Abstract
Pollen from 5 plant species (Lycopersicon pimpinellifolium Mill., Hermerocallis minor Mill., Galtonia condicans Decne., Camellia japonica L., and Lathyrus odoratus L.) representing 4 families germinated well in media containing trehalose as the sole carbon source. Data are presented indicating that pollen metabolized this disaccharide for germination and subsequent pollen-tube growth; the sugar was not merely an osmoregulator. An inhibitor of trehalase activity depressed germination in trehalose but not in sucrose. Phloridzin dihydrate, an inhibitor of glucose transport, depressed germination in both disaccharides.
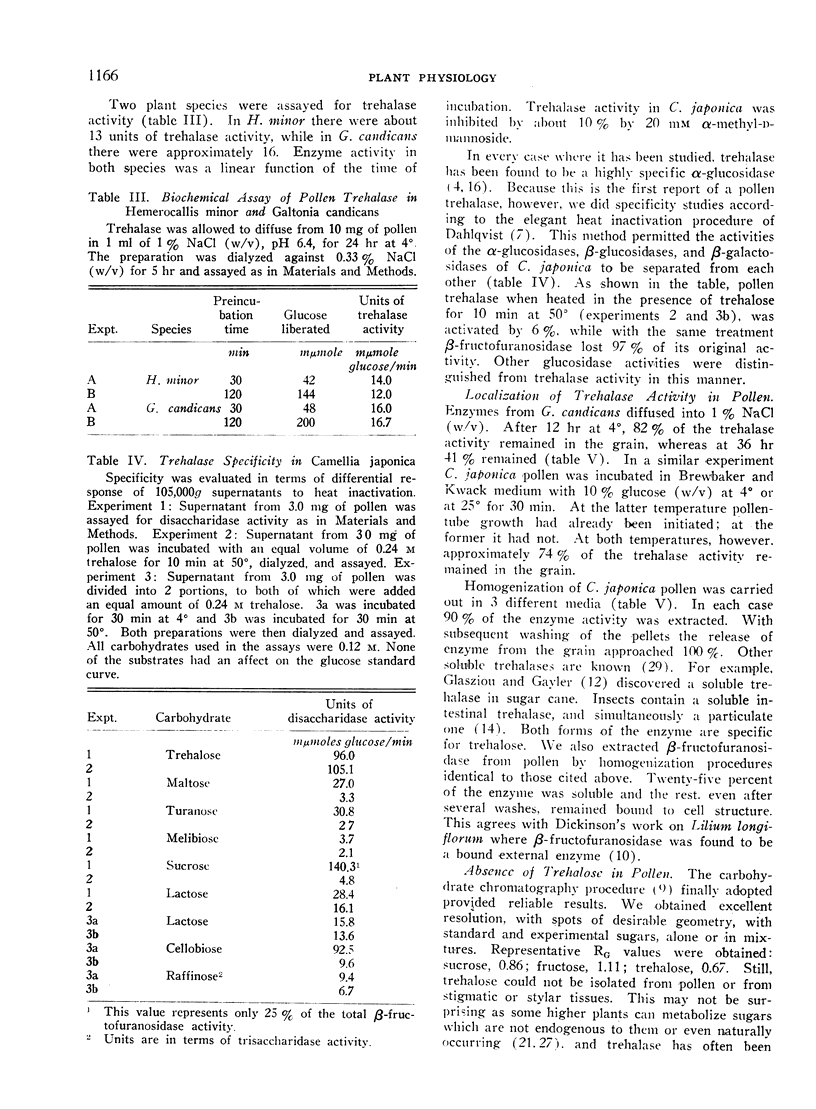
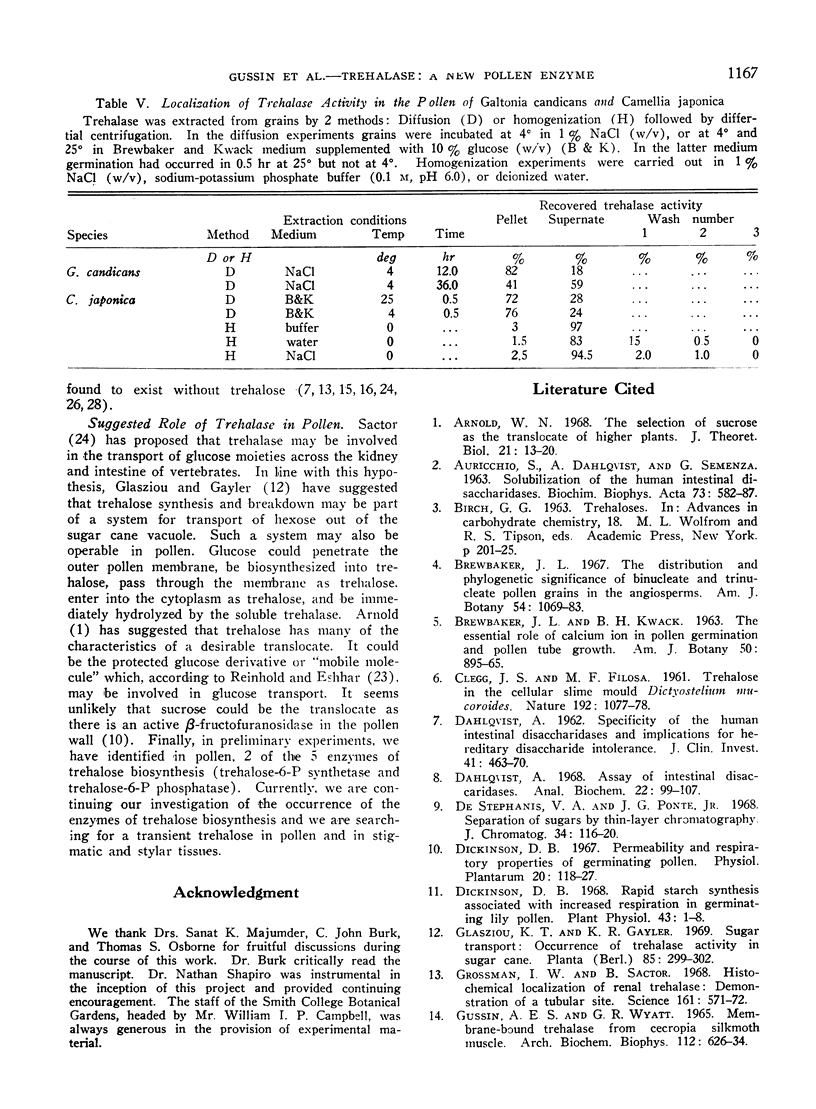
Biochemical tests demonstrated that a pollen extract was capable of hydrolyzing trehalose to its constituent glucose monomers. Heat inactivation experiments confirmed the presence of a distinct trehalase having a rigid specificity for its substrate. By this method, trehalase activity was completely distinguishable from the activities of other α- and β-glucosidases and β-galactosidases. Localization data indicated that the enzyme diffused from intact grains and was probably soluble. The presence of its substrate could not be demonstrated in pollen or in stigmatic or stylar tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., DAHLQVIST A., SEMENZA G. SOLUBILIZATION OF THE HUMAN INTESTINAL DISACCHARIDASES. Biochim Biophys Acta. 1963 Aug 6;73:582–587. doi: 10.1016/0006-3002(63)90329-9. [DOI] [PubMed] [Google Scholar]
- Arnold W. N. The selection of sucrose as the translocate of higher plants. J Theor Biol. 1968 Oct;21(1):13–20. doi: 10.1016/0022-5193(68)90056-8. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. J Clin Invest. 1962 Mar;41:463–470. doi: 10.1172/JCI104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. B. Rapid starch synthesis associated with increased respiration in germinating lily pollen. Plant Physiol. 1968 Jan;43(1):1–8. doi: 10.1104/pp.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman I. W., Sacktor B. Histochemical localization of renal trehalase: demonstration of a tubular site. Science. 1968 Aug 9;161(3841):571–572. doi: 10.1126/science.161.3841.571. [DOI] [PubMed] [Google Scholar]
- Gussin A. E., Wyatt G. R. Membrane-bound trehalase from cecropia silkmoth muscle. Arch Biochem Biophys. 1965 Dec;112(3):626–634. doi: 10.1016/0003-9861(65)90106-2. [DOI] [PubMed] [Google Scholar]
- Kerry K. R., Messer M. Intestinal glycosidases of three species of seals. Comp Biochem Physiol. 1968 May;25(2):437–446. doi: 10.1016/0010-406x(68)90352-6. [DOI] [PubMed] [Google Scholar]
- NAKAMURA S., WAKEYAMA T. Distribution of trypsin inhibitors in the sera of various animals. Nature. 1961 Dec 16;192:1077–1077. doi: 10.1038/1921077a0. [DOI] [PubMed] [Google Scholar]
- Reinhold L., Eshhar Z. Transport of 3-o-Methylglucose Into and Out of Storage Cells of Daucus carota. Plant Physiol. 1968 Jul;43(7):1023–1030. doi: 10.1104/pp.43.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B. Trehalase and the transport of glucose in the mammalian kidney and intestine. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1007–1014. doi: 10.1073/pnas.60.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R., KALE G. F. The chemistry of insect hemolymph. II. Trehalose and other carbohydrates. J Gen Physiol. 1957 Jul 20;40(6):833–847. doi: 10.1085/jgp.40.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]



