Abstract
Background
Intracranial arterial variations are a frequent finding in the general population. Knowledge of these vascular variations has significant clinical impact because some of them predispose patients to development of an aneurysm or cerebrovascular ischemic disease. The purpose of this study was to evaluate the frequency of intracranial vascular variations and associated vascular lesions on computed tomography angiography (CTA) examinations.
Material/Methods
CTA examinations performed by 16-detector computed tomography were prospectively reviewed in 455 patients for the presence of fenestrations, duplications, hypoplasia, aplasia, aneurysms, and other vascular lesions.
Results
Arterial fenestrations were found in 2.4% of patients, with the vertebrobasilar system as the most common location. The remaining fenestrations were located on the middle cerebral artery M1 segment (0.2%), anterior communicating artery (0.4%), and anterior cerebral artery A1 segment (0.6%). No associated aneurysms were noted in these patients. The prevalence of an azygos anterior cerebral artery was 1.5%. Bihemispheric anterior cerebral artery was found in 0.9%, hypoplastic A1 segment in 17.6%, and congenital absence of A1 segment in 0.4% of patients. Fetal origin of the posterior cerebral artery was found in 37% of cases. Hypoplastic vertebral artery terminating as posterior inferior cerebellar artery was observed in 9 patients, while transversal anastomosis between vertebral arteries was seen in only 1 patient.
Conclusions
CTA precisely demonstrates the diversity of intracranial arterial variations, whose overall frequency in this study is similar to previous radiological reports. Furthermore, our results do not show significant association between the frequency of aneurysms and cerebral arterial anomalies.
Keywords: Cerebral Angiography, computed tomography angiography, Intracranial Aneurysm – diagnosis, Cerebral Arteries – abnormalities
Background
Anatomical cerebral arterial variations are a very frequent finding in the general population due to the complex embryology of intracranial circulation. The most common normal variants of cerebral circulation include: fenestrations and duplications, persistent primitive fetal arteries, hypoplasia, and aplasia of arterial segments [1]. However the clinical significance of these variants is quite different. Thus, the presence of fenestrations and duplications may predispose patients to aneurysm development [2]. Moreover, the occlusion of an azygos or bihemispheric anterior cerebral artery (ACA) may result in ischemia of both hemispheres. Furthermore, patients with fetal origin of posterior cerebral artery (PCA) and concomitant atherosclerotic disease of the carotid artery are prone to ischemic events in the PCA territory [3]. Although the majority of normal variations have no major clinical impact, their appreciation may aid in surgical planning and can be useful in preventing complications during endovascular treatment.
Although digital subtraction angiography remains the standard reference procedure for detection of intracranial vascular variations, it is an invasive technique with potential complications [4]. Computed tomography angiography (CTA) has emerged as an important noninvasive diagnostic tool in the evaluation of intracranial circulation. The main drawback of CTA is radiation exposure of the patient. Previous studies investigating radiation dose in CTA examination reported the effective dose ranges between 0.6 and 1.2 mSv depending on the scanning protocol [5,6]. This is slightly higher than the dose a patient receives during DSA. However, local doses in CTA examination do not reach thresholds for the development of cataract formation, induction of thyroid malignancy, or hair loss [6]. Moreover, further developments, including reduced tube voltage and reducing scanning time, may provide a decrease in effective dose.
CTA allows reliable evaluation of intracranial arterial pathology, including aneurysms, stenosis, and occlusions. Moreover, CTA provides useful information about anatomical variations of cerebral circulation, with reported high sensitivity and specificity (81–90% and 93%, respectively), approaching the diagnostic accuracy of digital subtraction angiography [7]. At present, there are several reports regarding the use of CTA for the assessment of intracranial arterial variations [1,8,9]. However, these reports were mostly focused on fenestrations, duplications, and their association with aneurysm formation. Nevertheless, the prevalence of some of the normal variants, such as azygos ACA, hypoplastic vertebral artery terminating as posterior inferior cerebellar artery, and bihemispheric ACA, has not been previously reported using CTA. Thus, the purpose of this study was to provide analysis of the prevalence and characteristic features of intracranial arterial variations and insight into associated vascular lesions.
Material and Methods
This prospective study was conducted between January 2012 and May 2013 and included 517 patients who underwent CTA. Most patients had or were suspected to have subarachnoid hemorrhage, ischemic cerebrovascular disease, or prolonged unexplained headache. Sixty-two patients were excluded from the study due to suboptimal image quality, leaving 455 CTA studies for detailed analysis. There were 202 men and 253 women (mean age 51±12 years). The study was approved by an institutional review board and written informed consent was obtained from all participants.
CTA examinations were performed at GE Medical System (Waukesha, WI, USA). Images were obtained from C3 to the vertex using the following scanning parameters: detector rows, 16; collimation, 0.625 mm; pitch, 1.375; gantry rotation time, 1.0 s; slice thickness, 0.625 mm; tube load, 380 mA; and tube voltage, 120 kV. A total volume of 80–100 mL of Ultravist 370 was injected at a rate of 4.0–4.5 mL/s through an antecubital vein. The scan delay time was determined by a bolus tracking technique with a region of interest at 1 internal carotid artery. As soon as a threshold of 100 HU was exceeded, the spiral scan was automatically started. Coronal and sagittal multiplanar reformatted, maximum intensity projections and 3D volume-rendered images were created at a GE Advantage Workstation.
The demographic data, including sex, age at the time of presentation, and indication for the CTA, were recorded. A prospective evaluation of CT examinations patients was performed independently by 2 radiologists (A.S. 3 years of experience and D.S. 15 years of experience) using axial and multiplanar reformatted images with attention to the presence of intracranial vascular variations: fenestrations, duplications, hypoplasia, aplasia, and persistent carotid-basilar anastomosis. The examined variables were presented as frequencies. Independent-samples t-test was used for statistical evaluation. A value of p less than 0.05 was considered as significant.
Results
Arterial fenestrations and duplications
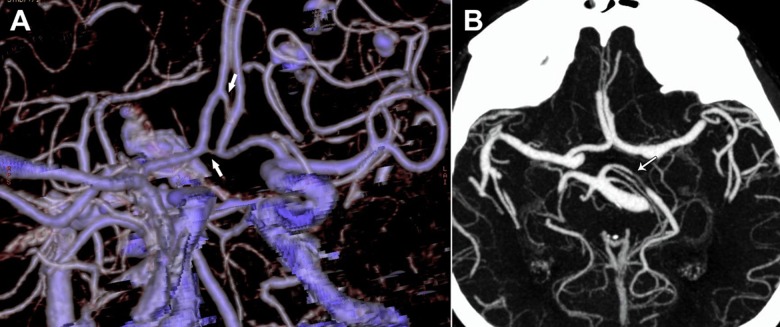
Intracranial arterial fenestrations were found in 11 (2.4%) patients. The most common location was the vertebrobasilar system, with 5 (1.1%) fenestrations. The fenestrations of the basilar artery were observed in 4 patients (0.8%) and were located at the proximal segment with small slit-like configuration (Figure 1A). The median length of the fenestrated segment was 11.6 mm, with 2 channels of 2.0 mm and 3.2 mm. The associated intracranial arterial variations were observed in 1 patient, who had ACA trifurcation and bilateral fetal PCA. Vertebral artery fenestration was found in 1 patient (0.2%) (Figure 1B), and was located at the V4 segment, with a length of 5.6 mm. The remaining fenestrations were found at the middle cerebral artery M1 segment in 1 patient (0.2%), anterior communicating artery (ACoA) in 2 patients (0.4%), and ACA A1 segment in 3 patients (0.6%) (Figure 2A–2C). The patient with fenestration of the middle cerebral artery had fetal PCA as an associated variation. No associated aneurysms were noted in patients with fenestrations. Arterial duplication was observed in 2 patients (0.4%), who had duplicated ACoA and duplicated PCA P1 segment, respectively (Figure 3A, 3B).
Figure 1.
Vertebrobasilar system fenestration. Three-dimensional volume-rendered image shows basilar artery fenestration immediately above the vertebrobasilar junction (thick arrow). Trifurcation of the anterior cerebral artery (arrowhead) and fetal right posterior cerebral artery (thin arrow) are seen as associated anomalies (A). Axial maximum intensity projection image shows fenestration of left vertebral artery C4 segment (arrow in B).
Figure 2.

Oblique axial volume-rendered projection shows M1 segment right middle cerebral artery fenestration (arrow in A). Three-dimensional volume-rendered image shows fenestration of anterior communicating artery (thick arrow) and right fetal posterior cerebral artery (thin arrow) as associated anomalies (B). Three-dimensional volume-rendered image shows anterior cerebral artery A1 segment fenestration (arrow in C).
Figure 3.
Cerebral arteries duplication. Three-dimensional volume-rendered image shows anterior communicating artery duplication with each vessel originating separately from an anterior cerebral artery (arrows in A). Three-dimensional volume-rendered image shows duplication of P1 segment left posterior cerebral artery (arrow). Dolichoectasia of the basilar artery is seen as an associated anomaly (B).
Anterior cerebral artery variants
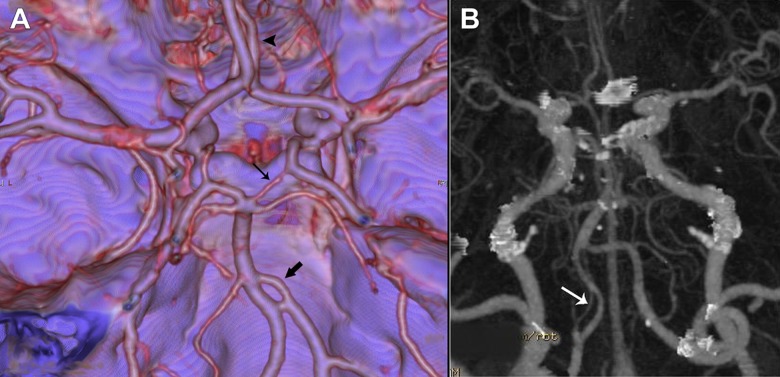
The prevalence of an azygos ACA was 1.5% (7 patients) (Figure 4A). Three patients had an associated giant aneurysm of the internal carotid artery C7 segment. Other associated vascular variations were fetal PCA in 3 patients, and hypoplasia of an ACA A1 segment in 2 patients. Bihemispheric ACA was noted in 4 patients (0.9%) (Figure 4B). In 3 cases the right ACA was dominant. Hypoplasia of the A1 segment was found in 17.6% of patients (Figure 4A, 4B), while congenital absence of A1 segment was observed in 2 patients (0.4%) (Figure 5A). Among patients whit hypoplastic A1 segment, 25% had an associated aneurysm of ACoA. The prevalence of ACA trifurcation was 1.9% (Figure 5B). Seven patients had associated vascular variants: 4 patients had hypoplastic A1 segment, and 3 patients had fetal PCA.
Figure 4.
Anterior cerebral artery variations. Three-dimensional volume-rendered image shows azygos anterior cerebral artery (thick arrow), and hypoplastic A1 segment of right anterior cerebral artery (thin arrow in A). Three-dimensional volume-rendered image shows bihemispheric anterior cerebral artery (thick arrow). Hypoplastic left A1 segment (arrowhead) and anterior communicating artery aneurysm (thin arrow) are seen as associated anomalies (B).
Figure 5.

Anterior cerebral artery variations. Three-dimensional volume-rendered image shows absence of right A1 segment (thick arrow) in association with right middle cerebral artery aneurysm (thin arrow in A). Three-dimensional volume-rendered image shows anterior cerebral artery trifurcation (thick arrow). Hypoplastic right A1 segment is also seen (thin arrow in B).
Posterior cerebral artery variants
Fetal origin of PCA was found in 106 patients (23.3%), 51 cases on the left and 37 cases on the right, while bilateral location was present in 18 patients (Figure 6). Associated aneurysm was detected in 27 patients: 18 patients had an aneurysm of the middle cerebral artery, 4 patients of the internal cerebral artery C7 segment, and 5 patients of the ACoA. This vascular variation was more common in men (p<0.05).
Figure 6.

Posterior cerebral artery variations. Three-dimensional volume-rendered image shows fetal right posterior cerebral artery (thick arrow) and hypoplastic A1 segment of right anterior cerebral artery (thin arrow).
Intracranial vertebral arteries variants
Hypoplastic vertebral artery terminating as posterior inferior cerebellar artery was observed in 9 cases (1.9%) (Figure 7A). One patient had an associated aneurysm of ACoA. All patients were men (39–68 years). This anatomical variation was more common on the right. Transversal anastomosis between vertebral arteries was seen in 1 patient (0.2%) (Figure 7B).
Figure 7.
Vertebral arteries variations. Three-dimensional volume-rendered image shows hypoplastic right vertebral artery ending-like posterior inferior cerebellar artery (arrow in A). Three-dimensional volume rendered image shows intervertebral transversal anastomosis (arrow in B).
Persistent carotid-basilar anastomosis
A lateral Saltzman type 2 persistent trigeminal artery was found in 1 patient (0.2%) (Figure 8).
Figure 8.

Persistent trigeminal artery. Three-dimensional volume-rendered image shows lateral Saltzman type 2 persistent trigeminal artery (thick arrows) and characteristic hypoplastic appearance of the basilar artery (thin arrow) proximal to its anastomosis with the persistent trigeminal artery.
Arterial variations and intracranial aneurysms
In our study population, 115 aneurysms were detected. Three patients had multiple aneurysms. The aneurysms were located at ACoA (n=39), middle cerebral artery (n=44), internal cerebral artery C7 segment (n=28), ACA (n=2), and basilar artery (n=2). Among patients with aneurysms, 60 had an associated vascular variation. The rate of vascular variations was not significantly different in patients who had and did not have aneurysms (p>0.05). Similarly, there was no significant difference in the rate of aneurysms among patients who had and did not have vascular variants (p>0.05).
Discussion
Normal variants of intracranial circulation are frequent incidental finding on CTA studies. Although most of these variations have no significant clinical importance, some may predispose the patient to development of aneurysms or ischemic events. Moreover, their recognition on CTA examinations is important for surgical and endovascular treatment planning.
Fenestration and duplication of intracranial arteries are among the most frequent vascular variations [2]. Fenestration consists of 2 arterial channels that fuse at the distal end and develop as a consequence of fusion failure during early gestation. The most common site of fenestration is the vertebrobasilar system, with high prevalence reported in previous studies [8–10]. Baratha et al. [9] reported a 2.8% overall rate of intracranial vertebrobasilar fenestrations and observed intracranial vertebral artery fenestrations in 0.40% of patients, consistent with the 0.38% found in the current study. However, the current study result of 0.8% BA fenestrations is significantly lower than the previously observed prevalence, and was closer to the results of the conventional angiographic studies (0.6%) reported by Takahashi et al. [11]. This discrepancy might be attributable to the larger study population and different clinical diagnoses of selected patients in prior CT and MR angiographic studies. Due to the complex embryology and anatomy of the anterior communicating system, fenestrations and duplications in this region are frequent findings. CT angiographic prevalence of anterior communicating region fenestrations was reported to be 5.3–6.9% [8,9], but the frequency of anterior communicating region fenestration in our study was much lower (1.2%). The difference is likely due to the different inclusion criteria concerning clinical indications, and inclusion of more patients with normal CTA findings in the present study. To our knowledge, the prevalence of duplication of ACoA has not been previously reported on CTA. Although the observed frequency of this variation in previous anatomic reports [12] is very high (18%), it was detected in only 1 patient (0.2%) in our study population.
Earlier studies have reported high incidence of aneurysms in association with arterial fenestrations. Pathologically, it could be explained by defects in the vessel wall at each end of the fenestration, which results in hemodynamic stress and structural degenerative changes in the vessel [13]. In this regard, Bayrak et al. [8] found that 27.5% of patients with fenestrations had aneurysms, but only 2 patients had an aneurysm at the fenestration site. Furthermore, no significant difference was found in the rate of aneurysms between patients with and without fenestrations. Similar results were reported by Baratha et al. [9]. In the present study, aneurysms were detected in 29% of patients. Among these patients, 52% had associated vascular variations, but none had fenestrations. On the basis of these results, it could be assumed that although fenestration is recognized as a risk for aneurysmal development, significant association could not be established.
Previous studies concerning the frequency and clinical significance of an azygos ACA are numerous [14–16]. This vascular variation represents a single midline A2 trunk that supplies blood to both hemispheres [15]. The reported prevalence of this anomaly was 1.3–2.0% in MR angiographic studies [16,17]. CT angiographic findings have not been previously published, but closely correlate with these results according to the present study (1.2%). Since there is a single A2 trunk, its occlusion may result in ischemia of both hemispheres. The same clinical significance has bihemispheric ACA, where one A2 segment is hypoplastic, and the contralateral A2 segment supplies the medial portions of both hemispheres [1]. To date, the prevalence of this vascular variant has not been reported on CTA, and was found to be 1% in our study, which is in accordance with prior anatomic reports (18). Among all ACA variations, A1 segment hypoplasia is the most frequent, observed in 10% of autopsies [18]. Similarly, the present study detected it in 17.6% of patients. Moreover, we found an associated ACoA aneurysm in 25% of these patients. Since an ACoA aneurysm frequently occurs in patients with these patients, it is of great clinical importance to carefully examine contralateral the ACA-ACoA junction on CTA examinations. Only 2 patients in the current study had unilateral A1 aplasia. Our results are consistent with the findings of anatomic studies, reporting that the A1 segment is seldom absent. The least significant ACA variation is its trifurcation or persistent median artery of the corpus callosum, and was observed in 2.2% of our patients. The results from the present study are in accordance with those of Uchino et al. [16], who found this anomaly in 3% of their study population.
Fetal origin of PCA is a very common vascular variant, in which the caliber of the PCA is the same or greater than that of the ipsilateral P1 segment [19]. It occurs when embryonic PCA does not regress, resulting in the persistence of internal carotid artery blood supply to the occipital lobe. Consistent with previous reports [4], the prevalence of this arterial variant was 37% in our study, occurring more commonly on the right side and with a slight male predominance. Such a fetal anastomosis may predispose patients to an ischemic event in PCA, since it allows thrombo-embolic material from the carotid artery to pass into the PCA.
The intracranial part of vertebral arteries is frequently the site of various vascular variations, with fenestrations, duplications, and aneurysms being the most common [20]. Although the prevalence of vertebrobasilar system fenestrations and duplications has been previously extensively studied, the number of CT and MR angiographic reports describing other vascular anomalies is very limited. In the current study, a transversal intervertebral anastomosis beneath the vertebrobasilar junction was observed in 1 patient. To the best of our knowledge, this is the first report of such a case diagnosed by CTA. This unusual variant could be the result of fusion of anterior spinal artery branches, as hypothesized in previous anatomic studies [21]. Although the prevalence of anterior spinal artery originating from transverse intervertebral anastomosis was reported to be 6.3% [20], it was not detected in our study. However, due to the small diameter of this artery, the visualization of anterior spinal artery is not an obligatory finding on CTA.
Hypoplastic vertebral artery terminating as posterior inferior cerebellar artery is a rare variation, detected in 0.2–1.3% of the population [20]. Since there is no connection between vertebral arteries, the compression or occlusion of one of these arteries can lead to ischemia of the cerebellum or lateral medulla. Although this vascular variant has many clinical implications, its significance and prevalence have not been previously reported in CT and MR angiography studies. In the present study, hypoplastic vertebral artery terminating as posterior inferior cerebellar artery was observed in 2.5% of patients. All patients were young men examined due to a first epileptic attack. However, more clinical studies are needed to clarify this association.
The main limitation of this study was the absence of conventional angiography findings as a standard reference procedure. Moreover, most of examined patients had or were thought to have ischemic or hemorrhagic cerebrovascular disease, which could have introduced some selection bias. In addition, the relatively small number of patients included in the study precludes generalized conclusions.
Conclusions
The results of our study provide comprehensive evaluation of intracranial vascular variations using CTA, reporting the prevalence of a few of them for the first time. Furthermore, this study demonstrated that the rate of aneurysms was not significantly different in patients who had and did not have vascular variants.
Footnotes
Source of support: Departmental sources
References
- 1.Dimmick SJ, Faulder KC. Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics. 2009;29:1027–43. doi: 10.1148/rg.294085730. [DOI] [PubMed] [Google Scholar]
- 2.Sanders WP, Sorek PA, Mehta BA. Fenestration of intracranial arteries with special attention to associated aneurysms and other anomalies. Am J Neuroradiol. 1993;14:675–80. [PMC free article] [PubMed] [Google Scholar]
- 3.Linn FHH, Chang HM, Caplan LR. Carotid artery disease: a rare cause of posterior cerebral artery territory infarction. J Neurovasc Dis. 1997;2:31–34. [Google Scholar]
- 4.van der Lugt A, Buter TC, Govaere F, et al. Accuracy of CT angiography in the assessment of a fetal origin of the posterior cerebral artery. Eur Radiol. 2004;14:1627–33. doi: 10.1007/s00330-004-2333-1. [DOI] [PubMed] [Google Scholar]
- 5.Sabarudin A, Yusof MZ, Mohamad M, Sun Z. Radiation dose associated with cerebral CT angiography and CT perfusion: an experimental phantom study. Radiat Prot Dosimetry. 2013 doi: 10.1093/rpd/nct280. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Cohnen M, Wittsack HJ, Assadi S, et al. Radiation exposure of patients in comprehensive computed tomography of the head in acute stroke. Am J Neuroradiol. 2006;27(8):1741–45. [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology. 2004;230:510–18. doi: 10.1148/radiol.2302021465. [DOI] [PubMed] [Google Scholar]
- 8.Bayrak AH, Senturk S, Akay HO, et al. The frequency of intracranial arterial fenestrations: a study with 64-detector CT-angiography. Eur J Radiol. 2011;77:392–96. doi: 10.1016/j.ejrad.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Bharatha A, Aviv RI, White J, et al. Intracranial arterial fenestrations: frequency on CT angiography and association with other vascular lesions. Surg Radiol Anat. 2008;30:397–401. doi: 10.1007/s00276-008-0340-7. [DOI] [PubMed] [Google Scholar]
- 10.Uchino A, Saito N, Okada Y, et al. Fenestrations of the intracranial vertebrobasilar system diagnosed by MR angiography. Neuroradiology. 2012;54:445–50. doi: 10.1007/s00234-011-0903-x. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Tamakawa Y, Kishikawa T, et al. Fenestration of the basilar artery. Report of three cases and review of the literature. Radiology. 1973;109:79–82. [PubMed] [Google Scholar]
- 12.Serizawa T, Saeki N, Yamaura A. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery. 1997;40:1211–16. doi: 10.1097/00006123-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Black SPW, Ansbacher LE. Saccular aneurysm associated with segmental duplication of the basilar artery. A morphological study. J Neurosurg. 1984;61:1005–8. doi: 10.3171/jns.1984.61.6.1005. [DOI] [PubMed] [Google Scholar]
- 14.Cinnamon J, Zito J, Chalif D, et al. Aneurysm of the azygos pericallosal artery: diagnosis by MR imaging and MR angiography. Am J Neuroradiol. 1992;13:280–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Okahara M, Kiyosue H, Mori H, et al. Anatomic variations of the cerebral arteries and their embryology: a pictorial review. Eur Radiol. 2002;12:2548–61. doi: 10.1007/s00330-001-1286-x. [DOI] [PubMed] [Google Scholar]
- 16.Uchino A, Nomiyama K, Takase Y, et al. Anterior cerebral artery variations detected by MR angiography. Neuroradiology. 2006;48:647–52. doi: 10.1007/s00234-006-0110-3. [DOI] [PubMed] [Google Scholar]
- 17.Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, et al. Circle of Willis: morphologic variation on threedimensional time-of-flight MR angiograms. Radiology. 1998;207:103–11. doi: 10.1148/radiology.207.1.9530305. [DOI] [PubMed] [Google Scholar]
- 18.Perlmutter D, Rhoton AL., Jr Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204–8. doi: 10.3171/jns.1978.49.2.0204. [DOI] [PubMed] [Google Scholar]
- 19.Jongen JC, Franke CL, Soeterboek AA, et al. Blood supply of the posterior cerebral artery by the carotid system on angiograms. J Neurol. 2002;249:455–60. doi: 10.1007/s004150200039. [DOI] [PubMed] [Google Scholar]
- 20.Songur A, Gonul Y, Ozen OA, et al. Variations in the intracranial vertebrobasilar system. Surg Radiol Anat. 2008;30:257–64. doi: 10.1007/s00276-008-0309-6. [DOI] [PubMed] [Google Scholar]
- 21.Govsa F, Aktan ZA, Arisoy Y, et al. Origin of the anterior spinal artery. Surg Radiol Anat. 1996;18:189–93. doi: 10.1007/BF02346126. [DOI] [PubMed] [Google Scholar]