Abstract
Objective
Several studies have reported the association between methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms and nonsyndromic cleft lip with or without palate (NSCL/P) in Asian populations. However, findings have been conflicting. In order to investigate the association, a meta-analysis was performed.
Methods
We searched Pubmed, MedLine and EmBase database to selected eligible studies. The pooled odds ratios (ORs) with 95% confidence intervals (95%CIs) were calculated using fixed effects model or random effects model to assess the association between MTHFR polymorphisms and NSCL/P in both Asian children and mothers.
Results
Finally, nine case-control studies were included. Overall, the MTHFR C677T polymorphism and NSCL/P showed pooled ORs (95%CI) of 1.41(1.23–1.61) in Asian children, and 1.70(1.19–2.42) in Asian mothers. Subgroup analyses by geographical locations further identified the association in Eastern Asian children, Western/Central Asian children and mothers, but not in Eastern Asian mothers. However, no significant relationship between MTHFR A1298C polymorphism and NSCL/P was found in this meta-analysis.
Conclusions
The MTHFR 677T allele was associated with an increased risk of NSCL/P in Asian populations.
Introduction
Nonsymdromic cleft lip with or without palate (NSCL/P) is one of the most common congenital malformations of the head and neck area in the world, occurring in approximately 1 in every 700 live births [1]. Around the globe, the distribution of prevalence rate of NSCL/P is not homogeneous, and higher values are found in parts of Asia (China, Japan) and Latin America, whereas Israel, South Africa and Southern Europe showed the lowest values [2]. The etiology of NSCL/P is considered to be multifactorial, causing by both hereditary factors and environmental factors. Epidemiologic studies revealed that mothers who used multivitamins containing folic acid showed a lower risk of having an offspring with NSCL/P compared with mothers did not use multivitamins [3]. However, it is still unknown which ingredients in multivitamins contribute to this risk reduction. It is widely accepted that folic acid is a key factor in the development of craniofacial structures. Several studies have reported a reduced risk of NSCL/P when mothers used either folic acid supplements or dietary folate during pregnancy [4], [5]. Some other studies, however, have provided variable or even contradictory results [6]–[8]. Therefore, genetic variations in folate metabolism gene were believed to affect individual susceptibility to NSCL/P.
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in the metabolism of folate. This enzyme irreversibly catalyzes the reaction of 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the primary circulating form of folate [6]. The most common single nucleotide polymorphism (SNP) is MTHFR C677T, which results in an alanine to valine exchanges and is associated with reduced enzyme activity. MTHFR A1298C, another common functional polymorphism, also affects enzyme activity [7], [8]. The two SNPs have been studied as candidate genetic factors for NSCL/P risk [9]–[12].
The first report evaluating the role of MTHFR gene polymorphisms in the development of NSCL/P was conducted by Tolarova et al.(1998) [13]. The findings suggested that MTHFR C677T variant genotype increased the risk of NSCL/P. They also found a significant association between MTHFR A1298C polymorphism and NSCL/P. Since then, a great number of studies have been conducted, but the results were inconsistent [14]–[16]. In order to elucidate the role of MTHFR C677T and A1298C polymorphisms in NSCL/P, several meta-analyses were performed [17]–[20], but the relationships especially among Asian subjects remained unclear. Recently, some new case-control studies with large sample size have been published [21]–[23]. Hence, we performed this meta-analysis to derive a more precise estimation of the association of MTHFR C677T and A1298C polymorphisms with NSCL/P in Asian populations.
Materials and Methods
Literature search and selection
Eligible literatures were screened from PubMed, MedLine and EmBase database (up to May 31, 2013). We used the following keywords and subject terms: “methylenetetrahydrofolate reductase” or “MTHFR” and “cleft lip” or “cleft palate”. Furthermore, we manually searched references in the eligible articles. There was no language limitation. And the search results were limited to humans.
Criteria of inclusion and exclusion
Inclusion criteria were showed as following: (1) evaluation the association between MTHFR C677T and/or A1298C polymorphisms and NSCL/P risk in Asian populations; (2) case-control studies; (3) the frequencies of all genotype distribution or other available data for estimating the OR (95% CI). If overlapping cases or controls were presented in multiple studies, the most recent publication or the largest study was included. The exclusion criteria were: (1) not using a case-control study design; (2) had no detailed data on genotype distribution; (3) not in Asian populations.
Data extraction
The following information was carefully and independently collected from each eligible study by two authors: first author's name, year of publication, country, population of cases and controls, genotyping method, and numbers of each genotype. The difference was settled by reaching an agreement among all authors.
Statistical analysis
To assess the study quality, Hardy–Weinberg equilibrium (HWE) in controls in each study was calculated by chi-squared test. P value<0.05 was considered a departure from HWE. The associations between MTHFR C677T and A1298C gene polymorphisms and NSCL/P risk were estimated by the odds ratios (ORs), together with the 95% confidence interval (95%CI). The significance of the pooled OR was determined by the Z test, with P<0.05 considered significant. Heterogeneity between studies was assessed by Q test. If P<0.05, the heterogeneity was considered statistically significant. The I2 values were used to quantify the percentage of the total variation among studies when heterogeneity was assessed. The I2 value ranged from 0 to 100%. 25%, 50%, 75% expressed low, moderate, high heterogeneity, respectively. When I2<50%, a fixed effects model was applied to estimate the pooled results. Otherwise, the random-effect model was used.
Publication bias was investigated visually in a funnel plot of log (OR) against its standard error (SE). An asymmetric plot suggested possible publication bias. And the degree of asymmetry was assessed by Egger's test. Sensitivity analysis was performed by omitting each study in turn to assess the results stability.
Software STATA version 11.0 was used for all analyses.
Results
Selection of studies
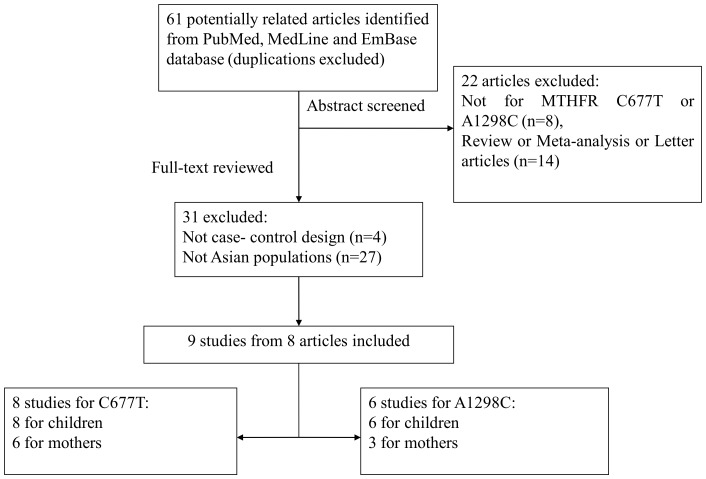
A flow chart summarizing the process of study selection is shown in Figure 1. Based on the inclusion and exclusion criteria, eight articles were included after full-text reviewing [14], [16], [21]–[26]. Worthy of note, a study by Aida et al.(2012) consisted of two groups of control was treated as two studies [21]. Thus, a total of nine studies from eight articles were included in this meta-analysis. Among them, nine studies provided date on MTHFR C677T polymorphism (eight studies were carried out in Asian children and six in Asian mothers), and 6 studies on A1298C polymorphism (six in Asian children and three in Asian mothers). The main characteristics of selected studies were summarized in Table 1.
Figure 1. Flow chart explaining the selection of eligible studies included in the meta-analysis.
Table 1. The characteristics of eligible studies included in the meta-analysis.
| Anthor(year) | Country | Geographical location | Population of cases | Source of control | Subjects | Sample size | P of HWE | ||
| Case | Control | 677 | 1298 | ||||||
| Kumari et al. (2013) | India | Western/Central Asia | Cases having family history of any congenital malformation, kidney-related diseases and other severe diseases were excluded. Mean age was 5 years. | Mixed | Children | 467 | 469 | 0.518 | 0.134 |
| Aida et al. (2012) | Turkey | Western/Central Asia | Cases were excluded due to alternative diagnosis of their existing syndromic conditions. Age range not stated. | Mixed | Children | 56 | 76/93a | 0.105/0.779 | 0.187 |
| Mothers | 54 | 76/93 | |||||||
| Wang et al. (2012) | China | Eastern Asia | Not stated. | HB | Mothers | 89 | 64 | 0.070 | |
| Han et al. (2011) | China | Eastern Asia | Cases with recognized congenital anomalies or syndromes were excluded. Mean age was 9.57±0.72 years. | HB | Children | 187 | 213 | 0.236 | 0.545 |
| Guo et al. (2009) | China | Eastern Asia | Cases having family history of any congenital malformation were excluded. Mean age was 9.04 years. | HB | Children | 97 | 104 | 0.277 | |
| Mothers | 97 | 104 | |||||||
| Ali et al. (2009) | India | Western/Central Asia | Syndromic patients as well as families with a syndromic member other than NSCL/P probands were not included. Age range for patients 4 months-24 years, for mothers 19–45 years. | PB | Children | 323 | 214 | 0.916 | 0.395 |
| Mothers | 116 | 214 | |||||||
| Wan et al. (2006) | China | Eastern Asia | Cases having family history of any congenital malformation were excluded. Age range not stated. | HB | Children | 48 | 60 | 0.075 | 0.210 |
| Shotelersuk et al. (2003) | Thailand | Eastern Asia | All syndromic cases were excluded. Age range not stated. | PB | Children | 109 | 202 | 0.478 | 0.876 |
| Mothers | 67 | 202 | |||||||
HWE Hardy-Weinberg equilibrium; HB hospital based; PB population based.
two groups of control.
Meta analysis
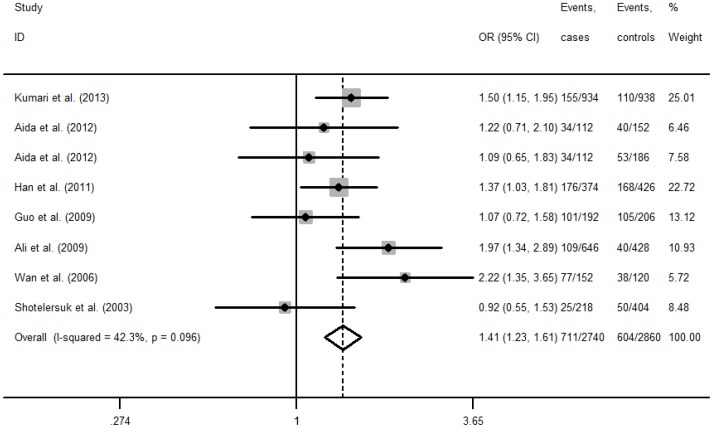
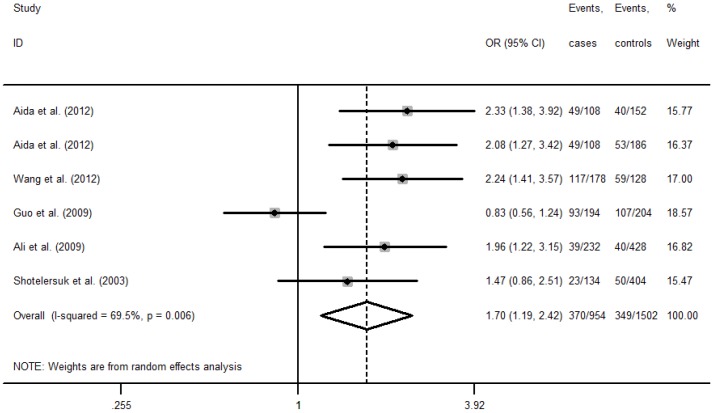
The association between MTHFR C677T and NSCL/P risk was shown in Table 2. Significant heterogeneity between studies was found in the majority of the genetic comparisons, except for allele comparison (T vs. C: I2 = 42.3%), homozygote comparison (TT vs. CC: I2 = 28.3%) and recessive comparison (TT vs. CC/CT: I2 = 17.4%) in Asian children, and recessive comparison (TT vs. CC/CT: I2 = 49.8%) in Asian mothers. Overall, the variant T allele of MTHFR C677T increased risk of NSCL/P, when compared with the wild-type C allele. The OR (95%CI) was 1.41(1.23–1.61, P<0.001, Figure 2) in Asian children, and 1.70(1.19–2.42, P = 0.003, Figure 3) in Asian mothers, respectively. In the subgroup analyses of geographical location, the similar associations were also observed in Eastern Asian children, Western/Central Asian children and mothers, but not in Eastern Asian mothers.
Table 2. Overall and subgroup results of the association between MTHFR C677T polymorphism and NSCL/P.
| Study(n) | T vs. C | TT vs. CC | CT vs. CC | CT/TT vs. CC | TT vs. CC/CT | |||||
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Overall | ||||||||||
| Children(n = 8) | 1.41(1.23–1.61) | <0.001 | 1.85(1.30–2.63) | 0.001 | 1.61(1.21–2.14) | 0.001 | 1.61(1.22–2.11) | 0.001 | 1.36(1.00–1.86) | 0.052 |
| Mothers(n = 6) | 1.70(1.19–2.42) | 0.003 | 2.58(1.09–6.09) | 0.031 | 1.66(1.08–2.55) | 0.021 | 1.83(1.17–2.88) | 0.008 | 1.95(1.32–2.86) | 0.001 |
| Eastern Asia | ||||||||||
| Children(n = 4) | 1.31(0.95–1.80) | 0.099 | 1.79(1.16–2.76) | 0.009 | 1.68(0.88–3.22) | 0.117 | 1.66(0.89–3.10) | 0.111 | 1.26(0.87–1.83) | 0.217 |
| Mothers(n = 3) | 1.39(0.75–2.54) | 0.293 | 2.19(0.46–10.44) | 0.326 | 1.09(0.72–1.65) | 0.688 | 1.28(0.64–2.59) | 0.487 | 2.06(0.64–6.61) | 0.224 |
| Western/Central Asia | ||||||||||
| Children(n = 4) | 1.50(1.25–1.81) | <0.001 | 1.96(1.08–3.55) | 0.026 | 1.56(1.25–1.95) | <0.001 | 1.61(1.30–1.99) | <0.001 | 1.44(0.53–3.95) | 0.477 |
| Mothers(n = 3) | 2.11(1.58–2.81) | <0.001 | 3.66(1.78–7.52) | <0.001 | 2.40(1.64–3.50) | <0.001 | 2.53(1.75–3.64) | <0.001 | 2.38(1.22–4.66) | <0.001 |
Figure 2. Forest plot of association between MTHFR C677T polymorphism and NSCL/P risk in children (T vs. C).
Figure 3. Forest plot of association between MTHFR A1298C polymorphism and NSCL/P risk in mothers (T vs. C).
For MTHFR A1298C polymorphism, the main results were shown in Table 3. Sound homogeneity between studies was seen, except for allele comparison (C vs. A: I2 = 71.0%), heterozygote comparison (AC vs. AA: I2 = 76.0%) and dominant comparison (AC/CC vs. AA: I2 = 74.8%) in Asian children. However, no significant associations between MTHFR A1298C polymorphism and NSCL/P were found under any of genetic model, even in the subgroup analyses by geographical location, neither in Asian children nor in Asian mothers.
Table 3. Overall and subgroup results of the association between MTHFR A1298C polymorphism and NSCL/P.
| Study(n) | T vs. C | TT vs. CC | CT vs. CC | CT/TT vs. CC | TT vs. CC/CT | |||||
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | |
| Overall | ||||||||||
| Children(n = 6) | 1.03(0.79–1.35) | 0.819 | 1.08(0.78–1.48) | 0.652 | 1.01(0.68–1.48) | 0.982 | 1.03(0.71–1.48) | 0.881 | 1.09(0.80–1.48) | 0.578 |
| Mothers(n = 3) | 0.94(0.73–1.20) | 0.593 | 0.78(0.40–1.50) | 0.452 | 0.98(0.71–1.35) | 0.905 | 0.95(0.70–1.30) | 0.751 | 0.79(0.41–1.50) | 0.467 |
| Eastern Asia | ||||||||||
| Children(n = 3) | 1.05(0.61–1.82) | 0.855 | 0.97(0.47–1.99) | 0.930 | 1.16(0.44–3.06) | 0.767 | 1.13(0.49–2.61) | 0.768 | 0.96(0.48–1.93) | 0.907 |
| Mothers(n = 1) | 0.224 | |||||||||
| Western/Central Asia | ||||||||||
| Children(n = 3) | 1.04(0.73–1.49) | 0.819 | 1.23(0.53–2.85) | 0.626 | 0.94(0.76–1.16) | 0.551 | 0.98 (0.66–1.44) | 0.901 | 1.23(0.60–2.53) | 0.568 |
| Mothers(n = 2) | 0.83(0.62–1.12) | 0.218 | 0.81(0.20–3.27) | 0.767 | 0.81(0.54–1.20) | 0.283 | 0.79(0.54–1.15) | 0.218 | 0.88(0.24–3.17) | 0.839 |
Sensitivity analysis and publication bias
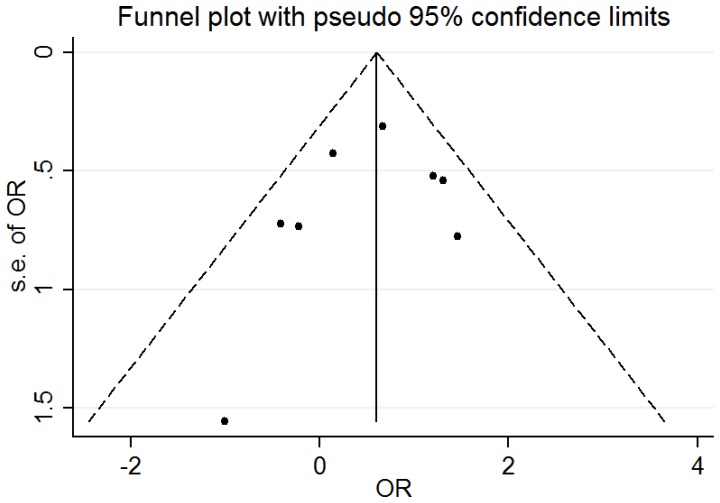
Elimination of each study made no qualitative difference on the pooled OR values, which indicated that the final results of the meta-analysis were stable. The publication bias of the included studies was assessed by the Funnel plot and Egger's test. The shapes of Funnel plot in each genotype comparison indicated no obvious asymmetry (Figure 4). And Egger's test provided statistical evidence for the funnel plot symmetry. No significant publication bias was found in the studies.
Figure 4. Funnel plot analysis to detect publication bias.
Discussion
In this meta-analysis, we investigated the association between MTHFR C677T and A1298C polymorphisms and NSCL/P, with a total of nine case-control studies included. The pooled results indicated that there was an obvious association between MTHFR C677T polymorphism and NSCL/P risk in Asian children under all models: allele contrast (T vs. C), homozygote (TT vs. CC), heterozygote (CT vs. CC) and dominant (TT/CT vs. CC) models, except for recessive (TT vs. CT+CC) model. Meanwhile, the variant T allele of Asian mothers was significantly associated with a 1.70-fold increased risk of having a NSCL/P offspring. Subgroup analyses by geographical location further identified this association in Eastern Asian children, Western/Central Asian children and mothers, but not in Eastern Asian mothers. Regarding MTHFR A1298C polymorphism, the pooled results revealed that A1298C was not related to children's or mothers' NSCL/P susceptibility under any of the genetic model, even in the subgroup analyses by geographical location. The above results suggested that MTHFR C677T polymorphism was a risk factor in the development of NSCL/P in Asian populations.
As far as we know, there has been four published meta-analyses regarding MTHFR polymorphisms and NSCL/P risk. According to Verkleij-Hagoort et al.(2007) [19] and Johnson and Little (2008) [20], no significant associations between MTHFR C677T and A1298C polymorphisms and NSCL/P were acquired. The pooled results of Luo Y et al. (2012) [17] indicated that maternal MTHFR 677TT genotype was related to increased risk of having a NSCL/P offspring. The more recently meta-analysis conducted by Pan Y et al. (2012) [18] showed that MTHFR C677T polymorphism contributed to elevated risk of NSCL/P among Asians. Compared with the previous meta-analyses, we updated this meta-analysis by adding newly published studies, which were not included in the previous meta-analyses. Finally, we achieved consistent conclusions with Luo Y et al. (2012) and Pan Y et al. (2012).
Some limitations of this meta-analysis should be acknowledged. Firstly, the sources of control among the studies were different from each other. Some studies were population-based studies, and others were hospital-based studies. Secondly, our results were based on unadjusted OR values that lack the original data from the eligible studies, which could lead to relatively weak power to estimate the real relationship. Thirdly, our analyses were based on single-factor estimates, which overlooked the interactions of gene-gene and gene-environment in the development of NSCL/P. Finally, the sample size was relatively small to investigate the association between MTHFR C677T and A1298C polymorphisms and NSCL/P risk.
In conclusion, our meta-analysis suggested that MTHFR C677T polymorphism was related with an increased NSCL/P risk in Asian populations. In the future, large sample studies should be warranted to investigate the association between MTHFR C677T and A1298C polymorphisms and NSCL/P, and to examine the potential gene-gene and gene-environment interactions.
Supporting Information
(DOC)
Funding Statement
This study was supported by grant no.81272293 and no.81102194 from National Natural Science Foundation of China, grant no.LS2010168 from Liaoning Provincial Department of Education, and grant no.00726 from China Medical Board.The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health O (2003) Global registry and database on craniofacial anomalies: Report of a WHO registry meeting on craniofacial anomalies. Geneva: World Health Organization Report nr ISBN 651656510. [Google Scholar]
- 2. Mossey PA, Modell B (2012) Epidemiology of oral clefts 2012: an international perspective. Front Oral Biol 16: 1–18. [DOI] [PubMed] [Google Scholar]
- 3. Schutte BC, Murray JC (1999) The many faces and factors of orofacial clefts. Hum Mol Genet 8: 1853–1859. [DOI] [PubMed] [Google Scholar]
- 4. Tolarova M (1982) Periconceptional supplementation with vitamins and folic acid to prevent recurrence of cleft lip. Lancet 2: 217. [DOI] [PubMed] [Google Scholar]
- 5. Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA (2000) Folic acid antagonists during pregnancy and the risk of birth defects. New England journal of medicine 343: 1608–1614. [DOI] [PubMed] [Google Scholar]
- 6. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Genetics 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 7. Bailey LB, Gregory JF 3rd (1999) Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: metabolic significance, risks and impact on folate requirement. The Journal of nutrition 129: 919–922. [DOI] [PubMed] [Google Scholar]
- 8. Van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, et al. (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? American journal of human genetics 62: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mills JL, Molloy AM, Parle-McDermott A, Troendle JF, Brody LC, et al. (2008) Folate-related gene polymorphisms as risk factors for cleft lip and cleft palate. Birth Defects Research Part A: Clinical and Molecular Teratology 82: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandalize AP, Bandinelli E, Borba JB, Félix TM, Roisenberg I, et al. (2007) Polymorphisms in genes MTHFR, MTR and MTRR are not risk factors for cleft lip/palate in South Brazil. Brazilian Journal of Medical and Biological Research 40: 787–791. [DOI] [PubMed] [Google Scholar]
- 11. van Rooij IA, Vermeij-Keers C, Kluijtmans LA, Ocké MC, Zielhuis GA, et al. (2003) Goorhuis-Brouwer SM, van der Biezen JJ, Kuijpers-Jagtman AM, RP S-T: Does the interaction between maternal folate intake and the methylenetetrahydrofolate reductase polymorphisms affect the risk of cleft lip with or without cleft palate? American journal of epidemiology 157: 583–591. [DOI] [PubMed] [Google Scholar]
- 12. Boyles AL, Wilcox A, Taylor JA, Meyer K, Fredriksen A, et al. (2008) Folate and one-carbon metabolism gene polymorphisms and their associations with oral facial clefts. Am J Med Genet A 146A: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tolarova M, Van Rooij IALM, Pastor M, Van der Put NM, Goldberg AC, et al. (1998) A common mutation in the MTHFR gene is a risk factor for nonsyndromic cleft lip and palate anomalies. Am J Hum Genet 63: A27. [Google Scholar]
- 14. Shotelersuk V, Ittiwut C, Siriwan P, Angspatt A (2003) Maternal 677CT/1298AC genotype of the MTHFR gene as a risk factor for cleft lip. J Med Genet 40: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaspar DA, Matioli SR, de Cássia Pavanello R, Araújo BC, Alonso N, et al. (2004) Maternal MTHFR interacts with the offspring's BCL3 genotypes, but not with TGFA, in increasing risk to nonsyndromic cleft lip with or without cleft palate. Eur J Hum Genet 12: 521–526. [DOI] [PubMed] [Google Scholar]
- 16. Ali A, Singh SK, Raman R (2009) MTHFR 677TT alone and IRF6 820GG together with MTHFR 677CT, but not MTHFR A1298C, are risks for nonsyndromic cleft lip with or without cleft palate in an Indian population. Genet Test Mol Biomarkers 13: 355–360. [DOI] [PubMed] [Google Scholar]
- 17. Luo YL, Cheng YL, Ye P, Wang W, Gao XH, et al. (2012) Association between MTHFR polymorphisms and orofacial clefts risk: a meta-analysis. Birth Defects Res A Clin Mol Teratol 94: 237–244. [DOI] [PubMed] [Google Scholar]
- 18. Pan Y, Zhang W, Ma J, Du Y, Li D, et al. (2012) Infants' MTHFR polymorphisms and nonsyndromic orofacial clefts susceptibility: a meta-analysis based on 17 case-control studies. Am J Med Genet A 158A: 2162–2169. [DOI] [PubMed] [Google Scholar]
- 19. Verkleij-Hagoort A, Bliek J, Sayed-Tabatabaei F, Ursem N, Steegers E, et al. (2007) Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: a meta-analysis. Am J Med Genet A 143A: 952–960. [DOI] [PubMed] [Google Scholar]
- 20. Johnson CY, Little J (2008) Folate intake, markers of folate status and oral clefts: is the evidence converging? Int J Epidemiol 37: 1041–1058. [DOI] [PubMed] [Google Scholar]
- 21. Semiç-Jusufagiç A, Bircan R, Çelebiler Ö, Erdim M, Akarsu N, et al. (2012) Association between C677T and A1298C MTHFR Gene Polymorphism and Nonsyndromic Orofacial Clefts in the Turkish Population: A Case-Parent Study. Turk J Pediatr 54: 617–625. [PubMed] [Google Scholar]
- 22. Wang SM, Wang JH, Yu JC, Wei B, Wang KH, et al. (2012) Association between parental MTHFR gene polymorphism 677C/T and nonsyndromic cleft lip and palate in offspring. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 29: 464–467. [DOI] [PubMed] [Google Scholar]
- 23. Kumari P, Ali A, Sukla KK, Singh SK, Raman R (2013) Lower incidence of nonsyndromic cleft lip with or without cleft palate in females: is homocysteine a factor? J Biosci 38: 21–26. [DOI] [PubMed] [Google Scholar]
- 24. Han Y, Pan Y, Du Y, Tong N, Wang M, et al. (2011) Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and nonsyndromic orofacial clefts susceptibility in a southern Chinese population. DNA and Cell Biology 30: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 25. Guo JZ, Song XM, Wang Y, Zhu WL, Li SQ, et al. (2009) Relationship between genetic polymorphisms of MTHFR C677T and nonsyndromic cleft lip with or without palate. Beijing Da Xue Xue Bao 41: 432–436. [PubMed] [Google Scholar]
- 26. Wan WD, Wang LJ, Zhou XP, Zhou DL, Zhang QG, et al. (2006) Relationship between nonsyndromic cleft lip with or without cleft palate (NSCL/P) and genetic polymorphisms of MTHFR C677T and A1298C. Zhonghua Zheng Xing Wai Ke Za Zhi 22: 8–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)