Abstract
Background
Human beta-defensin-1 (hBD-1) has recently been considered as a candidate tumor suppressor in renal and prostate cancer. The aim of this study was to investigate the role of hBD-1 in the progression of oral squamous cell carcinoma (OSCC) and its potential as diagnostic/prognostic biomarker and therapeutic target for OSCC.
Methods
HBD-1 expression in tissues at different stages of oral carcinogenesis, as well as OSCC cell lines was examined. HBD-1 was overexpressed in HSC-3, UM1, SCC-9 and SCC-25 cells and subjected to cell growth, apoptosis, migration and invasion assays. Tissue microarray constructed with tissues from 175 patients was used to examine clinicopathological significance of hBD-1 expression in OSCC.
Results
HBD-1 expression decreased from oral precancerous lesions to OSCC and was lower in OSCC with lymph node metastasis than those without metastasis. In vitro, the expression of hBD-1 was related to the invasive potential of OSCC cell lines. Induction of exogenous expression of hBD-1 inhibited migration and invasion of OSCC cells, probably by regulation of RhoA, RhoC and MMP-2; but had no significant effect on proliferation or apoptosis. In a cohort of patients with primary OSCC, cases with no expression of hBD-1 had more chance to be involved in lymph node metastasis. Eventually, the positive expression of hBD-1 was associated with longer survival of patients with OSCC, and multivariate analysis and ROC curve analysis confirmed hBD-1 positivity to be an independent prognostic factor of OSCC, especially OSCC at early stage.
Conclusions
Overall, these data indicated that hBD-1 suppressed tumor migration and invasion of OSCC and was likely to be a prognostic biomarker and a potential target for treatment of OSCC.
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common oral and maxillofacial malignancies, causing around 130,000 deaths each year worldwide [1]. Despite the advances in early detection of oral precancerous lesions and treatment of OSCC in recent years[2]–[5], the overall survival rate of OSCC is still not promising, probably due to the lack of understanding of the molecular mechanisms of carcinogenesis. Therefore, it is necessary to search for novel targets for treatment of this disease and to identify molecular markers associated with the progression of this disease.
Human beta-defensin-1 (hBD-1) is a small cationic peptide encoded by human DEFB1 gene located on the short arm of the chromosome 8 [6]. It is mainly expressed in epithelial tissues [7]–[12]. Recently, it was reported that other than its antimicrobial and chemotactic activities [13], [14], hBD-1 also showed characteristics as a potential tumor-suppressor. Gene mutation of DEFB1 or cancer-specific loss of hBD-1 expression has been reported in basal cell carcinoma, renal cell carcinoma and prostate cancer [15]–[17]. Transduction of DEFB1 gene or treatment with recombinant hBD-1 led to inhibition of cell growth and apoptosis of tumor cells [17], [18]. Therefore, the researchers proposed that hBD-1 might be a potential tumor suppressor. Similarly, DEFB1 gene expression was observed in normal oral mucosa [7], [19]–[21], while in some precancerous lesions, OSCC tissues and cell lines, the expression was decreased [21]–[23]. In vitro studies showed that recombinant hBD-1 had inhibitory effect on the growth of some OSCC cell lines [24]. Taken together, these studies indicate that hBD-1 may also have some suppressive effect on oral carcinogenesis, but the effect of hBD-1 on other behaviors of OSCC cells and the clinicopathological significance of hBD-1 in OSCC is still unclear.
In the current study, we investigated the expression of hBD-1 protein in oral epithelia at different stages of oral carcinogenesis, the impact of hBD-1 on migration, invasion, proliferation and apoptosis of OSCC cells, as well as the clinicopathological significance of hBD-1 in a large group of patients with primary OSCC. We have shown that hBD-1 suppresses tumor migration and invasion of OSCC and is likely to be a prognostic biomarker and a potential target for treatment of OSCC.
Materials and Methods
Clinical tissue samples
This study was carried out under the approval and supervision of the Ethnic Committee of Sichuan University and written informed consent was obtained from each patient. Sixty-two formalin-fixed paraffin-embedded tissue blocks was collected in order to detect hBD-1 expression in normal oral mucosa, precancerous lesion oral leukoplakia (OLK) and primary OSCC. Among them, 30 OSCC samples and 17 OLK samples were collected during surgery or biopsy, 15 normal oral mucosa samples were obtained from extra tissue from orthognathic or implant surgery. Primary OSCC samples used for hBD-1 staining on tissue microarrays were obtained from 175 patients who underwent curative surgery between 2002 and 2009 in West China Hospital of Stomatology, Sichuan University (Chengdu, China), with a mean follow-up time of 35 months (median, 33 months; range 2∼102 months). All tumors were staged according to the TNM classification system of the International Union against Cancer [25]. The survival time of each patient was calculated from the day of surgery until the time of cancer-related death or end of the follow-up, while death due to other reasons was considered as censored data.
Immunohistochemistry
A mouse monoclonal antibody against hBD-1 (Abcam) was used for immunohistochemistry. The specificity of the antibody was tested using dot blot (Figure S1). Sections were rehydrated and antigen retrieved with Tris-EDTA buffer (pH 9.0). Slides were peroxidase blocked with 3% hydrogen peroxide solution for 10 minutes and then blocked using 5% bovine serum albumin (Sigma) for 30 minutes. Slides were subsequently incubated with primary antibody against hBD-1 (Mouse monoclonal, Abcam, 1∶200) or IgG negative control (Mouse IgG, Abcam, 1∶20) for 30 minutes and detected with ChemMate DAKO EnVision Detection Kit (DAKO). Slides were counterstained with haematoxylin, dehydrated and mounted. Human skin and salivary gland samples were used as positive control for hBD-1 [8], [11].
The staining was scored by three independent investigators without any knowledge of the clinicalpathological data. The following criteria was used to score the staining: negative, no detectable staining; weak, brown staining in less than 50% of tumor cells; strong, brown staining in more than 50% of tumor cells. The staining intensity was acceptable if two or more investigators independently defined it as such.
Cell lines and cell culture
Four human OSCC cell lines, HSC-3, UM1, SCC-9 and SCC-25 were used in this study. HSC-3 (JCRB0623) was purchased from the cell bank of Japanese Collection of Research Bioresource (JCRB, Shinjuku, Japan). UM1 [26] was provided by Dr. Xiaofeng Zhou (Center for Molecular Biology of Oral Diseases, College of Dentistry, University of Illinois at Chicago). SCC-9 (CRL-1629) and SCC-25 (CRL-1628) were purchased from American Type Culture Collection (ATCC, Manassas, USA). Human immortalized oral keratinocyte cell line HOK16E6E7 was provided by Dr. Xuan Liu (Charles R. Drew University of Medicine and Science) [27]. All cells were cultured in appropriate media with 10% fetal bovine serum (PAA Laboratories) in a humidified incubator at 37°C with 5% CO2.
Semi-quantitative real-time PCR
Total RNA was extracted from cultured cells using Trizol reagent (Invitrogen) and reversed transcribed using PrimeScript RT reagent kit (Takara). Semi-quantitative real-time PCR was done using the SYBR Premix Ex Taq II kit (Takara) on a 7300 real-time PCR system (Applied Biosystems) with DEFB1 gene-specific primers (5′-CCTTCTGCTGTTTACTCTCTGC-3′ and 5′-GAATAGAGACATTGCCCTCC- AC-3′) or with glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) specific primers (5′-CTTTGGTATCGTGGAAGGACTC-3′ and 5′-GTAGAGGCAGGGATG- ATGTTCT-3′) as an internal control.
Enzyme-linked immunosorbent assay
HBD-1 expression in cell culture supernatant was determined in triplicates by enzyme-linked immunosorbent assay (ELISA) using Human BD-1 ELISA Development Kit (Peprotech) according to the manufacturer's protocol. Data was read with a spectrophotometer (Thermo) at 450 nm.
Migration and Invasion assay
5×104 cells of different groups were re-suspended with serum-free Dulbecco's modified eagle medium (DMEM, Gibco, Invitrogen) and then seeded in a cell culture insert (pore size 8 µm, BD Biosciences) for 24-well plate in triplicates. DMEM containing 10% fetal bovine serum was added to the lower chamber. 24 hours later, cells in the upper chamber were wiped using a cotton stick and cells migrated to the other side of the chamber were fixed with 70% methanol and stained by haematoxylin.
Procedures of invasion assay were similar to migration assay except for that BioCoat Matrigel Invasion Chambers (BD Biosciences) were used instead of uncoated inserts, and cell number and incubation time were doubled.
Plasmids and lentiviral infection
HSC-3, UM1, SCC-9 and SCC-25 cells with stable expression of hBD-1 were generated using a lentivirus-delivered eukaryote expression plasmid. Human DEFB1 (NM_005218) cDNA was cloned to the pGC-FU-3FLAG-IRES-Puromycin Vector, then lentivirus delivering the DEFB1 gene (pGCHBD-1) or empty vector (pGCcontrol) was prepared (provided by Genechem Corporation Ltd., Shanghai, China). HSC-3 and UM1 cells were infected with either pGCHBD-1 or pGCcontrol, after selection in puromycin, expression of hBD-1 was verified by real-time PCR and ELISA.
Cell proliferation, colony formation and apoptosis assay
HSC-3, UM1, SCC-9 and SCC-25 cells stably transfected with pGCHBD-1 or pGCcontrol plasmids were seeded in 96-well plate at the concentration of 1×103 cells/well. Every 24 hours after seeding, relative number of living cell was assessed using Cell Counting Kit-8 (CCK-8, Dojindo). Meanwhile, HSC-3, UM1, SCC-9 and SCC-25 cells were treated with 50 µg/ml recombinant hBD-1 (Peprotech), and cell viability with or without hBD-1 treatment was also assessed by CCK-8 assay.
For colony formation assay, cells were seeded in 6-well plate at the concentration of 3×102 cells/well. 14 days after seeding, cells were fixed with 4% paraformldehyde and then stained with crystal violet. Colony formation rate = colony number/cells seeded×100%.
The effect of exogenous expression of hBD-1 on apoptosis of HSC-3, UM1, SCC-9 and SCC-25 cells was assessed by Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) double staining. Briefly, 48 hours after transfection with pGCHBD-1 or pGCcontrol plasmids, 2×105 trypsinezed cells from each group were collected and stained according to the instructions of the Annexin V-FITC Apoptosis Detection Kit (R&D Systems) and analyzed by a flow cytometer (Beckman Coulter). The data was presented as dot plots showing fluorescence intensity of Annexin-V FITC and PI. The percentage of apoptotic cells (Annexin-V+/PI− and Annexin-V+/PI+) was calculated.
Western blot
Proteins from each group were extracted and applied to western blot analysis as described elsewhere [28], with primary antibodies specific for vimentin (mouse monoclonal, 1∶500, Abcam), RhoA (mouse monoclonal, 1∶250, Abcam), RhoC (rabbit polyclonal, 1∶500, Abcam) and MMP-2 (rabbit polycolonal, 1∶500, Abcam). GAPDH antibody (rabbit polycolonal, 1∶5,000, Trevigen) was used as an internal control.
Tissue microarray
In order to verify the clinicopathological significance of hBD-1 expression in a large group of OSCC patients, tissue microarrays (TMA) were constructed with formalin-fixed, paraffin-embeded tissue blocks from 175 patients with primary OSCC. In brief, the area for tissue cores was selected under microscope with a corresponding section with Hematoxylin & Eosin staining. Tissue cores (diameter, 1.5 mm) were taken from each donor block and then placed into a receiver paraffin block using a special tissue arrayer. The TMA block was then cut into 4 µm sections and used for immunohistochemical staining of hBD-1 protein as described above.
Statistical analysis
Statistical Package for Social Science (SPSS) version 17.0 for windows was used to analyze the data. Student's t test was used to compare the difference of migration, invasion, proliferation, colony formation of cells from different groups. Fisher's exact test was used to analyze the independence of hBD-1 expression and clinicopathological characteristics. The Kaplan-Meier survival analysis was applied to estimate cancer-specific survival rates, the difference between different groups were examined using log-rank test. Factors associated with the prognosis were analyzed using Cox proportional hazard regression model with a step-down mode. Receiver operating characteristic (ROC) curve analysis was used to determine the discrimination of hBD-1 expression as a predictor for 3-year survival of patients with OSCC. For all the statistical analysis, P value less than 0.05 was considered significant.
Results
HBD-1 expression decreased from oral precancerous lesions to OSCC and was lower in OSCC with lymph node metastasis than those without metastasis
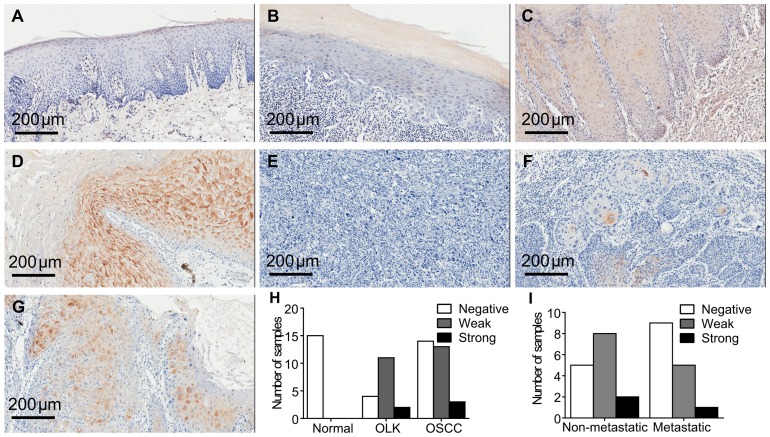
To confirm the expression pattern of hBD-1 in different stages of oral carcinogenesis, we first examined the expression of hBD-1 protein in 15 normal oral mucosa, 17 OLK and 30 OSCC specimens by immunohistochemistry. None of the normal oral mucosa samples showed any detectable hBD-1 expression; while cytoplasmic staining of hBD-1 was detected in some OLK and OSCC samples (Figure 1A–G). There was a higher percentage of hBD-1 staining in OLK (13 in 17 samples) than in OSCC (16 in 30 samples) (P = 0.0.284; Figure 1 H). Furthermore, OSCC without metastasis also had slightly higher percentage of hBD-1 staining (10 in 15 samples) than OSCC with metastasis (6 in 15 samples) (P = 0.338; Figure 1 I).
Figure 1. Immunostaining of hBD-1 protein in different tissue samples.
A) Examples of typical images of hBD-1 in normal oral mucosa (negative); B) OLK (negative); C) OLK (weak); D) OLK (strong); E) OSCC (negative); F) (weak); G) OSCC (strong). H) Number of samples with negative, weak and strong staining of hBD-1 in normal oral mucosa, OLK and OSCC. I) Percentage of negative, weak and strong staining of hBD-1 in OSCC with or without metastasis.
HBD-1 expression in OSCC cell lines was negatively correlated with the migration and invasion ability of OSCC cells
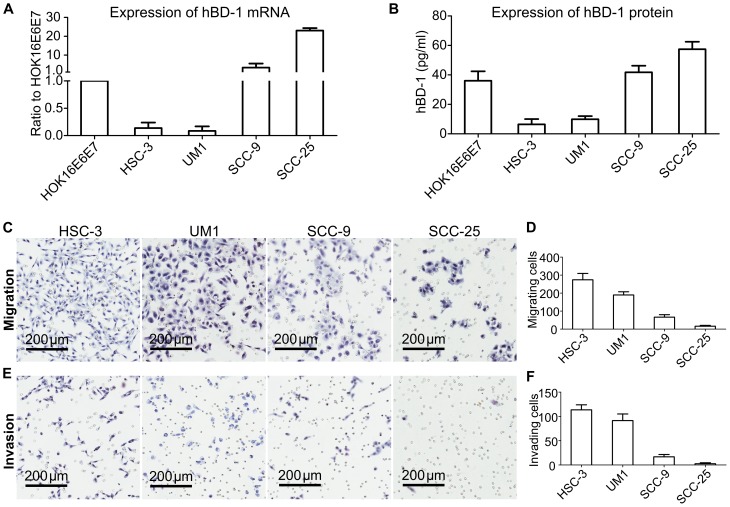
As OSCC samples without metastasis tended to have higher expression of hBD-1, the relationship of hBD-1 with migration and invasion of OSCC cells was suspected. The expression of hBD-1 in four OSCC cell lines with different invasive potentials was examined (Figure 2 A, B), human immortalized oral keratinocyte HOK16E6E7 was used as control. Two OSCC cell lines, HSC-3 and UM1, showed low expression of hBD-1, while cell lines SCC-9 and SCC-25 had relatively higher expression of hBD-1. The migration and invasion potential of these four OSCC cell lines was then analyzed. The results showed that cell lines with higher expression of hBD-1 tended to have lower potential of migration (Figure 2 C, D) and invasion(Figure 2 E, F) in vitro.
Figure 2. OSCC cell lines with low hBD-1 expression tended to have higher invasive potential.
A) hBD-1 mRNA expression level in OSCC cell lines. B) hBD-1 protein expression level in the supernatant of OSCC cell lines. Immortalized oral keratinocyte HOK16E6E7 was used as control. C–D) Assays showing the relative migration ability of OSCC cell lines. Haematoxylin staining (C) and the relative numbers of cells migrated to the lower surface of the cell culture insert (D). E–F) Assays showing the relative invasion ability of OSCC cell lines. Haematoxylin staining (E) and the relative numbers of cells migrated to the lower surface of the matrigel-coated cell culture insert (F). All assays were carried out three times in triplicates.
Exogenous expression of hBD-1 inhibited migration and invasion of OSCC cells but had no significant effect on the proliferation and apoptosis on OSCC cells in vitro
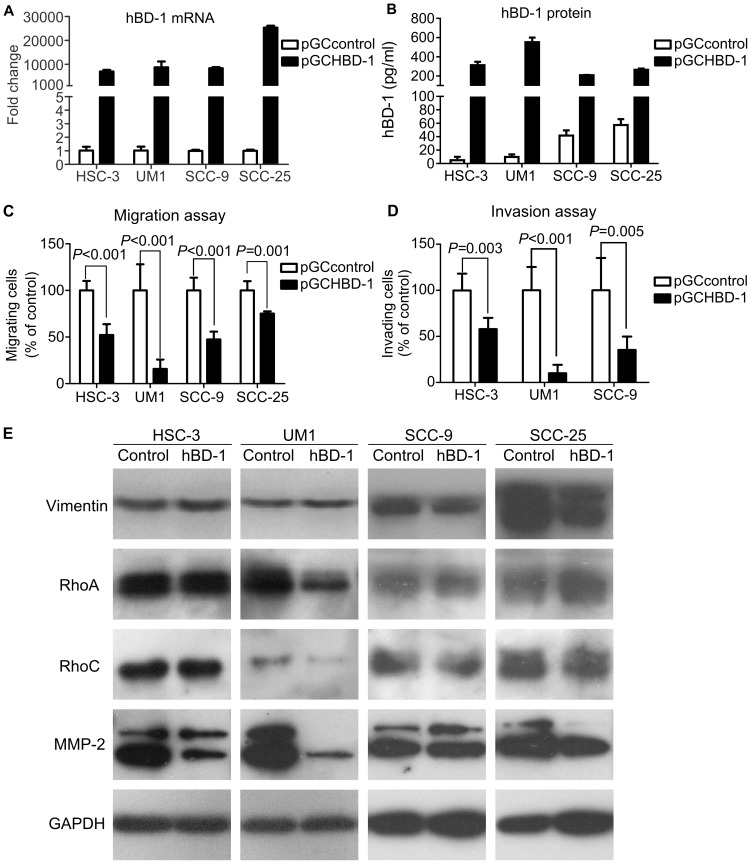
To verify whether hBD-1 would regulate the migration or invasion of OSCC cells in vitro, exogenous expression of hBD-1 was induced by lentivirus-delivered plasmid in HSC-3, UM1, SCC-9 and SCC-25. All 4 cell lines infected by pGCHBD-1 showed significantly increased hBD-1 expression compared with cells infected by pGCcontrol (Figure 3A–B). Migration and invasion assay showed that exogenous expression of hBD-1 significantly suppressed migration of all 4 cell lines (Figure 3 C) and invasion of HSC-3, UM1 and SCC-9 cell lines (Figure 3D). As shown in Figure 2F, SCC-25 cells had very low invasion ability. After infection by pGCHBD-1 or pGCcontrol, very few cells (less than 5 cells per chamber) invaded to the lower surface of the cell culture insert in either group, these data were not included in further analysis.
Figure 3. Effect of exogenous expression of hBD-1 on the migration and invasion of HSC-3, UM1, SCC-9 and SCC-25 cells.
Expression of hBD-1 mRNA (A) and protein (B) was enhanced after transfection with lentivirus-delivered pGCHBD-1 plasmid. C–D) HSC-3, UM1, SCC-9 and SCC-25 cells transfected by pGCHBD-1 displayed inhibited migration (C) and invasion (D). All assays were carried out three times in triplicates. E) Western blotting of tumor migration and invasion associated vimentin, RhoA, RhoC and MMP-2, GAPDH was used as an internal control. After exogenous expression of hBD-1, decreased expression of MMP-2 was observed in HSC-3, UM1 and SCC-25 cell lines, while decreased expression of RhoA and RhoC was only observed in UM1. No change of vimentin expression was observed in these cell lines. Representative blots from three independent experiments.
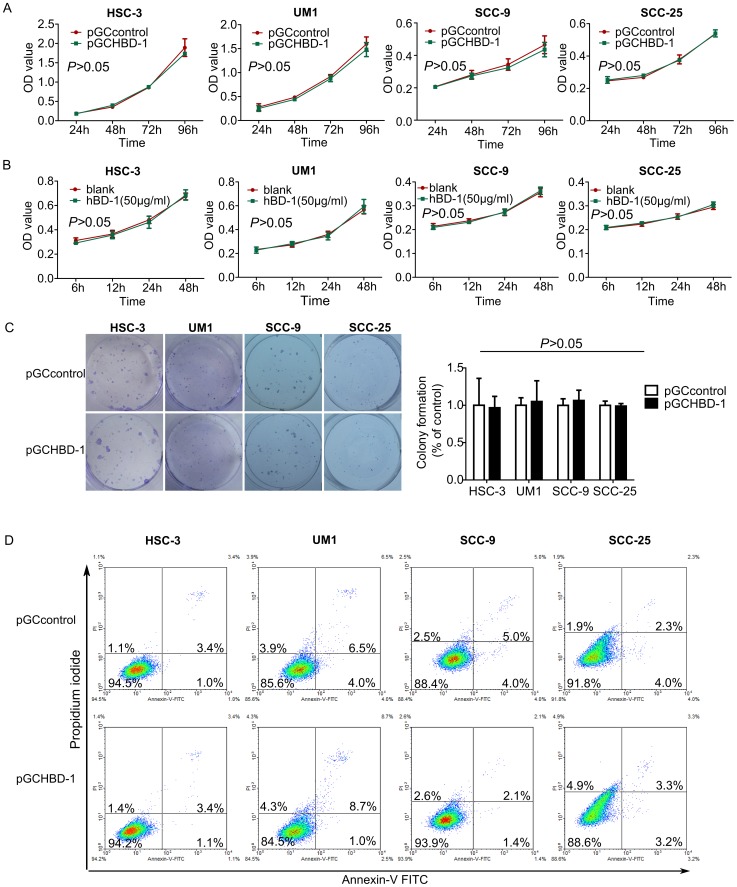
To confirm whether the inhibitory effect of hBD-1 on the migration and invasion of OSCC cells was due to suppression of cell proliferation or induction of apoptosis, the effects of hBD-1 on proliferation and apoptosis of these 4 cell lines were also examined. However, neither exogenous expression of hBD-1 (Figure 4 A) nor treatment by recombinant hBD-1 protein (50 µg/ml) (Figure 4 B) had any significant effect on the proliferation of any cell line. There was no significant difference of colony formation ability between hBD-1 over-expressing cells and control cells (Figure 4 C). HBD-1 expression didn't have any significant influence on apoptosis of these cell lines, either (Figure 4 D).
Figure 4. hBD-1 overexpression showed no significant effect on proliferation of HSC-3, UM1, SCC-9 and SCC-25 cells in vitro.
A) OSCC cells transfected with pGCHBD-1 plasmid showed similar viability compared with control cells in a span of 96 hours. B) Treatment with recombinant hBD-1 protein (50 µg/ml) did not have any significant effect on the viability of HSC-3, UM1, SCC-9 or SCC-25 cells over a period of 48 hours. C) Exogenous expression of hBD-1 did not have any significant effect on colony formation ability of these two cell lines, either (left panel: crystal violet staining; right panel, relative colony number). D) Flow cytometry analysis of cell apoptosis by Annexin-V FITC and PI double staining after transfection with pGCHBD-1 or control plasmid.
To further explore the possible mechanism of the inhibitory effect of hBD-1 on migration and invasion of OSCC cells, a pilot study was carried out to determine the expression of tumor migration and invasion associated proteins after exogenous expression of hBD-1 by western blot. In the current study, vimentin, RhoA, RhoC and MMP-2 was examined (Figure 3 E). UM1 cells transfected with hBD-1 showed decreased expression of RhoA, RhoC and MMP-2 while HSC-3 and SCC-25 cells only showed decreased expression of MMP-2 compared with control cells. There was no substantial change of vimentin expression after exogenous expression of hBD-1.
HBD-1 protein expression in OSCC tissue was an excellent predictor of cancer-specific survival of OSCC patients
Finally, we investigated the prognostic value of hBD-1 expression in a tumor tissue microarray containing tumor tissues from 175 patients with primary OSCC (Figure 5 A). The mean follow-up time of these patients was 35 months (median, 33 months; range 2∼102 months). As shown in Table 1, hBD-1 expression is significantly associated with lymph node status (P = 0.043), but had no association with the tumor size (P = 0.717). Meanwhile, hBD-1 expression was also associated with differentiation status (P<0.001) and smoking history (P = 0.029), but was not associated with gender or age of the patients.
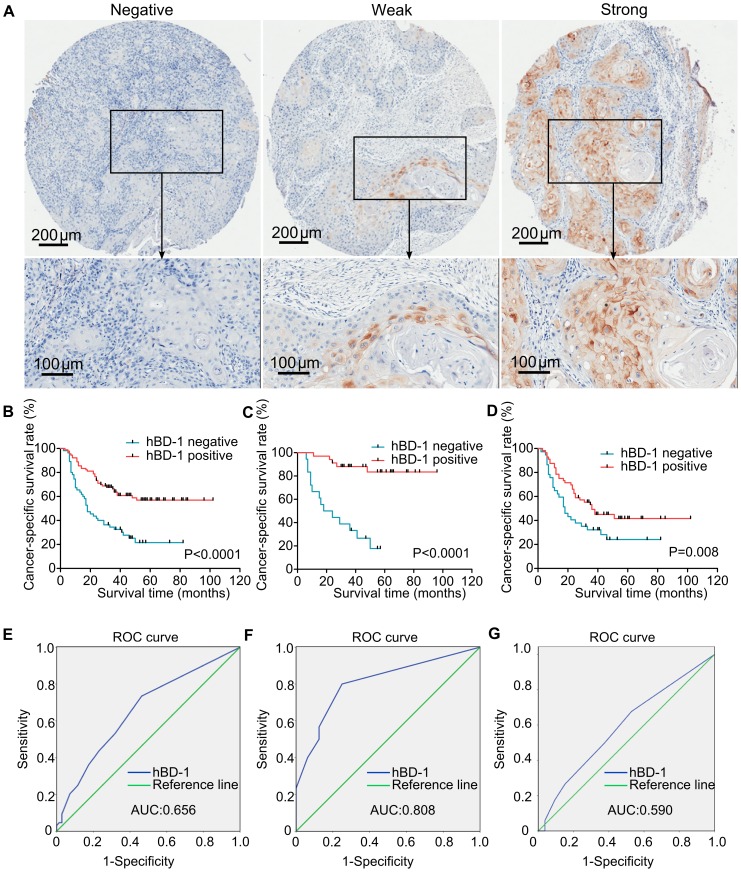
Figure 5. Association of hBD-1 expression with prognosis of OSCC.
A) Examples of negative, weak and strong staining of hBD-1 protein in tissue core from the tissue microarray. The lower panels displayed a magnified vision of the area in the corresponding black box. B–D) Kaplan-Meier analysis displayed cancer-specific survival time of patients with OSCC, and hBD-1 expression was associated with higher survival rate in all OSCC patients (B) as well as subsets of patients with stage I–II OSCC (C) and stage III–IV OSCC (D). E–G) ROC curve analysis of hBD-1 expression as a predictor for 3-year survival of all patients with OSCC (E), patients with stage I–II OSCC (F) and patients with stage III–IV OSCC (G). An area under the ROC curve (AUC) of 0.7–0.9 is considered excellent discrimination, whereas an AUC of 0.5–0.7 is considered moderate discrimination. The ROC curve showed that hBD-1 expression had excellent discrimination for the prediction of 3-year survival in patients with early stage OSCC (stage I–II).
Table 1. Association of hBD-1 expression in OSCC with patients' clinicopathological characteristics (n = 175).
| Total n | Negative n (percentage) | Weak n (percentage) | Strong n (percentage) | P value | |
| Gender | |||||
| Female | 50 | 15 (30.0%) | 24 (48.0%) | 11(22.0%) | 0.065 |
| Male | 125 | 58 (46.4%) | 53 (42.4%) | 14 (11.2%) | |
| Age (year) | |||||
| <60 | 85 | 38 (44.7%) | 39 (45.9%) | 8 (9.4%) | 0.198 |
| ≥60 | 90 | 35 (38.9%) | 38 (42.2%) | 17 (18.9%) | |
| Smoking status | |||||
| Smoker | 97 | 49 (50.5%) | 38 (39.2%) | 10 (10.3%) | 0.029 |
| Nonsmoker | 78 | 24 (30.8%) | 39 (50.0%) | 15 (19.2%) | |
| Differentiation | |||||
| Well | 107 | 29 (27.1%) | 54 (50.5%) | 24 (22.4%) | <0.001 |
| Medium/Poor | 68 | 44 (64.7%) | 23 (33.8%) | 1 (1.5%) | |
| pT | |||||
| T1–T2 | 90 | 35 (38.9%) | 41 (45.5%) | 14 (15.6%) | 0.717 |
| T3–T4 | 85 | 38 (44.7%) | 36 (42.4%) | 11 (12.9%) | |
| pN | |||||
| N0 | 110 | 39 (35.4%) | 51 (46.4%) | 20 (18.2%) | 0.043 |
| N1–Nx | 65 | 34 (52.3%) | 26 (40.0%) | 5 (7.7%) | |
| Tumor stage | |||||
| I | 17 | 5 (29.4%) | 8 (47.1%) | 4 (23.5%) | 0.138 |
| II | 45 | 16 (35.5%) | 21 (46.7%) | 8 (17.8%) | |
| III | 46 | 21 (45.6%) | 20 (43.5%) | 5 (10.9%) | |
| IV | 67 | 31 (46.3%) | 28 (41.8%) | 8 (11.9%) | |
The cumulative survival rate of OSCC patients with weak/strong positive staining of hBD-1 was significantly higher than those with negative hBD-1 staining (P<0.0001 by log-rank test, Figure 5 B). Cox proportional hazard model was used to assess association between cancer-specific survival of OSCC and clinicopathological factors. The hazard ratio (HR) and related 95% confidence interval (CI) of each factor was listed in Table 2. Differentiation, lymph node stage and hBD-1 expression were significantly related with prognosis in univariate analysis and were then selected in a multivariate analysis. In the multivariate analysis, the prognostic value of lymph node status (HR, 2.017; 95% CI, 1.273–3.194; P = 0.003) and hBD-1 expression (HR, 0.382; 95% CI, 0.238–0.615; P<0.001) was confirmed while the others were determined to not be prognostic factors in this cohort of OSCC patients. In order to find out whether the prognostic value of hBD-1 expression was different in different stages of OSCC, a subset analysis was done according to the UICC stages of the patients (stage I–II vs. stage III–IV). Kaplan-Meier analysis showed that OSCC patients with positive hBD-1 expression had longer survival rate in both subgroups (Figure 5 C & D, P<0.001, P = 0.008, respectively.)
Table 2. Cox's Proportional hazard model analysis of prognostic factors in patient with OSCC.
| Variables | HR (95% CI) | Unfavorable/favorable | P |
| Univariate analysis | |||
| Gender | 0.957(0.584–1.570) | Male/Female | 0.863 |
| Age (year) | 0.914(0.731–1.144) | ≥60/<60 | 0.448 |
| Smoking status | 0.841(0.537–1.316) | Smoker/Nonsmoker | 0.448 |
| Differentiation | 1.765(1.121–2.779) | Medium or Poor/Well | 0.014 |
| pT | 1.482(0.944–2.325) | T3–T4/T1–T2 | 0.087 |
| pN | 2.040(1.302–3.196) | N1–Nx/N0 | 0.002 |
| hBD-1 | 0.371(0.236–0.583) | Negative/Positive | <0.001 |
| Multiviriate analysis | |||
| Differentiation | 1.161(0.716–1.882) | Medium or Poor/Well | 0.546 |
| pN | 2.017(1.273–3.194) | N1–Nx/N0 | 0.003 |
| hBD-1 | 0.382(0.238–0.615) | Negative/Positive | <0.001 |
To further validate the prognostic value of hBD-1 expression in OSCC, we next performed ROC curve analysis to determine the discrimination of hBD-1 expression as a predictor for 3-year survival of OSCC patients. The area under the ROC curve (AUC) was determined from the plot of sensitivity and (1-specificity) and was considered as a measure of the predictability of a test. The sensitivity of a test was defined as the true positive rate (patient would be alive after 3 years when the test was positive), while the specificity was defined as the true negative rate (patient would die after 3 years when the test was negative). AUC between 0.7 and 0.9 was considered excellent discrimination, AUC between 0.5–0.7 was considered as moderate discrimination, while AUC of 0.5 was considered as no discrimination [29]. The AUC of hBD-1 was 0.657 (Figure 5 E), which was considered as moderate discrimination. In the subset analysis according to the UICC stages, the AUC of hBD-1 expression was 0.779 for patients with stage I–II OSCC (Figure 5 F); whereas for patients with stage III–IV OSCC, the AUC of hBD-1 expression was 0.589 (Figure 5 G), indicating that hBD-1 expression had an excellent value of discrimination in patients with stage I–II OSCC but only a moderate discrimination in stage III–IV OSCC.
Discussion
HBD-1 is an antimicrobial peptide that is mainly expressed in epithelial tissues. Recently, it has been considered as a potential tumor suppressor in renal cell carcinoma and prostate cancer [17], [18].
In the current study, it was the first time that expression of hBD-1 protein had been analyzed in the oral mucosa at different stages of oral carcinogenesis. Unexpectedly, we did not find a continuous decreasing pattern of hBD-1 expression from normal oral mucosa to OSCC, as displayed in other cancer types [15]–[18]. Instead, there wasn't any detectable expression of hBD-1 protein in all the 15 samples of normal oral mucosa, indicating that hBD-1 was not expressed in normal oral mucosa, or at least was expressed at such a low level that could not be detected by immunohistochemistry. These results were quite different from several previous studies, in which hBD-1 was reported to be constitutively expressed in oral mucosa [7], [19], [21]. However, in most of these studies, only the expression of hBD-1 mRNA was examined, which could not precisely reflect the expression level of hBD-1 protein. Though the expression of hBD-1 in normal oral mucosa was not detectable, it was observed in some of the OLK and OSCC samples, with a decreasing proportion of positive hBD-1 staining from OLK to OSCC. The reason why hBD-1 expression rate increased in precancerous lesions like OLK but then decreased in OSCC remained unclear to us. A previous study by Joly et al. showed that, although hBD-1 expression in oral keratinocytes could not be induced by pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-2, IL-6, IL-8, IL-12 and tumor necrosis factor-α, it could be induced by interferon-γ (IFN-γ) [30]. Other studies using different cell lines also demonstrated that hBD-1 expression could be increased by stimulation of IFN-γ or lipopolysaccharide [31], [32]. Therefore, a possible speculation is that expression of hBD-1 in OLK and OSCC may be a response of the body to inflammatory stimulus. Further study is warranted to prove this hypothesis.
Moreover, the proportion of hBD-1 positive samples in OSCC with metastasis was lower than those without metastasis; hBD-1 expression in OSCC cell lines differed from each other and was negatively associated with the invasive potential of the OSCC cells. Taken together, these findings raised the suspect that hBD-1 might have some regulatory effect on the migration and invasion of OSCC cells. In fact, exogenous expression of hBD-1 by lentivirus-delivered plasmid led to inhibition of migration and invasion of four OSCC cell lines in vitro. The inhibitory effect of hBD-1 on cancer cells, as well as the induction of apoptosis by hBD-1 in a caspase- related way had been reported by Sun [17] and Bullard [18], [33]. However, in the current study, we hadn't observed any significant effect of hBD-1 on the proliferation or apoptosis of OSCC cell lines HSC, UM1, SCC-9 and SCC-25. This is in accord with a previous report, in which Nishimura and colleagues [34] showed that hBD-1 had no effect on the proliferation of OSCC cell line KB (which is actually a derivative of Hela cells [35]) and SCC-9. Although in another report a mild inhibitory effect of hBD-1 on OSCC cell line BHY was observed [24], we could at least inferred from our results that hBD-1 did not have universal inhibitory effect on the proliferation of OSCC cells. These findings were then supported by the clinicopathological data, in which hBD-1 expression was significantly related with lymph node status but not tumor size.
The precise mechanism of how hBD-1 regulates the migration and invasion of OSCC cells is still un-known. In our pilot study, we detected the expression of 4 proteins which were closely related with the invasive potential of tumor cells, including vimentin, RhoA, RhoC and MMP-2 [36]–[40]. Decreased expression of MMP-2 was observed in HSC-3, UM1 and SCC-25 cell lines after exogenous expression of hBD-1, while decreased expression of RhoA and RhoC was only observed in UM1 cells. There wasn't any significant change of the expression of vimentin. These findings suggest that MMP-2, probably RhoA and RhoC may be associated with the regulatory effect of hBD-1 on the migration and invasion of OSCC cells, but further validation is needed.
Another main finding of this study is the significant correlation of hBD-1 expression with cancer-specific survival of OSCC patients. This is the first time that hBD-1 has been reported as a predictor for the prognosis of OSCC, or any type of cancer. Clinicopathological evidence from our TMA indicated that OSCC patients with hBD-1-positive tumors had higher survival rate and longer cancer-specific survival time. Multivariate analysis confirmed hBD-1 expression as an independent prognostic factor of cancer-specific survival besides lymph node status for patients with stage I–II OSCC. The prognostic value of hBD-1 as a predictor for OSCC survival was further validated by ROC curve analysis, in which area under the ROC curve was used as a measure of the predictability of the predictor [29]. Although hBD-1 expression showed only moderate discrimination for the prediction of 3-year survival of OSCC patients as a whole, it did show excellent discrimination in patients with stage I–II OSCC. Taken together, these data suggested that hBD-1 expression was associated with cancer-specific survival of OSCC, and it was a potential predictor for the survival of patients with primary OSCC, especially OSCC at early stage.
In summary, we reported about a decreasing pattern of hBD-1 expression from oral precancerous lesions to OSCC, a relationship between hBD-1 expression and invasive potential of OSCC and an inhibitory effect of exogenous expression of hBD-1 on the migration and invasion of OSCC cells. Moreover, our study is the first to show that hBD-1 expression is associated with cancer-specific of OSCC and can be a potential predictor of survival of early stage OSCC. These results suggested that hBD-1 might inhibit the progression of OSCC by suppressing the migration and invasion of OSCC cells and could be further investigated as a potential therapeutic target as well as a prognostic marker in patients with OSCC.
Supporting Information
Dot blot indicating specificity of anti-hBD-1 antibody. 200 ng of synthetic hBD-1 and hBD-2 were blotted onto the polyvinylidene difluoride (PVDF) membrane (Millipore). Following protein transfer, the membrane was incubated in 5% bovine serum albumin containing anti-hBD-1 antibody (mouse monoclonal, Abcam) followed by horseradish peroxidase conjugated goat anti-mouse antibody and visualized with an ECL detection system (Pierce). The result indicates positive reaction of this antibody against synthetic hBD-1 peptide without cross reaction with hBD-2 peptide.
(TIF)
Acknowledgments
We would like to thank Dr. Xiaofeng Zhou (Center for Molecular Biology of Oral Diseases, College of Dentistry, University of Illinois at Chicago) for providing UM1 cell line and Dr. Xuan Liu (Charles R. Drew University of Medicine and Science) for providing HOK16E6E7 cell line. We also thank Dr. Yuanyuan Liu (West China School of Public Health, Sichuan University) for her help with statistical analysis.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81321002, 30772424, 81200791, 81102060, 81072218), the Doctoral Program of the Ministry of Education of China (No. 20110181110055, 20120181120011) and the ISTCPC (2012DFA31370). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Messadi DV (2013) Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci 5: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Y, Zhu X, Lu R, Dan H, Wang F, et al. (2012) Vesicular stomatitis virus matrix protein (VSVMP) inhibits the cell growth and tumor angiogenesis in oral squamous cell carcinoma. Oral Oncol 48: 110–116. [DOI] [PubMed] [Google Scholar]
- 4. Li J, Gong C, Feng X, Zhou X, Xu X, et al. (2012) Biodegradable thermosensitive hydrogel for SAHA and DDP delivery: therapeutic effects on oral squamous cell carcinoma xenografts. PLoS One 7: e33860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ai JY, Smith B, Wong DT (2012) Bioinformatics advances in saliva diagnostics. Int J Oral Sci 4: 85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J (2006) Human beta-defensins. Cell Mol Life Sci 63: 1294–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA (1998) Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun 66: 4222–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahasrabudhe KS, Kimball JR, Morton TH, Weinberg A, Dale BA (2000) Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J Dent Res 79: 1669–1674. [DOI] [PubMed] [Google Scholar]
- 9. Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, et al. (1998) Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A 95: 14961–14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, et al. (1998) Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 101: 1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ali RS, Falconer A, Ikram M, Bissett CE, Cerio R, et al. (2001) Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J Invest Dermatol 117: 106–111. [DOI] [PubMed] [Google Scholar]
- 12. Zhao C, Wang I, Lehrer RI (1996) Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 396: 319–322. [DOI] [PubMed] [Google Scholar]
- 13. Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, et al. (1997) Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88: 553–560. [DOI] [PubMed] [Google Scholar]
- 14. Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, et al. (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286: 525–528. [DOI] [PubMed] [Google Scholar]
- 15. Donald CD, Sun CQ, Lim SD, Macoska J, Cohen C, et al. (2003) Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas. Lab Invest 83: 501–505. [DOI] [PubMed] [Google Scholar]
- 16. Gambichler T, Skrygan M, Huyn J, Bechara FG, Sand M, et al. (2006) Pattern of mRNA expression of beta-defensins in basal cell carcinoma. BMC Cancer 6: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun CQ, Arnold R, Fernandez-Golarz C, Parrish AB, Almekinder T, et al. (2006) Human beta-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res 66: 8542–8549. [DOI] [PubMed] [Google Scholar]
- 18. Bullard RS, Gibson W, Bose SK, Belgrave JK, Eaddy AC, et al. (2008) Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol Immunol 45: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bissell J, Joly S, Johnson GK, Organ CC, Dawson D, et al. (2004) Expression of beta-defensins in gingival health and in periodontal disease. J Oral Pathol Med 33: 278–285. [DOI] [PubMed] [Google Scholar]
- 20. Dale BA, Kimball JR, Krisanaprakornkit S, Roberts F, Robinovitch M, et al. (2001) Localized antimicrobial peptide expression in human gingiva. J Periodontal Res 36: 285–294. [DOI] [PubMed] [Google Scholar]
- 21. Wenghoefer M, Pantelis A, Dommisch H, Reich R, Martini M, et al. (2008) Decreased gene expression of human beta-defensin-1 in the development of squamous cell carcinoma of the oral cavity. Int J Oral Maxillofac Surg 37: 660–663. [DOI] [PubMed] [Google Scholar]
- 22. Abiko Y, Mitamura J, Nishimura M, Muramatsu T, Inoue T, et al. (1999) Pattern of expression of beta-defensins in oral squamous cell carcinoma. Cancer Lett 143: 37–43. [DOI] [PubMed] [Google Scholar]
- 23. Joly S, Compton LM, Pujol C, Kurago ZB, Guthmiller JM (2009) Loss of human beta-defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol Immunol 24: 353–360. [DOI] [PubMed] [Google Scholar]
- 24. Winter J, Pantelis A, Reich R, Martini M, Kraus D, et al. (2011) Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest 29: 196–201. [DOI] [PubMed] [Google Scholar]
- 25. Sobin LH, Compton CC (2010) TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 116: 5336–5339. [DOI] [PubMed] [Google Scholar]
- 26. Nakayama S, Sasaki A, Mese H, Alcalde RE, Matsumura T (1998) Establishment of high and low metastasis cell lines derived from a human tongue squamous cell carcinoma. Invasion Metastasis 18: 219–228. [DOI] [PubMed] [Google Scholar]
- 27. Nishitani J, Chen Z, Qin M, Liu F, Chen H, et al. (2002) Identification of genes required for immortalization in human papillomavirus-infected human oral keratinocytes. Cell Mol Biol (Noisy-le-grand) 48 Online Pub: OL331-341. [PubMed] [Google Scholar]
- 28. Wang Z, Jiang L, Huang C, Li Z, Chen L, et al. (2008) Comparative proteomics approach to screening of potential diagnostic and therapeutic targets for oral squamous cell carcinoma. Mol Cell Proteomics 7: 1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Zhang B, Jiang L, Zeng X, Chen Y, et al. (2009) RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J Cancer 45: 490–496. [DOI] [PubMed] [Google Scholar]
- 30. Joly S, Organ CC, Johnson GK, McCray PB, Guthmiller JM (2005) Correlation between beta-defensin expression and induction profiles in gingival keratinocytes. Mol Immunol 42: 1073–1084. [DOI] [PubMed] [Google Scholar]
- 31. Sørensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, et al. (2005) Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol 174: 4870–4879. [DOI] [PubMed] [Google Scholar]
- 32. Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH (2002) Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 106: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bose SK, Gibson W, Bullard RS, Donald CD (2009) PAX2 oncogene negatively regulates the expression of the host defense peptide human beta defensin-1 in prostate cancer. Mol Immunol 46: 1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishimura M, Abiko Y, Kurashige Y, Takeshima M, Yamazaki M, et al. (2004) Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci 36: 87–95. [DOI] [PubMed] [Google Scholar]
- 35. Masters JR (2002) HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer 2: 315–319. [DOI] [PubMed] [Google Scholar]
- 36. Hu L, Lau SH, Tzang CH, Wen JM, Wang W, et al. (2004) Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene 23: 298–302. [DOI] [PubMed] [Google Scholar]
- 37. Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, et al. (2008) RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res 68: 7613–7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam M, Lin G, Brenner JC, Pan Q, Merajver SD, et al. (2009) RhoC expression and head and neck cancer metastasis. Mol Cancer Res 7: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Struckhoff AP, Rana MK, Worthylake RA (2011) RhoA can lead the way in tumor cell invasion and metastasis. Front Biosci 16: 1915–1926. [DOI] [PubMed] [Google Scholar]
- 40. Mendes O, Kim HT, Lungu G, Stoica G (2007) MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis 24: 341–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dot blot indicating specificity of anti-hBD-1 antibody. 200 ng of synthetic hBD-1 and hBD-2 were blotted onto the polyvinylidene difluoride (PVDF) membrane (Millipore). Following protein transfer, the membrane was incubated in 5% bovine serum albumin containing anti-hBD-1 antibody (mouse monoclonal, Abcam) followed by horseradish peroxidase conjugated goat anti-mouse antibody and visualized with an ECL detection system (Pierce). The result indicates positive reaction of this antibody against synthetic hBD-1 peptide without cross reaction with hBD-2 peptide.
(TIF)