Abstract
ALS is associated with RNA processing impairments involving the RNA-binding protein TDP-43. Pioneering a novel RNA beacon to illuminate RNA trafficking in neurons, Alami et al. discover a novel cytoplasmic function for TDP-43, suggesting a new disease mechanism.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a devastating and universally fatal neurodegenerative disorder characterized by the selective loss of motor neurons from the spinal cord and cortex. In recent years there has been a seismic shift in understanding of the causes and mechanisms of ALS, with RNA-binding proteins and alterations in RNA metabolic processes emerging as powerful contributors to disease pathogenesis (Ling et al., 2013). This shift has been fueled by the initial discovery that the RNA-binding protein TDP-43 is a major component of the pathological inclusions found in the degenerating motor neurons of ALS patients (Neumann et al., 2006) and that mutations in the gene encoding TDP-43 cause some cases of familial and sporadic ALS (Chen-Plotkin et al., 2010). The conjunction of genetics and pathology clearly implicates TDP-43 as central to ALS and now the race is on to unravel the mechanisms by which it contributes to disease. What is its normal function? How do disease-associated mutations or pathological mislocalization affect that function? Since TDP-43 contributes broadly to ALS (depleted from the nucleus and mislocalized to cytoplasmic inclusions in virtually all ALS cases), deciphering TDP-43's normal cellular role and its role in disease could help to suggest novel and broadly applicable therapeutic strategies.
TDP-43 is a ubiquitously expressed DNA- and RNA-binding protein, originally identified as a transcriptional repressor and splicing regulator. TDP-43 localizes primarily to the nucleus but can also be found in the cytoplasm, as it shuttles between the two compartments (Chen-Plotkin et al., 2010). In the cytoplasm it has been shown to associate with mRNP granules, known as stress granules, which are transient sites of translational repression of mRNAs that form upon exposure to diverse environmental stresses (Li et al., 2013). ALS-linked mutations in TDP-43 can impair stress granule dynamics (Liu-Yesucevitz et al., 2010) and alterations in stress granule form and function (e.g., assembly and disassembly) per se may well be a major component of ALS and related neurodegenerative diseases (Li et al., 2013; Ramaswami et al., 2013). However, stress granules are not cell-type-specific and are present in all cell types, including neurons. Neurons also contain specialized types of mRNP granules that transport specific mRNA cargos from the cell body to axons and dendrites. In highly polarized neurons, with axons and dendrites that often terminate long distances from the soma, the transport of cellular supplies, including mRNA, to distal locations is incredibly important for normal cell function and defects in this process can lead to neuronal dysfunction. Given its already described role in stress granules, an intriguing question arises: could TDP-43 function in other types of mRNP granules, such as neuronal RNA transport granules and could this role explain its involvement in ALS pathogenesis and motor neuron degeneration? TDP-43 had previously been shown to associate with multiple proteins that are part of mRNP granules (e.g., staufen, FMRP, SMN, HuD), including neuronal transport granules (Freibaum et al., 2010). TDP-43 has also been shown to bind to the 3' UTRs of many target mRNAs, further evidence for a role in regulating the stability or transport of those mRNAs (Tollervey et al., 2011). However, the mechanisms by which TDP-43 regulates the spatial distribution of target mRNAs and the impact of disease mutations remain unresolved.
To define the role of TDP-43 in the cytoplasm, Alami et al. start out by using transgenic Drosophila that they engineered to express wild type or mutant TDP-43 in motor neurons. Consistent with previous reports by others, they found wild type TDP-43 was mainly localized to the nucleus. But they also found TDP-43 present in cytoplasmic granules that were distributed throughout the axon, even to distal parts of the axon and into the neuromuscular junction (NMJ). Interestingly, two independent disease-associated TDP-43 mutations impaired TDP-43's localization, preventing it from reaching the distal axons and NMJs. Because these TDP-43 transgenes were fluorescently labeled, they were next able to use in vivo imaging of Drosophila motor neurons to visualize TDP-43-containing granules transported up and down axons. These granules would move bidirectionally, often for long distances, pause for a bit, and then continue their journey. Compared to wild type, the ALS-linked mutant TDP-43-containing granules were transported less efficiently; their movements were interrupted with more pauses, resulting in more retrograde movement. This impaired anterograde movement of the mutant TDP-43 granules explains their diminished ability to reach distal axons and the NMJ.
Alami et al. next extend their findings from Drosophila to primary mouse cortical neurons. Again, using fluorescently-tagged wild type and mutant TDP-43, they visualized TDP-43 associating with cytoplasmic RNA transport granules and trafficking down the axons in a net anterograde direction. This process was less efficient (more pauses and direction reversals) with the TDP-43 mutants. The authors provide evidence that this transport is microtubule-dependent (instead of actin-dependent) and, interestingly, does not seem to be due to general defects in axonal transport because microtubule-dependent transport processes (e.g., mitochondria transport) are unaffected by TDP-43 in their system. Thus, the ability of TDP-43 to associate with cytoplasmic transport granules and traffic to distal parts of the axon in a net anterograde direction is conserved from flies to mice and disease-associated mutations cause a partial loss of this function.
Alami et al. next devised a clever strategy to visualize the actual mRNA within the transport granules. Harnessing a recent technological advancement for mRNA detection in live cells, the authors engineered a fluorescent “mRNA beacon”, essentially a fluorescently labeled oligonucleotide, which hybridizes to Neurofilament-L (Nefl) mRNA, a known target of TDP-43 and a well established component of cytoplasmic RNA granules. Using this reagent, they visualized Nefl mRNA frequently localizing to TDP-43-containing granules within axons. But sometimes TDP-43 was absent from the Nefl-positive mRNP granules, allowing the authors to compare the movement of TDP-43-positive and –negative granules within the same axon. Remarkably, even though both types of granules contained Nefl mRNA, the TDP-43-containing granules exhibited predominantly anterograde movement, whereas the ones without TDP-43 moved in more of a retrograde fashion. Thus it seems that TDP-43 selectively associates with granules involved in the anterograde transport of mRNA cargos within axons. Because the ALS-linked mutations affect this process, TDP-43 might also functionally participate in this transport rather than simply being a marker of the mRNP granules.
The final piece to the puzzle was for the authors to validate their findings in human patient-derived cells. Alami et al. obtained induced pluripotent stem (iPS) cells from ALS patients harboring three different TDP-43 mutations (G298S, A315T, and M337V) along with control cells from healthy individuals. Using established protocols, they differentiated these cells into motor neurons and compared the kinematics of mRNA granule transport (using their novel mRNA “beacon” approach). Just like in the fly and mouse experiments, neurons harboring TDP-43 mutations exhibited impairments in the anterograde transport of NEFL-containing mRNP granules. These results reveal a specific cellular phenotype associated with TDP-43 mutation, and suggest that, at least in part, one mechanism by which TDP-43 mutations can cause ALS is by loss of its cytoplasmic function. Additional gain-of-function and loss of nuclear function effects have been proposed and perhaps all contribute to disease to some extent (Figure 1).
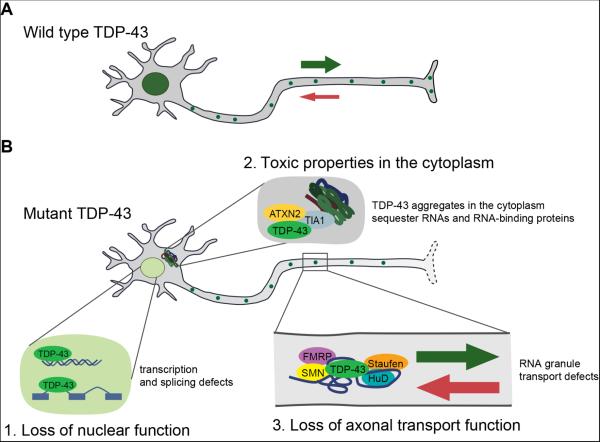
Figure 1.
Multiple potential mechanisms by which TDP-43 mutations could conspire to cause ALS. A) In healthy neurons, wild type TDP-43 localizes primarily to the nucleus and also to cytoplasmic mRNP granules, including neuronal transport granules, where it promotes their anterograde movement. B) ALS-causing TDP-43 mutations (or pathological mislocalization of the wild type protein) result in several possible cellular defects: 1. Depletion of TDP-43 from the nucleus causes a loss of its nuclear function resulting in massive changes in gene expression at the level of transcription and alternative splicing (e.g., Polymenidou et al., 2011). 2. Accumulation of TDP-43 aggregates in the cytoplasm can have toxic gain of function effects (e.g., Johnson et al., 2009), perhaps by sequestering RNAs and RNA-binding proteins. 3. Alami et al. propose a new mechanism in which TDP-43 mutations cause a loss of cytoplasmic function by impairing its ability to promote the anterograde transport of mRNA-containing cytoplasmic transport granules from the soma to distal parts of the axon.
Using three diverse model systems (fly, mouse primary neurons, and neurons derived from human ALS patient iPS cells) Alami et al. have discovered an unexpected and powerful requirement for TDP-43 in the transport of RNA granules. They also develop a novel technique to visualize transport of specific TDP-43 mRNA cargos up and down axons in living neurons. Although it has been noted previously that TDP-43 can localize to the cytoplasm and interact with RNA-binding proteins involved in mRNP granule transport, the direct role, if any, for TDP-43 in the cytoplasm has remained enigmatic. The authors have overcome this problem by discovering that TDP-43 functions in the cytoplasm to selectively transport mRNP granules in an anterograde manner and showing how impairments in this function might contribute to neurodegeneration. RNA granules – assemblies of RNAs and RNA-binding proteins – are emerging as key regulators of diverse RNA metabolic processes. Thus, dysfunction in RNA granule assembly or dynamics may be sufficient to cause neurodegeneration. If so, therapeutic strategies aimed at modulating RNA granule assembly, dynamics or disassembly may be efficacious for ALS and related neurodegenerative diseases (Kim et al., 2013; Li et al., 2013; Ramaswami et al., 2013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alami, et al. 2014.
- Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 2010;6:211–220. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum BD, Chitta RK, High AA, Taylor JP. Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res. 2010;9:1104–1120. doi: 10.1021/pr901076y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Snead D, Lee JJ, McCaffery JM, Shorter J, Gitler AD. TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity. J Biol Chem. 2009;284:20329–20339. doi: 10.1074/jbc.M109.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Raphael AR, Ladow ES, McGurk L, Weber RA, Trojanowski JQ, Lee VM, Finkbeiner S, Gitler AD, Bonini NM. Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2013 Dec 15; doi: 10.1038/ng.2853. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bilgutay A, Zhang YJ, Vanderweyde T, Citro A, Mehta T, Zaarur N, McKee A, Bowser R, Sherman M, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, et al. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154:727–736. doi: 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, Konig J, Hortobagyi T, Nishimura AL, Zupunski V, et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]