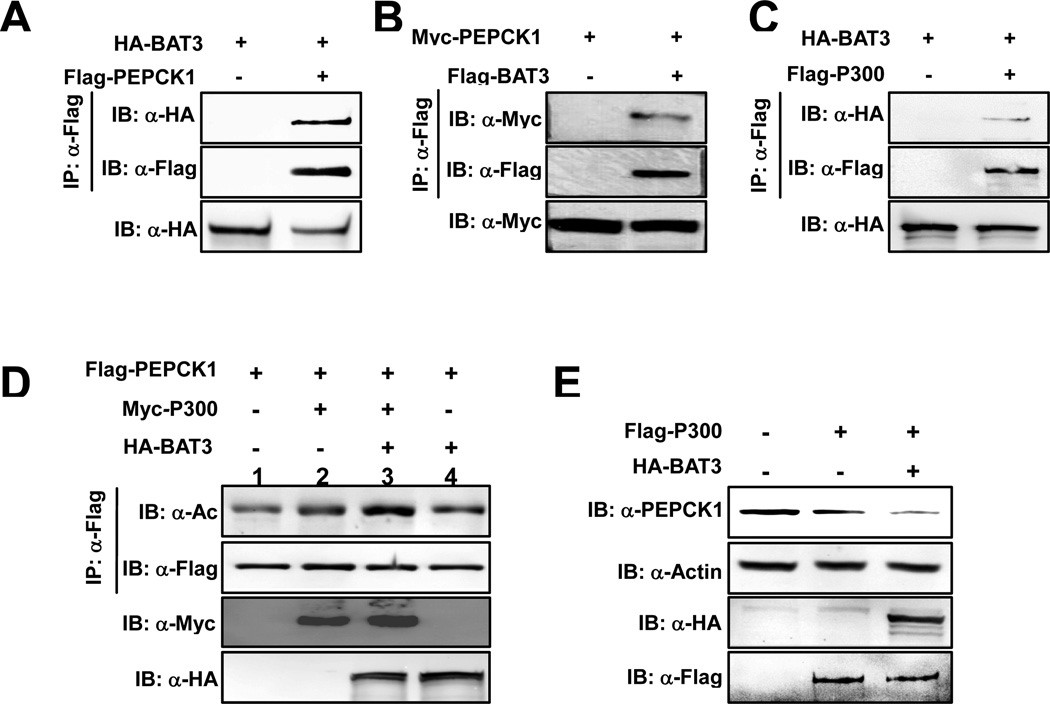
BAT3 enhances PEPCK1 acetylation and destabilizes PEPCK1
BAT3 enhances P53 acetylation by forming a complex with P300 and recruiting P53 to P300 (Mantelingu et al., 2007; Sasaki et al., 2007). To test whether BAT3 enhances PEPCK1 acetylation, we first tested interactions among P300, BAT3 and PEPCK1. We found that PEPCK1 interacted with both BAT3 and P300 individually (Figures 4A, 4B, 4C, 3D). These results indicate that BAT3, and P300 can both exist in the PEPCK1 protein complex, suggesting the possibility that BAT3 could be an enhancer of PEPCK1 acetylation. Furthermore, when either BAT3 or P300 was co-expressed with PEPCK1, there was an increase in PEPCK1 acetylation level (Figure 4D, lanes 1, 2, 4); moreover, when both BAT3 and P300 were co-expressed, PEPCK1 acetylation level was further increased by more than 2-fold (Figure 4D, lanes 1, 3), indicating a synergistic effect of BAT3 and P300 to promote PEPCK1 acetylation. Consistent with the acetylation results, expression of P300 in HEK293T cells caused a significant decrease in endogenous PEPCK1 level and co-expression of BAT3 further decreased the steady state PEPCK1 protein level (Figure 4E). These results collectively support a model that BAT3 enhances the ability of P300 to acetylate PEPCK1 and therefore, contributes to PEPCK1 protein level regulation.
Figure 4. BAT3 enhances PEPCK1 acetylation and promotes its degradation.
(A,B) BAT3 interacts with PEPCK1. Interactions between BAT3 and PEPCK1 were determined when proteins were co-expressed with each other in HEK293T cells. (C) BAT3 interacts with P300. Interactions between co-expressed BAT3 and P300 were analyzed. (D) BAT3 enhances PEPCK1 acetylation. Acetylation levels of PEPCK1 expressed and purified from 293T cells and 293T cells co-expressing P300 and P300 plus BAT3 were determined. (E) BAT3 overexpression decreases PEPCK1 level. Steady state PEPCK1 levels of 293T cells and 293T cells overexpressing P300 and P300 plus BAT3 were determined.
Sirt2 deacetylates PEPCK1
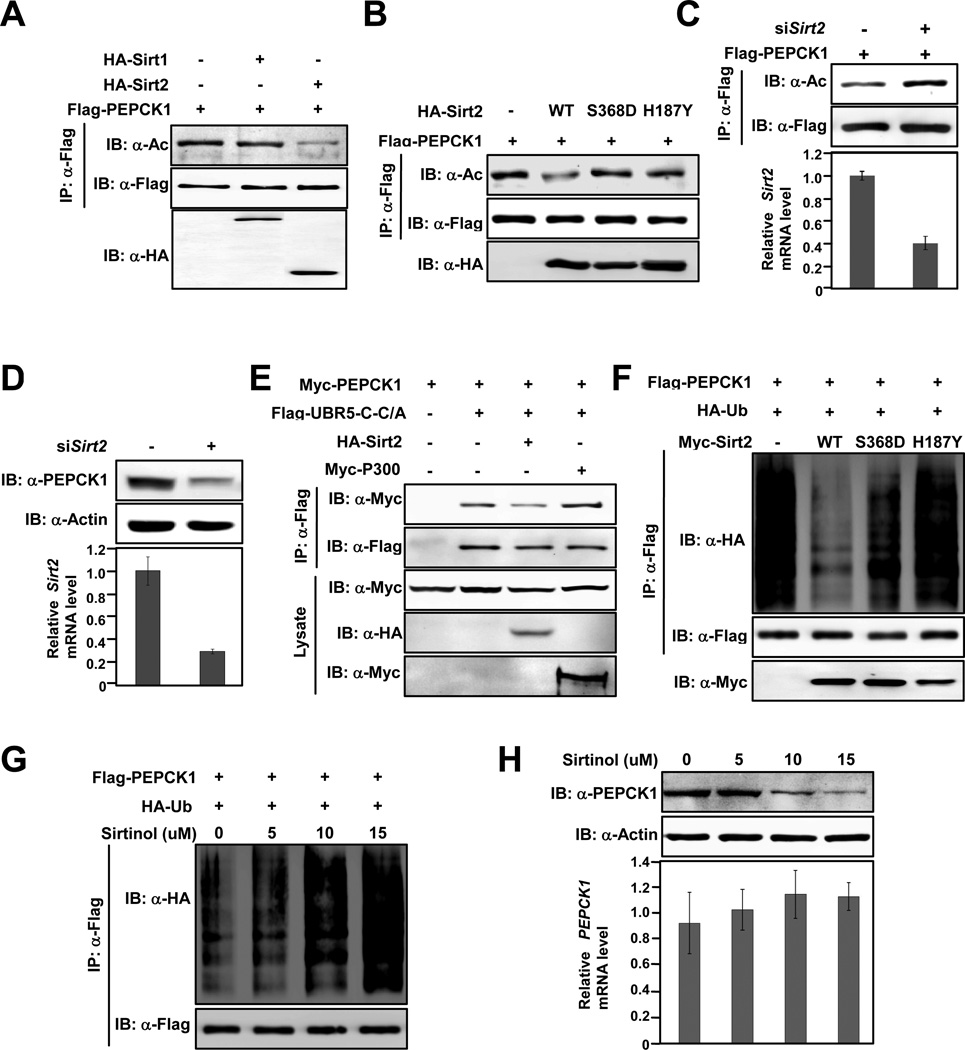
Next, the deacetylase responsible for PEPCK1 regulation was investigated. Taking advantage that TSA and NAM inhibit certain type of deacetylases, we first measured PEPCK1 acetylation levels in cells treated with TSA, a class I, II and IV deacetylases inhibitor, and NAM, an NAD+ dependent class III deacetylases inhibitor (Xu et al., 2007). NAM treatment led to more than a 300% increase in PEPCK1 acetylation while TSA treatment caused negligible acetylation level change (Figure S3A), suggesting that PEPCK1 is deacetylated by a class III enzyme. There are 7 known class III deacetylases in mammalian cells, namely Sirt1-7 among which Sirt1 and Sirt2 are the two major cytosolic members. Since PEPCK1 is a cytosolic protein, we thus examined the possibility of Sirt1 and Sirt2 being the deacetylase for PEPCK1. When PEPCK1 was co-expressed with Sirt1 and Sirt2 in 293T cells, Sirt2 interacted strongly with PEPCK1 while Sirt1 showed no interaction (Figure S3B), suggesting a possible role of Sirt2 in PEPCK1 deacetylation. Moreover, co-expressing Sirt2, but not Sirt1, with PEPCK1 in HEK293T cells caused an approximate 70% decrease of PEPCK1 acetylation level (Figure 5A), supporting a role of Sirt2 in PEPCK1 deacetylation. Moreover, co-expressing Sirt2 with wild type PEPCK1, but not with PEPCK13K/R, decreased PEPCK1 acetylation level by 40% (Figure S3C). Furthermore, when wild type Sirt2, but not its catalytic inactive S368D and H187Y mutants (North et al., 2003; North and Verdin, 2007), was coexpressed with PEPCK1, a 65% decrease of PEPCK1 acetylation level was observed (Figure 5B), connecting PEPCK1 deacetylation directly to Sirt2 catalytic activity. Consistently, a 50% increase in steady state endogenous PEPCK1 level was observed by overexpression of wild type Sirt2, but not its catalytic inactive S368D and H187Y in HEK293T cells (Figure S3D). Together, protein-protein interaction and Sirt2 overexpression results all support that Sirt2 is the deacetylase of PEPCK1. This notion was also tested under Sirt2 knock down conditions. PEPCK1 acetylation level increased by more than 100% when PEPCK1 was expressed in HEK293T cells with endogenous Sirt2 is knocked down by siRNA (Figure 5C); moreover, knock down of Sirt2 by siRNA caused more than a 70% decrease in steady state endogenous PEPCK1 level (Figure 5D), consistent with our model that acetylation destabilizes PEPCK1 (Figure 1A). Taken together, all results support a conclusion that Sirt2 is the major deacetylase that acts on PEPCK1.
Figure 5. Sirt2 deacetylates and stabilizes PEPCK1.
(A) Sirt2 deacetylates PEPCK1. Acetylation levels of PEPCK1 expressed and purified from 293T cells and 293T cells co-expressing Sirt1 and Sirt2 were detected. (B) Sirt2 deacetylates PEPCK1. Acetylation levels of Flag-PEPCK1 expressed and IP purified from HEK293T cells and HEK293T cells co-expressing Sirt2, Sirt2S368D and Sirt2H187Y, respectively, were determined. (C) Sirt2 knockdown increases PEPCK1 acetylation level. Acetylation levels of PEPCK1 expressed from HEK293T cells and HEK293T cells with Sirt2 knocked down were probed by panacetyllysine antibody. Sirt2 knockdown efficiency was monitored by realtime PCR. (D) Sirt2 knockdown decreases PEPCK1 protein level. Endogenous PEPCK1 levels of HEK293T cells and HEK293T cells with Sirt2 knocked down were determined by anti-PEPCK antibody. (E) Overexpression Sirt2 decreases PEPCK1-UBR5-C binding but overexpression P300 increases the binding. Myc-PEPCK1 and UBR5-C-C/A were expressed and purified from HEK293T cells or HEK293T co-expressing either Sirt2 or P300, interaction between PEPCK1 and UBR5-C-C/A were analyzed by the amount of PEPCK1 co-immunoprecipitated with UBR5-C-C/A. (F) Sirt2 increases ubiquitination level of PEPCK1. Flag-PEPCK1, HA-Ub and Myc-Sirt2 or its catalytic mutants were co-expressed in HEK293T cells. PEPCK1 was purified by IP. Ubiquitination level of PEPCK1 was probed by anti-HA antibody. (G) Sirtinol increases the ubiquitination level of PEPCK1. Flag-PEPCK1 and HA-Ub were co-expressed in HEK293T cells maintained under different concentrations of Sirtinol. IP purified PEPCK1 ubiquitination levels were detected. (H) Sirtinol decreases steady state PEPCK protein level. HEK293T cells were cultured at different concentrations of Sirtinol. Steady state cellular PEPCK1 level as well as PEPCK1 gene transcription level was determined. See also Figure S3.
Sirt2 decreases PEPCK1 ubiquitinylation
Since acetylation enhances the interaction between UBR5 and PEPCK1 (Figures 2D, 2F), a predicted consequence of overexpression of Sirt2 would be that Sirt2 will decrease the association between PEPCK1 and UBR5 by decreasing acetylation of PEPCK1 and subsequently stabilize PEPCK1. Indeed, overexpressing Sirt2 in 293T cells decreased interaction between PEPCK1 and UBR5-C-C/A (Figure 5E). That Sirt2 decreases UBR5-PEPCK1 interaction by deacetylating PEPCK1 is further evidenced by the result that expression of Sirt2 significantly decreased PEPCK1 ubiquitination (Figure 5F) and moreover, by an observation that overexpression of the catalytic inactive Sirt2 S368D and H187Y mutants did not decrease PEPCK1 ubiquitinylation (Figure 5F). Contrary to increased Sirt2 activity by overexpression, we also tested the consequence of decreasing Sirt2 activity. We treated cells with Sirtinol, a chemical that inactivates Sirt2 catalytic activity specifically (Outeiro et al., 2007), and observed that Sirtinol treatment caused a dramatic increase in ubiquitinylation of PEPCK1, a result that is opposite to Sirt2 overexpression, in a dose dependent manner (Figure 5G). When steady state endogenous PEPCK1 levels of Sirtinol treated cells were determined, we found that PEPCK1 level decreased with increased Sirtinol concentration (Figure 5H), consistent to an increased ubiquitinylation level by Sirtinol treatment. Notably, the transcription level of PEPCK1 was not affected by Sirtinol treatment (Figure 5H), excluding the possibility that altered PEPCK1 level by Sirtinol treatment was a result of altered PEPCK1 transcription. Collectively, the above data show that Sirt2 decreases PEPCK1 ubiquitinylation and increases protein stability.
PEPCK1 acetylation and ubiquitinylation are regulated by nutrients
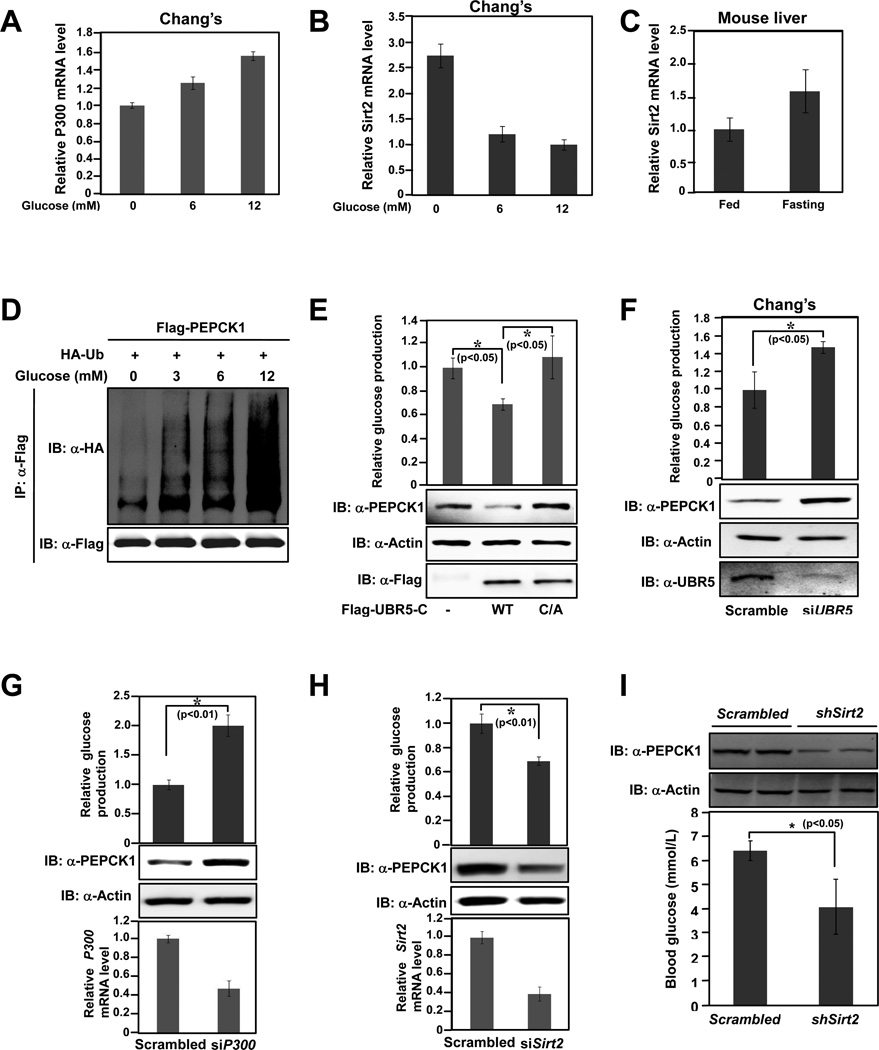
Being the key enzyme that controls gluconeogenesis, PEPCK1 activity is tightly regulated by cellular glucose levels. Consistent with a previous report that p300 transcription was up-regulated by glucose (Chen et al., 2010), we found glucose mildly activates transcription of P300 (Figure 6A). In contrast, Sirt2 mRNA level was decreased with elevated glucose concentrations (Figure 6B). Consistently, Sirt2 mRNA levels in starved mice hepatocytes increased about 50% (Figure 6C). These results support a notion that high glucose increases PEPCK1 acetylation through combined effects of increasing P300 transcription and decreasing Sirt2 transcription. We next tested whether PEPCK1 ubiquitination was regulated by glucose, which is known to induce PEPCK1 acetylation. A progressive increase of PEPCK1 ubiquitination was observed with increasing glucose concentrations (Figure 6D). This glucose-induced PEPCK1 ubiquitination is through increased PEPCK1 acetylation because non-acetylation mutant PEPCK3K/R ubiquitination is not responsive to elevated glucose concentrations (Figure S4A). Notably, when 293T cells were maintained in glucose free medium with different concentrations of amino acids, PEPCK1 ubiquitination level decreased with increasing amino acid concentrations (Figure S4B), consistent with the previous finding that amino acids decrease PEPCK1 acetylation (Zhao et al., 2010) and the notion that stability of PEPCK1 is regulated by its acetylation. Together, these results suggest that acetylation of PEPCK1 is regulated by different nutrients and controls PEPCK1 degradation according to the requirement of physiological needs.
Figure 6. PEPCK1 degradation is controlled by glucose level and can be manipulated by control degradation related factors.
(A)-(C), results are average values of triplicate q-RT-PCR assays with SDs. (A) P300 transcription is unregulated by glucose. P300 mRNA levels of 293T cells cultured under different glucose concentrations were determined. (B) Sirt2 transcription is down regulated by glucose. Sirt2 mRNA levels of 293T cells cultured under different glucose concentrations were determined. (C) Fasting reduces Sirt2 transcription. Sirt2 mRNA levels of mice liver cells were determined. (D) PEPCK1 ubiquitination response to glucose concentration. Flag-tagged PEPCK1 co-expressed with HA tagged ubiquitin in HEK293T cells maintained under various glucose concentrations were purified by IP and ubiquitination of purified proteins were determined by anti-HA antibody. (E)-(H), Secreted glucose levels of cells maintained in glucose free medium under conditions as indicated. Shown are average values of triplicate measurements with SDs. (E) Overexpression of UBR5-C decreases glucose production. HEK293T cells and HEK293T cells overexpressing UBR5-C and UBR5-C-C/A were analyzed. (F) UBR5 knockdown increases glucose production. Chang’s cells and Chang’s cells with UBR5 knocked down by siRNA were analyzed. (G) P300 knockdown increases glucose production. HEK293T cells and HEK293T cells with P300 knocked down by siRNA were analyzed. (H) Sirt2 knockdown decreases glucose production. HEK293T cells and HEK293T cells with Sirt2 knocked down by siRNA were analyzed. (I) Sirt2 knockdown decrease PEPCK1 and gluconeogenic rate in mice. Mice were tail vein injected by Sirt2 shRNA. Liver PEPCK1 level and blood glucose concentrations were measured. Shown are representative PEPCK1 protein levels and average blood concentrations with SD (n=4). See also Figure S4.
Changing acetylation and ubiquitination affects gluconeogenesis
Lastly, the biological relevance of PEPCK1 acetylation and degradation in gluconeogenesis was investigated. When UBR5-C was overexpressed in 293T cells maintained in glucose free medium, it caused an approximate 50% decrease in cellular PEPCK1 level and a 35% decrease in glucose production (Figure 6E), suggesting that gluconeogenesis can be controlled by manipulating the PEPCK1 E3 ligase activity. Unexpectedly, when the catalytically inactive UBR5-C-C/A was overexpressed in 293T cells, we observed an increase of approximately 40% in cellular PEPCK1 protein level and a mild increase in glucose production (Figure 6E). This is likely through a dominant negative effect of UBR5-C-C/A, whereby a high concentration of UBR5-C-C/A competes with endogenous UBR5 for binding to PEPCK1, further confirming the involvement of UBR5 in PEPCK1 degradation and suggesting that inhibition of UBR5 activity could lead to elevated glucose production. Indeed, when cellular UBR5 mRNA levels were each knocked down to about 20% of its original level in both Chang’s and 293T cells, there was a roughly 200% increase of cellular PEPCK1 protein levels and 60% increase in glucose production (Figures 6F, S4C). We then confirmed that PEPCK1 protein levels and gluconeogenesis rate could be modulated by altering PEPCK1 acetylation. Knocking down of P300, a manipulation that would decrease PEPCK1 acetylation, caused about 200% and 50% cellular PEPCK1 increase in 293T cells and Chang’s cells, respectively; and resulted in a 90% and 50% increase in glucose production in these two cells, respectively (Figures 6G, S4D). In contrast, knocking down Sirt2, which increase PEPCK1 acetylation level, caused 80% decrease in PEPCK1 level and 35% decrease in glucose production in HEK293T cells (Figure 6H). Consistently, inhibition of Sirt2 by Sirtinol decreases steady state PEPCK1 level by 60% and decreases the glucose production by 40% in Chang’s liver cells (Figure S4E).
Conversely, overexpressing Sirt2 in Chang’s liver cells caused a 50% increase in steady state PEPCK1 level and 43% increase in glucose production (Figure S4F). Finally, we investigated whether PEPCK1 level and gluconeogenic rate can be regulated by altering Sirt2 level in mice. Tail vein injection of adenovirus packed with Sirt2 shRNA in mice effectively reduced Sirt2 levels of mice liver by approximately 45% (Figure S4G) and decreased PEPCK1 level by about 70% (Figures 6I upper panel and S4H). Sirt2 knock down didn’t cause Pepck1 mRNA level change (Figure S4I), excluding the possibility that decreased liver Pepck1 levels were due to altered transcription. Supporting a decrease in liver Pepck1 levels by Sirt2 knockdown, average blood glucose levels of mice with Sirt2 knocked down decreased about 35% (Figures 6I lower panel and S4J). Together, these results showed that modulation of PEPCK1 acetylation or ubiquitinylation, hence protein levels, may serve an important mechanism to regulate the rate of cellular gluconeogenesis.
DISCUSSION
Virtually all intermediate metabolic enzymes are acetylated and many examples have shown an important role of acetylation in metabolic enzyme activity/function regulation. In this study, we have uncovered a biochemical mechanism presenting how acetylation controls metabolic enzyme stability and providing a physiological role of acetylation in regulation of gluconeogenesis. We propose that acetylation plays a critical role in coordinating the level of PEPCK1, hence the gluconeogenesis rate, with the availability of nutrients, such as glucose and amino acids. When glucose is sufficient, gluconeogenesis should be suppressed.
This is achieved in part by glucose-induced degradation of PEPCK1. High glucose concentration increases PEPCK1 acetylation, which promotes its interaction with the UBR5 E3 ubiquitin ligase. As a result, the acetylated PEPCK1 is ubiquitinylated and subsequently degraded by proteasomes. Therefore, gluconeogenesis is suppressed. In contrast, when glucose level is low, PEPCK1 is stabilized, hence gluconeogenesis is enhanced. Our data show that both P300 and Sirt2 are major enzymes responsible for PEPCK1 acetylation and deacetylation, respectively. Therefore, PEPCK1 stability is controlled by a regulatory network including P300, Sirt2, and UBR5 in response to glucose status.
Although our study provides insights into the cellular regulation of gluconeogenesis in response to nutrient availability, it also raises questions regarding how cells sense glucose levels to regulate PEPCK1 acetylation and what the molecular basis of acetylation is in promoting the interaction between PEPCK1 and UBR5. Sirt2 uses NAD+ as a co-factor and has been implicated in metabolism regulation and possibly cellular energy response. The NAD+/NADH ratio is an indicator for cellular energy status. It is possible that Sirt2 may sense cellular NAD+ levels, to influence PEPCK1 and thus gluconeogenesis, which requires energy and reducing power to produce glucose. Acetyl-coA, an essential substrate for protein acetyltransferases, is a key metabolic intermediate. Therefore, it is not surprising that nature may use acetyl-CoA and acetylation to modulate metabolism because the level of acetyl-CoA may serve as an indicator of cellular metabolic status.
The regulation of gluconeogenesis is unique in two aspects. First, this process consumes much energy; a quick shut down of this process when it is not needed is required. On the other hand, when physiological glucose level is low, a quick turn on of gluconeogenesis is required to supply glucose for the brain or other important organs. Both aspects need prompt change of cellular PEPCK1 activity since it is the rate limiting enzyme in gluconeogenesis. Compared to a translational control on PEPCK1 that usually takes hours to happen, proteosomal degradation can occur within minutes and thus could serve as a better way to rapidly respond to physiological glucose variation. Future study is of interest to demonstrate if acetylation-induced protein degradation is a general mechanism in regulation of metabolic enzymes.
EXPERIMENTAL PROCEDURES
Plasmids
Full-length PEPCK1, P300 and Sirt2 were cloned to a Flag-, Myc- or HA-tagged destination vectors according to different needs. Point mutations for PEPCK1, UBR5, P300, and Sirt2 were generated by site-directed mutagenesis. Mapping of UBR5 was made by cloning PCR fragments into Flag- or Myc-tagged vectors.
Antibodies
Rabbit anti-pan-acetyllysine antibodies were produced as previously described. Antibodies to PEPCK1 (Santa Cruz), Flag (Sigma), Myc (Santa Cruz), HA (Santa Cruz), P300 (NeoMarkers), and UBR5 (Bethyl Laboratories) were purchased commercially.
Cell Culture and Treatment
All experiments were carried out in HEK293T cells unless specified. Cells were culture in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% newborn bovine serum. The deacetylase inhibitor TSA was added 18 hours before harvest at the final concentration of 10uM, while 5mM NAM was added into medium 4–6 hours before harvest. Glucose or amino acid only medium was prepared with DMEM base and supplemented with glucose or amino acids of different concentration as indicated. For ubiquitination assays, the protease inhibitor MG132 was added 3 hours before harvest.
Tandem Affinity Purification
293T cells were transfected with pMCB-SBPFlag-PEPCK containing a puromycin-resistance marker. The PEPCK-positive stable cells were lysed on ice in 0.1%NP40 buffer (20mM Tris-HCl, pH8.0, 150mM NaCl) containing a protease inhibitor cocktail for 30 min. After removal of insoluble cell debris by centrifugation, cell lysates were incubated with streptavidin-Sepharose beads (GE Healthcare) for 3 hours at 4°C. The precipitates were washed 3 times with NP-40 buffer and 3 times with 50mM NH4CO3. On-beads tryptic digestion was performed at 37°C overnight. The peptides in supernatant were dried in a speed vacuum (Eppendorff) and re-dissolved in NH4CO3 buffer containing 0.1% formic acid and 5% acetonitrile before being subjected to Mass Spectrometry.
Cell transfections, immunoprecipitation and immunoblotting
Plasmid transfections were carried out by the calcium phosphate method. For immunopreciptation, cells were lysed with 0.1%NP-40 buffer as described above. Cell lysates were incubated with Flag beads (Sigma) for 3 hours at 4°C The binding complexes were washed with NP-40 buffer and then subjected to SDS-PAGE. For western blotting of tagged proteins, conventional procedures were employed. For acetylation Western Blotting, 50 mM Tris (pH 7.5) with 10% (V/V) Tween-20 and 1% peptone (AMRESCO) was used for blocking and 50 mM Tris (pH 7.5) with 0.1% peptone was used to prepare primary and secondary antibodies.
In vivo ubiquitination assay
36 hours after transfection, cells were lysed in 1% SDS buffer (Tris pH7.5, 0.5mM EDTA, 1mM DTT) and boiled for 10 minutes. For immunoprecipitation, the lysates were diluted 10-fold in Tris-HCl buffer. Analyses of ubiquitination were performed by anti-HA blotting.
RNA interference
Control and siSirt2 adenovirus were purchased from Vector Biolabs. P300, UBR5 and Sirt2 knocking down were carried out by using synthetic siRNA oligonucleotides synthesized from Genepharma, Shanghai. For each target gene, we employed two effective target sequences to exclude off-target effects. Transfections were performed by using Lipofectamine 2000 (Invitrogen). The knock down efficiency was verified by q-RT-PCR. The following target sequences were used.
Sirt2 siRNA-1: 5’-GAGGCCAUCUUUGAGAUCA
Sirt2 siRNA-2: 5’-AUGACAACCUAGAGAAGUA
UBR5 siRNA-1: 5’-CAACUUAGAUCUCCUGAAA
UBR5 siRNA-2: 5’- AAUUGGGUACGAUACUGUAUC
P300 siRNA-1: 5’-UGACACAGGCAGGCUUGAC
P300 siRNA-2: 5’-AACAGAGCAGUCCUGGAUUAG
Glucose production assay
293T and Chang’s cells expressing siRNA or plasmids were cultured in DMEM. The medium was replaced with 1 ml of DMEM base supplemented with 2 mM sodium pyruvate and 20 mM sodium lactate. After 3 hr incubation, medium was collected and the glucose concentration was measured with a colorimetric glucose assay kit (GAGO20; Sigma-Aldrich). The readings were normalized to the total protein content determined from the whole-cell extracts.
Nuclear / Cytoplasma Isolation
Cells were lysed in harvest buffer (10mM HEPES, 50mM NaCl, 0.5M Sucrose, 0.1mM EDTA, 0.5% Triton100, 1mM DTT, pH 7.9). The lysates were centrifuged at 1000rpm. The supernatant (cytoplasmic extract) were collected. The pellets (nuclear extracts) were washed 3 times and then boiled in 1% SDS for western analysis.
Animal Experiments
All animal experiments conformed to protocols approved by animal care and use committees at Fudan University. Experiments were performed in 6- to 8-week-old male BALB/c mice, purchased from Shanghai Medical School of Fudan University. Animals were housed in a humidity- and temperature-controlled room with a 12:12 h dark/light cycle. Adenovirus infections were performed by tail vein injection with 0.5×108 infectious particles per mouse. At Day 6, mice were fasted for 6 hours before sacrifice. Livers were removed and whole-cell homogenates were made with NP40 buffer and used for western blot analysis. Liver mRNA was isolated using Trizol reagent (Invitrogen) and used for quantitative PCR. Blood was collected from tail vein for glucose detection (Roche).
Statistic Method
Statistics were performed with a two-tailed unpaired Student's t-test.
Supplementary Material
Highlights.
Glucose destabilizes PEPCK1 by inducing its acetylation
P300 and SIRT2 regulates PEPCK1 acetylation
UBR5 ubiquitinates the acetylated PEPCK1
ACKNOWLEDGMENTS
We thank members of Fudan MCB laboratory for their support throughout this study and Dr. Ronald Somerville for the critical reading of the manuscript. We thank Dr. Subbareddy Maddika for providing UBR5 constructs. This work is supported by the 985 program from the Chinese Ministry of Education; state key development programs of basic research of China (2009CB918401, 2009CB918600), Chinese National Science Foundation grants (31030042, 30971485/C0706) and NIH grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Browner MF, Fletterick RJ. Phosphorylase: a biological transducer. Trends Biochem Sci. 1992;17:66–71. doi: 10.1016/0968-0004(92)90504-3. [DOI] [PubMed] [Google Scholar]
- Callaghan MJ, Russell AJ, Woollatt E, Sutherland GR, Sutherland RL, Watts CK. Identification of a human HECT family protein with homology to the Drosophila tumor suppressor gene hyperplastic discs. Oncogene. 1998;17:3479–3491. doi: 10.1038/sj.onc.1202249. [DOI] [PubMed] [Google Scholar]
- Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab. 2010;298:E127–E137. doi: 10.1152/ajpendo.00432.2009. [DOI] [PubMed] [Google Scholar]
- Granner DK, O'Brien RM. Molecular physiology and genetics of NIDDM. Importance of metabolic staging. Diabetes Care. 1992;15:369–395. doi: 10.2337/diacare.15.3.369. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Patel YM. Phosphoenolpyruvate carboxykinase (GTP): the gene and the enzyme. Adv Enzymol Relat Areas Mol Biol. 1994;69:203–281. doi: 10.1002/9780470123157.ch6. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Tojo M, Matsuzaki K, Anan T, Matsumoto M, Ando M, Saya H, Nakao M. Cooperation of HECT-domain ubiquitin ligase hHYD and DNA topoisomerase II-binding protein for DNA damage response. J Biol Chem. 2002;277:3599–3605. doi: 10.1074/jbc.M104347200. [DOI] [PubMed] [Google Scholar]
- Johnson LN. Glycogen phosphorylase: control by phosphorylation and allosteric effectors. FASEB J. 1992;6:2274–2282. doi: 10.1096/fasebj.6.6.1544539. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, Tao SC, Qian J, Zhao Y, Boeke JD, et al. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang L, Zhao K, Thompson PR, Hwang Y, Marmorstein R, Cole PA. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008;451:846–850. doi: 10.1038/nature06546. [DOI] [PubMed] [Google Scholar]
- Mantelingu K, Reddy BA, Swaminathan V, Kishore AH, Siddappa NB, Kumar GV, Nagashankar G, Natesh N, Roy S, Sadhale PP, et al. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol. 2007;14:645–657. doi: 10.1016/j.chembiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. Mitotic regulation of SIRT2 by cyclin-dependent kinase 1-dependent phosphorylation. J Biol Chem. 2007;282:19546–19555. doi: 10.1074/jbc.M702990200. [DOI] [PubMed] [Google Scholar]
- Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem. 2008;283:27565–27574. doi: 10.1074/jbc.M804393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, et al. Sirtuin 2 inhibitors rescue alpha-synucleinmediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Panin LE, Kolosova IE, Nechaev Iu S. Change in the activity of the key gluconeogenesis enzymes in the rat liver and kidneys during the action of subextreme and extreme factors on the body. Biull Eksp Biol Med. 1979;87:544–547. [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H. HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev. 2007;21:848–861. doi: 10.1101/gad.1534107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DK, O'Doherty RM, Stafford JM, Newgard CB, Granner DK. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J Biol Chem. 1998;273:24145–24151. doi: 10.1074/jbc.273.37.24145. [DOI] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Srivastava AK, Bass MA, Khatra BS. Phosphorylation and inactivation of glycogen synthase by phosphorylase kinase. Proc Natl Acad Sci U S A. 1979;76:2536–2540. doi: 10.1073/pnas.76.6.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell. 143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- Tannen RL. Ammonia metabolism. Am J Physiol. 1978;235:F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.