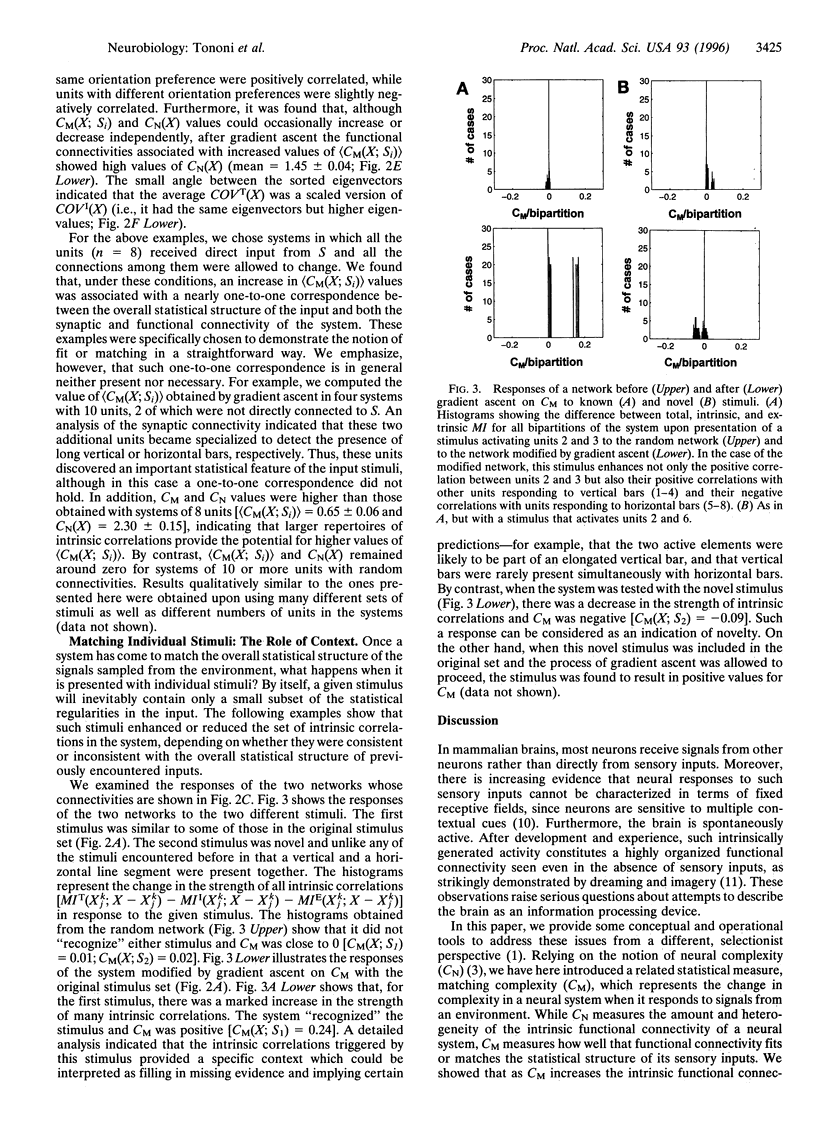
Abstract
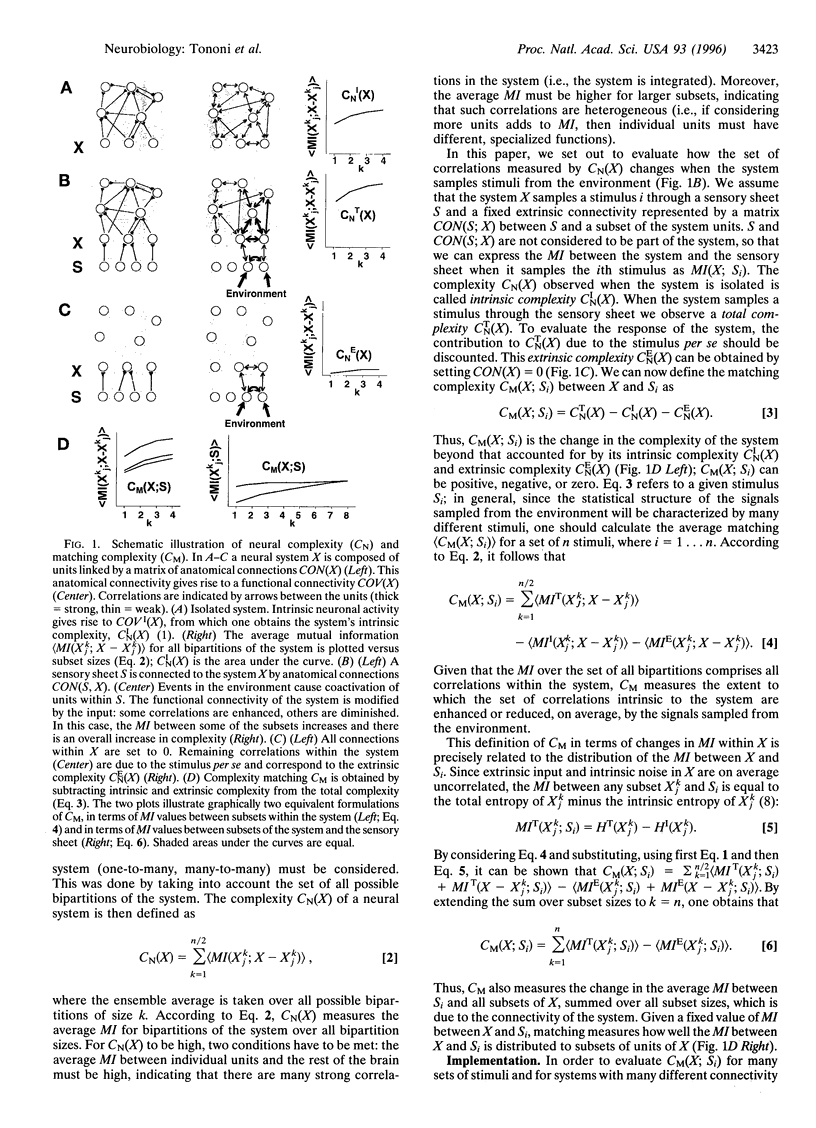
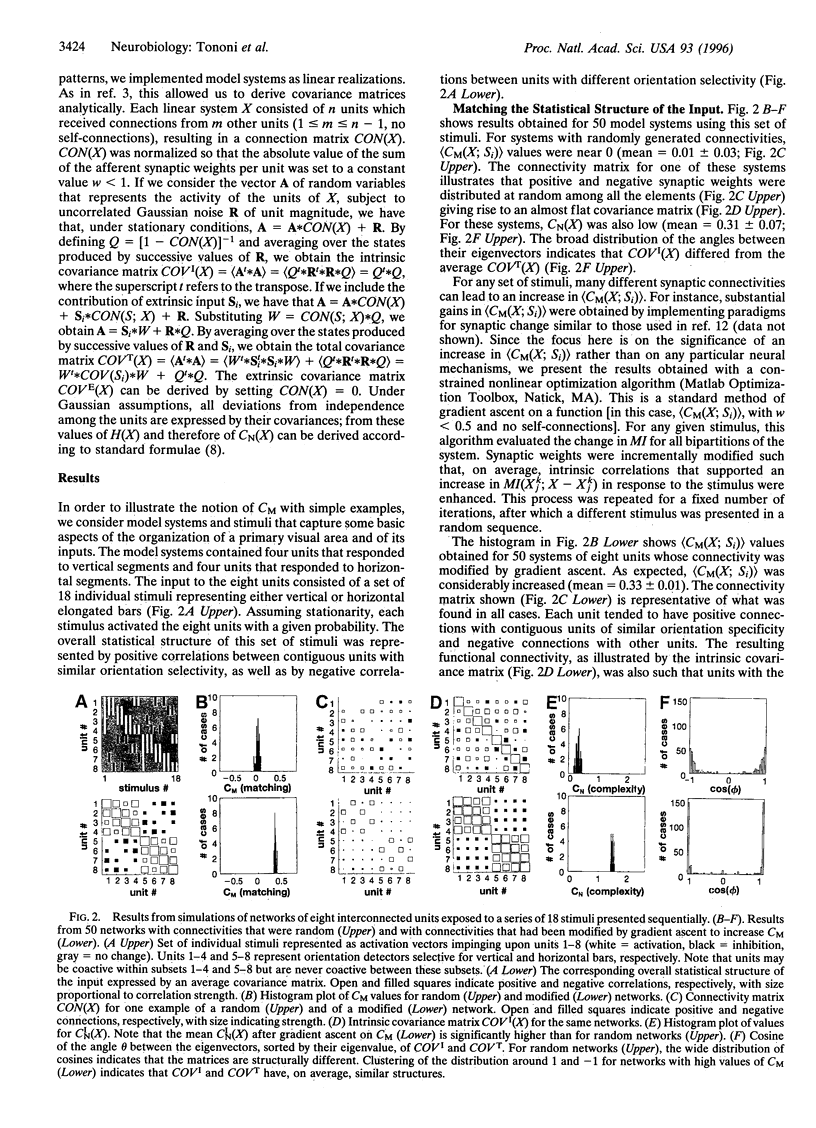
We have previously derived a theoretical measure of neural complexity (CN) in an attempt to characterize functional connectivity in the brain. CN measures the amount and heterogeneity of statistical correlations within a neural system in terms of the mutual information between subsets of its units. CN was initially used to characterize the functional connectivity of a neural system isolated from the environment. In the present paper, we introduce a related statistical measure, matching complexity (CM), which reflects the change in CN that occurs after a neural system receives signals from the environment. CM measures how well the ensemble of intrinsic correlations within a neural system fits the statistical structure of the sensory input. We show that CM is low when the intrinsic connectivity of a simulated cortical area is randomly organized. Conversely, CM is high when the intrinsic connectivity is modified so as to differentially amplify those intrinsic correlations that happen to be enhanced by sensory input. When the input is represented by an individual stimulus, a positive value of CM indicates that the limited mutual information between sensory sheets sampling the stimulus and the rest of the brain triggers a large increase in the mutual information between many functionally specialized subsets within the brain. In this way, a complex brain can deal with context and go "beyond the information given."
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callaway E. M., Katz L. C. Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990 Apr;10(4):1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne T. J., Richmond B. J. How independent are the messages carried by adjacent inferior temporal cortical neurons? J Neurosci. 1993 Jul;13(7):2758–2771. doi: 10.1523/JNEUROSCI.13-07-02758.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44(3):521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Sporns O., Tononi G., Edelman G. M. Modeling perceptual grouping and figure-ground segregation by means of active reentrant connections. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):129–133. doi: 10.1073/pnas.88.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G. M. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G. M. Reentry and the problem of integrating multiple cortical areas: simulation of dynamic integration in the visual system. Cereb Cortex. 1992 Jul-Aug;2(4):310–335. doi: 10.1093/cercor/2.4.310. [DOI] [PubMed] [Google Scholar]