Abstract
Histone deacetylase inhibitors (HDACis) are known to exert immunomodulatory effects. We have recently demonstrated that the therapeutic efficacy of HDACis against aggressive B-cell lymphoma and colon carcinoma relies on a functional immune system, in particular on the production of interferon γ (IFNγ). Our findings provide a rationale for the combination of HDACis with immunotherapeutic agents in the clinic.
Keywords: anticancer immunity, anticancer therapy, epigenetic regulatory agent, HDACi, immunogenic cell death, immunotherapy
Histone deacetylase inhibitors (HDACis) are currently employed in the clinic for the treatment of a wide panel of solid and hematological malignancies. In particular, vorinostat (Zolinza®) and romidepsin (Istodax®) have been approved by the US Food and Drug Administration for the treatment of cutaneous T-cell lymphoma.1,2 HDACis mediate a range of different antitumor effects, one or more of which may be responsible for their clinical efficacy. The proposed anticancer activities of HDACis include: (1) the induction of apoptosis and cell cycle arrest; (2) the inhibition of angiogenesis; and (3) the stimulation of cancer cell differentiation. Importantly, HDACis have been shown to enhance the immunogenicity of cancer cells. Several groups have reported the upregulation of natural killer (NK)-cell activating ligands, MHC class I and II molecules, components of the machinery for antigen presentation, and co-stimulatory molecules on the surface of cancer cells exposed to HDACis.3-5 In addition, the pre-treatment of malignant cells with HDACis has been employed to generate an effective anticancer vaccine for therapeutic use, and HDACi-treated malignant cells exhibit an increased propensity to be taken by dendritic cells (DCs).3,6 Finally, we have recently demonstrated that colon cancer cells treated with vorinostat undergo immunogenic cell death, as documented by the translocation of calreticulin on the cell surface as well as by the release of ATP and high mobility group box 1 (HMGB1) into the extracellular milieu.7
Despite such clear indicators of the immunostimulatory activity of HDACis, these agents are also being associated with paradoxical effects. For instance, the administration of HDACis to wild-type (WT) mice led to the upregulation of forkhead box P3 (FOXP3) and hence to the expansion of regulatory T cells (Tregs), while HDACi-treated DCs exhibited a limited ability to release cytokines in response to lipopolysaccharide.8,9 HDACis can also limit pathological immune responses in the context of systemic lupus erythematosus (SLE) by decreasing the activity of autoimmune CD4+ T cells.10 Indeed, the use of HDACis for the treatment of autoimmune and inflammatory diseases has provided encouraging results in recent clinical trials.9,10 It appears that HDACis may influence the activity of the host immune system in a manner that highly depends on the underlying disease. It may be hypothesized that deregulated cells are the most susceptible to HDAC inhibition in a pathological state, for example, malignant cells in cancer patients, and autoimmune CD4+ T cells in individuals with SLE. We sought to further understand this paradox by examining the role of the immune system during HDACi-based anticancer therapy in vivo.
We demonstrated that the HDACis vorinostat and panobinostat are significantly less efficient against aggressive Eμ-Myc-driven B-cell lymphomas and colon carcinomas in mice lacking a functional immune system (Rag2−/−γc−/− mice) than in their WT counterparts. Indeed, tumor-bearing Rag2−/−γc−/− mice succumbed to lymphoma significantly earlier than WT mice, despite ongoing treatment with HDACis. The decreased survival of tumor-bearing Rag2−/−γc−/− mice treated with a HDACi was not due to adverse side effects, the lack of drug-target interactions, or the inability of the HDACi to trigger the apoptotic demise of cancer cells. The non-immunogenic chemotherapeutic agent etoposide was able to enhance the survival of WT and immunocompromised tumor-bearing mice to similar extents. To our knowledge, ours is the first comprehensive demonstration that the therapeutic efficacy of HDACis in vivo relies on an intact immune system. These data are in line with our previous findings demonstrating that the efficacy of HDACis can be significantly enhanced by the concurrent administration of immunostimulatory monoclonal antibodies that operate as CD137 and CD40 agonists.6
By investigating in detail the immunological mechanisms triggered by HDACis in immunocompetent mice, we found that interferon γ (IFNγ) is critical for the therapeutic activity of these agents. Contrarily to our initial hypothesis, we found that IFNγ acts on malignant cells to induce anticancer effects concurrently with HDACis. Moreover, the administration of HDACis was found to sensitize malignant cells to the antineoplastic effects of IFNγ, as signal transduction via the IFNγ receptor 1 (IFNGR1) was increased by HDACis, as were the levels of MHC class I and II molecules expressed on the surface of cancer cells. In order to confirm our findings, we overexpressed a non-functional dominant negative variant of the IFNGR1 in Eμ-Myc-driven lymphoma cells, and found that mice bearing these genetically engineered malignant cells succumbed to lymphoma significantly earlier than those bearing WT tumors, in spite of vorinostat treatment. Finally, we demonstrated that the co-administration of a potent immune adjuvant and IFNγ inducer, namely α-galactosylceramide (α-GalCer), with vorinostat significantly prolonged the survival of tumor-bearing mice as compared with either agent alone. Our findings indicate that the combination of HDACis with immunotherapy is a promising strategy for the treatment of cancer.
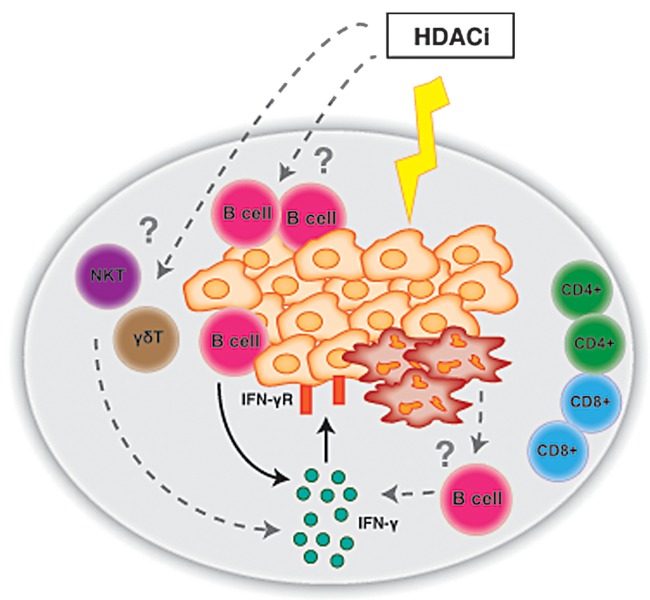
We next sought to determine the origin of IFNγ secreted in the course of treatment with vorinostat. Surprisingly we found that NK cells, CD8+ T cells and CD4+ T cells are not required for the therapeutic efficacy of vorinostat. Conversely, we demonstrated a critical role for B cells in the anticancer effects of HDACis. Moreover, B cells isolated from vorinostat-treated, tumor-bearing mice were found to produce IFNγ. While the role of B cells in oncogenesis and tumor progression is controversial and still under investigation, the localization of Eμ-Myc-driven lymphoma cells within the B-cell niche of lymphoid organs strongly suggest that HDACis may induce an antitumor B-cell immune response (Fig. 1).
Figure 1. Immunomodulatory effects of HDAC inhibitors in cancer therapy. Histone deacetylase inhibitors (HDACis) such as vorinostat and panobinostat are highly efficient against cancer cells of multiple types, including Eμ-Myc-driven lymphoma cells (orange) infiltrating the spleen (gray). HDACis can directly induce the apoptotic demise of malignant cells (red cells). For HDACis to induce a sustained therapeutic responses against lymphoma, B cells and interferon γ (IFNγ) are required. In this setting, lymphoma cells are the target of IFNγ, which in tumor-bearing mice treated with vorinostat is produced by B cells. However it is not known whether HDACis also influence the anticancer activity of B cells in a direct fashion. Along similar lines, it remains unclear whether additional cell types (such as natural killer T or γδ T cells) are required for the full-blown antineoplastic effects of HDACis against lymphoma.
In conclusion, we have recently shown that the immune system is a critical component of the antitumor effects of HDACis. These findings confirm previous in vitro data demonstrating that HDACis increase the immunogenicity of cancer cells. Our study will provide additional impetus to combine HDACis with immunotherapeutic agents, including immune adjuvants such as α-GalCer and immunostimulatory monoclonal antibodies, in the clinic.
Disclosure of Potential Conflicts of Interest
The R.W.J. laboratory has collaborative research grants from Merck and Co and Novartis for studies involving vorinostat and panobinostat, respectively. M.J.S. acknowledges the support of a NH&MRC Australia Fellowship.
Glossary
Abbreviations:
- DC
dendritic cell
- HDACi
histone deacetylase inhibitor
- HMGB1
high mobility group box 1
- IFN
interferon
- NK
natural killer
- NKT
natural killer T
- SLE
systemic lupus erythematosus
- WT
wild-type
Citation: West AC, Smyth MJ, Johnstone RW. The anticancer effects of HDAC inhibitors require the immune system. OncoImmunology 2013; 2:e27414; 10.4161/onci.27414
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27414
References
- 1.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–15. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 2.Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, Scarisbrick J, Reddy S, Robak T, Becker JC, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–91. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 3.Khan ANH, Magner WJ, Tomasi TB. An epigenetic vaccine model active in the prevention and treatment of melanoma. J Transl Med. 2007;5:64. doi: 10.1186/1479-5876-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–24. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 5.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–45. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen AJ, West A, Banks KM, Haynes NM, Teng MW, Smyth MJ, Johnstone RW. Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc Natl Acad Sci U S A. 2011;108:4141–6. doi: 10.1073/pnas.1011037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West AC, Mattarollo SR, Shortt J, Cluse LA, Christiansen AJ, Smyth MJ, Johnstone RW. An Intact Immune System Is Required for the Anticancer Activities of Histone Deacetylase Inhibitors. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0890. [DOI] [PubMed] [Google Scholar]
- 8.Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Donà G, Fossati G, Sozzani S, Azam T, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao R, de Zoeten EF, Özkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 10.Mishra N, Brown DR, Olorenshaw IM, Kammer GM. Trichostatin A reverses skewed expression of CD154, interleukin-10, and interferon-gamma gene and protein expression in lupus T cells. Proc Natl Acad Sci U S A. 2001;98:2628–33. doi: 10.1073/pnas.051507098. [DOI] [PMC free article] [PubMed] [Google Scholar]