Abstract
Heterometallic Mn–Ca and Mn–Sr complexes have been prepared and employed as model complexes for Ca and Sr EXAFS spectral comparisons with the Oxygen-Evolving Complex (OEC) of Photosystem II (PS II); these have revealed similarities that support the presence of at least one O atom bridge between the Mn and Ca/Sr in the OEC.
The Oxygen-Evolving Complex (OEC) in Photosystem II (PS II) catalyzes the oxidation of H2O to O2 in green plants, algae and cyanobacteria.1 This four-electron light-driven process involves various oxidation levels of the OEC (the Si states, i = 0 to 4).2 The complex is a tetranuclear, oxo-bridged Mn4 cluster containing primarily MnIII and MnIV ions. In addition, one Ca plays a crucial role in OEC activity; without Ca, the OEC does not advance to the S3 state. This Ca can be substituted with major retention of activity (∼40%) only with Sr, either chemically or biosynthetically.3
Several studies in the last few years have shown that Ca is an integral part of the OEC.1,3–7 Ca/Sr EXAFS (extended X-ray absorption fine structure) studies of the OEC show a Mn⋯Ca(Sr) distance of 3.4(3.5) Å.4,5 Recent crystallographic studies at 3.5 Å6 and 3.0 Å7 resolution have also detected the presence of Ca in the OEC using anomalous diffraction data. Although there is considerable uncertainty about the Mn4Ca structure obtained from crystallography (XRD) due to the current resolution and the radiation damage during X-ray data collection,8 there is little doubt that the OEC is a heterometallic [Mn4CaOx] cluster on the basis of XRD6,7 and EXAFS studies.4,5,9
To date, various multinuclear Mn complexes have been synthesized as models of the OEC.10 In contrast, there have been very few synthetic MnIII/IVCa11,12 and no MnIII/IVSr mixed-metal complexes. The Mn–Ca(Sr) distances of ∼3.4(3.5) Å in the OEC detected by EXAFS studies4,5 are rather short and are in the range expected for a Ca intimately associated with the Mn4 portion of the OEC.11 In fact, this short distance strongly suggests that the Ca/Sr atom is bridged to Mn via a single atom bridge, probably oxygen. Here we discuss two complexes that contain such single O-atom bridges between MnIII/MnIV and Ca/Sr atoms, and present a comparison of their EXAFS spectra with that of the OEC; such model compound studies are important for better interpretation of data on the native system.5,9 The complexes are the recently reported [Ca2Mn13O10(OH)2(OMe)2(O2CPh)18(H2O)4] (1·10MeCN; MnIVMnIII10MnII2) (Fig. 1A),11 and the first MnSr molecular complex [SrMn14O11(OMe)3(O2CPh)18(MeCN)2] (2·12MeCN; MnIII13MnII) (Fig. 1B). The structures of 1 and 2 are similar but not identical, and both complexes contain single O-atom bridges between Mn and Ca(Sr).
Fig. 1.
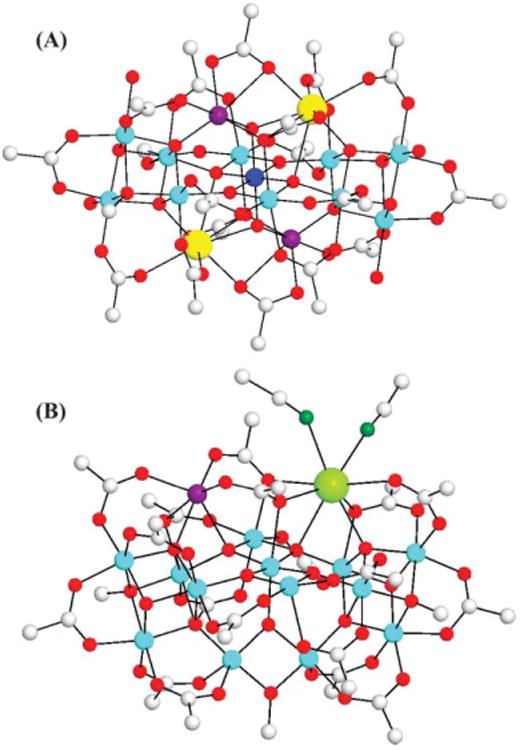
The structures of complexes 1 (A)11 and 2 (B), with only the ipso C atoms of benzoate rings shown for clarity. Color code: blue, MnIV; cyan, MnIII; purple, MnII; yellow, Ca; green, Sr; red, O; dark green, N; gray, C. H atoms have been omitted for clarity.
Treatment of (NBun4)[MnIII4O2(O2CPh)9(H2O)] with 1 equiv. of Sr(ClO4)2·xH2O in MeCN–MeOH (10 : 1 v/v) resulted in the subsequent isolation of 2·12MeCN in 55% yield; this procedure is very similar to that used for the synthesis of 1·10MeCN.11 Complex 2·12MeCN crystallizes‡ in the triclinic space group P1̄, and its structure consists of a [SrMn14O11(OMe)3]18+ core whose Mn ions are mixed valent (MnIII13MnII) and bridged by six μ4-O2−, five μ3-O2−, two μ3-MeO−, and one μ2-MeO− ions. Peripheral ligation is provided by fourteen doubly- and four triplybridging benzoates, as well as two terminal MeCN molecules on the Sr2+ ion. The core (Fig. 2B) can be described as two [Mn4O3(OMe)] cubanes attached, via oxide bridges, on either side of a central, near linear and planar [Mn3O4] unit. To the latter are also attached the MnII (Mn8) and SrII atoms above the central unit, and a [Mn2O(OMe)] rhombus below it. The core of 2 is thus overall fairly similar to the [Mn13Ca2O10(OH)2(OMe)2]18+ core of 1 (Fig. 2A), the main difference being the single SrII atom in 2. The Ca2+ atoms in 1 are eight-coordinate with the Ca–O bonds in the range 2.30–3.04 Å. The closest Ca⋯Mn separation is 3.51 Å to Mn5. The Sr in 2 is also eight-coordinate, with six Sr–O bonds in the range 2.50–2.77 Å and two Sr–N bonds of 2.71 and 2.76 Å, and the closest Sr⋯Mn separation is 3.29 Å to Mn3.
Fig. 2.
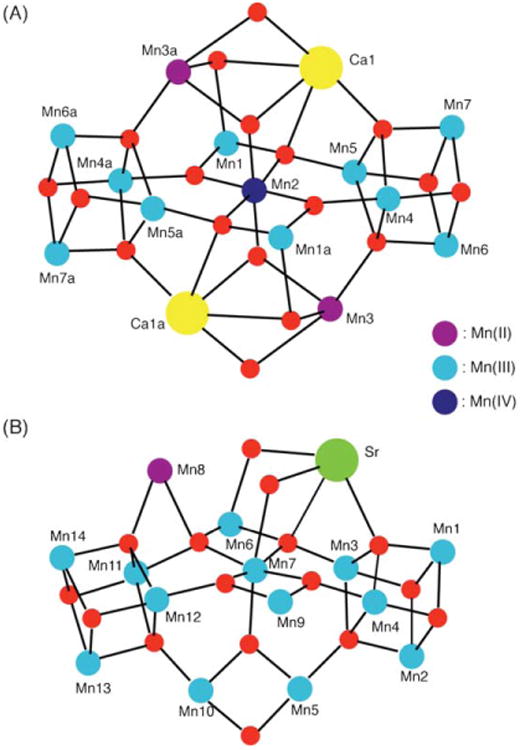
(A) The centrosymmetric core structure of complex 1. (B) The core structure of complex 2. Color code: blue, MnIV; cyan, MnIII; purple, MnII; yellow, Ca; green, Sr; red, O.
Mn4Ca and Mn4Sr structural sub-units present within complexes 1 and 2 have some similarity with the proposed structures of the OEC of PS II.4,5–7,9 The Mn3Ca sub-units (also see Fig. 3) in the center of 1 (Mn1, Mn2, Mn3a, and Ca1) possess a Mn3O4Ca cubane-like topology as proposed by Ferreira et al.6 Similarly, the distorted tetrahedral sub-unit topologies adopted by Ca1, Mn4, Mn5, Mn7 in complex 1, and Sr, Mn1, Mn3, Mn4 in complex 2 are also present in one of the most recently proposed structures for the OEC Mn4Ca site on the basis of single-crystal EXAFS studies of PS II by Yano et al.9 We therefore decided to carry out analogous Ca and Sr EXAFS studies on 1 and 2 to allow comparisons with the available Ca and Sr EXAFS spectra of the native Ca-containing and Sr-reconstituted OEC. The resulting comparative EXAFS spectra are presented in Fig. 4, and the Fourier transforms (FTs) of the EXAFS spectra are shown in Fig. 5. For all further discussions regarding EXAFS, we shall refer to Fig. 5.
Fig. 3.
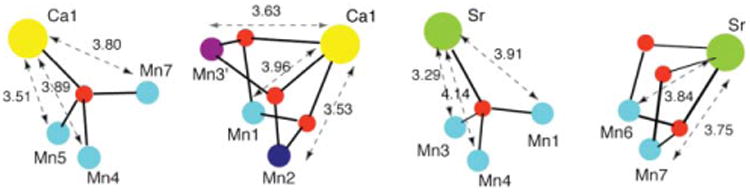
Selected Mn–Ca and Mn–Sr sub-units present within the structures of complexes 1 and 2.
Fig. 4.
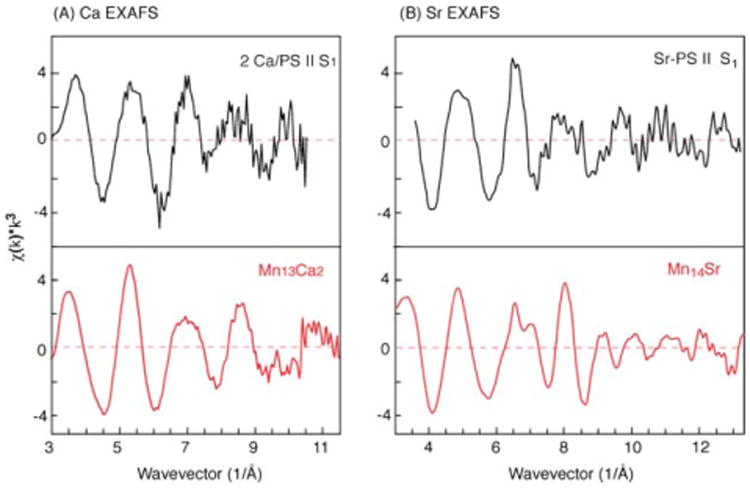
(A) Ca EXAFS spectra of the Mn13Ca2 complex 1 (red) and PS II S1 state (black),4 and (B) Sr EXAFS spectra of the Mn14Sr complex 2 (red) and Sr-reconstituted PS II S1 state (black).5
Fig. 5.
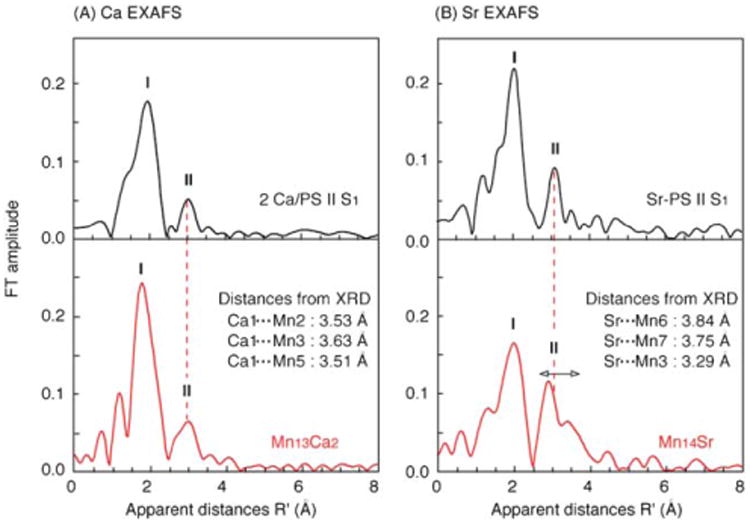
(A) Fourier transforms of the Ca EXAFS spectra of the Mn13Ca2 complex 1 (red) and PS II S1 state (black),4 and (B) Fourier transforms of the Sr EXAFS spectra of the Mn14Sr complex 2 (red) and Sr-reconstituted PS II S1 state (black).5 Note that there are two Ca per PS II, including one Ca extraneous to the OEC. Also, note that the apparent distances (R′) of the FT peaks are shorter by ∼0.5 Å compared to the actual distances due to a phase shift induced by the potentials of the absorber–backscatterer pair on the photo-electron. The distances in the inset are from the X-ray crystal structures (XRD).
Mn⋯Ca/Sr and Ca/Sr-ligand Fourier peaks (I and II) observed in the Ca/Sr EXAFS spectra of the model complexes and the OEC are in a similar distance range (Fig. 5). Peak I corresponds to the first shell of Ca/Sr-ligands: these are O for 1, O and N for 2, and most probably O and N for the native OEC of PS II and Sr-reconstituted PS II. Peak II is due to the Ca/Sr⋯Mn interactions. FT peak II from PS II was clearly identified with backscattering from Mn in both the Ca and Sr EXAFS because it was absent when the OEC was disrupted by treatment with NH2OH. Peak II is at a similar position in both the PS II OEC and complexes 1 and 2 (Fig. 5, dashed line), indicating some similarity in the bridging environment around the metal atoms of the model complexes and the biological sites.
Fits of the data for complex 1 improve significantly when FT peak I (Fig. 5A) was fit with two Ca–O distances (R = ∼2.4 and ∼2.8 Å), in accord with the X-ray data (see Supplementary Material). One broad FT peak observed at the apparent distance R′ = 3.0 Å in the FT peak II region is primarily the mixture of three Ca⋯Mn distances in the range of 3.51–3.63 Å. For complex 2, the FT peak I is best fit with 7 ± 2 light atoms (O and N) at 2.53 ± 0.02 and 2.66 ± 0.02 Å, which is in good agreement with the X-ray structure (see Supplementary Material). Although there are three Sr⋯Mn separations (3.3, 3.7 and 3.8 Å; Fig. 5) in the Mn14Sr structure, the shorter vector (3.3 Å) will be the dominant feature in the FT peak II region due to the weaker EXAFS oscillation of longer absorber–backscatterer interactions (>3.5 Å). These short Mn⋯Ca/Sr distances are in contrast to the longer values expected for metal pairs bridged only by carboxylate ligands.10
The structural sub-units extracted from the cores of the Mn–Ca(Sr) complexes (Fig. 3) all contain a monoatomic O bridge between Mn and Ca/Sr relevant to the proposed OEC structure. In spite of the presence of a monoatomic O bridge, there is a distribution of Mn–Ca(Sr) distances in these complexes in the range of 3.3 to 4.0 Å, depending on the other bridging ligands. In fact, preliminary Ca and Sr EXAFS studies on the OEC of cyanobacteria grown on Ca- or Sr-containing media suggest such a Mn–Ca(Sr) distance heterogeneity in the range of 3.4 to 4.0 Å, as a function of the OEC S-state (unpublished data). It should also be noted that the similar overall structures of the Ca and Sr model complexes 1 and 2 support the fact that the Ca of the OEC can be replaced with Sr without a major perturbation of the OEC structure. However, the reduced activity of the Sr-substituted OEC (∼40%) may suggest that the small structural changes are nevertheless sufficient to affect the enzyme activity, possibly through the alteration of bridging interactions with Mn, the hydrogen-bonding network, redox potentials, the pKa of bound water, and even the accessibility of water to the OEC.
This work has provided the first Ca and Sr EXAFS data on mixed-metal model complexes and their comparison with similar data from the OEC. Note that although the overall metal nuclearities of the model complexes are much higher than the OEC, the presence of two well-separated, symmetry-equivalent Ca atoms in 1 and a single Sr atom in 2 permit a ‘single Ca(Sr) site’ analysis. This allows comparisons of their Ca and Sr EXAFS data with those of the corresponding Ca and Sr OEC. The work has provided an important yardstick for assisting in the interpretation of data from the native site.4,5 Synthetic efforts in progress are providing additional examples of mixed-metal Mn–Ca complexes,11–13 with different structural topologies, and these are allowing an extension of such comparative studies. Detailed X-ray absorption spectroscopy on these and other complexes synthesized by our group will be reported in a full paper.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Science Foundation (CHE-0414555), the National Institutes of Health (GM 55302), and by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences of the Department of Energy (DOE) under Contract DE-AC02-05CH11231. Synchrotron radiation facilities were provided by the Advanced Light Source (BL 10.3.2), Berkeley, and Stanford Synchrotron Radiation Laboratory (BL 9-3), Stanford, operated by the U.S. DOE and by the NIH.
Footnotes
Electronic supplementary information (ESI) available: CIF for complex 2·12MeCN; EXAFS data collection details, and fit results. See DOI: 10.1039/b701355h
Vacuum-dried solid analyzed as solvent-free. Elemental analysis (%) calcd. for 2 (C133H105Mn14Sr1N2O50): C, 47.15; H, 3.12; N, 0.83; found: C, 46.92; H, 2.92; N, 0.63%. Crystal structure data for complex 2·12MeCN: SrMn14C157H141N14O50, M = 3880.62, dark brown plates, triclinic, P1̄, a = 18.4417(16), b = 19.0430(16), c = 25.447(2) Å, α = 69.789(2), β = 103.6350(10), γ = 73.792(2)°, V = 8001.2(12) Å3, 52550 reflections collected; 1750 parameters were refined in the final cycle of refinement using 35042 reflections (I > 2σ(I)); R1 = 0.0896, wR2 = 0.2069 (based on F2 and all data). CCDC 283564. Also see CCDC 249919 for complex 1·10MeCN. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b701355h
References
- 1.Debus RJ. Biochim Biophys Acta. 1992;1102:269. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]; Yachandra VK, Sauer K, Klein MP. Chem Rev. 1996;96:2927. doi: 10.1021/cr950052k. [DOI] [PubMed] [Google Scholar]; Wydrzynski T, Satoh S. Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. Springer; Dordrecht: 2005. [Google Scholar]
- 2.Kok B, Forbush B, McGloin M. Photochem Photobiol. 1970;11:457. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghanotakis DF, Babcock GT, Yocum CF. FEBS Lett. 1984;167:127. [Google Scholar]; Boussac A, Rutherford AW. Biochemistry. 1988;27:3476. [Google Scholar]; Boussac A, Rappaport F, Carrier P, Verbavatz JM, Gobin R, Kirilowsky D, Rutherford AW, Sugiura M. J Biol Chem. 2004;279:22809. doi: 10.1074/jbc.M401677200. [DOI] [PubMed] [Google Scholar]
- 4.Cinco RM, Holman KLM, Robblee JH, Yano J, Pizarro SA, Bellacchio E, Sauer K, Yachandra VK. Biochemistry. 2002;41:12928. doi: 10.1021/bi026569p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco RM, Robblee JH, Rompel A, Fernandez C, Yachandra VK, Sauer K, Klein MP. J Phys Chem B. 1998;102:8248. doi: 10.1021/jp981658q. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cinco RM, Robblee JH, Messinger J, Fernandez C, Holman KM, Sauer K, Yachandra VK. Biochemistry. 2004;43:12271. doi: 10.1021/bi036308v. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim SH, Gregor W, Peloquin JM, Brynda M, Britt RD. J Am Chem Soc. 2004;126:7228. doi: 10.1021/ja030614e. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 7.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Nature. 2005;438:1040. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 8.Yano J, Kern J, Irrgang K, Latimer MJ, Bergmann U, Glatzel P, Pushkar Y, Biesiadka J, Loll B, Sauer K, Messinger J, Zouni A, Yachandra VK. Proc Natl Acad Sci U S A. 2005;102:12047. doi: 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dau H, Liebisch P, Haumann M. Phys Chem Chem Phys. 2004;6:4781. [Google Scholar]
- 9.Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK. Science. 2006;314:821. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Mandal SK, Bhaduri S, Armstrong WH. Chem Rev. 2004;104:3981. doi: 10.1021/cr0206014. [DOI] [PubMed] [Google Scholar]; Milios CJ, Prescimone A, Mishra A, Parsons S, Wernsdorfer W, Christou G, Perlepes SP, Brechin EK. Chem Commun. 2007:153. doi: 10.1039/b611174b. [DOI] [PubMed] [Google Scholar]
- 11.Mishra A, Wernsdorfer W, Abboud KA, Christou G. Chem Commun. 2005:54. doi: 10.1039/b413680b. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt IJ, Tang JK, Madhu NT, Clérac R, Buth G, Anson CE, Powell AK. Chem Commun. 2006:2650. doi: 10.1039/b518026k. [DOI] [PubMed] [Google Scholar]
- 13.Jerzykiewicz LB, Utko J, Duczmal M, Sobota P. Dalton Trans. 2007:825. doi: 10.1039/b613320g. [DOI] [PubMed] [Google Scholar]; Wang W, Zhang X, Chen F, Ma C, Chen C, Liu Q, Liao D, Li L. Polyhedron. 2005;24:1656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


