Abstract
Phthalates are extensively used as plasticizers in a variety of daily-life products, resulting in widespread distribution in aquatic environments. However, limited information is available on the endocrine disrupting effects of phthalates in aquatic organisms. The aim of the present study was to examine whether exposure to mono-(2-ethylhexyl) phthalate (MEHP), the hydrolytic metabolite of di-(2-ethylhexyl) phthalate (DEHP) disrupts thyroid endocrine system in fish. In this study, zebrafish (Danio rerio) embryos were exposed to different concentrations of MEHP (1.6, 8, 40, and 200 μg/L) from 2 h post-fertilization (hpf) to 168 hpf. The whole-body content of thyroid hormone and transcription of genes involved in the hypothalamic-pituitary-thyroid (HPT) axis were examined. Treatment with MEHP significantly decreased whole-body T4 contents and increased whole-body T3 contents, indicating thyroid endocrine disruption. The upregulation of genes related to thyroid hormone metabolism (Dio2 and UGT1ab) might be responsible for decreased T4 contents. Elevated gene transcription of Dio1 was also observed in this study, which might assist to degrade increased T3 contents. Exposure to MEHP also significantly induced transcription of genes involved in thyroid development (Nkx2.1 and Pax8) and thyroid hormone synthesis (TSHβ, NIS and TG). However, the genes encoding proteins involved in TH transport (transthyretin, TTR) was transcriptionally significantly down-regulated after exposure to MEHP. Overall, these results demonstrate that acute exposure to MEHP alters whole-body contents of thyroid hormones in zebrafish embryos/larvae and changes the transcription of genes involved in the HPT axis, thus exerting thyroid endocrine toxicity.
Introduction
Phthalate esters are a group of industrial chemicals extensively used as plasticizers in a variety of commercial products, such as polyvinyl chloride (PVC) floors, food packaging, clothing, toys, films, paints, adhesives, lubricants, cosmetics, electronics, ink printers and biomedical devices (e.g., blood transfusion bags) [1], [2]. A recent study estimated that the worldwide production of phthalates reached 11 billion pounds every year [3]. Because they are not chemically bonded to the polymer, phthalates tend to release from the matrix with time and use [4]. Various phthalate esters have been detected in all environmental compartments, including indoor and ambient air, indoor dust, water sources, and sediments [5]. Moreover, phthalates have tendency to bioaccumulate in fish and, eventually entering the food chain [6].
Of the various phthalates, di-(2-ethylhexyl) phthalate (DEHP) is one of the most frequently used phthalates, and it accounts for approximately 50% of total plasticizer production [7]. Due to its large-scale production and widespread use, the general population is continuously exposed to DEHP, mainly via inhalation, ingestion, dermal contact and intravenous routine throughout their whole lifetime, including in the intrauterine environment during pregnancy [8], [9]. Once enters into human body, DEHP is readily metabolized by esterases in the gut to mono-(2-ethylhexyl) phthalate (MEHP), which is more toxic than its parent compound [2]. In addition, MEHP might also be formed out of DEHP by abiotic and biotic processes in natural environment [10]–[13]. For example, MEHP has been detected in intravenous solutions stored in medical grade PVC bags [11], serum and plasma products packed into plastic containers and water from medical grade PVC tubing [12]. Therefore, MEHP is an environmental contaminant of its own, and have been detected in many environmental media. For instance, Suzuki et al., observed MEHP in the Tama River in Tokyo at concentrations of 0.01–1.3 μg/L (2001). A recent study demonstrated that MEHP was detected in all sediment and soil samples ranged from 13.0 to 166.7 ng/g [14]. Moreover, MEHP has been widely detected in human biological samples such as milk, urine, saliva, and serum [15]–[19]. Therefore, the potential health risks caused by DEHP/MEHP are receiving growing public concern.
Since thyroid hormones (THs) are essential for fetal development of the brain and involved in numerous physiological processes, the impact of environmental chemicals on the thyroid endocrine system has received increasing attention in recent years [20]. Chemicals may exert thyroid disrupting effects through disturbing the THs synthesis, secretion, transport, binding, action, or elimination [21]. Studies investigating the association between exposure to DEHP/MEHP and thyroid function are limited. An in vitro study demonstrated that DEHP enhances iodine uptake by modulating the sodium/iodide symporter in rat thyroid cell line [22]. In animal studies, rats administered diets containing DEHP showed lower plasma thyroxine (T4) and histological changes in the thyroid such as reduced follicle size and colloid density, [23]–[26], while rats intravenously receiving DEHP displayed increased concentrations of serum triiodothyronine (T3) and T4 [27]. In humans, increased urinary concentrations of MEHP were associated with decreased concentrations of total T3 and free T4 in the serum of adult men [28], [29]. Similar results were also found in a cross-sectional study conducted in Danish children, which demonstrated that urinary DEHP metabolites were inversely related to total and free T3 levels [30].
Thyroid endocrine disruption are of particular concern to amphibians and teleosts as these animals may be exposed to waterborne contaminants during a portion or the entirety of their life span. In teleosts and amphibians, the homeostasis of circulating THs is regulated through a finely tuned negative feedback mechanism by the hypothalamic-pituitary-thyroid (HPT) axis [31]. In many aspects, the thyroid system of zebrafish is similar to the mammalian or the amphibian thyroid system, and zebrafish are widely used as a vertebrate model in several areas of research with the prospect of extrapolating findings to other vertebrates and humans [32]–[35]. It has been shown that developing zebrafish embryos/larvae were a reliable model to assess chemical disruption of the thyroid endocrine system [36]–[38]. However, the potential thyroid endocrine disrupting effects of MEHP in fish, especially during the early developmental stages has not been fully elaborated up to now. In the present study, we investigated the effects of MEHP on mRNA expression involved in the HPT axis using zebrafish embryos/larvae. Moreover, an enzyme-linked immunosorbent assay (ELISA) was employed to measure whole-body thyroid hormone contents after MEHP exposure.
Materials and Methods
Chemicals
MEHP (CAS No: 4670-20-9, 99%) was purchased from AccuStandard (New Haven, CT, USA). Stock solution of MEHP was prepared by dilution in dimethyl sulfoxide (DMSO) (purity>99%), which was obtained from Amresco (Solon, OH, USA), and was stored at 4°C. 3-Aminobenzoic acid ethyl ester or methane-sulfonate salt (MS-222) was obtained from Sigma (St. Louis, MO, USA). All other chemicals used in this study were of analytical grade.
Fish maintenance and experimental design
Adult zebrafish (Danio rerio) of the wild-type (AB strain) were maintained in a flow-through system in charcoal-filtered tap water at a constant temperature (28±1°C), with a photoperiod 14∶10 (light:dark). Fish were fed a commercial food pellet (Trea, Germany) and newly hatched brine shrimp (Artemia nauplii) two times daily in a quantity that was consumed within 5 minutes. Zebrafish embryos were obtained from spawning adults in a ratio of 2∶1 (male: female) in tanks overnight. Spawning was triggered in the morning when the light was turned on and was usually completed within 30 min. At 2 h post-fertilization (hpf), embryos were examined under a dissecting microscope, and those embryos that had developed normally and reached the blastula stage were selected for subsequent experiments. Fertilization rate of the batch of eggs used was at least 90%. Normal embryos (approximately 400) were randomly distributed into separate glass beakers containing 500 mL of exposure solution (0, 1.6, 8, 40, and 200 μg/L MEHP) containing 0.2 mM Ca (NO3)2, 0.13 mM MgSO4, 19.3 mM NaCl, 0.23 mM KCl and 1.67 mM HEPES [39] until 168 hpf. The selected exposure concentrations were previously ascertained by performing a range-finding study, and the lowest concentration employed in this study was based on an environmental investigation [10]. During the experimental period, the exposure solutions were renewed daily, and both the control and exposure groups with 6 replicates in each exposure concentration were treated with 0.01% (v/v) DMSO. After exposure, zebrafish larvae were anesthetized in 0.03% MS-222 and randomly sampled for body length and weight determination, mRNA expression analysis and thyroid hormones measurement. The hatching, malformation, growth and survival were also recorded.
Ethics Statement
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the 305 Hospital of PLA of China.
Thyroid hormone extraction and measurement
Procedures for thyroid hormone extraction and measurement were performed as described by Yu et al. [38] and Wang et al. [40]. Briefly, approximately 200 larvae for each treatment were homogenized in 0.2 mL ELISA buffer provided in the kits. The samples were then disrupted for 10 min on ice by spasmodic sonication. After centrifugation at 5000 × g for 10 min at 4°C, the supernatants were collected for T3 and T4 measurement using commercial ELISA kits (Uscnlife, Wuhan, China) following the manufacture's instructions. The detection limits for T3 and T4 were 0.1 ng/mL and 1.2 ng/mL, respectively. Intra-assay and inter-assay variations were below 15% in this study. No significant cross-reactivity or interference was observed for each kit.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from 30 homogenized larvae using RNAiso Plus reagent (Takara Biochemicals, Dalian, China), all procedures followed the manufacturer's instruction and the published protocols. The concentration of total RNA was measured at 260 nm using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, Wilmington, DE). The RNA quality was examined by measuring the 260/280 nm ratios and 1% agarose-formaldehyde gel electrophoresis with ethidium bromide staining. Approximately 1 μg total RNA was retrotranscribed by PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Biochemicals, Dalian, China) according to the manufacturer's instructions. Quantitative real-time PCR was analyzed on an ABI 7300 System (PerkinElmer Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR kit (Takara Biochemicals, Dalian, China). The primer sequences of the selected genes were obtained by using the online Primer 3 program (http://frodo.wo.mit.edu/) and are listed in Table 1 . The PCR reaction comprised an initial denaturation step at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, 60 °C for 15 s, and 72 °C for 45 s. A melting temperature-determining dissociation step was performed at 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 60 s at the end of the amplification phase. The dissociation curve was used to check the specificity of PCR products. All the samples were analyzed in triplicate and the mean value of these triplicate measurements were used for the calculations of the mRNA transcriptions. The housekeeping gene ribosomal protein L8 (rpl8) did not vary upon chemical exposure (data not shown) and was used as internal control. The expression levels of genes were normalized to rpl8 mRNA contents using the 2−ΔΔCt method.
Table 1. Primer sequences for the genes tested in the present study.
| Gene name | Primer Sequence (5′-3′) | Accession Number | |
| Forward | Reverse | ||
| Rpl8 | ttgttggtgttgttgctggt | ggatgctcaacagggttcat | NM_200713 |
| TSHβ | gcagatcctcacttcacctacc | gcacaggtttggagcatctca | AY135147 |
| Nkx2.1a | aggacggtaaaccgtgtcag | caccatgctgctcgtgtact | NM_131589 |
| Pax8 | gaagatcgcggagtacaagc | ctgcactttagtgcggatga | AF072549 |
| NIS | ggtggcatgaaggctgtaat | gatacggcatccattgttgg | NM_001089391 |
| TG | ccagccgaaaggatagagttg | atgctgccgtggaatagga | XM_001335283 |
| TTR | cgggtggagtttgacacttt | gctcagaaggagagccagta | BC081488 |
| Dio1 | gttcaaacagcttgtcaaggact | agcaagcctctcctccaagtt | BC076008 |
| Dio2 | gcataggcagtcgctcattt | tgtggtctctcatccaacca | NM_212789 |
| UGT1ab | ccaccaagtctttccgtgtt | gcagtccttcacaggctttc | NM_213422 |
Statistical analysis
Normality and homogeneity of data were analyzed using the Kolmogorov-Smirnov test, and Levene's test, respectively. All data were shown as means ± standard error (SEM). One-way analysis of variance (ANOVA) was applied to calculate statistical significance followed by Dunnett's test as a post hoc test to independently compared each exposure group to the control group. The LSD test was used as a post hoc test for multiple comparisons between groups. For hatching, survival and malformation rates, as these data were presented as proportions, they were square root arcsine-transformed before analysis of variance. All the analyses were conducted with SPSS statistical software version 13.0 (SPSS, Inc., Chicago, IL, USA). A p < 0.05 was considered statistically significant.
Results
Developmental toxicity
There were no significant effects on hatching, malformation, survival, body length and weight after exposure to MEHP (1.6, 8, 40 and 200 μg/L) relative to the control until 168 hpf ( Table 2 ).
Table 2. Development index of zebrafish larvae after exposure to MEHP (0, 1.6, 8, 40 and 200 μg/L) until 168 hpf.a .
| MEHP (μg/L) | 0 | 1.6 | 8 | 40 | 200 |
| Hatchinga (%) | 93.5±1.2 | 91.9±2.6 | 94.4±0.7 | 90.8±0.7 | 93.2±0.4 |
| Malformationa (%) | 2.08±0.42 | 3.02±0.87 | 2.04±0.43 | 2.57±0.25 | 3.01±0.38 |
| Survivala (%) | 75.2±1.3 | 73.2±3.4 | 76.5±1.0 | 72.6±0.1 | 74.9±0.2 |
| Lengtha (mm) | 3.41±0.13 | 3.41±0.05 | 3.52±0.11 | 3.32±0.06 | 3.11±0.13 |
| Weighta (mg) | 0.36±0.03 | 0.37±0.01 | 0.36±0.03 | 0.35±0.04 | 0.34±0.01 |
The values represent mean ± standard error (SEM) of six replicate groups.
Whole-body TH content
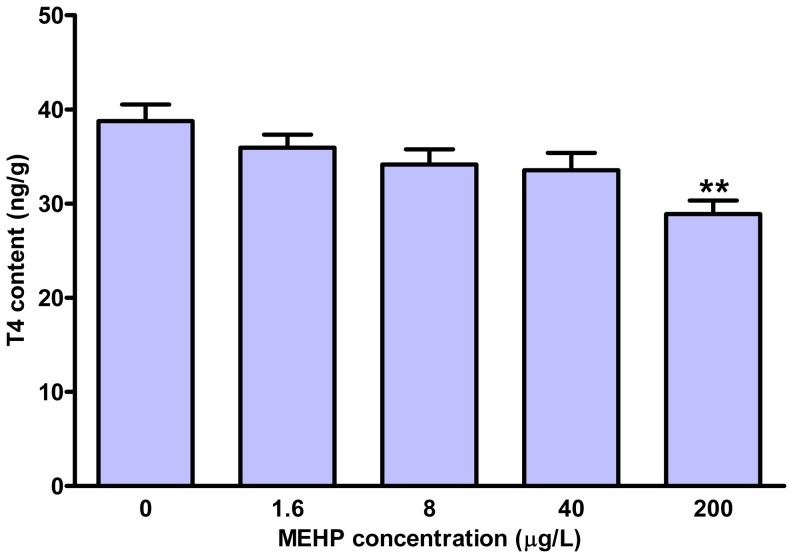
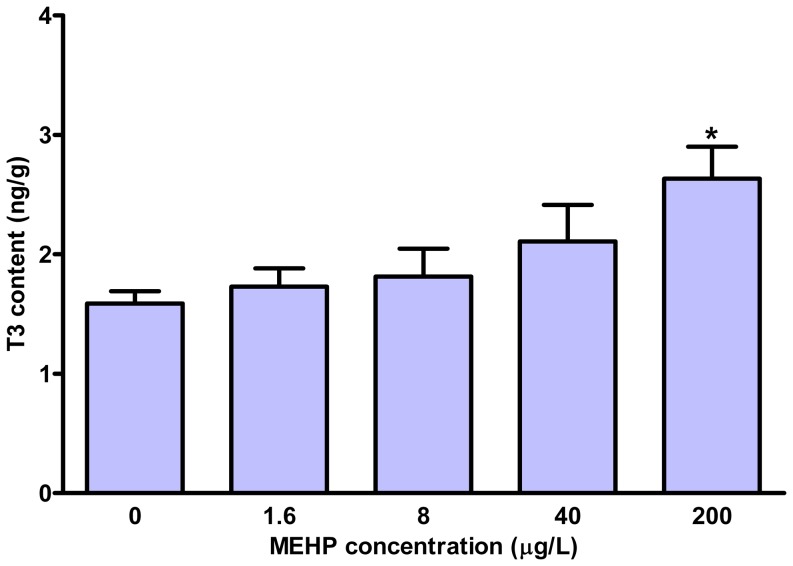
The whole-body TH contents were measured in the larvae at 168 hpf. As shown in Fig. 1 , MEHP exposure caused a concentration-dependent reduction in whole-body T4 contents. The total T4 contents were significantly decreased by 25.5% in 200 μg/L exposure group relative to the control. However, we observed an increase of total T3 contents in the exposure groups, which was significant in the 200 μg/L MEHP group (63.4%) ( Fig. 2 ).
Figure 1. Whole-body thyroxine (T4) contents in zebrafish larvae exposed to different concentrations of MEHP until 168 hpf.
Values represent mean±SEM (n = 6). Significant difference from the control group is indicated by ** P<0.01.
Figure 2. Whole-body triiodothyronine (T3) contents in zebrafish larvae exposed to different concentrations of MEHP until 168 hpf.
Values represent mean±SEM (n = 6). Significant difference from the control group is indicated by * P<0.05.
Gene transcription profile
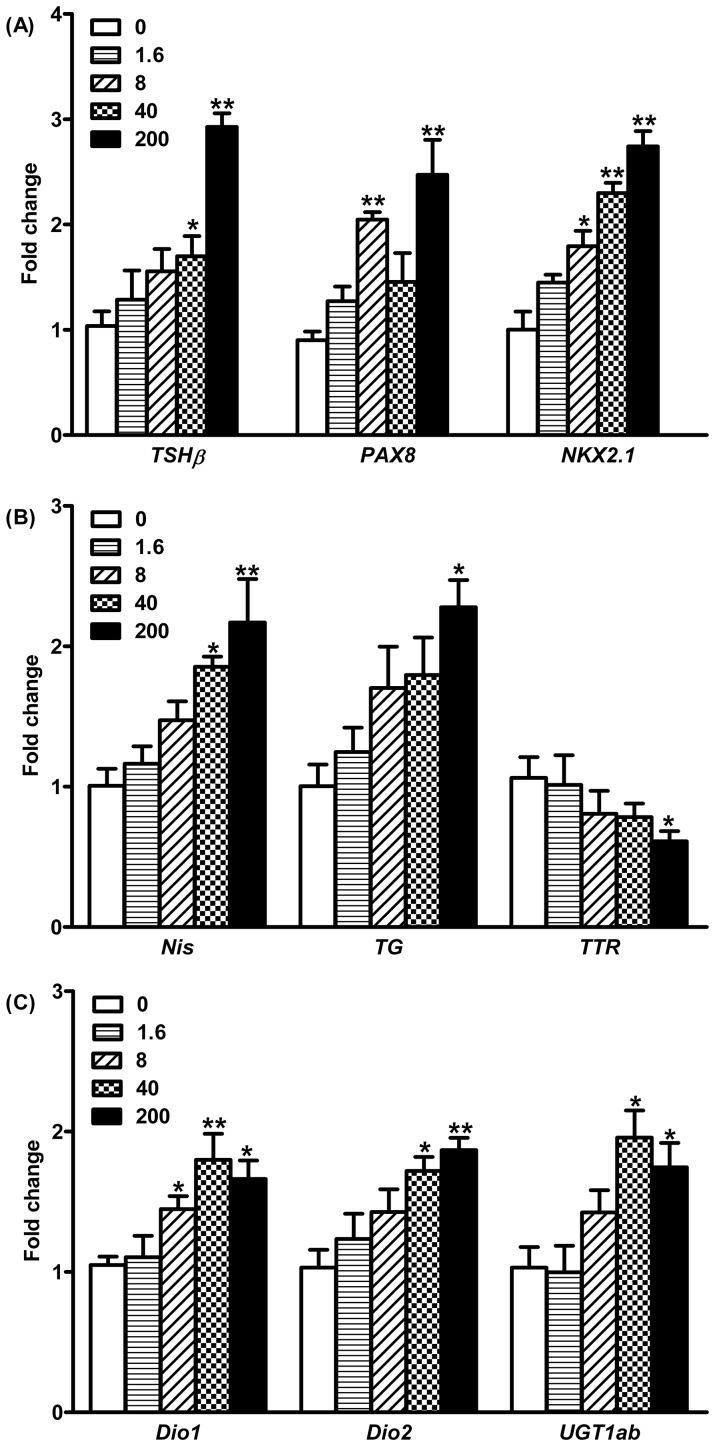
Several genes involved in the HPT axis of zebrafish were examined in this study. The transcription of the thyroid stimulating hormone (TSHβ) gene was significantly increased by 1.7- and 2.9-fold after exposure to 40 and 200 μg/L MEHP as compared to that in the control ( Fig. 3A ). In identifying the marker genes involved in thyroid growth and development, Pax8 was transcriptionally significantly induced by 2.0- and 2.5-fold in the 8 and 200 μg/L MEHP exposure groups, respectively ( Fig. 3A ), while Nkx2.1 was up-regulated by 1.8-, 2.3-, and 2.7-fold in a concentration-dependent manner after exposure to 8, 40, and 200 μg/L MEHP ( Fig. 3A ). The mRNA expression of gene encoding sodium/iodide symporter (NIS) was significantly increased 1.9- and 2.2-fold after treatment with 40 and 200 μg/L MEHP, respectively ( Fig. 3B ). Upon treatment with 200 μg/L MEHP, the thyroglobulin (TG) was significantly up-regulated transcriptionally by 2.3-fold ( Fig. 3B ). However, the transthyretin (TTR) gene transcription was significantly down-regulated 1.7-fold after exposure to 200 μg/L MEHP ( Fig. 3B ). In this study, two deiodinase isoforms (Dio1 and Dio2) have been examined. The transcription of Dio1 gene was significantly induced by 1.4-, 1.8-, and 1.7-fold in the 8, 40, and 200 μg/L MEHP groups, respectively ( Fig. 3C ), while the mRNA expression of Dio2 after treatment with 40 and 200 μg/L of MEHP was significantly increased 1.7- and 1.9-fold, respectively ( Fig. 3C ). In addition, the gene involved in the metabolism of TH (uridinediphosphate glucoronosyltransferases, UGT1ab) was transcriptionally significantly up-regulated by 2.0- and 1.7-fold after exposure to 40 and 200 μg/L of MEHP, respectively ( Fig. 3C ).
Figure 3. Gene transcription in hypothalamic-pituitary-thyroid (HPT) axis in zebrafish larvae after exposure to different concentrations of MEHP until 168 hpf.
Data expressed as mean±SEM (n = 6). Significant difference from the control group is indicated by * P<0.05, and ** P<0.01.
Discussion
Studies exploring the association between MEHP exposure and thyroid endocrine disrupting effects are limited, however, growing human studies have indicated that MEHP has the potential to alter thyroid hormone levels [28]–[30]. In the present study, developing zebrafish embryos/larvae were employed to assess effects of MEHP on thyroid hormone contents (T4 and T3) and mRNA expression related to thyroid hormones synthesis, secretion, transport and metabolism. The results showed that MEHP changed whole-body thyroid hormone (T4 and T3) contents and expression of genes involved in the HPT axis, thus clearly demonstrating its thyroid endocrine disrupting activity.
It has been suggested that MEHP, the active metabolites of DEHP, is related to the toxic effects of its parent compound [2]. In the present study, a significant decrease in T4 contents and increase in T3 contents were observed with MEHP exposure. This result was, to a certain extent, in agreement with previous findings, in which oral exposure to DEHP was found to decrease the plasma T4 levels in rats [23], [41]. On the contrary, the serum T4 levels were increased in rats after intravenously receiving DEHP [27]. The inconsistent responses reported in these studies are likely due to different exposure scenarios and concentrations. A recent epidemiological study also demonstrated that increased urinary concentrations of MEHP were negatively correlated with free T4 concentrations in the serum of adult men [29], which is consistent with the results of our study.
The pituitary gland regulates thyroid activity through the secretion of TSH, and in turn TSH secretion is triggered by changes in the concentrations of circulating THs via feedback mechanisms [42]. TSH is a glycoprotein complex that composed of two subunits (α and β), and the β subunit (TSHβ) plays pivotal roles in the HPT axis. It has been proposed that assessment of TSHβ gene transcription can be used to determine whether environmental pollutants give rise to thyroid dysfunction. In the present study, transcription of TSHβ gene was significantly up-regulated after MEHP treatment. Reductions of T4 and accompanying up-regulation of TSHβ gene transcription have been previously observed in fathead minnows (Pimephales promelas) and zebrafish larvae after chemical exposure [40], [43], [44].
We also examined genes involved in thyroid growth and development (Nkx2.1 and Pax8) and TH synthesis (NIS and TG) in the present study. Exposure to MEHP significantly increased transcription of Nkx2.1 and Pax genes. It has been demonstrated that Nkx2.1 (TTF1) and Pax8 are the main transcription factors regulating the expression of NIS and TG in the thyroid system [45], [46]. Therefore, elevated gene transcription of Nkx2.1 and Pax8 may give rise to increased NIS and TG, which is in accordance with our findings. Similar results have also been observed for zebrafish embryos/larvae exposed to various pollutants [40], [44], [47].
TTR, an important transport protein for THs, plays an important role in the thyroid axis in fish by regulating the supply of the hormone to various target tissues [48]. In this study, MEHP exposure led to decrease of TTR gene transcription, which may reduce the amount of TTR binding to free THs. Consequently, the excess uncombined T4 would be more susceptible to hepatic catabolism, resulting in a greater elimination and a reduction in circulating TH concentrations [49].
Deiodinases play a key role in the regulation of circulating and peripheral TH levels in vertebrates. In teleosts, there are three types of deiodinases: type 1 (Dio1), type 2 (Dio2), and type 3 (Dio3), and each of them has different functions. Dio1 has a considerable influence on iodine recovery and thyroid hormone degradation [50]. Dio2 exclusively catalyzes outer-ring deiodination of T4 into active T3, consequently controls the intracellular concentration of T3, and Dio3 is a purely inactivating enzyme [51]. In this study, both Dio1 and Dio2 were significantly induced after MEHP exposure, consistent with previous study demonstrating that hypothyroidism increases Dio1 and Dio2 activities and mRNA expression [51]. Combining with previous studies, we suggest that induction in the transcription of Dio2 may be, at least partly, responsible for the reduction of T4 contents, and increased transcription of Dio1 may assist to degrade the elevated T3 contents as a compensatory mechanism. Besides deiodinases, uridinediphosphate glucuronosyltransferase (UGT) also plays an important role in TH metabolism, via the major pathway for T4 conjugation [52]. Previous studies conducted on rats and zebrafish have demonstrated that T4 concentrations were negatively associated with UGT activities or gene transcription after chemicals treatment [38], [40], [47], [53]–[55]. In agreement with these previous studies, a significant induction of UGT1ab mRNA expression was also observed in our study. This negative association between whole-body T4 contents (reduction) and UGT1ab expression (induction) suggests that UGT1ab might play a role in reducing circulating T4 concentrations.
In conclusion, our results demonstrated that exposure of MEHP to zebrafish larvae decreased the T4 contents but increased the T3 contents by changing a series of genes transcription involved in the HPT axis. These results clearly showed that MEHP has the potential to disrupt thyroid endocrine system in fish. Furthermore, the observed effective concentrations of MEHP in causing thyroid endocrine-disrupting activity in this study were several orders of magnitude greater than those reported in water [10]. Therefore, MEHP alone at concentrations commonly found in environment and during short-term exposures may not cause thyroid endocrine disrupting effects in fish species. To gain more complete toxicological profiles of MEHP, further research are require to examine the effects on adult fish and a long-term exposure. Taking into account the extensive use of DEHP and its biotransformation to MEHP, the thyroid endocrine toxicity of MHEP should be paid for more attention.
Funding Statement
The authors have no support or funding to report.
References
- 1. Latini G, Del Vecchio A, Massaro M, Verrotti A, De Felice C (2006) Phthalate exposure and male infertility. Toxicology 226: 90–98. [DOI] [PubMed] [Google Scholar]
- 2. Shea KM (2003) Pediatric exposure and potential toxicity of phthalate plasticizers. Pediatrics 111: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 3.Lowell Center for Sustainable Production (2011) U.L. Phthlates and their Alternatives: Health and Environmental Concerns. Lowell Center for sustainalble Production, University of Massachusetts, Lowell. Available: http://www.sustainableproduction.org/downloads/PhthalateAlternatives-January2011.pdf.
- 4. Fromme H, Kuchler T, Otto T, Pilz K, Muller J, et al. (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36: 1429–1438. [DOI] [PubMed] [Google Scholar]
- 5. Magdouli S, Daghrir R, Brar SK, Drogui P, Tyagi RD (2013) Di 2-ethylhexylphtalate in the aquatic and terrestrial environment: A critical review. J Environ Manage 127C: 36–49. [DOI] [PubMed] [Google Scholar]
- 6. Cheng Z, Nie XP, Wang HS, Wong MH (2013) Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ Int 57-58C: 75–80. [DOI] [PubMed] [Google Scholar]
- 7. Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994) 43: 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210: 623–634. [DOI] [PubMed] [Google Scholar]
- 9. Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V (2013) Fetal origin of endocrine dysfunction in the adult: the phthalate model. J Steroid Biochem Mol Biol 137: 5–17. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Yaguchi K, Suzuki S, Suga T (2001) Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ Sci Technol 35: 3757–3763. [DOI] [PubMed] [Google Scholar]
- 11. Arbin A, Östelius J (1980) Determination by electron-capture gas chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous solutions stored in poly(vinyl chloride) bags. J Chromatogr A 193: 405–412. [Google Scholar]
- 12. Shintani H (1985) Determination of phthalic acid, mono-(2-ethylhexyl) phthalate and di-(2-ethylhexyl) phthalate in human plasma and in blood products. J Chromatogr 337: 279–290. [DOI] [PubMed] [Google Scholar]
- 13. Babu B, Wu JT (2010) Production of phthalate esters by nuisance freshwater algae and cyanobacteria. Sci Total Environ 408: 4969–4975. [DOI] [PubMed] [Google Scholar]
- 14. Wang AL, Cai XD, Chi J (2012) Determination of Trace Phthalic Acid Monoesters in Sediments and Soils by GC-MS. Soil Sediment Contam 21: 996–1005. [Google Scholar]
- 15. Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE (2009) Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ Health Perspect 117: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, et al. (2013) Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ 461–462: 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim S, Kang S, Lee G, Lee S, Jo A, et al. (2014) Urinary phthalate metabolites among elementary school children of Korea: Sources, risks, and their association with oxidative stress marker. Sci Total Environ 472: 49–55. [DOI] [PubMed] [Google Scholar]
- 18. Fromme H, Gruber L, Seckin E, Raab U, Zimmermann S, et al. (2011) Phthalates and their metabolites in breast milk - Results from the Bavarian Monitoring of Breast Milk (BAMBI). Environ Int 37: 715–722. [DOI] [PubMed] [Google Scholar]
- 19. Liu L, Bao H, Liu F, Zhang J, Shen H (2012) Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ Int 42: 78–83. [DOI] [PubMed] [Google Scholar]
- 20. Boas M, Feldt-Rasmussen U, Main KM (2012) Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355: 240–248. [DOI] [PubMed] [Google Scholar]
- 21. Kloas W, Lutz I (2006) Amphibians as model to study endocrine disrupters. J Chromatogr A 1130: 16–27. [DOI] [PubMed] [Google Scholar]
- 22. Wenzel A, Franz C, Breous E, Loos U (2005) Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol 244: 63–71. [DOI] [PubMed] [Google Scholar]
- 23. Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, et al. (1986) Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect 70: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price SC, Chescoe D, Grasso P, Wright M, Hinton RH (1988) Alterations in the thyroids of rats treated for long periods with di-(2-ethylhexyl) phthalate or with hypolipidaemic agents. Toxicol Lett 40: 37–46. [DOI] [PubMed] [Google Scholar]
- 25. Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, et al. (1997) Subchronic oral toxicity of di-n-octyl phthalate and di(2-Ethylhexyl) phthalate in the rat. Food Chem Toxicol 35: 225–239. [DOI] [PubMed] [Google Scholar]
- 26. Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH (2001) Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol Lett 121: 35–43. [DOI] [PubMed] [Google Scholar]
- 27. Gayathri NS, Dhanya CR, Indu AR, Kurup PA (2004) Changes in some hormones by low doses of di (2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J Med Res 119: 139–144. [PubMed] [Google Scholar]
- 28. Meeker JD, Calafat AM, Hauser R (2007) Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115: 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meeker JD, Ferguson KK (2011) Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect 119: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, et al. (2010) Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect 118: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr JA, Patino R (2011) The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen Comp Endocrinol 170: 299–312. [DOI] [PubMed] [Google Scholar]
- 32. Briggs JP (2002) The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol 282: R3–9. [DOI] [PubMed] [Google Scholar]
- 33. Parng C, Seng WL, Semino C, McGrath P (2002) Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol 1: 41–48. [DOI] [PubMed] [Google Scholar]
- 34. Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, et al. (2013) Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ Health Perspect 121: 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scholz S (2013) Zebrafish embryos as an alternative model for screening of drug-induced organ toxicity. Arch Toxicol 87: 767–769. [DOI] [PubMed] [Google Scholar]
- 36. Thienpont B, Tingaud-Sequeira A, Prats E, Barata C, Babin PJ, et al. (2011) Zebrafish Eleutheroembryos Provide a Suitable Vertebrate Model for Screening Chemicals that Impair Thyroid Hormone Synthesis. Environ Sci Technol 45: 7525–7532. [DOI] [PubMed] [Google Scholar]
- 37. Chan WK, Chan KM (2012) Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat Toxicol 108: 106–111. [DOI] [PubMed] [Google Scholar]
- 38. Yu L, Deng J, Shi X, Liu C, Yu K, et al. (2010) Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat Toxicol 97: 226–233. [DOI] [PubMed] [Google Scholar]
- 39.Westerfield M (1995) The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish (Danio rerio)3rd Edition. Eugene, OR, USAUniversity of Oregon Press
- 40. Wang Q, Liang K, Liu J, Yang L, Guo Y, et al. (2013) Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol 126: 207–213. [DOI] [PubMed] [Google Scholar]
- 41. Erkekoglu P, Giray BK, Kizilgun M, Hininger-Favier I, Rachidi W, et al. (2012) Thyroidal effects of di-(2-ethylhexyl) phthalate in rats of different selenium status. J Environ Pathol Toxicol Oncol 31: 143–153. [DOI] [PubMed] [Google Scholar]
- 42. Ji C, Jin X, He J, Yin Z (2012) Use of TSHbeta:EGFP transgenic zebrafish as a rapid in vivo model for assessing thyroid-disrupting chemicals. Toxicol Appl Pharmacol 262: 149–155. [DOI] [PubMed] [Google Scholar]
- 43. Lema SC, Dickey JT, Schultz IR, Swanson P (2009) Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. J Endocrinol 202: 43–54. [DOI] [PubMed] [Google Scholar]
- 44. Yu L, Chen M, Liu Y, Gui W, Zhu G (2013) Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat Toxicol 138–139: 35–42. [DOI] [PubMed] [Google Scholar]
- 45. Zoeller RT, Tan SW, Tyl RW (2007) General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol 37: 11–53. [DOI] [PubMed] [Google Scholar]
- 46. Kambe F, Nomura Y, Okamoto T, Seo H (1996) Redox regulation of thyroid-transcription factors, Pax-8 and TTF-1, is involved in their increased DNA-binding activities by thyrotropin in rat thyroid FRTL-5 cells. Mol Endocrinol 10: 801–812. [DOI] [PubMed] [Google Scholar]
- 47. Chen Q, Yu L, Yang L, Zhou B (2012) Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol 110–111: 141–148. [DOI] [PubMed] [Google Scholar]
- 48. Power DM, Elias NP, Richardson SJ, Mendes J, Soares CM, et al. (2000) Evolution of the thyroid hormone-binding protein, transthyretin. Gen Comp Endocrinol 119: 241–255. [DOI] [PubMed] [Google Scholar]
- 49. Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, et al. (2005) Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius). Toxicol Sci 88: 375–383. [DOI] [PubMed] [Google Scholar]
- 50. Van der Geyten S, Byamungu N, Reyns GE, Kuhn ER, Darras VM (2005) Iodothyronine deiodinases and the control of plasma and tissue thyroid hormone levels in hyperthyroid tilapia (Oreochromis niloticus). J Endocrinol 184: 467–479. [DOI] [PubMed] [Google Scholar]
- 51. Orozco A, Valverde RC (2005) Thyroid hormone deiodination in fish. Thyroid 15: 799–813. [DOI] [PubMed] [Google Scholar]
- 52. Hood A, Klaassen CD (2000) Differential effects of microsomal enzyme inducers on in vitro thyroxine (T(4)) and triiodothyronine (T(3)) glucuronidation. Toxicol Sci 55: 78–84. [DOI] [PubMed] [Google Scholar]
- 53. Zhou T, Ross DG, DeVito MJ, Crofton KM (2001) Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci 61: 76–82. [DOI] [PubMed] [Google Scholar]
- 54. Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, et al. (2009) Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci 107: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hallgren S, Darnerud PO (2002) Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology 177: 227–243. [DOI] [PubMed] [Google Scholar]