Abstract
To develop magnetic resonance imaging (MRI) indicators to predict trismus outcome for post-operative oral cavity cancer patients who received adjuvant intensity-modulated radiation therapy (IMRT), 22 patients with oral cancer treated with IMRT were studied over a two-year period. Signal abnormality scores (SA scores) were computed from Likert-type ratings of the abnormalities of nine masticator structures and compared with the Mann-Whitney U-test and Kruskal–Wallis one-way ANOVA test between groups. Seventeen patients (77.3%) experienced different degrees of trismus during the two-year follow-up period. The SA score correlated with the trismus grade (r = 0.52, p<0.005). Patients having progressive trismus had higher mean doses of radiation to multiple structures, including the masticator and lateral pterygoid muscles, and the parotid gland (p<0.05). In addition, this group also had higher SA-masticator muscle dose product at 6 months and SA scores at 12 months (p<0.05). At the optimum cut-off points of 0.38 for the propensity score, the sensitivity was 100% and the specificity was 93% for predicting the prognosis of the trismus patients. The SA score, as determined using MRI, can reflect the radiation injury and correlate to trismus severity. Together with the radiation dose, it could serve as a useful biomarker to predict the outcome and guide the management of trismus following radiation therapy.
Introduction
Trismus is one of the sequela for head and neck cancer patients. The prevalence of trismus after head and neck oncology treatment could be as high as 42% [1]. It has been described as any type of restriction in the opening of the mouth including radiation and conditions after trauma, surgery, or tetanus [2], [3], [4]. Radiation therapy involving the temporomandibular joint (TMJ), pterygoid muscles, and the temporalis or the masseter muscle is most likely to result in trismus [5], [6]. Moreover, there may be scar tissue from radiation, surgery, nerve damage, or a combination of these factors to cause trismus [6], [7]. Further, doses of radiotherapy (RT) in excess of 60 Gy [8] or the configuration of the radiation field increasing [9] are more likely to cause trismus.
Recently, extensive data suggest intensity-modulated radiation therapy (IMRT) is safe and efficacious in the adjuvant setting for oral cavity cancer (OCC) [10], [11], [12]. Hsiung et al. [13] and Chen et al. [14] noted radiation induced trismus for nasopharyngeal carcinoma patients progressed over time and improved by IMRT. Louise et al. [15] noted when doses of massetor or pterygoid muscles larger than 55 Gy were given, the incidences of trismus for head and neck patients were as high as 45%. With every additional 10 Gy to the pterygoid muscle, the increase in the probability of trismus was 24% [5].
Magnetic resonance imaging (MRI) can display abnormal image findings in masticator structures for patients with trismus developing after radiotherapy for nasopharyngeal carcinoma (NPC) [6]. However, in this report, the image findings showed no correlation between the severity of trismus and radiation dosage [6]. Moreover, aggressive interventions should be given to the patient whose trismus does not improve as time passes. However, there is still no good indicator to predict the outcome of trismus developing after radiotherapy.
The purpose of this study is to use MRI to predict the severity of trismus and evaluate the correlation of images and radiation dosage between related structures concerning trismus. We also tried to find the prognostic factors of trismus developing after the operation and IMRT for oral cancer patients.
Materials and Methods
Patient characteristics
This retrospective study was approved by the Institutional Review Board (IRB) of the Far Eastern Memorial Hospital (FEMH-IRB-101127-F) and Clinical Trials government with number of NCT02004639. Patient consents were specifically waived because the data were analyzed anonymously and approval was given by the IRB. Patient confidentiality and privacy were protected according to national standards. Between December 2006 and December 2012, patients with OCC squamous cell carcinoma (SCC) who had undergone surgery followed by postoperative IMRT with or without chemotherapy at Far Eastern Memorial Hospital were enrolled. Patients who were treated for recurrent SCC of the oral cavity (including neck recurrences), follow-up time less than 2 years, incomplete trismus grading records or incomplete MRI image studies were excluded from the analysis. The disease was staged according to the American Joint Committee on Cancer Staging Classifications 6th edition, which was based on the pathological findings after radical surgery.
Radiation therapy
A 7-filed IMRT or helical tomotherapy, image-guided IMRT, with daily fractions of 1.8 or 2 Gy in five consecutive days were used. It encompassed the preoperative gross tumor and postoperative flap plus a 0.8- to 1-cm margin, including the resection bed with soft-tissue invasion by the tumor or extra-capsular extension (ECE) that received 60–66 Gy in 30–33 fractions; 64–66 Gy was delivered to high-risk OCC patients and 60 Gy was delivered to intermediate-risk OCC patients. For the high-risk subclinical area, 59.4–60 Gy/30–33 fractions were delivered and for the low-risk area of potential subclinical disease, 51.2–54 Gy/30–33 fractions were delivered [16]. The grading of trismus in the clinical results was from grade one to three according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The medial and lateral pterygoid, masseter and temporalis muscles, parotid gland, mandibular rami and temporomandibular joints (TMJ) were targeted and calculated by dose-volume histogram for those enrolled patients retrospectively (Fig. 1).
Figure 1. Describe the trismus related muscles and structures that were targeted by colors in one of enrolled patients.
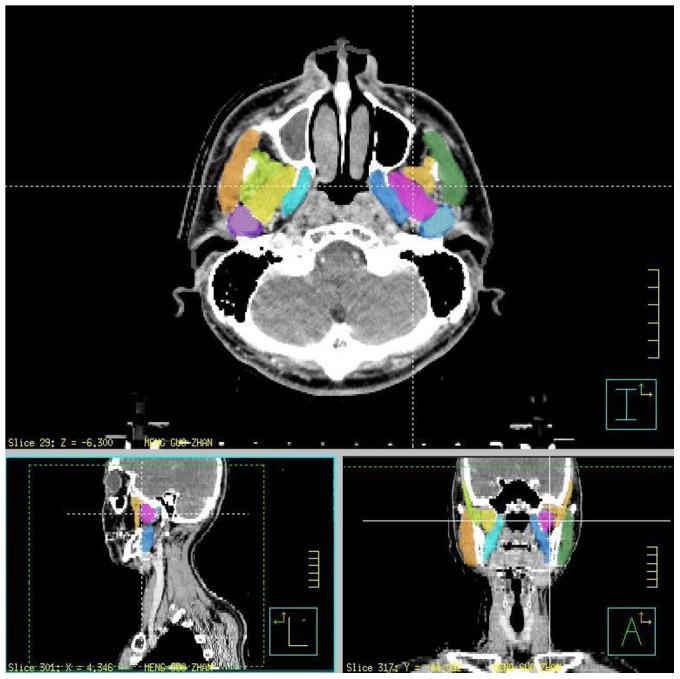
Light blue: left side medial pterygoid muscle; Pink: left side lateral pterygoid muscle; Light orange: left side temporalis; Forest green: left side masseter; Steel blue: left side temporomandibular joints. Sky blue: right side medial pterygoid muscle; Yellow: right side lateral pterygoid muscle; Yellow green: right side temporalis; Orange: right side masseter; Purple: right side temporomandibular joints.
Evaluation of MRI examinations
MRI results at 6th months, 12th months and 24th months after initiation of radiation treatment were selected for analysis. These data were assessed by two radiologists blind to their clinical information and trismus severity. The MRI imaging protocols applied at our institution for patients with oral cancer include axial T1 weighted image (T1W), coronal T2 weighted image (T2W), axial T2W with fat saturation, sagittal, coronal T1W post-gadolinium and axial T1W post-gadolinium with fat saturation sequences. TIW and T2W are both conventional image sequences applied in daily MRI examination. In practice, T1W and T2W image are where the contrast depends predominantly on the differences in the T1 times and T2 times between tissues e.g. fat and water, respectively [17]. Tl describes the interaction of excited nuclei with the surroundings (spin-lattice relaxation), while T2 describes the interaction of excited nuclear spins with the spins of other nuclei (spin-spin relaxation) [18].
Signal abnormality scores (SA score) were rated with a Likert scoring system [19]. A three-point Likert item SA score was used in the current study, and the structures analyzed were listed in table 1. For evaluation of the masticator muscles, we rated the signal change as “0” = normal; “1” = T2 signal change only; “2” = 1 plus muscle atrophy or obvious abnormal enhancement on T1W post-gadolinium sequences. While rating the perimasticator space, “0” = normal; “1” = T2 signal change only, “2” = 1 plus fibrosis tissue formation or abnormal enhancement. For chronic injury of the mandibular division of the trigeminal nerve, which was inferred if there was atrophy of all ipsilateral masticator muscles as well as of the mylohyoid and anterior belly of the digastric muscles, we rated atrophy of part of these muscles as “1” and complete atrophy of all muscles as “2”. In the parotid gland, only signal change was rated as “1” and obvious gland atrophy was rated as“2”. These abnormalities were differentiated from residual tumor by their signal characteristics and static appearance on serial imaging.
Method of analysis
At first, groups according to trismus grade in CTCAE v3.0 were separated and compared using Kruskal–Wallis one-way ANOVA test without assuming parametric distribution. The correlation between the SA score and trismus grade, or the SA score and the radiation dose of individual masticator structures were tested by Spearman's correlation test. Statistically significant SA scores were used as independent variables, and determined as useful in predicting the severity of trismus. Using the SA scores found to be useful, the function of logistic regression was calculated and the receiver-operating characteristic (ROC) curve was generated to find an optimum cut-off for predicting trismus severity.
After that, the patients were re-separated into two groups according to their trismus condition. One was the “good prognosis, GP” group if patient's trismus grade improved in time sequence or as normal. The other was “poor prognosis, PP” group if patient's trismus remained stable or got worsened during the two year follow up. The SA scores at 6th months, 12th months, the radiation dose of all and individual masticator structures, along with the product of the SA score with mean radiation dose of masticator muscles (SA score × mean radiation dose of masticator muscles for specific interesting) were all compared between these two groups. All data were evaluated with the non-parametric Mann-Whitney U-test without assuming parametric distribution.
The statistically significant variables were used as independent factors, and only the ones showing statistical significance were determined as useful in predicting the incidence of poor prognosis trismus. Utilizing the function of logistic regression, propensity score was sought for estimating the incidence of poor prognosis trismus among all the subjects during the follow-up period [18].
All the above statistical analyses were performed using Prism (release 6.0, GraphPad Software Inc. La Jolla, CA, USA) and SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY) and a P value equal or less than 0.05 was considered statistically significant.
Results
Totally, 87 patients were enrolled but sixty-five cases were excluded due to incomplete clinical information or incomplete imaging data. Twenty-two cases with a total of sixty-six MRI exams were enrolled in this study. Table 2 lists the characteristics of the patients. The mean age was 50 years old, consisting of twenty men and two women. The dominant subgroups were buccal cancer (54.5%) and tongue cancer (36.4%). Ninety percent were stage III and IV. The median radiation dose was 64 Gy. Seventeen patients (77.3%) experienced different degrees of trismus during two year follow up after IMRT.
Table 1. Signal Abnormality Score in different masticator structures.
| Anatomy | SA Score | ||
| 0 | 1 | 2 | |
| Masticator Muscles | |||
| Medial pterygoid | |||
| Lateral Pterygoid | |||
| Masseter | Normal | T2 signal change | T2 signal change with abnormal enhancement |
| Temporalis | |||
| Masticator atrophy | Normal | Part of masticator muscle atrophy | All muscles atrophy |
| Temporomandibular Joint | |||
| Deformity | Normal | Mild | Severe |
| Ramus ORN | Normal | Signal change | Bone destruction |
| Other Structures | |||
| Perimasticator space | Normal | Inflammation change | Fibrosis |
| Parotid gland | Normal | Abnormal enhancement | Atrophy |
Abbreviation.
SA score = Signal Abnormality score; ORN = Osteoradionecrosis.
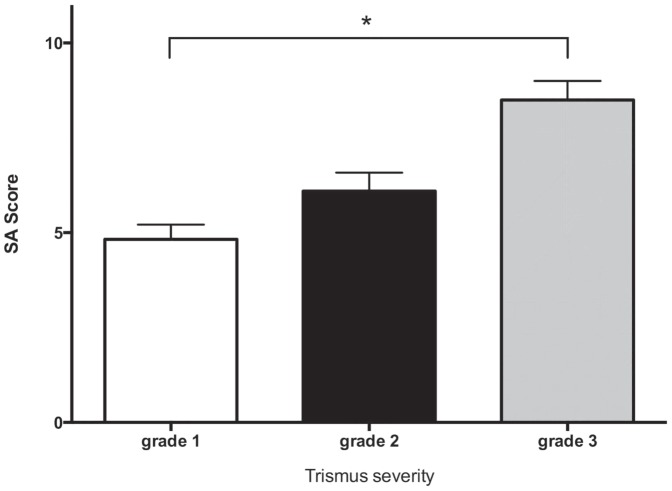
Imaging analysis revealed all patients had various degrees of masticator muscle signal abnormality (Fig. 2a and 2b). Within them, denervation atrophy of the masticator muscles secondary to mandibular nerve damage (6/22, 9.0%), mild osteoradionecrosis change of mandibular rami (10/22, 15.2%), and perimasticator fibrosis extending into the masticator space totaled six (9.0%, Fig. 2c). Post RT damage of the parotid glands was 63.6% (Fig. 2d). The mean SA scores of the grade 1, 2 and 3 group are 4.87±1.9, 6.1±3.2 and 8.5±0.7, respectively. Significant differences between groups (Fig. 3, p = .03, one-way ANOVA test) were noted. Additionally, there was a significant positive correlation between SA score and trismus grading (r = 0.52, p<0.005) to predict the severity of trismus. In addition, the SA score of the trismus group (6.20±3.1, group of grade 2 and grade 3) was higher than that of the non-trismus group (4.87±1.9, grade 1 group, p = .04). The ROC curve was then generated using the values of the SA scores from the non-trismus (grade 1) and trismus (grade 2 plus grade 3) groups. The best cut-off values of the SA score that maximizes (sensitivity + specificity) were 5.5, with 64% sensitivity and 61% specificity.
Figure 2. Illustrated T1W MRI post-contrast enhancement of trismus patient after RT.
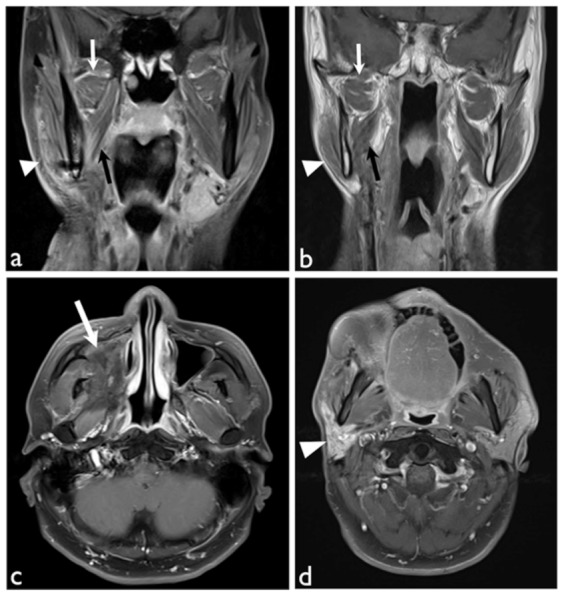
(a) Coronal view with fat saturation showed radiation effects in right lateral (white arrows), medial (black arrows) pterygoid muscles and masseter muscle (white arrowhead) with increased enhancement. (b) Coronal view showed atrophic change of the right lateral (white arrows), medial (black arrows) pterygoid muscles and masseter muscle (white arrowhead). (c) Axial view with fat saturation showed remarkable fibrotic tissue (white arrow) occupying right maxillary sinus and pterygoid space. (d) Axial view with fat saturation showed increased enhancement with atrophic change of the right parotid gland. All these findings were rated as point 2 according to our Likert scoring system of signal abnormality (SA score).
Figure 3. Comparison of signal abnormality scores between different groups of trismus severity.
Significant difference in the SA scores between groups is noted (p = .03). The grade 3 group (most severe one) has the highest score and the grade 1 (least severe one) group has the lowest score.
There was a positive correlation between the dose of the individual masticator structures and the two year SA scores summation (r = 0.48, p<0.0001; Fig. 4a). Furthermore, the SA score gradually decreased along with clinical trismus grade and time sequence (Fig. 4b).
Figure 4. Correlation of SA score with radiation dose and the time trend of trismus development.
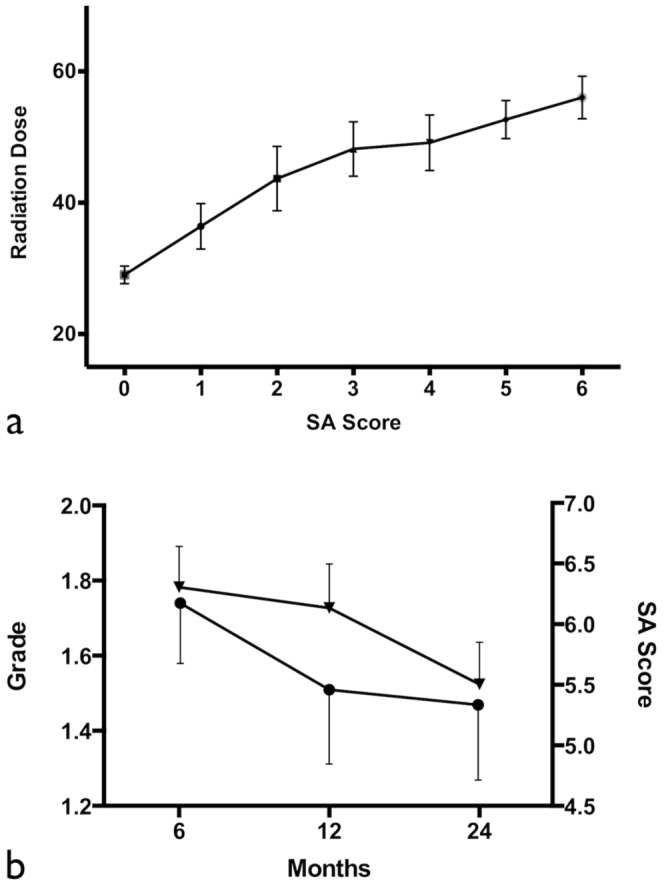
(a) Correlation of the received radiation dose of individual masticator structures with their two year SA scores summation (r = 0.48, p<0.0001). (b) Evolution of the SA scores and the trismus grade over time. Gradual decrease of trismus severity grade (▾) and SA scores (□) is noted within 24 months.
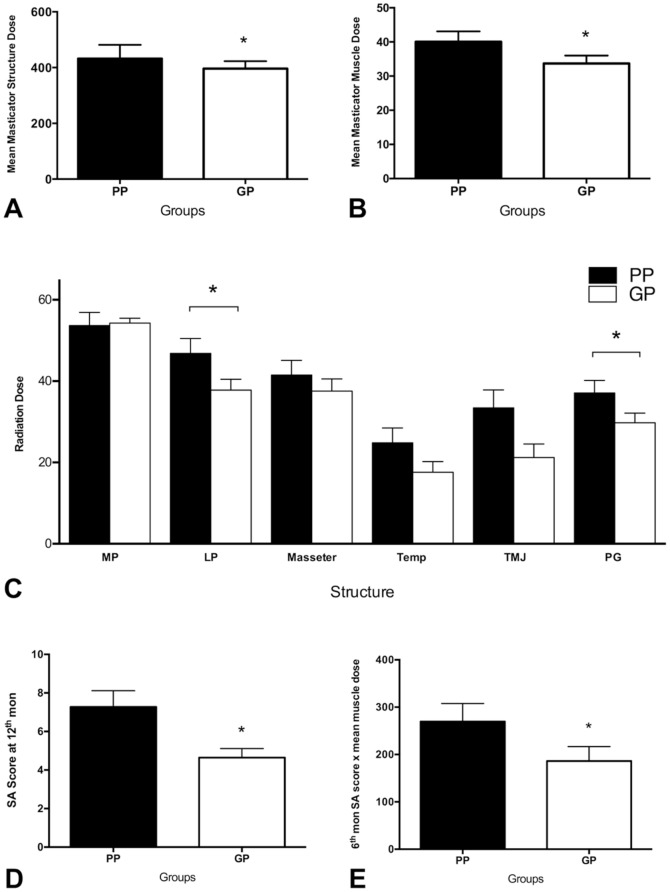
There were significant differences between PP and GP groups in the mean dose of masticator structures (39.6±9.3 vs 33.3±7.1, p = .04; Fig. 5a), masticator muscles (40.0±10.1 vs 33.7±7.4, p = .05; Fig. 5b), lateral pterygoid muscle (46.8±17.3 vs 37.8±11.8, p = .04; Fig. 5c), parotid gland (37.1±14.3 vs 29.8±10.7, p = .01; Fig. 5c), and the product of six month SA scores with mean masticator muscle dose (269.6±126 vs 186.2±97.0, p = .04; Fig. 5d) and twelve month SA scores (7.3±2.8 vs 4.6±1.6, p = .01; Fig. 5e). There was no significant difference in six month SA scores, radiation dose of medial pterygoid muscle, masseter muscle, and the temporalis muscle between the PP and GP groups (Fig. 5c). However, there was a trend for the mean dose of TMJ to influence both groups (p = .06).
Figure 5. Comparison of radiation doses and SA scores between good prognosis and poor prognosis trismus groups.
A significant difference is noted in the mean dose of masticator structures (p = .04; Fig. 5a), mean dose of masticator muscles (p = .05; Fig. 5b), mean dose of lateral pterygoid muscles (p = .04; Fig. 5c), mean dose of parotid glands (p = .01; Fig. 5c), the product of the six month SA score with mean masticator muscles dose (p = .04; Fig. 5d) and the twelve month SA score (p = .01; Fig. 5e). (MP: medial pterygoid muscle; LP: lateral pterygoid muscle; Temp: temporalis muscle; TMJ: temporomandibular joint; PG: parotid gland; SA score: signal abnormality score; GP: good prognosis; PP: poor prognosis).
The best cut-off values of the above data that maximized sensitivity and specificity were obtained and listed in table 3. The functional formula for predicting the incidence of poor prognosis trismus, i.e. propensity score, came out as follows:
Table 2. Patient characteristics.
| Enrolled patients (No. = 22) | |
| Variable | No. of patients (%) |
| Age (years) | |
| Mean | 50 |
| Range | 38–71 |
| Gender | |
| Male | 20 (94.3%) |
| Female | 2 (5.7%) |
| Subsite | |
| Oral tongue | 8 (36.4%) |
| Buccal mucosa | 12 (54.5%) |
| Lip | 1 (4.5%) |
| Gum | 1 (4.5%) |
| Pathology | |
| Squamous cell carcinoma | 22 (100%) |
| Tumor stage | |
| Stage I | 1 (4.5%) |
| Stage II | 1 (4.5%) |
| Stage III | 7 (31.8%) |
| Stage IVA | 13 (59.1%) |
| Stage IVB | 0 |
| Primary tumor stage | |
| T1 | 2 (9.1%) |
| T2 | 5 (22.7%) |
| T3 | 7 (31.8%) |
| T4a | 8 (36.4%) |
| T4b | 0 |
| Regional lymph node stage | |
| N0 | 9 (40.9%) |
| N1 | 4 (18.2%) |
| N2a | 0 |
| N2b | 8 (36.4%) |
| N2c | 1 (4.5%) |
| N3 | 0 |
| Adjuvant concurrent chemotherapy | |
| Yes | 20 (90.9%) |
| No | 2 (9.1%) |
| RT dose | |
| Median (range) | 64 Gy (59.8–70 Gy) |
| Mean dose of | |
| Right temporomandibular joint | 24.8±16.9 (Gy) |
| Right masseter muscle | 37.3±15.9 (Gy) |
| Right temporalis | 19.8±14.0 (Gy) |
| Right medial pterygoid muscle | 52.6±13.1 (Gy) |
| Right lateral pterygoid muscle | 40.8±16.3 (Gy) |
| Right parotid gland | 30.1±11.4 (Gy) |
| Left temporomandibular joint | 28.3±21.6 (Gy) |
| Left masseter muscle | 39.4±18.4 (Gy) |
| Left temporalis | 21.2±16.9 (Gy) |
| Left medial pterygoid muscle | 52.6±13.1 (Gy) |
| Left lateral pterygoid muscle | 41.85±17.0 (Gy) |
| Left parotid gland | 34.9±14.9 (Gy) |
Table 3. The incidence of poor prognosis trismus predicts by cut-off values of masticator structures, time schedual SA score and propensity score.
| Cutoff Values from ROC curve results | |||
| Data Set | Cutoff Value | Sensitivity (%) | Specificity (%) |
| Mean Radiation Dose of masticator structures | 42.14 | 63.64 | 90 |
| Mean Radiation Dose of masticator muscles | 42.54 | 63.64 | 90 |
| Mean Radiation Dose of LP | 42.03 | 72.73 | 70 |
| Mean Radiation Dose of PG | 27.99 | 80.95 | 60 |
| 6th month SA score × mean masticator muscle dose | 240.6 | 72.73 | 90 |
| 12th month SA score | 5.5 | 72.73 | 72.73 |
| Propensity Score* | 0.38 | 100 | 91 |
Abbreviation.
LP = lateral pterygoid muscle; PG = parotid gland; ROC curve = receiver operating characteristic curve.
*The propensity score was generated from the above six parameters with logistic regression analysis.
Propensity score = 1/(1+1/exp (mean dose of masticator structures ×3.576+ mean dose of masticator muscles ×−3.284+ lateral pterygoid muscle dose × 0.138+ parotid gland dose ×−0.296+ the product of 6th month SA scores with mean masticator muscle dose ×0.001+12th month SA scores ×1.281−12.654)).
The area-under-curve (AUC) was 0.93 for propensity score and the optimum cut-off points were 0.38. By applying the cut-off value, we achieved 100% sensitivity and 93% specificity to predict the prognosis of these trismus patients (Table 3).
Discussion
In the current study, there is a good correlation between the MRI SA score with trismus severity (r = 0.52, p<0.005) and the received radiation dose (r = 0.48, p<0.0001). Several prognostic factors of trismus were also detected with signifcant differences between trismus improving and worsening patients, which were the mean dose of the masticator structures (p = .04), masticator muscles (p = .05), lateral pterygoid muscle (p = .04), parotid gland (p = .01), the product of six month SA scores with the mean masticator muscle dose (p = .04), and twelve month SA scores (p = .01). With the optimum cut-off points as 0.38 for the propensity score, we achieved 100% sensitivity with 93% specificity to predict the prognosis of these trismus patients.
Trismus is a well known complication of head and neck cancer treatment [9], [20]. In a previous study, 35 NPC patients completed RT treatment with trismus with a dental gap less than 2.5 cm being studied by retrospective MRI analysis [6]. Fifty-four percent of them had abnormalities in several structures involved with mastication, without mentioning the correlation between RT doses. Several previous studies reported a direct correlation between RT dose of the masticator muscles and subsequent reductions in the dental gap [21], [22], [23]. Dijkstra et al [5] also reported reduced mouth opening of 18% in patients treated by RT involving the structures of the TMJ and/or pterygoid muscles. Similar conditions were also noted in our OCC patients who received RT or CCRT. In the current study, several abnormalities of the masticator muscles were noted by MRI, suggesting trismus is a multifactorial disease. Additionally, there was a strong correlation between the two year SA score summation with the radiation dose received by individual masticator structures (r = 0.48, p<0.0001). This meant our SA score can reflect the radiation dose received by each individual masticator structures and also implied the radiation-induced fibrosis or injury of OCC patients can be revealed by MRI and quantified by our scoring system.
The SA score based on the serial MRI findings proved to have good correlation (r = 0.52, p<0.005) with the clinical trismus severity, hinting that the severity of trismus could be predicted by imaging. According to the results of the ROC curve, patients experiencing some degree of trismus-related impaired eating could be predicted with an SA score higher than 5.5 (with 64% sensitivity and 61% specificity respectively). This suggests the SA score may serve as an imaging predictor of trismus and may also reflect the degree of underlying pathological changes. Moreover, the SA score gradually decreased along with clinical trismus grade and time sequence, which corresponds to our clinical experiences that most radiation-induced trismus patients after IMRT will gradually improve over time. This implies the SA score can be applied as an indicator of treatment response in trismus during follow up.
Our results also revealed some trismus subsided over time, but some did not. For the patient whose trismus will not improve as time goes by, a more aggressive treatment plan including rehabilitation exercise [24], Botox injection or even surgical intervention [25] should be suggested as early as possible. Therefore, it is critical to find the prognostic factors of trismus after RT to help physicians and patients overcome this sequela. In the current study, the six factors correlated with prognosis were the mean dose of masticator structures, masticator muscles, lateral pterygoid muscle, parotid gland, the product of six month SA scores with mean dose of masticator muscles and the twelve month SA scores. Here, we also found the radiation dose of the parotid glands plays a role in trismus prognosis. This may due to the fact that the anatomical correlation between parotid glands and masticator structures influences the dose distribution in RT plan. According to our model, applying the optimum cut-off point of the propensity score at 0.38, we are able to pick out poor-prognostic trismus patients with 100% sensitivity and 93% specificity, which is better than predicted by radiation dose (sensitivity and specificity around 63%/90%). So it considers more factors and thus has better prediction strength. Therefore, in terms of improving the validity of screening, we recommend using all six of these parameters together rather than any one of them alone to enhance the detection of poor prognosis trismus.
If the doses of massetor or pterygoid muscles are larger than 55 Gy, the incidences of trismus for head and neck patients were as high as 45% [15]. Additionally, there is an increased probability of trismus of 24% with every additional 10 Gy to the pterygoid muscle [5]. Practically, according to the previous report and data revealed here, the constraints of the masticator structures would be better below the cut-off values, emphasizing the lateral pterygoid muscle (42 Gy) and parotid gland (28 Gy). For those cases who cannot achieve this goal, the patients would experience a high risk of developing poor-prognosis trismus and some preventive management should be given. Further, if the product of six month SA scores with the mean dose of masticator muscles or the twelve month SA score itself exceeds the proposed cut-off at the 6th or 12th month follow-up after RT, aggressive treatment or intervention for the trismus should be prescribed to facilitate disease improvement.
There are some limitations to the current study. First, due to the nature of retrospective study, we were unable to match the age and gender in each of our groups. In addition, we did not measure the mean incisor distance of each patient and the trismus severity grading system we used may be affected by other factors. Finally, the patients enrolled in the current study should experience post-RT period for more than 2 years due to the deterioration of radiation-induced trismus occurring within 24 months after RT limited the number of patients. All these factors could possibly cause some bias in our results.
MRI SA score and the radiation dose of masticators muscles are useful in predicting trismus severity per se and its prognosis in OCC patients after RT or CCRT. Though more investigations are mandatory to validate our results, the radiation and imaging parameters may serve as a powerful tool to guide the management of trismus after radiation therapy. In addition, one may prevent the development of poor-prognostic trismus by the constraints suggested here, especially on the lateral pterygoid muscles and also parotid glands. If the radiation dose of specific structures or the SA scores at six and twelve months are higher than the proposed cutoffs, more aggressive treatment for trismus is suggested. Long-term follow-up and large prospective studies are needed to confirm these preliminary findings.
Acknowledgments
Special thanks to Dr. Chien-Hao Chen for his astute statistical consultation.
Funding Statement
This work was supported by the Far Eastern Memorial Hospital grants (FEMH-2012-C-055; FEMH 101-2314-B-418-010-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wang CJ, Huang EY, Hsu HC, Chen HC, Fang FM, et al. (2005) The degree and time-course assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope 115: 1458–1460. [DOI] [PubMed] [Google Scholar]
- 2. Johnson J, van As-Brooks CJ, Fagerberg-Mohlin B, Finizia C (2010) Trismus in head and neck cancer patients in Sweden: incidence and risk factors. Med Sci Monit 16: CR278–282. [PubMed] [Google Scholar]
- 3. Chua DT, Lo C, Yuen J, Foo YC (2001) A pilot study of pentoxifylline in the treatment of radiation-induced trismus. Am J Clin Oncol 24: 366–369. [DOI] [PubMed] [Google Scholar]
- 4. Qin DX, Hu YH, Yan JH, Xu GZ, Cai WM, et al. (1988) Analysis of 1379 patients with nasopharyngeal carcinoma treated by radiation. Cancer 61: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 5. Dijkstra PU, Kalk WW, Roodenburg JL (2004) Trismus in head and neck oncology: a systematic review. Oral Oncol 40: 879–889. [DOI] [PubMed] [Google Scholar]
- 6. Bhatia KS, King AD, Paunipagar BK, Abrigo J, Vlantis AC, et al. (2009) MRI findings in patients with severe trismus following radiotherapy for nasopharyngeal carcinoma. Eur Radiol 19: 2586–2593. [DOI] [PubMed] [Google Scholar]
- 7. Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, et al. (2010) A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer 18: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 8. Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, et al. (2008) Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck 30: 622–630. [DOI] [PubMed] [Google Scholar]
- 9. Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14: 199–212. [DOI] [PubMed] [Google Scholar]
- 10. Chen WC, Hwang TZ, Wang WH, Lu CH, Chen CC, et al. (2009) Comparison between conventional and intensity-modulated post-operative radiotherapy for stage III and IV oral cavity cancer in terms of treatment results and toxicity. Oral Oncol 45: 505–510. [DOI] [PubMed] [Google Scholar]
- 11. Gomez DR, Zhung JE, Gomez J, Chan K, Wu AJ, et al. (2009) Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys 73: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 12. Yao M, Chang K, Funk GF, Lu H, Tan H, et al. (2007) The failure patterns of oral cavity squamous cell carcinoma after intensity-modulated radiotherapy-the university of iowa experience. Int J Radiat Oncol Biol Phys 67: 1332–1341. [DOI] [PubMed] [Google Scholar]
- 13. Hsiung CY, Huang EY, Ting HM, Huang HY (2008) Intensity-modulated radiotherapy for nasopharyngeal carcinoma: the reduction of radiation-induced trismus. Br J Radiol 81: 809–814. [DOI] [PubMed] [Google Scholar]
- 14. Chen YY, Zhao C, Wang J, Ma HL, Lai SZ, et al. (2011) Intensity-modulated radiation therapy reduces radiation-induced trismus in patients with nasopharyngeal carcinoma: a prospective study with >5 years of follow-up. Cancer 117: 2910–2916. [DOI] [PubMed] [Google Scholar]
- 15. Louise Kent M, Brennan MT, Noll JL, Fox PC, Burri SH, et al. (2008) Radiation-induced trismus in head and neck cancer patients. Support Care Cancer 16: 305–309. [DOI] [PubMed] [Google Scholar]
- 16. Hsieh CH, Kuo YS, Liao LJ, Hu KY, Lin SC, et al. (2011) Image-guided intensity modulated radiotherapy with helical tomotherapy for postoperative treatment of high-risk oral cavity cancer. BMC Cancer 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Axel L (1987) Revised glossary of MR terms. Radiology 162: 874. [DOI] [PubMed] [Google Scholar]
- 18. Council on Scientific Affairs (1989) Musculoskeletal applications of magnetic resonance imaging. JAMA: the journal of the American Medical Association 262: 2420–2427. [PubMed] [Google Scholar]
- 19. Keeling AN, Flaherty JD, Davarpanah AH, Ambrosy A, Farrelly CT, et al. (2011) Coronary multidetector computed tomographic angiography to evaluate coronary artery disease in liver transplant candidates: methods, feasibility and initial experience. Journal of cardiovascular medicine 12: 460–468. [DOI] [PubMed] [Google Scholar]
- 20. Jansma J, Vissink A, Bouma J, Vermey A, Panders AK, et al. (1992) A survey of prevention and treatment regimens for oral sequelae resulting from head and neck radiotherapy used in Dutch radiotherapy institutes. Int J Radiat Oncol Biol Phys 24: 359–367. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Chen M, Zhao C, Lu LX, Han F, et al. (2007) [Radiation-induced temporomandibular joint damage in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy]. Ai zheng = Aizheng = Chinese journal of cancer 26: 64–67. [PubMed] [Google Scholar]
- 22. Steelman R, Sokol J (1986) Quantification of trismus following irradiation of the temporomandibular joint. Missouri dental journal 66: 21–23. [PubMed] [Google Scholar]
- 23. Goldstein M, Maxymiw WG, Cummings BJ, Wood RE (1999) The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 88: 365–373. [DOI] [PubMed] [Google Scholar]
- 24. van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Rasch CR, et al. (2011) A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: feasibility, compliance, and short-term effects. Dysphagia 26: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Israel HA, Ward JD, Horrell B, Scrivani SJ (2003) Oral and maxillofacial surgery in patients with chronic orofacial pain. J Oral Maxillofac Surg 61: 662–667. [DOI] [PubMed] [Google Scholar]