Abstract
Background
Standard treatment of colorectal cancer includes both cytostatic chemotherapy and targeted therapies. Bevacizumab, targeting the VEGF receptor, is one of the primary targeted therapies that achieve better response rate and survival rate as compared to combination chemotherapy. To the best of our knowledge, there is no established single marker that can be used as a predictive marker in bevacizumab therapy.
Material/Methods
We enrolled 24 patients with the diagnosis of metastatic colorectal cancer in our study. During the study, 2 blood samples were drawn from patients before the first cycle and after the sixth cycle of bevacizumab therapy. Serum levels of VEGF, ANG II, and NO were recorded.
Results
While the change across VEGF levels was found to be a statistically significant decreasing trend (p=0.009), this decrease was not found to be correlated with treatment response and hypertension development. Additionally, no statistically significant difference was found in terms of NO and ANG II levels.
Conclusions
This study showed a significant decrease in serum VEGF, but failed to show a significant change in NO and ANG II levels during bevacizumab treatment. Although no significant correlation was found between the presence of hypertension and markers, most patients (83%) had an increase in their blood pressure. Our results suggest that dynamic monitoring of NO and ANG II, along with VEGF, may not be useful as predictive markers for bevacizumab treatment in colorectal cancer.
Keywords: Angiotensin II – diagnostic use, Colorectal Neoplasms, Bevacizumab, Nitric Oxide – diagnostic use, Vascular Endothelial Growth Factors
Background
Colorectal cancer is the one of the most lethal cancers. On the other hand, significant improvement in survival has been achieved as a result of the introduction of cancer screening programs, the advancements in diagnostic methods in molecular oncology, and the implementation of more efficient cytostatic chemotherapies and targeted therapies in the past 2 decades.
Bevacizumab is a recombinant humanized monoclonal antibody with high binding specificity for vascular endothelial growth factor (VEGF). Bevacizumab recognizes and inhibits all of the important isoforms of VEGF. VEGF inhibition affects endothelial cells and tumor vascularization in several ways. Endothelial cells cannot mature in the absence of VEGF, thus the formation of new vessels is inhibited and existing vessels regress [1,2]. However, inhibition of VEGF could lead to severe hypertension (HT), which carries high risk of mortality, particularly if necessary precautions are not taken.
Although hypertension and cancer are 2 independent diseases, angiogenesis plays a significant role in the development of both diseases. When the balance between proangiogenic and anti-angiogenic factors shifts toward angiogenic factors, angiogenesis cannot be controlled [1,2] and excessive production of VEGF, nitric oxide (NO), and angiotensin II (Ang II) is observed. Inhibition of these pathways in clinical trials can improve survival. However, inhibition of angiogenesis can cause serious effects on microvascular structure and leads to specific side effects, known as ‘class side effects’, consisting primarily of bleeding and hypertension [3]. Many studies have shown that hypertension can depend on the dose and duration of treatment; it is observed in about 30% of the cases and the incidence of grade 3 and higher HT is about 8–10% [4–6].
In this study, we tried to evaluate the effect of VEGF inhibition on blood pressure and serum levels of VEGF, nitric oxide and angiotensin II to determine whether these biochemical markers can be used to predict long-term efficacy and toxicity of these specific treatments in advanced colorectal cancer patients.
Material and Methods
We enrolled 24 patients with metastatic colorectal cancer who were followed up between April 2010 and May 2011 at the outpatient clinic of the Division of Medical Oncology, Adana Practice and Research Center, Başkent University, Adana, Turkey. The main inclusion criteria were aged over 18 years, diagnosis with metastatic colorectal disease, ECOG PS under stage 3, no known renal disease, and any contraindication for bevacizumab combination chemotherapy. All patients gave written informed consent. The patients rested in sitting position for 5 minutes, then their basal blood pressure was measured using an appropriate size of cuff. The patients were taught how to measure and record their blood pressure at home 2 times per day and they were asked to record these measurements on a blood pressure evaluation form. Six cycles of combination chemotherapy consisting of 5 fluorouracil + leucovorin + irinotecan and bevacizumab were given to all patients every 14 days. In all patients, 2 blood samples of 2 cc were obtained, 1 before the treatment and the other during the 3rd month of therapy (after 6 cycles), and these samples were used to investigate the serum levels of VEGF, NO, and Ang II. For the quantitative measurement of the VEGF levels, ELISA was used (Human VEGF ELISA Kit, RayBiotech, Inc., Norcross, GA). Serum levels of NO were determined using colorimetric method (Roche Diagnostics GmbH, Mannheim, Germany). Ang II levels were determined using an ELISA kit (Human Angiotensin II ELISA kit, Cusabio Biotech, Wuhan, China).
Statistical methods
Statistical analysis of the data was performed using SPSS 17.0 software. Categorical measurements are expressed as number and percentage and continuous measurements are expressed as median and minimum-maximum. Intergroup comparison of the categorical measurements was done using the chi-square test. For the general intergroup comparison of continuous measurements, one-way analysis of variance was used if the hypotheses could be met and Kruskal-Wallis test was used if the hypotheses could not be met. Paired comparisons of the groups were done using Bonferroni or Scheffe tests. Because some continuous measurements did not meet the hypothesis of normal distribution, the correlation across these continuous measurements was examined using the Spearman coefficient of correlation. All tests were performed at 95% confidence level and with α=0.05 error value.
Results
Of 24 patients, 15 (62.5%) were male and mean age was 61 years (range: 37–75) years. The patients had diagnosed as colon and rectum cancer in 19 (79.2%) and 5 (20.8%), respectively. Initial presentation with metastatic disease was detected in 20 (83.3%) patients. During the initial evaluation, 19 of the patients (79.2%) were found normotensive. Demographic and clinicopathologic characteristics of the study patients are summarized in Table 1.
Table 1.
Demographical and clinicopathological characteristics of the study patients.
| Number (n) | Percentage (%) | |
|---|---|---|
| Number of patients | 24 | 100 |
| Age | ||
| Mean | 61 (range 37–75) | – |
| Gender | ||
| Female | 9 | 37.5 |
| Male | 15 | 62.5 |
| Tumor localization | ||
| Colon | 19 | 79.2 |
| Rectum | 5 | 20.8 |
| Histologic grade | ||
| Grade 2 | 9 | 37.5 |
| Grade 3 | 15 | 62.5 |
| History of hypertension at the time of admission | ||
| Yes | 5 | 20.8 |
| No | 19 | 79.2 |
| Presence of uncontrolled hypertension | 0 | 0 |
| Presence of diabetes at the time of admission | ||
| Yes | 3 | 12.5 |
| No | 21 | 87.5 |
| Presence of uncontrolled diabetes | 0 | 0 |
| Smoking | ||
| Yes | 13 | 54.2 |
| No | 11 | 45.8 |
| History of adjuvant chemotherapy | ||
| Yes | 4 | 16.7 |
| No | 20 | 83.3 |
Biochemical results obtained from the serum samples of the patients
Blood samples were drawn at baseline and at the end of 6 cycles for the investigation of serum levels of VEGF, NO, and ANGII. According to the result of the blood sample analysis, median levels of VEGF, NO, and ANGII were 197.7 pg/mL, 6.32 pg/mL, and 4.51 pg/mL at baseline and 87.5 pg/mL, 6.71 pg/mL, and 3.55 pg/mL at the end of 6 cycles, respectively. The change across VEGF levels was found to be a statistically significant decreasing trend (p=0.009). The results are given in Table 2.
Table 2.
Serum tumor marker, VEGF, NO and ANG II levels of the patients at baseline and at the end of 6th cycle of therapy.
| Baseline (median, min–max) | At the end of 6th cycle (median, min–max) | ||
|---|---|---|---|
| VEGF | 197.7 (8.2–681.6) | 87.1 (12.4–141.2) | P=0.009 |
| NO | 6.32 (2.5–21.5) | 6.7 (2.9–28.9) | |
| ANG II | 4.51 (1.2–10.8) | 3.55 (1.58–8.9) |
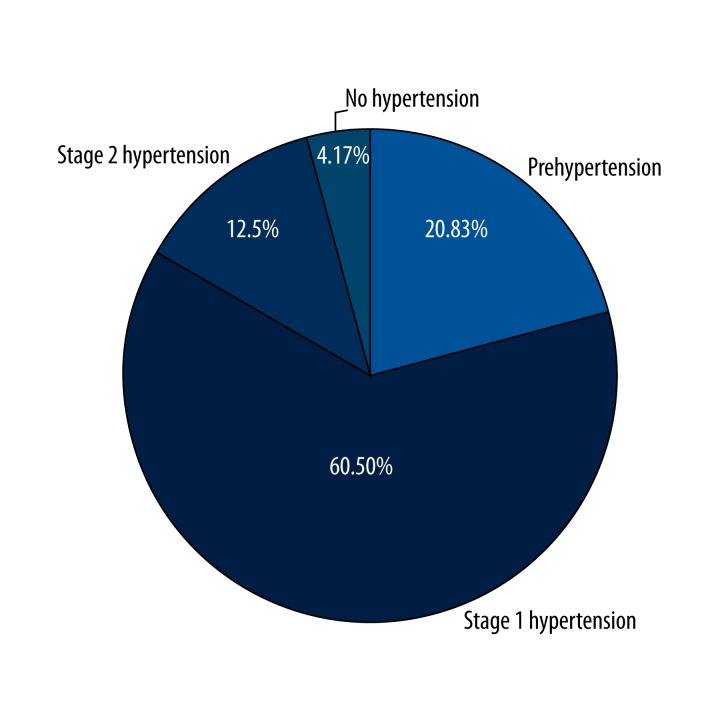
The patients were asked to measure and record their blood pressure 2 times per day using a digital blood pressure measurement device during their examination in the outpatient clinic and at home during the time interval between 2 chemotherapy courses. In addition, blood pressure of the patients was measured during the control visit. Accordingly, all measurements of the patients were recorded in the statistical software and analyzed for development of hypertension during their follow-up. One patient (4.2%) did not develop hypertension, 5 patients (20.8%) had prehypertension, 15 patients (62.5%) had Stage 1 hypertension, and 3 patients (12.5%) had Stage 2 hypertension. None of the 5 patients who had hypertension at baseline showed an imbalanced change in blood pressure levels. Three (12.5%) of the patients had no hypertension at baseline, which was needed for the initiation of antihypertensive drug treatment. In several cycles due to the development of Stage 2 hypertension and thereafter, the blood pressure of these patients were also controlled. Figure 1 shows the percentage of patients that developed hypertension after bevacizumab treatment.
Figure 1.
Hypertension development percentage after 6th cycles of bevacizumab treatment.
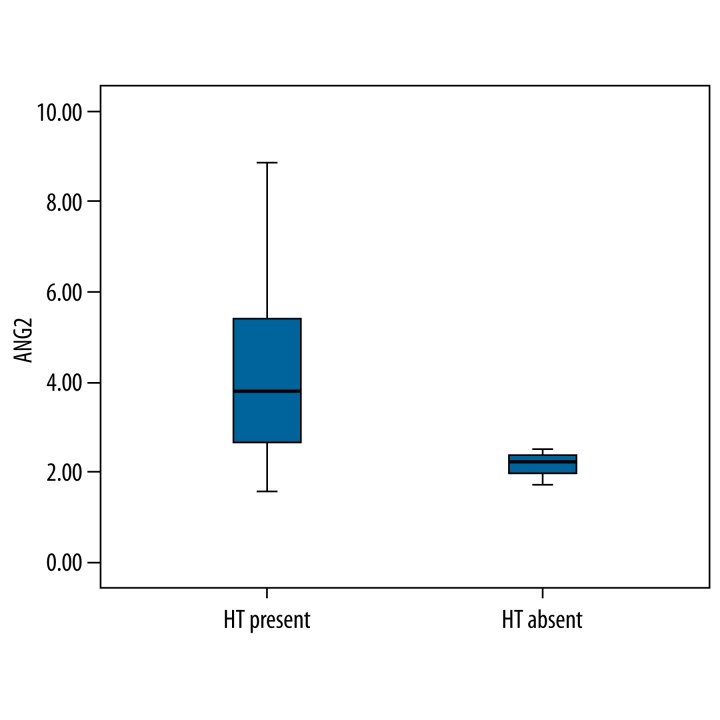
Statistical analysis failed to show a significant correlation between the development of hypertension and serum levels of VEGF, NO, and ANGII obtained at baseline at the end of the 6th cycle of the therapy. The levels of ANG II were found to be higher, although not significantly so, in the patients who developed hypertension when compared with patients who were normotensive at the end of the 6th cycle (p=0.09). However, the degree of decrease was not found to be correlated with response to the chemotherapy and hypertension development (r>0.1). Figure 2 shows the correlation between serum ANG II levels and presence of hypertension.
Figure 2.
Correlation between serum levels of ANG II and hypertension in the patients at the of 6th cycle of the therapy.
Discussion
The rationale of anti-angiogenic treatment is based on the hypothesis by Dr. Judah Folkman, which was published in an article titled ‘Tumor growth and metastasis is an angiogenesis-dependent process’ [7]. This hypothesis was validated in many clinical and experimental studies [8–10]. Bevacizumab, an IgG1-like monoclonal antibody developed against VEGF, is the most commonly used anti-angiogenic molecule and has been shown to have a statistically significant increase in response and survival rate in a wide range of tumors. Bevacizumab was approved for treatment of colon, breast, lung, and ovarian cancer, and also treatment of glioblastoma multiforme [11].
In our study, in 24 patients with complete follow-up, the levels of VEGF, NO, and ANG II and blood pressure were measured at baseline and at the end of 6 therapeutic cycles. Although, when changes in the level of VEGF, NO, and ANG II from baseline to the end of 6 cycles were examined, only VEGF levels showed a statistically significant decrease (p=0.009). Additionally, we found that all patients except 1 showed some degree of increased blood pressure.
The correlation between the use of VEGF inhibitors and serum levels of VEGF has been examined in many studies, some of which investigated the correlation between pre-treatment serum VEGF levels and response, whereas others examined the correlation between drug-dependent increase and decrease of serum VEGF levels. Clinical studies showed that VEGF levels increased with treatment using oral tyrosine kinase inhibitors with intrinsic anti-VEGF properties, whereas VEGF levels decreased with the treatment of bevacizumab [12–16]. Some studies have suggested that the effect of bevacizumab on VEGF levels can be affected by concomitant chemotherapeutics. Only 1 clinical study in which bevacizumab was initiated as a single agent or in combination with chemotherapy showed increased VEGF levels in the single-agent arm and decrease of VEGF levels in the combination arm [17]. Unfortunately, the only studies that showed the change of only VEGF levels with anti-angiogenic treatment had neither prognostic nor predictive value [18–21]. In our study, there was a statistically significant decrease in VEGF levels with bevacizumab therapy, but we did not find any correlation between degree of decrease and incidence of hypertension. We suggest that this result can be explained by the small number of patients in our study group.
Experimental studies in mice demonstrated that intravenous and infusional VEGF led to vasodilatation and lowered blood pressure by increasing endothelial and renal levels of NO and prostacyclines [22,23]. The authors in these studies hypothesized that VEGF inhibition may cause a decrease in NO levels and this decrease shows an inverse correlation with hypertension [24]. Bevacizumab was shown to be associated with decreased NO levels in 1 in vitro experimental study [25]. In the literature, there are a limited number of clinical studies that investigated the possible interplay between bevacizumab therapy and serum levels of NO and ANG II; they failed to show a significant decrease in these biochemical markers with Anti-VEGF therapy [26]. An experimental study performed by Facemire et al. reported a significant decrease in urinary excretion of renin mRNA and aldosterone levels with VEGF inhibition when compared with the control group [27]. A clinical study by Veronese et al. reported unchanged serum creatinine, aldosterone, and renin concentrations with sorafenib treatment [28]. Results of our study also failed to show a significant effect of bevacizumab on NO and ANG II levels.
In the literature, incidence of hypertension after initiation of bevacizumab was observed in studies at a rate of 30–80% for all stages and 10–15% for CTC Stage 3 or JNC 7 Stage 2 HT [29,30]. In our study, all patients except 1 developed hypertension. This high incidence rate of hypertension may be explained by the more frequent measurement of blood pressure due to study protocol.
In the present study, we aimed to evaluate alteration and interplay between serum levels of VEGF, NO, and ANG II during anti-angiogenic treatment and to use these biochemical markers to predict long-term efficacy and toxicity of this targeted therapy. We found a significant decrease in serum VEGF levels but failed to show a significant change in NO and ANG II. Also, our study failed to show interplay between VEGF, NO, and ANGII levels.
Conclusions
We suggest that more intensive follow-up of blood pressure during bevacizumab treatment may be needed. Dynamic measurement of NO and ANG II along with VEGF serum levels may not be used as predictive marker during bevacizumab treatment.
Footnotes
Source of support: This study was performed with a grant from the Turkish Medical Oncology Society
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Kramer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32:1–14. doi: 10.1111/j.1365-2710.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–93. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Wingo PA, Ries LA, Parker SL, et al. Long-term cancer patient survival in the United States. Cancer Epidemiol Biomarkers Prev. 2007;7:271–82. [PubMed] [Google Scholar]
- 5.Midgley R, Kerr D. Colorectal cancer. Lancet. 2004;353:391–99. doi: 10.1016/S0140-6736(98)07127-X. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–86. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 9.Abali H, Abalı G. Angiogenesis and Anti Angiogenesis in Neoplastic and Non Neoplastic Diseases. New Angiogenesis Research. 2005:235–55. [Google Scholar]
- 10.Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22:6549–56. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 11.Verheul HM, Pinedo HM. Vascular endothelial growth factor and its inhibitors. Drugs Today. 2003:81–93. [PubMed] [Google Scholar]
- 12.Jubb AM, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–27. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 13.Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 14.Ko AH, Dito E, Schillinger B, et al. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and lowdose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest. New Drugs. 2008;26:463–71. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 15.Dowlati A, Robertson K, Radivoyevitch T, et al. Novel phase I dose de-escalation design trial to determine the biological modulatory dose of the antiangiogenic agent SU5416. Clin Cancer Res. 2005;11:7938–44. doi: 10.1158/1078-0432.CCR-04-2538. [DOI] [PubMed] [Google Scholar]
- 16.Bernaards C, Hegde P, Chen D, et al. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: Analysis of phase III studies. J Clin Oncol. 2010:28. [Google Scholar]
- 17.Smerdel MP, Steffensen KD, Waldstrøm M, et al. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecologic Oncology. 2010;118:167–71. doi: 10.1016/j.ygyno.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–26. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu JF, Bruno R, Eppler S, et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 20.Loupakis F, Falcone A, Masi G, et al. Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol. 2007;25:1816–18. doi: 10.1200/JCO.2006.10.3051. [DOI] [PubMed] [Google Scholar]
- 21.Zahiragic L, Schliemann C, Bieker R, et al. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia. 2007;21:1310–12. doi: 10.1038/sj.leu.2404632. [DOI] [PubMed] [Google Scholar]
- 22.Henry TD, Rocha-Singh K, Isner JM, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142(5):872–80. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz JR, Rivard A, van der Zee R, et al. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997;17:2793–800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 24.Maitland ML, Kasza KE, Karrison T, et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–57. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335–45. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki S, Siragy HM, Gildea JJ, et al. Production and role of extracellular guanosine cyclic 3, 5 monophosphate in sodium uptake in human proximal tubule cells. Hypertension. 2004;43:286–91. doi: 10.1161/01.HYP.0000112421.18551.1e. [DOI] [PubMed] [Google Scholar]
- 27.Facemire CS, Nixon AB, Griffiths R, et al. Vascular Endothelial Growth Factor Receptor 2 Controls Blood Pressure by Regulating Nitric Oxide Synthase Expression. Hypertension. 2009;21:86–94. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006;24(9):1363–69. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 29.Pande A. Bevacizumab induced hypertension: a manageable toxicity. J Clin Oncol, ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2006;24:242. [Google Scholar]
- 30.Kozloff M, Hainsworth J, Badarinath S. Survival of patients with mCRC treated with bevacizumab in combination with chemotherapy: results from the BRİTE registry. American Society of Clinical Oncology Gastrointestinal Cancers Symposium; Orlando, FL, USA . 19–21 January 2007; Abstract 364. [Google Scholar]