Abstract
Acanthamoeba is a free-living amoeba commonly present in the environment and often found in human airway cavities. Acanthamoeba possesses strong proteases that can elicit allergic airway inflammation. To our knowledge, the aeroallergenicity of Acanthamoeba has not been reported. We repeatedly inoculated mice with Acanthamoeba trophozoites or excretory-secretory (ES) proteins intra-nasally and evaluated symptoms and airway immune responses. Acanthamoeba trophozoites or ES proteins elicited immune responses in mice that resembled allergic airway inflammation. ES proteins had strong protease activity and activated the expression of several chemokine genes (CCL11, CCL17, CCL22, TSLP, and IL-25) in mouse lung epithelial cells. The serine protease inhibitor phenyl-methane-sulfonyl fluoride (PMSF) inhibited ES protein activity. ES proteins also stimulated dendritic cells and enhanced the differentiation of naive T cells into IL-4-secreting T cells. After repeated inoculation of the protease-activated receptor 2 knockout mouse with ES proteins, airway inflammation and Th2 immune responses were markedly reduced, but not to basal levels. Furthermore, asthma patients had higher Acanthamoeba-specific IgE titers than healthy controls and we found Acanthamoeba specific antigen from house dust in typical living room. Our findings suggest that Acanthamoeba elicits allergic airway symptoms in mice via a protease allergen. In addition, it is possible that Acanthamoeba may be one of the triggers human airway allergic disease.
Introduction
An airway allergic reaction is characterized by the synthesis of allergen-specific immunoglobulin (Ig) E and Th2 cytokines, such as interleukin (IL)-4, IL-5, and IL-13, which lead to the recruitment and sensitization of effector cells such as eosinophils, basophils, and mast cells [1]. Antigen recognition and uptake by innate immune cells is the first step in the process of antigen presentation, which can lead to the initiation of the adaptive immune response [2]. The allergic cascade starts with the recognition of allergens by antigen presenting cells, mainly dendritic cells (DCs); leads to Th2 polarization and a switch to IgE production by B cells; and culminates in mast cell sensitization and triggering [2]. Antigens are recognized by a diverse set of pattern recognition receptors, such as Toll-like receptors, C-type lectin receptors, and protease-activated receptor (PAR) 2 on immune or non-immune cells [2], [3]. Allergic reactions are induced by various environmental allergens. Many allergens exhibit intrinsic protease activities, and some proteases from infectious agents, parasites, and fungi have been identified as potent allergens [4]–[8]. These protease allergens induce Th2 immune reactions by activating several chemokines and cytokines [8]. Thus, protease activities may be critical to the initiation of allergic responses.
Recently, some proteolytic allergens were shown to cause the breakdown of epithelial barriers through mechanisms mediated by PAR2. Activation of PAR promotes cytokine production and leukocyte activation [9], [10]. PARs belong to the recently described family of G protein-coupled seven transmembrane domain receptors [11], [12]. PARs are activated via the proteolytic cleavage of their N-terminal domain by proteinases. Cleavage generates a new N-terminal “tethered ligand,” which can autoactivate receptor activity [13]. Four members of the PAR family have been cloned thus far. PAR1, PAR3, and PAR4 are activated by thrombin, and PAR2 is activated by serine proteases, including trypsin, tissue kallikreins, coagulation factors VIIa and Xa, mast cell tryptase, and transmembrane serine proteases [14]. PAR1 and PAR2 are widely distributed in the airway, including within human nasal primary epithelial cells. PAR2 acts primarily as a pro-inflammatory molecule in the respiratory system, and it can be activated by exogenous proteinase allergens [15].
We introduced the possibility of free-living amoebae (FLA) as new aeroallergens. One FLA, Acanthamoeba, is an opportunistic protozoan widely distributed in the environment. Acanthamoeba can cause serious human infections, including blinding keratitis and fatal encephalitis [16]. Acanthamoeba species have been isolated from public water supplies, swimming pools, air-conditioning units (cooling towers), sewage, compost, sediments, soil, beaches, vegetables, surgical instruments, contact lenses and their cases, and the atmosphere [16]. In addition, Acanthamoeba species have been recovered from human nasal cavities, pharyngeal swabs, lungs tissues, and skin lesions [16]–[18]. Unsurprisingly, anti-Acanthamoeba antibodies have been identified in the majority of healthy individuals tested, indicating that exposure to FLA is common [19]. In addition, many proteases, including serine and cysteine proteases, have been isolated from Acanthamoeba [20]–[23]. A central role for these proteases in diverse processes, such as differentiation, host cell invasion and egress, catabolism of host proteins, cyto-adherence, and stimulation and evasion of host immune responses, has been proposed [23]–[25]. However, despite the ubiquitous presence of Acanthamoeba in the environment and its possession of proteases strong enough to elicit allergic airway inflammation, to our knowledge, no report on the role Acanthamoeba in allergic airway inflammation has been published to date.
In this study, we repeatedly inoculated mice intranasally with Acanthamoeba trophozoites or excretory-secretory (ES) proteins and evaluated symptoms and immune responses. In addition, we investigated the role of DCs and PAR2 in airway inflammation induced by Acanthamoeba.
Materials and Methods
Acanthamoeba cultivation and ES protein preparation
Acanthamoeba lugdunensis KA/WP6 strain, isolated from domestic tap water in Korea, was maintained in PYG medium [26]. The KA/WP6 strain has the same molecular characteristics as the A. lugdunensis L3A strain (ATCC 50240) [26]. To obtain ES proteins, live trophozoites were incubated in PYG medium for one week at 25°C. Following centrifugation at 12,000×g for 30 min, supernatants were concentrated using 3000-Da centrifugal filter units (Millipore Co., Billerica, Massachusetts, USA). HiTrap Desalting™ (GE Healthcare, Little Chalfont, United Kingdom) was performed to eliminate excess salts from the collected medium. The samples were then dialyzed against PBS. After obtaining ES proteins, the ToxinSensor Gel Clot Endotoxin Assay Kit (GenScript, Piscataway, New Jersey, USA) was used to eliminate endotoxins.
Animals and experimental design
Female C57BL/6 mice (six weeks old) were purchased from Samtako Co. (Gyeonggi-do, Korea). PAR2−/− mice (C57BL/6 background) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and were bred in a specific pathogen-free facility at the Institute for Laboratory Animals of Pusan National University. Mice in the infected group were administered 100 (low dose) or 5×104 (high dose) A. lugdunensis trophozoites intranasally on days 0, 2, 4, 6, 8, and 10. Using the same protocol, mice in the other groups were treated with 20 μg of ES proteins or PMSF pre-treated ES proteins under light anesthesia using Zoletil 50 (Virbac Laboratories, Courbevoie Cedex, France). On the day after the last infection, airway hypersensitivity reactions were measured, and animals were sacrificed. Serum was collected by cardiac puncture, and lung-draining lymph nodes (LLNs) were excised. Lymphocytes were isolated from LLNs as previously described [27]. Briefly, LLNs were disrupted and treated with ammonium-chloride-potassium (ACK) hypotonic lysis solution to lyse red blood cells. The remaining cells were filtered through a 100-μm mesh and plated in 48-well plates. For CD3 stimulation experiments, 0.5 μg/ml of CD3 antibody was added to the plated cells. These cells were incubated for 72 hr at 37°C in a 5% CO2 atmosphere. Following incubation, the culture medium were harvested and stored at −20°C for ELISA.
Analysis of bronchoalveolar lavage fluid (BALF) and differential cell counting
To obtain BALF, the tracheas of anesthetized mice were exposed and cut just below the larynx. A polyurethane flexible tube (outer diameter: 0.4 mm, length: 4 cm; BD Biosciences, San Jose, California, USA) attached to a blunt 24-gauge needle was placed in the trachea, and the lung was lavaged once with 800 μl of sterile, warm PBS. BALF samples were centrifuged for 5 min at 1500 rpm at 4°C. Supernatants were then decanted and immediately frozen at −70°C. Cell pellets were resuspended and washed twice in PBS. Total cell numbers were counted using a hemocytometer. To determine the differential cells counts, BALF cell smears were prepared with a cytospin apparatus and stained with Diff-Quik solution (Sysmex Co., Kobe, Japan), in accordance with conventional morphological criteria. At least 500 cells per slide were evaluated to obtain differential leukocyte counts.
ELISA
ELISA was used to determine the levels of total IgE and Acanthamoeba-specific IgE in sera and the levels of IL-4, IL-5, IL-13, IFN-γ and IL-17A in BALF, the culture supernatants of lymphocytes from LLNs, and the supernatants of naive T cells co-cultured with BMDCs, according to the manufacturer's instructions (eBioscience, San Diego, California, USA). To estimate the level of Acanthamoeba-specific IgE, 96-well plates (Nunc, Roskilde, Denmark) were coated with ES proteins (final concentration: 1 μg/ml) in 0.1 M sodium carbonate buffer, pH 9.6, at 37°C until dry. The absorbance was measured at 450 nm using an ELISA plate reader. To eliminate the possibility of cross-reactions between Acanthamoeba and known allergens, the sera were reacted with Der P1 and Aspergillus protease for 30 min before ELISA.
Lung histopathology
Histological analyses were conducted as described previously [28]. In brief, lung tissues were fixed with formaldehyde and embedded in paraffin. Thin sections of the embedded tissues were then stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) and examined microscopically.
Zymography for detection of protease activity
A previously described method was used for zymography analysis [23], [29]. Briefly, a gel containing 0.1% gelatin was prepared. ES proteins treated with or without protease inhibitors were loaded onto the gelatin gel and separated by electrophoresis with 1× Tris-glycine SDS running buffer (0.025 M Tris, 0.192 M glycine, pH 8.5, 0.1% SDS) at 125 V for 2 h. The gels were rinsed several times, incubated in renaturing buffer, and then stained with Coomassie brilliant blue, as described previously [29]. Clear bands against a stained background indicated regions of gelatin digestion by proteases.
Analysis of Th2 related gene expression in lung epithelial cells
Mouse lung epithelial cells (MLE12 cells) were obtained from ATCC (Manassas, VA, USA). Real-time PCR was performed using an iCycler™ (Bio-Rad, Hercules, California, USA) to determine the levels of CCL17 (thymus and activation-regulated chemokine, TARC), CCL22 (macrophage-derived chemokine, MDC), CCL11 (eotaxin), thymic stromal lymphopoietin (TSLP), and IL-25 mRNA. GAPDH was used as an internal reference. Primers and PCR conditions have been described previously [30]–[32].
Generation of bone marrow-derived DCs (BMDCs)
BMDCs were differentiated from bone marrow cells by methods previously reported, with modification [33], [34]. In brief, bone marrow cells were flushed from the femurs and tibias of 7-week-old C57BL/6 mice, washed, and cultured in complete RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 50 μM 2-mercaptoethanol, 2 mM glutamine, penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively; Invitrogen, Carlsbad, California, USA), and recombinant mouse granulocyte macrophage colony-stimulating factor (GMC-SF) and recombinant mouse IL-4 (10 ng/ml each; R&D Systems, Minneapolis, Minnesota, USA). Non-adherent granulocytes were removed after 24 hr of culture, and fresh complete medium was added every other day. All cultures were incubated at 37°C in 5% CO2. After 7 days of culture, >80% of the cells expressed characteristic DC-specific markers (CD11c+) as determined by flow cytometry.
In vitro BMDC stimulation assay
Immature BMDCs were cultivated in 6-well plates at 5×106 cells/well in 3 ml of complete RPMI 1640 medium enriched with 10% FBS and 40 ng/ml GM-CSF. BMDCs were stimulated with 1 μg/ml of LPS or 5 μg/ml of ES proteins for 48 hr. BMDCs stimulated with ES proteins were then incubated with the following anti-mouse antibodies: FITC-conjugated anti-CD11c and PE-conjugated anti-MHCII, -CD40, -CD80 and -CD86 (all from eBioscience). Flow cytometry was performed using a FACS Canto II cytometer (BD Biosciences) equipped with Canto software.
Naive T-cell differentiation and cytokine production
After BMDCs were stimulated with ES proteins or LPS, they were incubated with naive T cells. Th2 differentiation was analyzed by FACS and ELISA. BMDCs (100 μl) were seeded in round-bottom wells of 96-well culture plates at 2×105 cells/well and were stimulated with 5 μg/ml ES proteins or 1 μg/ml LPS for 48 hr at 37°C. Naive T cells were isolated from the spleens and lymph nodes of C57BL/6 mice using a CD4+CD62L+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The isolation of naive T cells was a two-step process. First, non-CD4+ T cells were depleted in a negative selection step by incubating cells with a cocktail of biotin-conjugated antibodies and anti-biotin microbeads. Second, CD4+CD62L+ T cells were labeled with CD62L microbeads and isolated by positive selection. Isolated naive T cells were co-cultured with BMDCs at a BMDC:T cell ratio of 1∶5 for 72 hr at 37°C. Subsequently, for CD3 stimulation experiments, 0.5 μg/ml of CD3 antibody was added to the cells. Cells were incubated for 72 hr at 37°C. T cells were then incubated with GolgiPlug (BD Biosciences), stained with FITC-labeled anti-CD4 (eBioscience) in accordance with the manufacturer's recommended protocol, fixed, permeabilized, and stained intracellularly with PE-Cy7-labeled anti-mouse IL-4. The Th2 differentiation rate was analyzed by flow cytometry. Total RNA isolation and cDNA synthesis from co-cultured naïve T cells and perform real-time PCR to determine expression levels of T-bet, GATA-3, ROR-γ and Foxp3. GAPDH levels were used to normalize RNA content of samples.
Evaluation of Acanthamoeba-specific IgE levels in asthma patients
To investigate the Acanthamoeba-specific IgE levels in asthma patients, 25 patients diagnosed with asthma and 9 non-atopic healthy controls were recruited to the study from January to December 2010. Asthma was diagnosed based on history and positive airway hyperresponsiveness to methacholine challenge. Airway hyperresponsiveness was expressed as the drug concentration required to cause a 20% decrease in forced expiratory volume (FEV1) (PC20) in non-cumulative units, as determined by methacholine challenge testing, and was regarded as positive if the PC20 was <8 mg/ml. The level of Acanthamoeba-specific IgE in the serum was measured using ELISA inhibition tests, in accordance with the manufacturer's instructions (eBioscience). All assays were performed in duplicate. The cut-off value for detecting Acanthamoeba-specific IgE levels in serum was determined from the mean plus the two-fold SD of the absorbance values from non-atopic healthy controls. Competitive ELISA inhibition tests were performed to determine the allergenic cross-reactivity between Acanthamoeba and known allergens.
Detection of Acanthamoeba-derived antigen in house dust
House dust was collected from a bed mattress and carpet in an apartment using a vacuum (vacuum pore size: 0.34 mm). The house dust was dissolved in filtered PBS and mixed by vortexing and shaking for 1 hr. The house dust solution was stored at −70°C. Acanthamoeba-specific antigen from the house dust was detected by ELISA. A 96-well plate (Nunc) was coated at 37°C until dry with 5 μg/ml of house dust solution. After 3 washes with PBS containing 0.1% Tween 20 (Sigma), diluted serum (1∶500) from Acanthamoeba-infected mice was added to the plate and incubated for 2 hr at 37°C. After incubation, the Acanthamoeba-specific IgG1 level was estimated by ELISA.
Statistical analysis
All experiments were performed in triplicate. Student's t-test or ANOVA was used to calculate the mean ± SD and to determine significant differences. Statistical analysis was performed with PASW 18.0.
Ethics Statement
All animal studies were approved by the Pusan National University Animal Care and Use Committee (Approval No. PNU-2010-000165). Human serum specimen was provided by national biobank of Korea, PNUH. Written informed consent was obtained from each participant before enrollment. Participation was entirely voluntary. Participants were free to refuse to participate or withdraw from the study at any time, and were informed that only the aggregate data would be reported. Participants were also told that there would be no disadvantages for canceling their participation. The study protocol using the sample was approved by the Ethics Committee of Pusan National University Hospital (IRB Approval No. PNUH-2010-5). We used the same dust samples of previous study [35]. Dust was collected from private apartment houses after getting permission from people who lived in each house.
Results
Treatment with Acanthamoeba trophozoites elicits strong allergic airway inflammation
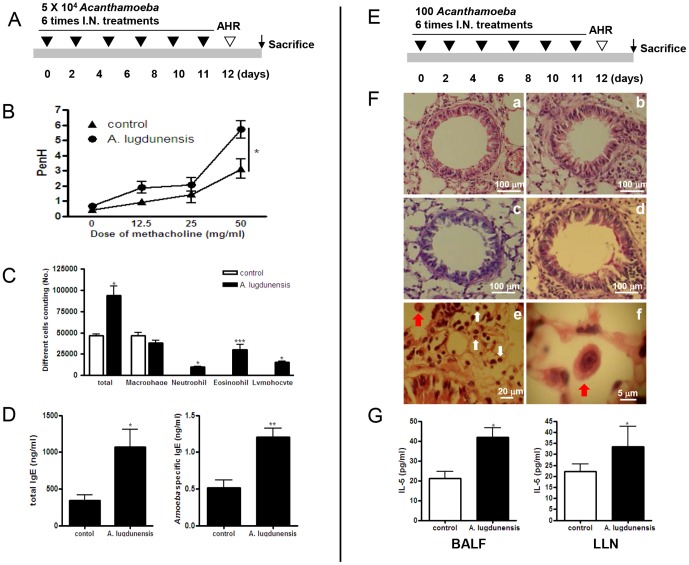
To demonstrate the effects of Acanthamoeba on allergic airway inflammation, we introduced Acanthamoeba trophozoites (5×104) into the airways of mice and assessed biological and pathological changes (Figure 1A). Dose-dependent increases in airway hyperresponsiveness to methacholine were observed in mice inoculated with Acanthamoeba trophozoites (Figure 1B). Inflammatory cell infiltration was also observed in the airways, and the numbers of eosinophils, neutrophils, and lymphocytes increased in BALF (Figure 1C). Histological examination of the lungs in the Acanthamoeba-treated group revealed massive inflammatory cell infiltration, bronchial epithelial cell hyperplasia, and goblet cell hyperplasia (Figure S1A). In addition, the levels of IL-4, -5, and -13 (Th2 cytokines) in BALF and in the culture medium of LLN T cells increased in the Acanthamoeba-infected group, compared with levels in the control group (Figure S1B). For Acanthamoeba-infected mice, the amount of IFN-γ level in LLNs increased, but IL-17 levels in BALF and LLNs did not change. Finally, marked increases in total serum IgE and Acanthamoeba-specific IgE were observed after Acanthamoeba infection (Figure 1D).
Figure 1. Acanthamoeba trophozoites induce allergic airway inflammation.
Allergic airway inflammation was induced by inoculation of mice with high (A–D) and low (E–G) doses of Acanthamoeba. (A) Intranasal inoculation schedule for the high-dose (4×105 Acanthamoeba trophozoites) model. (B) Airway resistance values in response to methacholine (0 to 50 mg/ml). (C) Differential cell count in 800 μl BAL after Diff-Quik staining. (D) Total and Acanthamoeba-specific IgE levels were measured in serum by ELISA. (E) Intranasal inoculation schedule for the low-dose (100 Acanthamoeba trophozoites) model. (F) Tissue inflammation observed on stained lung sections (a and c: PBS-treated; b and d: Acanthamoeba-infected; a and b: H&E-stained; c and d: PAS-stained; red arrows indicate Acanthamoeba trophozoites and white arrows indicate eosinophils). (G) Cytokine concentrations in BALF and in the culture medium of CD3-stimulated lymphocytes isolated from LLNs. (*p<0.05, **p<0.01, ***p<0.001; n = 7, three independent experiments).
To know the general infection dose of Acanthamoeba in nature system, we investigated previous reports about the average number of infected Acanthamoeba trophozoites in several environment sources. We determined infection dose of Acanthamoeba according to average number of Acanthamoeba trophozoites in 1 L contaminated domestic tap water [36]. Occasionally, we could find 1000 over Acanthmaoeba trophozoites in 1 L tap water. It might be possible that 100 Acanthamoeba trophozoites contact to normal person at one time who used Acanthamoeba contaminated tap water. To determine whether a low dose of Acanthamoeba elicited allergic airway inflammation, we intranasally administered 100 A. lugdunensis trophozoites into the airways of mice six times (Figure 1E). Histological examination showed mild infiltration of inflammatory cells into the lungs and hyperplasia of some bronchial epithelial cells after treatment with A. lugdunensis (Figure 1F-b). In addition, mucin production by goblet cells was slightly higher in infected mice than in control mice (Figure 1F-d). Some Acanthamoeba were observed in alveoli, and several eosinophils were detected around Acanthamoeba (Figure 1F-e and -f). PenH values slightly increased after methacholine administration in the low dose Acanthamoeba treated group, but it is not significant (Figure S2A). In airways, the proportion of immune cells increased slightly after Acanthamoeba administration; the number of eosinophils increased considerably (Figure S2B). In BALF and LLNs, IL-5 levels increased after Acanthamoeba administration (Figure 1G); however, IL-4 and IL-13 levels did not change (data not shown). Total serum IgE and Acanthamoeba-specific IgE did not increase after low-dose Acanthamoeba infection (Figure S2C).
The protease activity of ES proteins induces Th2 chemokine production in lung epithelial cells
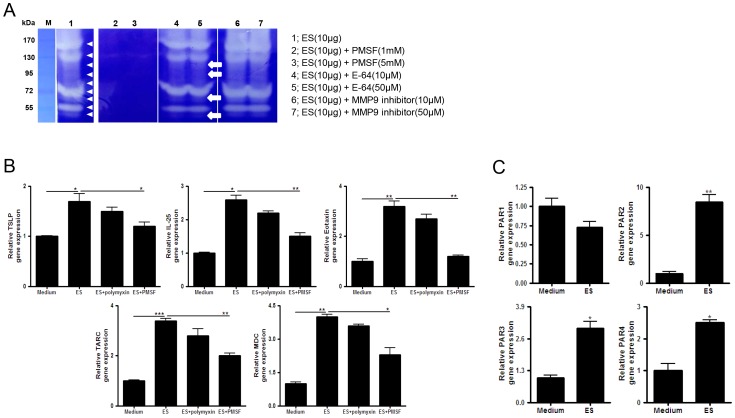
To evaluate the protease activities of ES proteins, we used zymogram analysis with several types of protease inhibitors. Several proteases in the ES protein population digested gelatin. Their activities were completely inhibited by the serine protease inhibitor PMSF. The strongest activity band was not affected by E-64 (cysteine protease inhibitor) treatment, but some weaker activity bands disappeared. The matrix metallopeptidase (MMP)-9 inhibitor did not inhibit the activity of most proteases (Figure 2A). Treatment with ES proteins increased TSLP, TARC, MDC, eotaxin, and IL-25 gene expression (known to be critical for the initiation and expansion of the Th2 response in lung epithelial cells) in MLE12 cells. The increase in gene expression was not inhibited by polymyxin B treatment but was significantly inhibited by serine protease inhibitors (Figure 2B). Treatment with ES proteins also increased PAR2, PAR3, and PAR4 mRNA levels in MLE12 cells. In particular, PAR2 expression was more than 8-fold higher in ES-treated cells than in control cells (Figure 2C).
Figure 2. ES proteins elicit Th2-related chemokine production in the absence of protease inhibition.
(A) ES proteins were treated with various protease inhibitors. The samples were incubated for 2 hr and were assayed by zymography on 0.1% gelatin SDS-PAGE gels (lane 1: 10 μg ES proteins; lane 2: with 1 mM PMSF; lane 3: with 5 mM PMSF; lane 4: with 10 μM E-64; lane 5: with 50 μM E-64; lane 6: with 10 μM MMP-9 inhibitor; lane 7: with 50 μM MMP-9 inhibitor; arrowhead: protease activity of ES proteins; arrow: protease activity inhibited by E64). (B) Th2-related chemokine gene expression (TSLP, TARC, MDC, eotaxin, and IL-25) was measured in MLE12 cells after incubation with 1 μg/ml of ES proteins (ES) for 2 hr or pre-treatment with 0.1 mM PMSF and 10 μg/ml polymyxin B (polymyxin) for 2 hr. (C) The fold-change in PAR mRNA levels in MLE12 cells treated with ES proteins relative to those treated with medium, as detected by real-time RT-PCR. (*p<0.05, **p<0.01, ***p<0.001; three independent experiments).
ES proteins accelerate the differentiation of Th2 cells into CD4+T cells by activating DCs
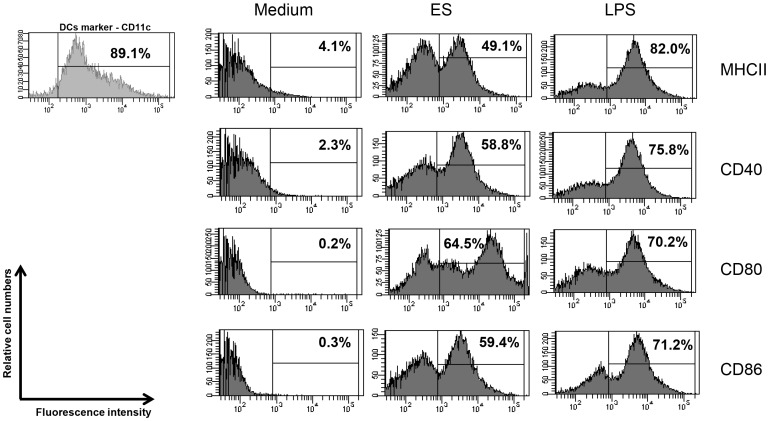
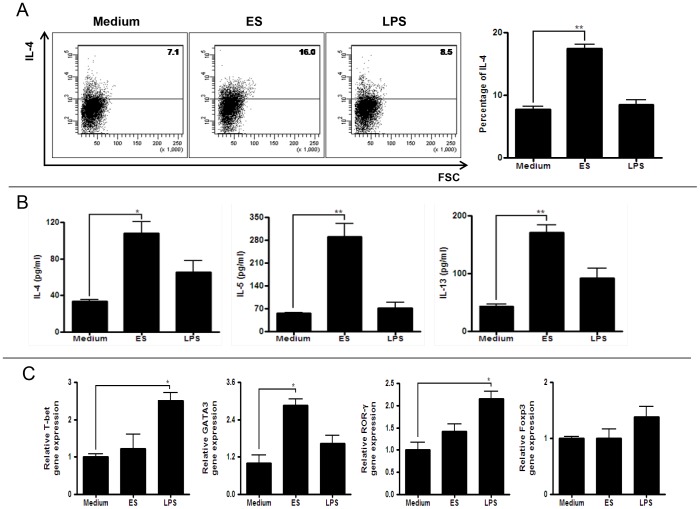
Activated DCs are characterized by increased expression of co-stimulatory molecules (e.g., CD80 and CD86), MHCII and CD40 on their surface [37]. We cultured DCs (89% cells expressed CD11c, maturation marker) in the presence or absence of ES proteins or LPS (positive control) and analyzed surface markers using flow cytometry. The results showed that ES proteins stimulated DCs: the expression of MHCII, CD40, CD80, and CD86 was higher than in DCs cultured in medium alone (Figure 3). When ES protein-activated DCs were co-cultured with naive T cells (CD4+CD25−CD62L+ T cells), the number of IL-4-secreting CD4+ T cells (Th2 cells) was higher than in the control group (medium-treated group) (Figure 4A). In addition, IL-4, IL-5, and IL-13 production by CD4+ T cells increased when the cells were co-cultured with ES protein-activated DCs (Figure 4B). Furthermore, co-cultured naïve T cells with ES proteins treated DCs highly expressed GATA-3 transcription factor. (Figure 4C).
Figure 3. Activation of BMDCs by ES proteins.
Expression of cell surface markers (MHCII, CD40, CD80, and CD86) on mouse BMDCs pulsed with ES proteins or LPS for 48 hr, compared with expression in untreated cells. (three independent experiments).
Figure 4. Differentiation of Th2 cells from naive T cells after co-cultivation with BMDCs activated by ES proteins.
(A) Naive T cells were cultured with stimulated BMDCs by ES proteins or LPS or non-stimulated BMDCs for 3 days in the presence of anti-CD3 antibody. After gated with CD4+ T cells, IL-4 producing T cells were counted. (Medium: naive T cell with non-stimulated BMDCs; ES: naive T cell with BMDCs stimulated with ES proteins; LPS: naive T cell with BMDCs stimulated with LPS). (B) Cytokine levels in the supernatants from naive T cell and BMDC co-cultures were measured by ELISA. (C) The gene level was evaluated by realtime-RT PCR from naive T cell and BMDC co cultures (*p<0.05, **p<0.01, ***p<0.001; three independent experiments).).
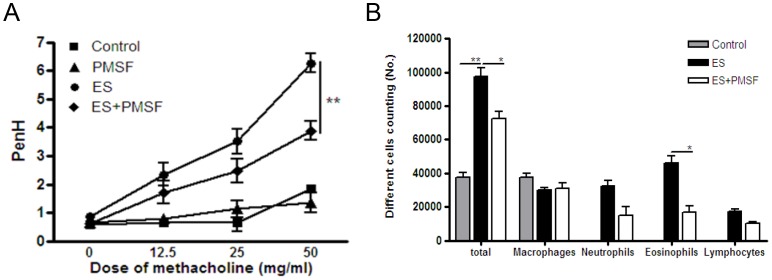
Serine protease dependant manner ES proteins
ES proteins induce severe allergic airway inflammation by serine protease dependant manner, it's effective in part via PAR2
In order to know in vivo effects of serine protease inhibitor on ES protein induced inflammation, we treated ES protein with 20PMSF and evaluated airway inflammation. Most of inflammation index by ES protein treatment was significantly decreased by PMSF treatment. The airway hyper responsiveness to methacholine for ES protein were significantly decreased by treatment with PMSF (Figure 5A). Also, the infiltration of immune cells decreased after treated with PMSF, compared with only ES proteins treatment group; especially, the number of eosinophils decreased significantly (Figure 5B).
Figure 5. ES protein-induced allergic airway inflammation was inhibited by PMSF treatment.
(A) Airway resistance values in response to methacholine (0 to 50 mg/ml). (B) Differential cell count in 800 μl BAL after Diff-Quik staining. (*p<0.05, **p<0.01, ***p<0.001; n = 15).
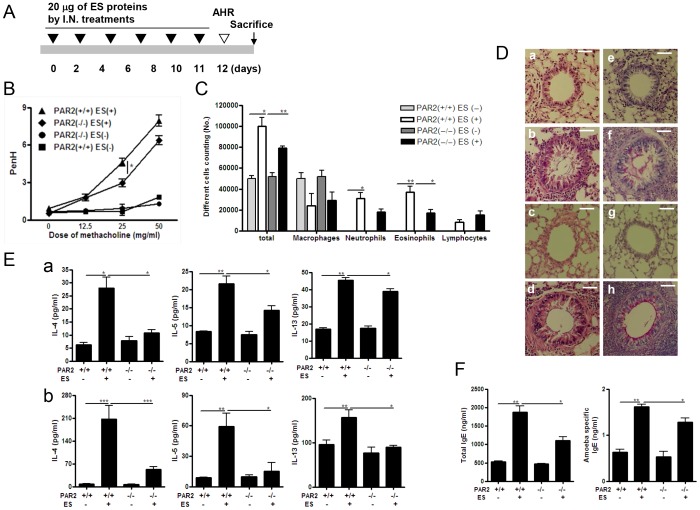
To determine whether PAR2 mediates the allergic airway inflammation induced by ES proteins of Acanthamoeba, we inoculated PAR2 knockout (KO) and wild-type (WT) mice with Acanthamoeba ES proteins six times (Figure 6A). For WT and PAR2 KO mice, PenH values increased after methacholine administration in the ES protein-treated group, compared with values in the control group (Figure 6B); however, responsiveness was slightly lower in the KO mice (Figure 6B). From WT and PAR2 KO mice, the numbers of inflammatory cells, particularly eosinophils and neutrophils, was significantly higher in the ES protein-treated group than in the control group (Figure 6C); however, the number of infiltrating eosinophils was lower in the KO mice (Figure 6C). The lungs of WT and KO mice administered ES proteins showed significant immune cell infiltration around the bronchial tracts, enhanced mucin production, and hyperplasia of lung epithelial cells and goblet cells (Figure 6D). The levels of IL-4, -5, and -13 in BALF and LLNs increased when mice were treated with ES proteins; however, the cytokine levels were lower in the PAR2 KO mice (Figure 6E). IL-17 and IFN-γ levels in BALF and LLNs were not affected by ES protein treatment in any of the experimental groups (data not shown). Finally, total serum IgE and Acanthamoeba-specific IgE levels were markedly higher when mice were treated with ES proteins, but amoeba-specific IgE levels in PAR2 KO mice were lower than in WT mice (Figure 6F).
Figure 6. ES protein-induced allergic airway inflammation is reduced in PAR2 KO mice.
(A) Intranasal inoculation schedule for the ES protein model. (B) Airway resistance values in response to methacholine (0 to 50 mg/ml). (C) Differential cell count in 800 μl BAL after Diff-Quik staining. (D) Tissue inflammation observed on stained lung sections (a and e: PBS-treated PAR2+/+ mice; b and f: ES protein-treated PAR2+/+ mice; c and g: PBS-treated PAR2−/− mice; d and h; ES protein-treated PAR2−/− mice; a, b, c and d: H&E-stained; e, f, g and h: PAS-stained). (E) Cytokine concentrations in BALF (a) and in the culture medium of CD3-stimulated lymphocytes isolated from LLNs (b) were measured. (F) Total and Acanthamoeba-specific IgE levels were measured in serum by ELISA. (*p<0.05, **p<0.01, ***p<0.001; n = 3∼5, three independent experiments).).
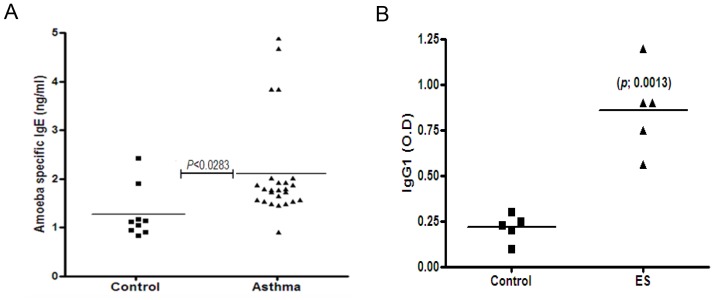
Asthma patients have a high level of Acanthamoeba-specific IgE
To determine whether Acanthamoeba causes airway allergic reactions in humans, we evaluated Acanthamoeba-specific IgE levels in patients with asthma. The average IgE level in asthma patients was significantly higher (p = 0.028) than that in healthy controls (Figure 7A).
Figure 7. Acanthamoeba-specific IgE levels in asthma patients and Acanthamoeba-specific antigens in house dust.
(A) After serum absorption with Der P1 and Aspergillus protease, the levels of Acanthamoeba-specific IgE in the sera of asthma patients were evaluated by ELISA. (B) Control: sera of 9 healthy individuals; Asthma: sera of 25 asthma patients. Immune-plates were coated with 5 μg/ml of house dust solution and then incubated with diluted serum (1:500) from Acanthamoeba-infected mice. Specific IgG1 levels were estimated by ELISA. (*p<0.05, **p<0.01, ***p<0.001).
Acanthamoeba-specific antigens are present in house dust
To assess whether ES proteins are present in domestic environments, we collected 5 house dust samples from typical living rooms. The house dust samples were incubated with serum from Acanthamoeba-infected mice or uninfected mice. After incubation with dust proteins, the IgG1 level in serum from Acanthamoeba-infected mice was higher than the IgG1 level in serum from of uninfected mice (Figure 7B).
Discussion
The free-living amoeba Acanthamoeba is abundantly present in our environment. Acanthamoeba produces strong proteases, indicating that the amoeba might be a new allergenic agent. However, there are no reports to this effect. In this study, we investigated the ability of Acanthamoeba to elicit allergic airway inflammation in vivo and assessed whether Acanthamoeba culture products (ES proteins) induce a Th2 response. Principally, we found that Acanthamoeba and its culture products elicited allergic airway inflammation responses in experimental mice. ES proteins included many proteases, which seemed to have a central role in the Th2-mediated airway inflammation response in vivo and in vitro. ES proteins promoted DC maturation and activation, and the activated DCs promoted the differentiation of T cells into a Th2 cell population.
The proteases of Acanthamoeba have been previously demonstrated; they are required to maintain the Acanthamoeba life cycle [20], [23], [38]. Serine protease, the most abundant Acanthamoeba ES protein, is crucial for the encystment and excystment of Acanthamoeba [23]. Subtilisin and elastase, which are asthma inducers, have also been identified as Acanthamoeba ES proteins [39], [40]. Subtilisin has been isolated from several other organisms, including Curvularia lunata, Bacillus subtilis, Epicoccum purpurascens, and Penicillium citrinum. Subtilisin elicits specific antibody production in mice and produce immediate and late onset allergic reactions in allergy patients [41]–[43]. In this study, using gelatin zymogram analysis, we also found that the major protease activity originated from serine protease (Figure 2). Inhibition of serine protease activity by specific inhibitors suppressed the expression of most chemokine genes with Th2 promoters, including eotaxin, MDC, and TARC, in lung epithelial cells (Figure 2). Our results show that ES proteins include several strong protease allergens that could elicit a Th2 response.
The induction of allergic airway inflammation by protease allergens occurs via several known mechanisms. In one mechanism, a protease allergen can activate DCs and induce IL-25, TSLP, and allergy-related chemokines [44]. In this study, Acanthamoeba ES proteins induced the activation of DCs, which accelerated the differentiation of naive CD4+ T cell into Th2 cells (Figure 3 & 4). Th2 cells are critical for the induction of allergic asthma via production of IL-4, IL-5, IL-13, and eotaxin [45], [46]. In addition, Th2 cells express CCR3, CCR4, and CCR8. In vitro studies have shown that MDC and TARC, high-affinity CCR4 ligands, induce the selective migration of Th2 cells [47] and IL-25-mediated Th2 responses in eosinophilia [45]. The results of this study show that Acanthamoeba contains ES proteins with serine protease activity that stimulate DCs and thereby promote the differentiation of naive T cells into Th2 cells (Figure 2, 3 & 4).
A second mechanism for the induction of allergic symptoms by protease allergens involves the digestion of cell surface molecules and tissue destruction. Der P1, a cysteine protease from the house dust mite, can cleave human cell surface molecules, including the low-affinity IgE receptor (CD23/FcεRII), the alpha-subunit of the IL-2 receptor (CD25), and the protease inhibitor alpha1-antitrypsin [48]. Proteolytic degradation of tight junctions in lung epithelium and the release of proinflammatory cytokines from bronchial epithelial cells, mast cells, and basophils in response to Der P1 have been reported [49]. In addition, the fungus Alternaria alternata is associated with asthma; its serine protease activity can disrupt the lung epithelial barrier [50]. However, we do not know whether Acanthamoeba ES proteins can cleave human cell surface molecules and/or cause tissue destruction in the manner of Der P1. Thus, further investigation is required to elucidate the exact mechanisms by which ES proteins induce the allergic response.
In a third mechanism for the induction of allergic symptoms by protease allergens, some proteases signal through PAR2 [3], [4], [11], [14], [51]. The effects of PAR2 activation on leukocyte motility, cytokine production, adhesion molecule expression, and a variety of other physiological or pathophysiological functions in vitro and in vivo have been described [4], [51], [52]. In in vivo experiments using PAR2 KO mice, we demonstrated that Acanthamoeba ES proteins elicited allergic airway inflammation via PAR2 (Figure 5). Production of Th2 cytokines in the spleen and LLNs and the eosinophil infiltration rate in the lung were significantly lower in ES protein-treated PAR2 KO mice than in ES protein-treated WT mice (Figure 5). In order to know how ES proteins interact with PAR2 molecule indirect/direct and what is key molecules in ES proteins on PAR2 activation, we need more detailed information from further studies. Although most clinical indices of allergic airway inflammation were reduced in PAR2 KO, few reached the basal level. In the present study, ES proteins upregulated PAR2 expression 8-fold over control levels. They also activated PAR3 and PAR4 gene expression in lung epithelial cells (Figure 2C). Thus, although signaling via PAR2 was blocked in PAR2 KO mice, ES proteins might have activated the Th2 response via PAR3, PAR4, or other receptors.
Finally, Acanthamoeba and ES proteins significantly induced also neutrophilic inflammation in airway, it has the possibility related with Th17 cell mediate steroid resistant airway inflammation. However, Th17 response in Acanthamoeba and ES treatment was not significantly increased and we have little information about therapeutic effects of steroids on Acanthamoeba induced asthma model. The detailed mechanisms of neutrophilic inflammation by Acanthamoeba airway inflammation model will be reviled in next studies.
The KA/WP6 Acanthamoeba strain used in this study was isolated from domestic tap water. Genetically similar strains have been isolated from contact lens storage cases, swimming pools, air conditioning systems, and the corneas of amoebic keratitis patients [26]. We also detected Acanthamoeba antigens in house dust (Figure 6B), indicating that Acanthamoeba can be encountered in domestic environments, similar to house dust mite allergens. Therefore, it is not surprising that most healthy individuals possess specific IgG against Acanthamoeba [53]. However, to our knowledge, the levels of anti-Acanthamoeba IgE in human serum have not been previously reported. Of particular interest, we observed significantly higher Acanthamoeba-specific IgE levels in asthma patients than in healthy controls (Figure 6A). These results indicate that Acanthamoeba might be a novel human allergen.
In summary, we found that Acanthamoeba trophozoites and ES proteins elicited asthma-like symptoms and promoted Th2 responses by activating DCs via PAR2 signaling in a mouse model. In addition, we observed that asthma patients had higher Acanthamoeba-specific IgE titers than healthy controls. In conclusion, we suggest that future studies attempt to identify specific major allergens among the ES proteins of Acanthamoeba, which could aid in the diagnosis of allergic airway conditions.
Supporting Information
High-dose Acanthamoeba trophozoite infection induces severe allergic airway inflammation. (A) Tissue inflammation observed on stained lung sections (a and c: PBS-treated; b and d: Acanthamoeba-infected; a and b, H&E-stained; c and d, PAS-stained). (B) Cytokine concentrations in BALF and in the culture medium of CD3-stimulated lymphocytes isolated from LLNs were measured. (*p<0.05, ** <0.01, ***p<0.001).
(PPT)
Eosinophils are recruited to the airway by low-dose Acanthamoeba infection. (A) Airway resistance values in response to methacholine (0 to 50 mg/ml). (B) Differential cell count in 800 μl BAL after Diff-Quik staining. (C) Total and Acanthamoeba-specific IgE levels were measured in serum by ELISA. (*p<0.05, **p<0.01, ***p<0.001).
(PPT)
Acknowledgments
We thanks to Prof. Yun-Keun Kim, he kindly provide dust samples to us.
Funding Statement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2042979). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Paul WE, Zhu J (2010) How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 10: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salazar F, Ghaemmaghami AM (2013) Allergen recognition by innate immune cells: Critical role of dendritic and epithelial cells. Front Immunol 4: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, et al. (2011) Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol 300: L605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho HJ, Lee HJ, Kim SC, Kim K, Kim YS, et al. (2012) Protease-activated receptor 2-dependent fluid secretion from airway submucosal glands by house dust mite extract. J Allergy Clin Immunol 129: : 529–35, 535.e1-5. [DOI] [PubMed] [Google Scholar]
- 5. Roelandt T, Heughebaert C, Hachem JP (2008) Proteolytically active allergens cause barrier breakdown. J Invest Dermatol 128: 1878–1880. [DOI] [PubMed] [Google Scholar]
- 6. Liang G, Barker T, Xie Z, Charles N, Rivera J, et al. (2012) Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. J Allergy Clin Immunol 129: 1377–1386.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P (2000) Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 105: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 8. Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, et al. (2002) A protease-activated pathway underlying th cell type 2 activation and allergic lung disease. J Immunol 169: 5904–5911. [DOI] [PubMed] [Google Scholar]
- 9. Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P (2000) Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 105: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 10. Vergnolle N (1999) Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol 163: 5064–5069. [PubMed] [Google Scholar]
- 11. Traynelis SF, Trejo J (2007) Protease-activated receptor signaling: New roles and regulatory mechanisms. Curr Opin Hematol 14: 230–235. [DOI] [PubMed] [Google Scholar]
- 12. Traynelis SF, Trejo J (2007) Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol 14: 230–235. [DOI] [PubMed] [Google Scholar]
- 13. Ossovskaya VS, Bunnett NW (2004) Protease-activated receptors: Contribution to physiology and disease. Physiol Rev 84: 579–621. [DOI] [PubMed] [Google Scholar]
- 14. Rothmeier AS, Ruf W (2012) Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol 34: 133–149. [DOI] [PubMed] [Google Scholar]
- 15. Kawabata A, Kawao N (2005) Physiology and pathophysiology of proteinase-activated receptors (PARs): PARs in the respiratory system: Cellular signaling and physiological/pathological roles. J Pharmacol Sci 97: 20–24. [DOI] [PubMed] [Google Scholar]
- 16. Khan NA (2006) Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol Rev 30: 564–595. [DOI] [PubMed] [Google Scholar]
- 17. Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16: 273–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez AJ (1996) Free-living amebas: Naegleria, acanthamoeba and balamuthia. In: Baron S, editor. Medical Microbiology. Galveston (TX): The University of Texas Medical Branch at Galveston. [PubMed]
- 19. Cursons RT, Brown TJ, Keys EA, Moriarty KM, Till D (1980) Immunity to pathogenic free-living amoebae: Role of humoral antibody. Infect Immun 29: 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serrano-Luna Jde J, Cervantes-Sandoval I, Calderon J, Navarro-Garcia F, Tsutsumi V, et al. (2006) Protease activities of Acanthamoeba polyphaga and Acanthamoeba castellanii. Can J Microbiol 52: 16–23. [DOI] [PubMed] [Google Scholar]
- 21. Mitro K, Bhagavathiammai A, Zhou OM, Bobbett G, McKerrow JH, et al. (1994) Partial characterization of the proteolytic secretions of acanthamoeba polyphaga. Exp Parasitol 78: 377–385. [DOI] [PubMed] [Google Scholar]
- 22. Kim WT, Kong HH, Ha YR, Hong YC, Jeong HJ, et al. (2006) Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from Acanthamoeba strains with different degree of virulence. Korean J Parasitol 44: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dudley R, Alsam S, Khan NA (2008) The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiol Lett 286: 9–15. [DOI] [PubMed] [Google Scholar]
- 24. Sissons J, Alsam S, Goldsworthy G, Lightfoot M, Jarroll EL, et al. (2006) Identification and properties of proteases from an acanthamoeba isolate capable of producing granulomatous encephalitis. BMC Microbiol 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh B, Fleury C, Jalalvand F, Riesbeck K (2012) Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev 36: 1122–1180. [DOI] [PubMed] [Google Scholar]
- 26. Jeong HJ, Lee SJ, Kim JH, Xuan YH, Lee KH, et al. (2007) Acanthamoeba: keratopathogenicity of isolates from domestic tap water in Korea. Exp Parasitol 117: 357–367. [DOI] [PubMed] [Google Scholar]
- 27. Park HK, Cho MK, Choi SH, Kim YS, Yu HS (2011) Trichinella spiralis: Infection reduces airway allergic inflammation in mice. Exp Parasitol 127: 539–544. [DOI] [PubMed] [Google Scholar]
- 28. Trujillo-Vargas CM, Mayer KD, Bickert T, Palmetshofer A, Grunewald S, et al. (2005) Vaccinations with T-helper type 1 directing adjuvants have different suppressive effects on the development of allergen-induced T-helper type 2 responses. Clin Exp Allergy 35: 1003–1013. [DOI] [PubMed] [Google Scholar]
- 29. Khan NA, Jarroll EL, Panjwani N, Cao Z, Paget TA (2000) Proteases as markers for differentiation of pathogenic and nonpathogenic species of Acanthamoeba. J Clin Microbiol 38: 2858–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C (2010) Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci 25: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ritter M, Goggel R, Chaudhary N, Wiedenmann A, Jung B, et al. (2005) Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem Biophys Res Commun 334: 254–262. [DOI] [PubMed] [Google Scholar]
- 32. Zhou X, Hu H, Balzar S, Trudeau JB, Wenzel SE (2012) MAPK Regulation of IL-4/IL-13 Receptors Contributes to the Synergistic Increase in CCL11/Eotaxin-1 in Response to TGF-beta1 and IL-13 in Human Airway Fibroblasts. J Immunol 188: 6046–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng T, Cong Y, Qin H, Benveniste EN, Elson CO (2010) Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol 185: 5915–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muccioli M, Pate M, Omosebi O, Benencia F (2011) Generation and labeling of murine bone marrow-derived dendritic cells with qdot nanocrystals for tracking studies. J Vis Exp (52). pii: : 2785. doi: 10.3791/2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, et al. (2013) Extracellular vesicles, especially derived from gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy 43: 443–454. [DOI] [PubMed] [Google Scholar]
- 36. Jeong HJ, Yu HS (2005) The role of domestic tap water in Acanthamoeba contamination in contact lens storage cases in Korea. Korean J Parasitol 43: 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merad M, Sathe P, Helft J, Miller J, Mortha A (2013) The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31: 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouyer S, Rodier MH, Guillot A, Hechard Y (2009) Acanthamoeba castellanii: Proteins involved in actin dynamics, glycolysis, and proteolysis are regulated during encystation. Exp Parasitol 123: 90–94. [DOI] [PubMed] [Google Scholar]
- 39. Ferreira GA, Magliano AC, Pral EM, Alfieri SC (2009) Elastase secretion in acanthamoeba polyphaga. Acta Trop 112: 156–163. [DOI] [PubMed] [Google Scholar]
- 40. Kim WT, Kong HH, Ha YR, Hong YC, Jeong HJ, et al. (2006) Comparison of specific activity and cytopathic effects of purified 33 kDa serine proteinase from acanthamoeba strains with different degree of virulence. Korean J Parasitol 44: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thorne PS, Hillebrand J, Magreni C, Riley EJ, Karol MH (1986) Experimental sensitization to subtilisin. I. production of immediate- and late-onset pulmonary reactions. Toxicol Appl Pharmacol 86: 112–123. [DOI] [PubMed] [Google Scholar]
- 42. Hillebrand JA, Thorne PS, Karol MH (1987) Experimental sensitization to subtilisin. II. production of specific antibodies following inhalation exposure of guinea pigs. Toxicol Appl Pharmacol 89: 449–456. [DOI] [PubMed] [Google Scholar]
- 43. Tripathi P, Nair S, Singh BP, Arora N (2011) Molecular and immunological characterization of subtilisin like serine protease, a major allergen of curvularia lunata. Immunobiology 216: 402–408. [DOI] [PubMed] [Google Scholar]
- 44. Kim HY, DeKruyff RH, Umetsu DT (2010) The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 11: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HY, DeKruyff RH, Umetsu DT (2010) The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat Immunol 11: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puxeddu I, Bader R, Piliponsky AM, Reich R, Levi-Schaffer F, et al. (2006) The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. J Allergy Clin Immunol 117: 103–110. [DOI] [PubMed] [Google Scholar]
- 47. Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, et al. (1999) Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 11: 81–88. [DOI] [PubMed] [Google Scholar]
- 48. Takai T, Kato T, Ota M, Yasueda H, Kuhara T, et al. (2005) Recombinant der p 1 and der f 1 with in vitro enzymatic activity to cleave human CD23, CD25 and alpha1-antitrypsin, and in vivo IgE-eliciting activity in mice. Int Arch Allergy Immunol 137: 194–200. [DOI] [PubMed] [Google Scholar]
- 49. Chapman MD, Wunschmann S, Pomes A (2007) Proteases as Th2 adjuvants. Curr Allergy Asthma Rep 7: 363–367. [DOI] [PubMed] [Google Scholar]
- 50. Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, et al. (2013) Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One 8: e71278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, et al. (2002) Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol 169: 5315–5321. [DOI] [PubMed] [Google Scholar]
- 52. Lewkowich IP, Day SB, Ledford JR, Zhou P, Dienger K, et al. (2011) Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir Res 12: 122–9921-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez AJ (1996) Free-Living Amebas: Naegleria, Acanthamoeba and Balamuthia. In: Baron S, editor. Medical Microbiology .4th ed. Galveston (TX). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High-dose Acanthamoeba trophozoite infection induces severe allergic airway inflammation. (A) Tissue inflammation observed on stained lung sections (a and c: PBS-treated; b and d: Acanthamoeba-infected; a and b, H&E-stained; c and d, PAS-stained). (B) Cytokine concentrations in BALF and in the culture medium of CD3-stimulated lymphocytes isolated from LLNs were measured. (*p<0.05, ** <0.01, ***p<0.001).
(PPT)
Eosinophils are recruited to the airway by low-dose Acanthamoeba infection. (A) Airway resistance values in response to methacholine (0 to 50 mg/ml). (B) Differential cell count in 800 μl BAL after Diff-Quik staining. (C) Total and Acanthamoeba-specific IgE levels were measured in serum by ELISA. (*p<0.05, **p<0.01, ***p<0.001).
(PPT)