Abstract
Objectives
Glycated hemoglobin (HbA1c) is associated with an increased risk of cardiovascular disease. The aim of this study was to examine the relationship between HbA1c levels and the complexity of coronary artery lesions among the older patients with diabetes mellitus (DM).
Methods
This retrospective study enrolled a total of 3805 consecutive type 2 DM patients aged 60 years and older who underwent their first elective coronary angiography and had their HbA1c levels measured at the Chinese PLA General Hospital between December 2005 and December 2012.The complexity of the coronary artery lesions was evaluated using the Syntax score, and the subjects were divided into three groups according to their HbA1c levels. Logistic regression and Pearson correlation were used to analyze the association between the measured HbA1c levels and Syntax score.
Results
The mean age was 72.3±10.6 years. The higher HbA1c levels were significantly associated with higher Syntax score (p<0.001). The unadjusted correlation coefficient of HbA1c levels and the Syntax score was 0. 371 (p<0.001). In addition, the higher HbA1c categories were able to independently predict patients with intermediate or high Syntax score (Syntax score ≥23) after adjustment for age, sex, hypertension, smoking, dyslipidemia and creatinine levels in the logistic regression analysis.
Conclusion
HbA1c is significantly associated with the complexity of coronary lesions among older patients with DM. A higher HbA1c value is an independent predictor of the prevalence of complex coronary lesions. Further prospective multi-centre studies are needed to confirm this finding.
Introduction
Diabetes mellitus (DM) is an important risk factor for coronary heart disease [1], and it is associated with a high prevalence of coronary artery disease (CAD) and an unfavorable prognosis [2]. Glycated hemoglobin (HbA1c) reflects the average blood glucose concentrations over the preceding 2 to 3 months [3]. Compared with the fasting blood glucose test, HbA1c has several advantages: it has higher repeatability [3], [4], can be assessed in the non-fasting state, and the committee highlighted that HbA1c is a more convenient test, with less biological variability and greater stability [5]. HbA1c levels may be of prognostic value with regard to future cardiovascular disease [6]. Several previous studies have demonstrated positive correlations of HbA1c with mortality and even subclinical cardiovascular disease in subjects without a history of diabetes [7]–[10]. Selvin et al. demonstrated that the HbA1c was also a strong predictor of future DM, cardiovascular disease, and all cause of mortality [11]. Moreover, DM is a major health problem for the aging population. Ageing is another factor that contributes to variance in the HbA1c and diabetes risk [12]. In elderly patients, the fear of iatrogenic hypoglycemia makes achievement of optimal glycemic control and HbA1c levels complex and generally only partially successful.
The Syntax score is an angiographic grading system based on the severity and complexity of the characteristics of coronary artery lesions [13], [14]. This system is widely accepted as a CAD complexity marker, and its prognostic value has been demonstrated in different clinical situations, and patients with the higher Syntax score have significantly more major adverse cardiac events (MACE) [15], [16].
The benefits of intensive therapy in an effort to lower HbA1c level should always be weighed against the greater risk of disabling and unpredictable hypoglycemia among older patients with DM, especially in elderly patients. No clinical trial data that exclusively represents the value of strict glycemic control in the geriatric population are available. Therefore, the aim of the present study was to evaluate the relationship between HbA1c levels and complexity of coronary artery lesions among older patients with DM.
Methods
Study population
We retrospectively reviewed the data of consecutive type 2 DM patients aged 60 years and older who underwent their first elective coronary angiography, to evaluate suspected CAD, at the Chinese PLA General Hospital between December 2005 and December 2012. Their medical history, lipid levels, creatinie levels, smoking status, demographic and angiographic data prior to undergoing coronary angiography were obtained from the hospital database. The indications for angiography in individuals in clinically stable condition were chest pain and/or noninvasive test results consistent with myocardial ischemia. We excluded patients whose HbA1c levels were not available during the hospitalization. The HbA1c of reference range for healthy nondiabetic individuals ranges from 4.1% to 6.5%. HbA1c was measured with an immunoassay (Bayer DCA-2000, Germany) during this time. Subjects with age <60 years, known type 1 DM, any previous history of coronary revascularization, or incomplete laboratory measurements were also excluded, resulting in a cohort of 3805 subjects for the present analysis. Patients were divided into three groups according to their HbA1c levels: 1535 patients with HbA1c levels of <6.4% (group 1), 1195 patients with HbA1c levels of 6.5% to 8.5% (group 2) and 1075 patients with HbA1c levels higher than 8.5% (group 3).
Ethics Statement
The written informed consents were obtained from all subjects or their designated relatives. The Institutional Review Board of the PLA General Hospital approved this retrospective study.
Syntax score and angiographic analysis
The severity of coronary artery lesions was quantified with the Syntax score. From the baseline diagnostic angiogram, each coronary lesion producing ≥50% diameter stenosis in vessels ≥1.5 mm was scored separately and added together to provide the overall Syntax score, which was calculated using the Syntax score algorithm[13], [14]. Coronary angiograms were analyzed by two experienced observers who were blinded to the identities and clinical information of the patients. Consistent with the SYNTAX trial, the low, intermediate and high Syntax score were defined as 0 to 22, 23 to 32 and 33 or more, respectively [17].
Statistical analysis
The continuous variables are presented as the means ± SDs. The categorical variables are presented as proportions (percentages). Intergroup differences of continuous variables were analyzed by one-way ANOVA. Categorical variables were compared with χ2 tests. Linear regression analysis with Pearson's coefficient was used to assess the strength of association between HbA1c and Syntax score. Logistic regression analysis was used to predict the prevalence of an intermediate or high Syntax score, with adjustment in the age, sex, hypertension, smoking, dyslipidemia, and creatinine levels. All data were processed using the PASW (version 18.0; SPSS, Chicago, IL). A p-value <0.05 was considered to be significant.
Results
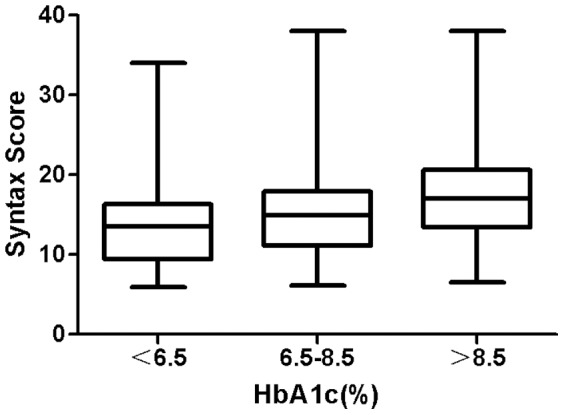
Of the 3805 patients included in this study population, the mean age was 72.3±10.6 years. The subjects were divided into three groups: group I (HbA1c <6.5%); group II (6.5%≤HbA1c≤8.5%); and group III (HbA1c>8.5%). Baseline characteristics and laboratory data of the patients are summarized in Table 1. There were no significant differences in the proportion of male, sex, smoking and creatinine found among the three groups. In contrast, Syntax score was significantly different among the HbA1c groups, p<0.001(Fig 1). In addition, the higher HbA1c category was significantly associated with age, a higher proportion of male sex and hypertention, higher low density lipoprotein (LDL) cholesterol and triglyceride, and lower high density lipoprotein (HDL) cholesterol.
Table 1. Baseline characteristics and laboratory data of the type 2 DM patients aged 60 years and older.
| HbA1c Groups | ||||
| I(<6.5%) | II (6.5%≤HbA1c≤8.5%) | III (>8.5%) | p Value | |
| n = 1535 | n = 1195 | n = 1075 | ||
| Age,years (mean±SD) | 66.4±7.9 | 73.3±10.2 | 79.4±10.5 | <0.001 |
| Sex,male/female | 862/673 | 643/552 | 468/407 | 0.3299 |
| Smoking,% | 38.6 | 34.9 | 36.3 | 0.1320 |
| Hypertention,% | 23.1 | 47.9 | 65.3 | <0.001 |
| Creatinine (mg/dl) | 1.34±0.36 | 1.37±0.46 | 1.37± 0.53 | 0.129 |
| LDL cholesterol (mg/dl) | 107.9±9.9 | 113.1±10.6 | 117.2±10.3 | <0.001 |
| HDL cholesterol (mg/dl) | 43.6±6.2 | 41.3±5.2 | 38.3±4.1 | <0.001 |
| Triglyceridea (mg/dl) | 148.7±9.2 | 151.5±9.2 | 154.8±9.0 | <0.001 |
| Syntax score | 13.3±4.3 | 14.8±4.9 | 17.4±5.7 | <0.001 |
LDL: low density lipoprotein; HDL: high density lipoprotein.
Figure 1. Syntax score according to HbA1c levels in type 2 DM patients aged 60 years and older.
The HbA1c levels and Syntax score were correlated (Fig 2) (r = 0.371; p<0.001). Moreover, the higher HbA1c categories were able to independently predict patients with intermediate or high Syntax score (Syntax score≥23) after the age, sex, hypertension, smoking, dyslipidemia, and creatinine levels were adjusted in the logistic regression analysis (Table 2).
Figure 2. Correlation between HbA1c levels and Syntax score in type 2 DM patients aged 60 years and older.
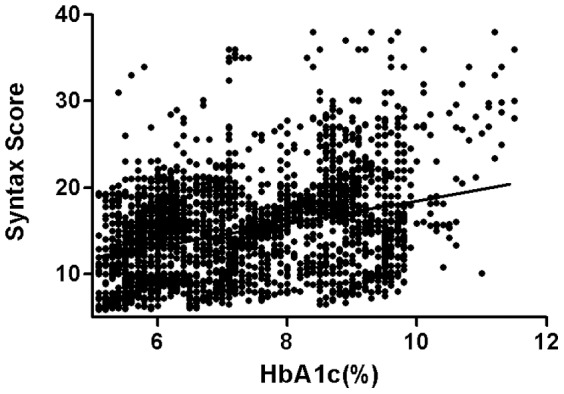
Table 2. Adjusted odds ratios for prediction of high Syntax score patients.
| Risk factors | Adjusted Odds Ratio | p Value |
| (95% Confidence Interval) | ||
| Age, years | 1.03(1.013–1.049) | 0.0005 |
| Sex, male/female | 0.92(0.689–1.240) | 0.598 |
| Smoking,% | 1.00(0.736–1.354) | 0.9907 |
| Hypertention,% | 0.91(0.599–1.381) | 0.6577 |
| Creatinine (mg/dl) | 0.89(0.672–1.175) | 0.406 |
| LDL cholestorol (mg/dl) | 0.98(0.965–0.999) | 0.0416 |
| HDL cholestorol (mg/dl) | 1.00(0.965–1.037) | 0.9847 |
| Triglyceridea (mg/dl) | 0.98(0.956–1.011) | 0.2234 |
| HbA1c,% | 4.95(3.625–6.757) | 0.0000 |
LDL: low density lipoprotein; HDL: high density lipoprotein.
Discussion
DM has been associated with the extent of coronary atherosclerosis measured by coronary angiography and coronary multi-detector computed tomography [18]–[20]. In light of the severity of CAD in diabetic patients, it is necessary to take measures to prevent or delay its occurrence and development. However, in older patients, the benefits of intensive therapy to lower HbA1c level must always be weighed against the greater risk of disabling and unpredictable hypoglycemia, considering that the geriatric population is less likely to benefit from reducing the risk of microvascular complications and more likely to suffer serious adverse effects from hypoglycemia [21].
The Syntax score, can be especially useful in deciding the optimal treatment strategy in patients with complex lesions [15]. In particular, it is a useful tool to guide decision-making in patients undergoing three-vessel disease and left main percutaneous coronary intervention [22]. Zhao et al. recommend the use the clinical SYNTAX score for routine clinical decision-making [23]. In addition, the Syntax score has been shown to be an independent predictor of mortality and MACE at long-term follow-up [15], [16], [24]–[30].HbA1c is a useful marker of cardiovascular risk, even more superior to fasting glucose for long-term macrovascular risk stratification [9].
The principal findings of our study indicate that HbA1c level is associated with coronary lesion complexity in older diabetic patients. Previous studies have found an association between elevated cardiovascular risk and elevated HbA1c level [7], [8], [31]–[37], independent of classical risk factors [38]. Chronic hyperglycemia is associated with an increased risk for cardiovascular outcomes and all-cause mortality among patients with type 2 diabetes [39]. Nishimura et al. suggested that the control of HbA1c, was necessary to reduce the cardiovascular risk in diabetic patients with elevated HbA1c [40]. However, some clinical trials have shown little benefit, and possibly some harm, of lowering the glycated hemoglobin value in patients with DM to prevent cardiovascular outcomes [41]–[46]. In addition, those studies did not investigate the coronary artery lesion morphology quantified with the Syntax score among the exclusive older DM patients. Therefore, the aim of the present study was to evaluate the relationship between HbA1c levels and complexity of coronary artery lesions among older patients with DM. Also, this retrospective, prevalence study is quite a stretch in view of the failure of large randomized trials to demonstrate a beneficial effect.
In the present study, we found that HbA1c is an independent predictor of the prevalence of complex coronary artery lesions (Syntax score ≥23) in older diabetic patients. We established that the HbA1c level is significantly associated with the complexity of coronary lesions, independent of age, sex, and other cardiovascular risk factors such as hypercholesterolemia, hypertension, and smoking. Our findings indicate that the severity of coronary artery lesions is also notably influenced by the HbA1c, irrespective of other cardiovascular risk factors, age, and gender. Even after adjustment for these factors, HbA1c was still significantly associated with Syntax score. Additionally, the multivariate regression analysis showed that HbA1c is independently associated with Syntax score. Our study therefore demonstrated that, among older DM patients, HbA1c values can be a predictor of the prevalence of complex coronary artery lesions. The patients with the lower HbA1c levels have a distinctly lower risk of complex coronary artery lesions. Therefore, diabetic patients with cardiovascular risk factors should pay more attention to their blood glucose levels and potential cardiovascular complications, even among older patients with DM. Furthermore, the relationship between the Syntax score and HbA1c demonstrated in this study supports using HbA1c as a simplified indicator of prognosis. This study suggest that an improvement in glycemic control results in more favorable intermediate or long-term clinical outcomes in diabetic patients aged 60 years and older. However, the relationship between glycemic control and coronary atherosclerosis is not a simple one. There are many unmeasured potentially important covariates that may play a role. Maybe, for example, the duration of the diabetic process leads to both worse coronary atherosclerosis and higher HbA1c. As reported in a study, they found that interwoven actions of poor glycemic control, low grade inflammation and low HDL-cholesterol on atherosclerotic processes in type 2 diabetes [47]. Therefore, further studies are necessary to investigate the relation between HbA1c and Syntax score among older diabetic patients.
There were several limitations in this study which should be considered. This study was a retrospective and non-randomized study. First, our patients are only those who underwent first elective coronary angiography. These patients comprise some patients with CAD and may not represent all patients with CAD. Second, several confounding factors may not have been properly accounted for in the analysis. Consequently, numerous patients were excluded, and selection bias may have affected the results. Third, the present study excluded patients with a history of PCI or CABG, therefore our findings may not apply to these excluded subjects who represent more severe cases. We also did not collect information on severe obesity, peripheral vascular disease, cerebrovascular disease, estimated glomerular filtration rate, or duration of diabetes, which were also associated with the burden of vascular atherosclerosis. Thus, we should interpret the results very carefully. Finally, our study is a single-center observational experience. Most of the patients came from Beijing and its neighboring areas, so their glucose metabolic state may not completely be in coincidence with population of other places. Further prospectively multiple centre studies are required to better quantify this finding. Although caution is necessary for the interpretation of our data, we consider it improbable that these limitations have influenced our main findings.
In conclusion, the HbA1c is significantly associated with the complexity of coronary lesions amongst type 2 DM patients aged 60 years and older. The present study reveals that higher HbA1c value is an independent predictor of the prevalence of complex coronary lesions, even after adjusting the age and other coronary atherosclerotic risk factors. HbA1c may be a useful indicator of type 2 DM in patients aged 60 years and older with greatest absolute risk of cardiovascular disease. HbA1c could be used as a simple prognostic value after primary PCI in coronary care unit, and it also helps for the strict control of complications and preventive strategies. Control of glycemic metabolism may play an important role in reducing the HbA1c levels. For patients without hypoglycemia, further study is necessary to evaluate the benefit of intensive treatment of glycometabolic disorder to reduce the HbA1c level.
Funding Statement
The authors have no support or funding to report.
References
- 1. Silbernagel G, Rosinger S, Grammer TB, Kleber ME, Winkelmann BR, et al. (2012) Duration of type 2 diabetes strongly predicts all-cause and cardiovascular mortality in people referred for coronary angiography. Atherosclerosis 221: 551–557. [DOI] [PubMed] [Google Scholar]
- 2. Saleh N, Petursson P, Lagerqvist B, Skúladóttir H, Svensson A, et al. (2012) Long-term mortality in patients with type 2 diabetes undergoing coronary angiography: the impact of glucose-lowering treatment. Diabetologia 55: 2109–2117. [DOI] [PubMed] [Google Scholar]
- 3. Selvin E, Crainiceanu CM, Brancati FL, Coresh J (2007) Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 167: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 4. Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, et al. (2002) Biological variation of glycohemoglobin. Clin Chem 48: 1116–1118. [PubMed] [Google Scholar]
- 5. The International Expert Committee (2009) International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, et al. (2005) Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 26: 650–661. [DOI] [PubMed] [Google Scholar]
- 7. Levitan EB, Liu S, Stampfer MJ, Cook NR, Rexrode KM, et al. (2008) HbA1c measured in stored erythrocytes and mortality rate among middle-aged and older women. Diabetologia 51: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, et al. (2008) CHARM Program Investigators. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med 168: 1699–1704. [DOI] [PubMed] [Google Scholar]
- 9. Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, et al. (2010) Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med 25 7: e1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNeely MJ, McClelland RL, Bild DE, Jacobs DR Jr, Tracy RP, et al. (2009) The association between A1C and subclinical cardiovascular disease: the multi-ethnic study of atherosclerosis. Diabetes Care 32: 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, et al. (2010) Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, et al. (2012) Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, et al. (2005) The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. Eurointervention 1: 219–227. [PubMed] [Google Scholar]
- 14. Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, et al. (2009) Assessment of the SYNTAX score in the Syntax study. Eurointervention 5: 50–56. [DOI] [PubMed] [Google Scholar]
- 15. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, et al. (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360: 961–972. [DOI] [PubMed] [Google Scholar]
- 16. Serruys PW, Onuma Y, Garg S, Vranckx P, De Bruyne B, et al. (2010) 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol 55: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 17. Morice MC, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, et al. (2010) Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation 121: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 18. Saely CH, Drexel H, Sourij H, Aczel S, Jahnel H, et al. (2008) Key role of postchallenge hyperglycemia for the presence and extent of coronary atherosclerosis: an angiographic study. Atherosclerosis 199: 317–322. [DOI] [PubMed] [Google Scholar]
- 19. Rizza S, Cardellini M, Martelli E, Porzio O, Pecchioli C, et al. (2010) Occult impaired glucose regulation in patients with atherosclerosis is associated to the number of affected vascular districts and inflammation. Atherosclerosis 212: 316–320. [DOI] [PubMed] [Google Scholar]
- 20. Yun CH, Schlett CL, Rogers IS, Truong QA, Toepker M, et al. (2009) Association between diabetes and different components of coronary atherosclerotic plaque burden as measured by coronary multidetector computed tomography. Atherosclerosis 2009 205: 481–485. [DOI] [PubMed] [Google Scholar]
- 21. Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, Teixeira AM, et al. (2012) Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Capodanno D, Tamburino C (2012) Does the SYNTAX score get on your nerves? Practical considerations on how and when avoiding it to maximize its usefulness with no waste of time. Int J Cardiol 159: 165–168. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Stampf S, Valina C, Kienzle RP, Ferenc M, et al. (2013) Role of euro SCORE II in predicting long-term outcome after percutaneous catheter intervention for coronary triple vessel disease or left main stenosis. Int J Cardiol http://dx.doi.org/10.1016/j.ijcard.2013.04. 136. [DOI] [PubMed]
- 24. Valgimigli M, Serruys PW, Tsuchida K, Vaina S, Morel MA, et al. (2007) Cyphering the complexity of coronary artery disease using the SYNTAX score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol 99: 1072–1081. [DOI] [PubMed] [Google Scholar]
- 25. Capodanno D, Di Salvo ME, Cincotta G, Miano M, Tamburino C, et al. (2009) Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv 2: 302–308. [DOI] [PubMed] [Google Scholar]
- 26. Garg S, Serruys PW, Silber S, Wykrzykowska J, van Geuns RJ, et al. (2011) The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All Comers trial. J Am Coll Cardiol. Cardiovasc Interv 4: 432–441. [DOI] [PubMed] [Google Scholar]
- 27. Wykrzykowska JJ, Garg S, Girasis C, de Vries T, Morel MA, et al. (2010) Value of the SYNTAX score for risk assessment in the all-comers population of the randomized multicenter LEADERS (Limus Eluted From a Durable Versus Erodable Stent Coating) trial. J Am Coll Cardiol 56: 272–277. [DOI] [PubMed] [Google Scholar]
- 28. Garg S, Sarno G, Serruys PW, Rodriguez AE, Bolognese L, et al. (2011) STRATEGY and MULTISTRATEGY Investigators. Prediction of 1-year clinical outcomes using the SYNTAX score in patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: a substudy of the STRATEGY (Single High-Dose Bolus Tirofiban and Sirolimus-Eluting Stent Versus Abciximab and Bare-Metal Stent in Acute Myocardial Infarction) and MULTISTRATEGY (Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction Study) trials. J Am Coll Cardiol Intv 4: 66–75. [DOI] [PubMed] [Google Scholar]
- 29. Garg S, Sarno G, Girasis C, Vranckx P, de Vries T, et al. (2011) A patientlevel pooled analysis assessing the impact of the SYNTAX (synergy between percutaneous coronary intervention with taxus and cardiac surgery) score on 1-year clinical outcomes in 6508 patients enrolled in contemporary coronary stent trials. JACC Cardiovasc Interv 4: 645–653. [DOI] [PubMed] [Google Scholar]
- 30. van Gaal WJ, Ponnuthurai FA, Selvanayagam J, Testa L, Porto I, et al. (2009) The Syntax score predicts peri-procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol 135: 60–65. [DOI] [PubMed] [Google Scholar]
- 31. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, et al. (2004) Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 141: 421–431. [DOI] [PubMed] [Google Scholar]
- 32. Silbernagel G, Grammer TB, Winkelmann BR, Boehm BO, März W (2011) Glycated hemoglobin predicts all-cause, cardiovascular, and cancer mortality in people without a history of diabetes undergoi0ng coronary angiography. Diabetes Care 34: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynn Fillipon NM, Kitkungvan D, Dani SS, Downey BC (2012) The relationship between glycosylated hemoglobin and myocardial perfusion imaging. Clin Cardiol 35: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikeda N, Iijima R, Hara H, Moroi M, Nakamura M, et al. (2012) Glycated hemoglobin is associated with the complexity of coronary artery disease, even in non-diabetic adults. J Atheroscler Thromb 19: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 35. Pradhan AD, Rifai N, Buring JE, Ridker PM (2007) Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med 120: 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brewer N, Wright CS, Travier N, Cunningham CW, Hornell J, et al. (2008) A New Zealand linkage study examining the associations between A1C concentration and mortality. Diabetes Care 31: 1144–1149. [DOI] [PubMed] [Google Scholar]
- 37. Lazzeri C, Valente S, Chiostri M, Picariello C, Attanà P, et al. (2011) The prognostic impact of glycated hemoglobin in diabetic ST-elevation myocardial infarction. Int J Cardiol 151: 250–252. [DOI] [PubMed] [Google Scholar]
- 38. Khaw KT, Wareham N (2006) Glycated hemoglobin as a marker of cardiovascular risk. Curr Opin Lipidol 17: 637–643. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Hu G, Yuan Z, Chen L (2012) Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One 7: e42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishimura R, Nakagami T, Sone H, Ohashi Y, Tajima N (2011) Relationship between hemoglobin A1c and cardiovascular disease in mild-to-moderate hypercholesterolemic Japanese individuals: subanalysis of a large-scale randomized controlled trial. Cardiovasc Diabetol 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Action to Control Cardiovascular Risk in Diabetes Study Group (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, et al. (2007) Rosiglitazone evaluated for cardiovascular outcomes-an interim analysis. N Engl J Med 357: 28–38. [DOI] [PubMed] [Google Scholar]
- 43. The ADVANCE Collaborative Group (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 44. Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471. [DOI] [PubMed] [Google Scholar]
- 45. Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, et al. (2008) Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 168: 2070–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singla A, Orshaw P, Boura J, Harjai KJ (2012) Glycosylated hemoglobin and outcomes in diabetic patients with acute myocardial infarction after successful revascularization with stent placement: findings from the guthrie health off-label stent (GHOST) investigators. J Interven Cardiol 25: 262–269. [DOI] [PubMed] [Google Scholar]
- 47. Pacilli A, De Cosmo S, Trischitta V, Bacci S (2013) Role of relationship between HbA1c, fibrinogen and HDL-cholesterol on cardiovascular disease in patients with type 2 diabetes mellitus. Atherosclerosis 228: 247–248. [DOI] [PubMed] [Google Scholar]


