Abstract
Antibody-mediated blockade of CTLA4 has been shown to be effective in treating a select group of patients with late-stage melanoma. The precise mechanism underlying the clinical activity of CTLA4 immunotherapy is poorly understood, although recent experimental findings indicate that antibody-mediated depletion of regulatory T cells (Tregs) in the tumor microenvironment plays a key role in efficacious antitumor responses. In the current study, we used an experimental model of pancreatic adenocarcinoma to compare the antitumor efficacy of peritumoral low-dose anti-CTLA4 monoclonal antibody (mAb) administration to that of a commonly utilized systemic high-dose anti-CTLA4 regimen. We selected pancreatic adenocarcinoma as it presents a particular challenge to clinicians due to its aggressive behavior, metastatic spread and limited treatment options. Furthermore, Fc gamma receptor (FcγR)-dense myeloid cells commonly infiltrate pancreatic tumors, such that these tumor types exhibit increased susceptibility to CTLA4 antibody-targeted Treg depletion via antibody-dependent cell-mediated cytotoxicity (ADCC). Locally administered anti-CTLA4 mAb effectively reduced tumor growth at a low dose and no additional anti-tumor effects were apparent when increasing the dose or number of injections. No significant difference in overall survival was seen when comparing locally administered low-dose with standard systemic high-dose CTLA4 blockade therapy, and both delivery routes led to increased tumor-infiltrating effector T cells and reduced Treg cells. As opposed to low-dose peritumoral treatment, high-dose systemic therapy stimulated the accumulation of Tregs in secondary lymphoid organs, an effect that could potentially counteract the antitumor immunotherapeutic benefit of CTLA4 blockade. Our study confirms previous findings that local administration of low-dose anti-CTLA4 antibody generates sustained antitumor effects and provides rationale to devise ultrasound-guided intratumoral anti-CTLA4 antibody injection regimens to treat patients with pancreatic adenocarcinoma and other types of solid tumors. In support, clinical relevancy could include reduced immune-related adverse events by limiting systemic antibody spread to immune cell-dense organs.
Keywords: local therapy, anti-CTLA4, checkpoint blockade, immunotherapy, pancreatic cancer
Introduction
Pancreatic adenocarcinoma continues to be one of the most aggressive forms of solid cancer, such that following diagnosis the 5-y survival rate is less than 5% and the median survival time is only 6 mo. In the United States, the incidence is approximately 44 000 new cases yearly with mortality as high as 85%.1,2A high metastatic tendency as well as a drug-resistance phenotype contributes to the poor prognosis for those afflicted with this devastating disease. Only 20% of patients qualify for surgical resection, a treatment that is rarely curative due to early relapse and cancer cell dissemination. Currently, therapeutic regimens based on the nucleoside analog, gemcitabine are first line treatments for advanced pancreatic cancer despite limited efficacy.2 Moreover, the pancreatic adenocarcinoma tumor microenvironment consists of a dense stroma that comprises inflammatory extracellular matrix proteins and tumor-associated immunosuppressive cells.3 Such immunomodulatory manifestations, including regulatory T cells (Tregs) and myeloid-derived suppressor cells could underlie failures in drug delivery and potentiate tumor progression and invasion.3
Monoclonal antibodies (mAb) and targeted therapies against numerous molecular pathways have yielded promising results in mouse model studies but have typically failed to effectively treat pancreatic cancer patients.4 Among antibody-based therapies in the clinic is Ipilimumab targeting cytotoxic T-lymphocyte antigen-4 (CTLA4), an immunoglobulin superfamily member commonly known for its inhibitory effect on activated cytotoxic T-lymphocytes and for its’ important role in maintaining peripheral tolerance.
Several mechanisms account for the immunosuppressive effects of CTLA-4 signaling. These include the expression levels of CD80 and CD86 on antigen presenting cells, ligands for both the T cell inhibitory CTLA4 and the T cell co-stimulatory receptor CD28, as well as the relatively higher affinity of these ligands for CTLA4. Inhibitory CTLA4-signaling functionally attenuates specific T cells within the T cell repertoire by interference with cell cycle progression as well as by activation of negative intracellular signaling cascades that lead to reduced transcription of interleukin-2 (IL2).5
Blockade of CTLA4 with antagonistic antibodies has been extensively studied for anticancer potential in a variety of experimental models6 and clinical trials have demonstrated therapeutic efficacy.7 Despite these beneficial effects, systemic anti-CTLA4 mAb therapy has also been associated with autoimmune adverse events mostly involving the gut and skin. However, several large trials have suggested a positive correlation between the occurrence of such immune-related adverse events and improved clinical outcome.7,8 In 2010, a Phase III trial enrolling more than 600 melanoma patients demonstrated improved overall survival for groups treated with the CTLA4-blocking mAb Ipilimumab (Yervoy ®)9 and the drug was subsequently approved as a first- or second-line treatment of patients with advanced malignant melanoma.10 Although Ipilimumab has most often been administered to patients with metastatic melanoma, treatment of patients harboring other types of malignancies are currently under investigation. Ipilimumab was initially tested as a single agent at 3 mg/kg in advanced pancreatic adenocarcinoma patients generating a minimal beneficial effect.11 Ipilimumab was more recently evaluated to treat pancreatic cancer patients at a higher dose of 10 mg/kg, either alone or in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transfected tumor cells as an immunostimulatory cancer vaccine.12 Although in this study, no statistically significant difference was observed between treatment groups, the increased median overall survival (3.6 vs. 5.7 mo) and 1-y survival rate (7% vs. 27%) favored the combinatorial therapy, a striking outcome for this extremely lethal tumor type. Patients in this trial surviving longer than 4.3 mo exhibited expanded mesothelin-specific T cells in both treatment arms indicating that even in late-stage pancreatic cancer patients, immune responses can be elicited by blockade of CTLA4 and that these immunological activities correlate with clinical efficacy.12
Recent independent findings suggest that a myriad of tumors may regress in response to local CTLA4 mAb therapy13-15 and that CTLA4 blockade-mediated antitumor responses are, at least partly, the result of selective Treg depletion in the tumor microenvironment.16-18 It has been postulated that co-administration of an adjuvant is necessary for the success of the anti-CTLA4 antibody injection strategy.19 Furthermore, we have previously demonstrated that localized anti-CD40 antibody administration—without the addition of other co-stimulatory factors—is superior and less toxic as compared with systemic approaches.20 Hence, we set out to assess anti-CTLA4 mAb treatment only, without accompanying agonistic antibody, synthetic oligonucleotides CpG, or mineral oil, in the context of localized peritumoral injection in a mouse model of pancreatic adenocarcinoma.
Pancreatic cancer represents a particularly pertinent tumor type for localized CTLA4 mAb therapy, since the neoplasm is heavily infiltrated by Tregs as well as FcγR-dense myeloid cells that could potentially mediate Treg depletion by antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent phagocytosis (ADP). In a murine model of pancreatic cancer, we find that locally injected and low-dose anti-CTLA4 antibody can effectively reduce intratumoral Treg levels and inhibit tumor growth without a corresponding systemic increase in Tregs, a complication observed at higher dosages. Further, higher local anti-CTLA4 mAb doses actually appeared to yield attenuated antitumor effects, possibly as a result of systemic Treg induction.
Results
Low-dose peritumoral administration of anti-CTLA4 mAb reduces tumor growth of experimental pancreatic adenocarcinoma
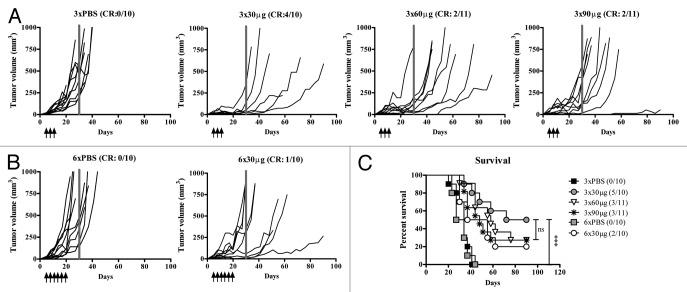
To establish the optimal dose for treating experimental pancreatic adenocarcinoma with localized administration of CTLA4 blocking antibody, a titration was performed assaying peritumoral (p.t.) injection of 30, 60, or 90 μg of anti-CTLA4 every third day for a total of 3 times, 5 days after Panc02 tumor cell inoculation. As illustrated in Figure 1A, 4 out of 10 animals were complete responders in the 30 µg group such that no palpable tumor mass was detected, and no further reduction in tumor incidence was observed upon dose escalation. In contrast, none of the PBS-treated control animals were complete responders with 100% tumor incidence and rapidly increasing tumor volumes. In comparison to 3 injections of 30 μg anti-CTLA4 mAb, no further increase in the incidence of complete responders was detected upon 3 additional injections (6 in total; compare Figure 1B to Figure 1A). Moreover, animals treated with 3 injections of 30 μg anti-CTLA4 mAb exhibited a significantly prolonged survival (median 81 d) as compared with PBS treatment (median 34 d; P = 0.0001; Figure 1C). Finally, all anti-CTLA4 mAb treatment groups exhibited a significantly increased percent survival relative to PBS control groups irrespective of dosage and injection frequency (Fig. 1C).
Figure 1. Localized low-dose anti-CTLA4 antibody therapy is efficacious. (A–C) Mice (n = 10–11 per group) were inoculated subcutaneously with 2.5 × 105 Panc02 cells and treated with anti-CTLA4 blocking antibody by peritumoral injections either 3 times (day 5, day 8, and day 11) or 6 times (day 5, day 8, day 11, day 14, day 17, and day 20), as indicated. (A and B) To determine the optimum dosage and injection frequency of locally administered anti-CTLA4 monoclonal antibody (mAb), a dose-response experiment was performed by 3 (A) 30 μg, 60 μg, and 90 μg or 6 (B) 30 μg peritumoral injections of anti-CTLA4 mAb. Tumor growth was measured with caliper and calculated by 4/3π*a(radius of length)*b(radius of width)*c(radius of depth). Lines indicate individual animals. Grey bar indicates d30. (C) Kaplan–Meier survival curve from data presented in (A and B). Cumulative results from 2 independent experiments. Statistical analysis of survival was performed by log-rank test with ***P < 0.001. CR, complete responder; ns, not significant
Comparison of local low-dose and systemic high-dose anti-CTLA4 mAb therapy
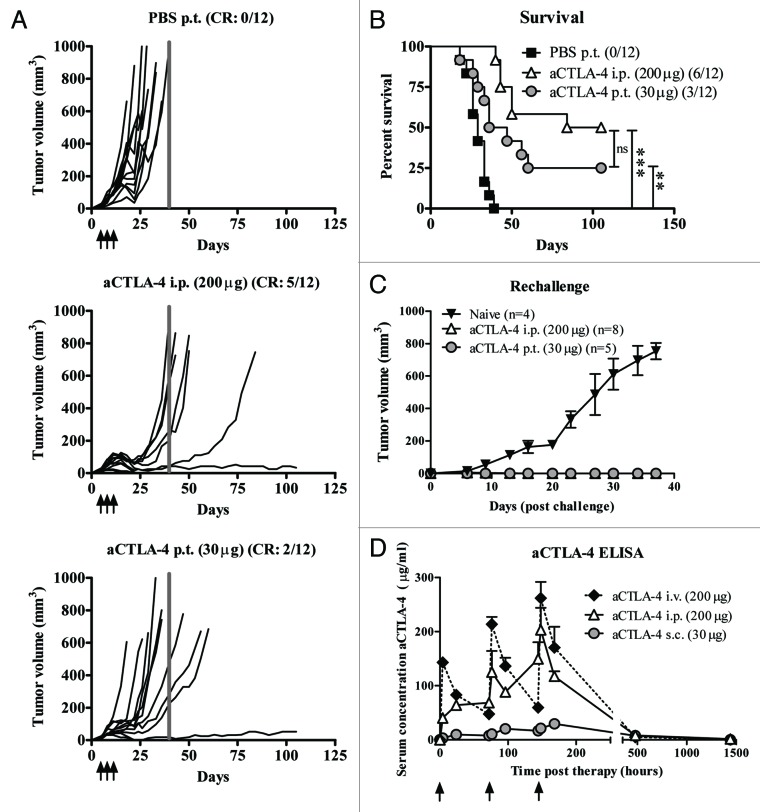
Considering that we found 30 μg of p.t. injected anti-CTLA4 mAb to be therapeutically beneficial, we compared the biological efficacy of this locally administered low dose with a systemically administered higher dose (200 µg) delivered via intraperitoneal (i.p.) injection, a route previously shown21,22 to generate effective antitumor responses. Both systemic high-dose (Fig. 2A, middle panel) and local low-dose (Fig. 2A, lower panel) CTLA4-blocking antibody injections reduced pancreatic tumor growth as compared with PBS-treated animals (Fig. 2A), eliciting a complete response in 2 (localized administration) or 5 (systemic delivery) animals relative to none in the PBS control group (n=12/group). Additionally, both administration routes significantly prolonged survival of tumor-bearing mice (Fig. 2B). No statistically significant difference was noted between the 2 treatment arms. However, the systemic high-dose group did exhibit a slightly improved overall percent survival. Of particular importance, tumor rechallenge of anti-CTLA4 mAb treated mice cured from Panc02-tumors confirmed complete tumor immunity in both therapy groups (Fig. 2C). Blood kinetic analysis demonstrated reduced serum anti-CTLA4 mAb in mice receiving low-dose subcutaneous (s.c.) injections compared with those receiving systemic high-dose administration (Fig. 2D). Since i.p. injections are most commonly used in preclinical models and intravenous (i.v.) administration in patients, we also investigated the blood kinetics of anti-CTLA4 mAb levels in mice responding to i.v. administration. As depicted in Figure 2D, i.v. injection of anti-CTLA4 mAb resulted in a larger variation in serum anti-CTLA antibody concentration compared with i.p. delivery, although both routes exhibited similar kinetics.
Figure 2. Antitumor efficacy and circulating serum levels of locally delivered vs. systemically administered anti-CTLA4 blocking antibody. (A–C) Mice (n = 12 per group) were inoculated subcutaneously with 2.5 × 105 Panc02 cells and treated with anti-CTLA4 blocking antibody by either peritumoral injections of 30 μg or intraperitoneal injection of 200 μg anti-CTLA4 monoclonal antibody or a phosphate-buffered saline (PBS) control day 5, day 8, and day 11. (A) Tumor growth was measured with caliper and calculated by 4/3π*a(radius of length)*b(radius of width)*c(radius of depth). Lines represent individual mice. Grey bar indicates d40. (B) Kaplan–Meier survival curve of mice in (A). Statistical analyses were performed by log-rank test with **P < 0.01, ***P < 0.001. (C) Tumor growth of naïve mice and complete responders from 2 independent experiments rechallenged with 2.5 × 105 Panc02 in the contralateral flank (n = 4-8). Averaged tumor volumes (measured as in A) are shown per group. (D) Naïve mice were locally (30 μg s.c.) or systemically (200 μg, i.p. or i.v.) treated with anti-CTLA4 mAb with 3 days intervals. The levels of circulating therapeutic anti-CTLA4 antibodies were monitored during the course of treatment and long-term in the serum of treated animals via enzyme-linked immunosorbant assay (n = 1–5 per time point, data shown are the mean ± SEM). CR, complete responders; ns, not significant; i.p., intraperitoneal; i.v., intravenous; p.t., peritumoral; s.c., subcutaneous.
Autoimmune adverse events commonly occur in patients after CTLA4 antibody-mediated blockade therapy but rarely occur in murine models. The plasma levels of the liver damage indicator alanine aminotransferase, but not those of aspartate aminotransferase or alkaline phosphatase, were slightly increased in mice treated with high-dose systemic injections (both i.p. and i.v.) 20 and 60 d after the initiation of anti-CTLA4 mAb therapy (data not shown). Histological analysis of various target organs (stomach, duodenum, colon, rectum, kidney, liver, pancreas, and salivary glands) was also performed by a small animal pathologist revealing elevated levels of leucocyte infiltration exclusively in the livers of animals in the systemic high-dose therapy group 60 d after the start of treatment (data not shown). Hematological analysis of the different treatment groups did not reveal conclusive patterns (data not shown).
Anti-CTLA-4 shifts the intratumoral effector to regulatory T cell balance but systemic Treg induction is exclusive to high-dose anti-CTLA4 mAb therapy
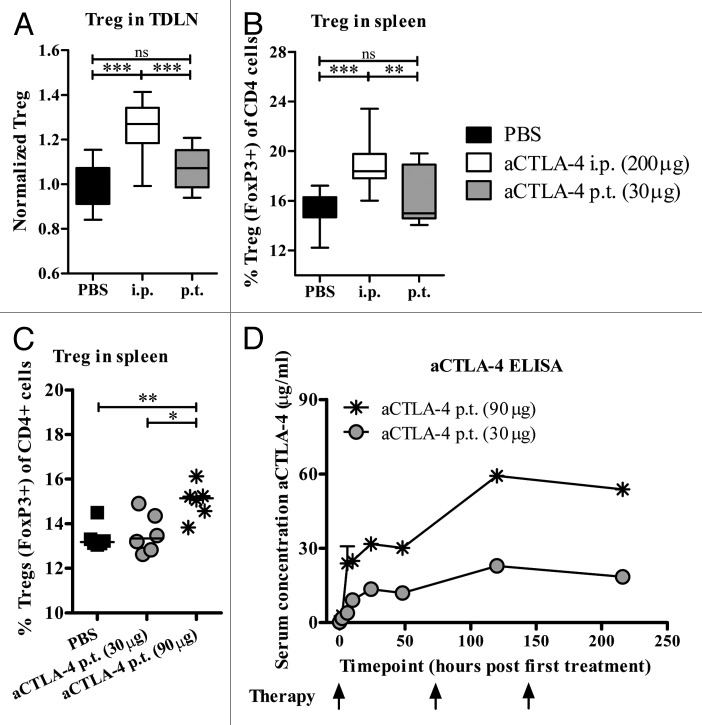
Considering that earlier studies had shown elevated Treg levels both in patients treated systemically with CTLA4 blocking antibody23 and in experimental models after prolonged i.p. delivery of anti-CTLA4 mAb therapy,6 we next sought to investigate differences in Treg levels between the local low-dose and systemic high-dose treatment groups. Fourteen days after Panc02 inoculation, Tregs (distinguished as CD4+FoxP3+) were significantly elevated in the tumor-draining lymph node (TDLN; Figure 3A, P < 0.001) and in the spleen of systemically treated mice (Fig. 3B, P < 0.001 and P < 0.01 relative to the PBS control and localized treatment groups, respectively). On the other hand, low-dose p.t. injections of anti-CTLA4 mAb did not significantly alter Treg levels in comparison to the levels in PBS control treated mice in either the TDLN or the spleen (Fig. 3A and B). As no therapeutic benefit had been observed in response to higher doses of localized antibody-mediated CTLA4 blockade therapy (refer to Figure 1A), we also investigated the frequency and function of Tregs in animals treated twice with peritumoral anti-CTLA4 mAb (30 μg or 90 μg) and a significant increase in Treg levels was noticed exclusively in animals treated with the higher dosage (Fig. 3C; PBS vs. 90 μg, P < 0.01; 30 μg vs. 90 μg P < 0.05). Co-culture Treg suppression assay demonstrated that the capacity of Tregs (CD4+CD25+), isolated by magnetic cell separation and confirmed as FoxP3+ by flow cytometry, to suppress the proliferation of responder cells (CD4+CD25- splenocytes from naïve mice) in vitro was nearly equivalent between all treatment groups, whereas control cells (CD4+CD25- from treated animals) did not display anti-proliferative effects (data not shown). Blood kinetic analysis of serum anti-CTLA4 mAb levels revealed dose-dependent antibody concentration differences present in the serum of peritumorally treated animals (Fig. 3D).
Figure 3. Systemic accumulation of Tregs after high-dose anti-CTLA4 mAb therapy. (A–D) Mice were inoculated subcutaneously with 2.5 × 105 Panc02 cells and treated with anti-CTLA4 blocking antibody (as indicated below) or a phosphate-buffered saline (PBS) control on day 5, day 8, and day 11. (A–C) The levels of Tregs (CD4+FoxP3+) residing in secondary lymphoid organs were evaluated by immunofluorescent staining and flow cytometry on day 14 after low-dose peritumoral (p.t.) or high-dose intraperitoneal (i.p.) anti-CTLA4 mAb therapy in animals carrying Panc02 tumors. (A) Data represent cumulative results from 3 independent experiments visualized as percent Tregs in the tumor-draining lymph node (TDLN) normalized against the PBS treated control (median, whiskers: min to max; n = 16–17). Statistical analysis was performed by ANOVA and the Bonferroni multiple comparison test. (B) Average percent FoxP3+ Tregs of total CD4+ cells present in the spleens of animals treated as indicated. Data represent cumulative results from 2 independent experiments (median, whiskers: min to max, n = 12–13). Statistical analysis was performed by ANOVA and the Bonferroni multiple comparison test. (C) Animals were treated twice with 30 μg or 90 μg local peritumoral anti-CTLA4 mAb, day 5 and day 8 after Panc02-tumor inoculation. The percentage of Tregs (CD4+ FoxP3+) present in the spleen was quantified by immunofluorescent staining and flow cytometry on day11 (median, n = 6). Statistical analysis was performed by unpaired Student’s t test. (D) The levels of circulating therapeutic anti-CTLA4 antibodies were monitored in serum via enzyme-linked immunosorbant assay (n = 2–5 per time point, data shown are the mean ± SEM) *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
Experiments previously conducted in the B16 melanoma model have reported that successful anti-CTLA4-mediated anticancer activity was associated with elevated levels of intratumoral effector T cells and a reduction in Tregs.6,24,25 Thus, we wanted to elucidate if this also holds true in our pancreatic cancer model and, more specifically, in animals treated via the localized low-dose administration route. Therefore, tumors collected from Panc02-bearing mice were dissociated and evaluated to quantify intratumoral CD4+ effector T (Teff) cells (CD4+FoxP3−), CD8+ Teff cells (CD8+FoxP3−) and Tregs (CD4+FoxP3+). Both local and systemic anti-CTLA4 mAb therapy increased the percentage of tumor-infiltrating CD4+ Teff cells (Fig. 4A) as compared with PBS-treated control animals. Irrespective of the treatment route, the levels of intratumoral CD8+ Teff cells were also markedly elevated in both groups treated with anti-CTLA4 mAb, but only the systemically treated arm demonstrated a statistically significant increase relative to control-treated animals (Fig. 4B). Finally, both administration routes decreased the levels of tumor-infiltrating Tregs as a percentage of the total tumor-infiltrating CD4+ lymphocytes (Fig. 4C).
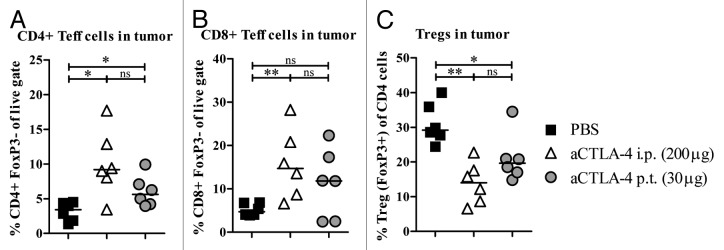
Figure 4. Anti-CTLA-4 shifts the balance between tumor-infiltrating Teff cells and Tregs. Tumor-infiltrating effector T (Teff) cells and regulatory T cells (Tregs) were evaluated on day 14 after low-dose p.t. and high-dose i.p. aCTLA-4 therapy in animals carrying Panc02 tumors. Data are visualized as (A) percent CD4+ Teff cells (CD4+FoxP3-), (B) percent CD8+ Teff cells (CD8+FoxP3-) and (C) percent Tregs (CD4+FoxP3+) in tumor tissue (data shown are the median; n = 6). Statistical analysis was performed by Mann–Whitney. *P < 0.05, **P < 0.01; ns, not significant.
Discussion
Pancreatic adenocarcinoma patients have a poor prognosis due to the proclivity of this particular malignancy to metastasize, the rapidity of disease progression and limited treatment options. In 1997, gemcitabine became the standard treatment for patients with advanced disease. The efficacy of this treatment modality is limited even in adjuvant settings, but nevertheless modestly improves overall survival.26 Consequently, new therapies to treat this devastating disease are urgently needed. CTLA4-targeting blocking antibody immunotherapy has recently been demonstrated to be dependent upon tumor-infiltrating myeloid cells as they mediate FcγR cross-linking and subsequent cytolytic or phagocytic depletion of CTLA4 antibody-decorated Tregs.16-18 One tumor type commonly known to be rich in myeloid cell infiltrates as well as Tregs is pancreatic adenocarcinoma,3 suggesting that this tumor type could potentially respond more robustly to CTLA4 antibody-based therapy. As demonstrated by others, CTLA4 antagonists can be administrated locally to maintain tumor inhibition with reduced adverse events.13-15 The attenuation of non-desirable side effects taken together with the presence of a poised tumor-directed Treg depleting mechanism makes the local approach even more attractive as a means of tumor inhibition. However, the local approach has been implicated to work best together with an immune adjuvant.19 Fransen et al., previously demonstrated that tumor-site directed anti-CTLA4 antibody suspended in a slow-release matrix eradicated both primary and distant MC38-OVA tumor growth, despite reduced serum antibody levels.13 Here, we demonstrate that, at least in some tumor contexts, local low-dose anti-CTLA4 monotherapy can be successfully utilized to treat cancer without a slow-release formulation or adjuvant (mineral oil or CpG).
Specifically, we investigated the therapeutic potential of local administration of CTLA4 blocking antibodies in an experimental model of pancreatic adenocarcinoma. Local low-dose anti-CTLA4 mAb therapy inhibited tumor growth, but frequent dosing or increased dose levels did not improve tumor regression. This could be explained by the fact that higher dosages induced systemic Treg expansion that counteracted antitumor T-cell responses. Nevertheless, when comparing the efficacy of a commonly used systemic high-dose (200 μg) with peritumoral administration of low-dose (30 μg) anti-CTLA4 mAb, a small, but insignificant difference in overall survival favoring the high-dose systemic treatment was detected.
A larger proportion of patients treated with systemic anti-CTLA4 therapy, such as Ipilimumab, experience immune-related adverse events, mostly affecting the gastrointestinal tract and skin.7,8 If gastrointestinal Treg depletion is partly responsible for these adverse events (and this remains to be established), a slow-release formula or antibody design aiming to increase antibody retention at the tumor site could potentially limit such side effects. Evidently, mice do not respond with the same toxicity profile as patients after CTLA4-blockade immunotherapy. One possible underlying explanation could be that the gut microbiota of experimental animals differs from that of humans as the mice are housed in clean environments that could affect the density of myeloid cells and/or the FcγR-expressing immune cell repertoire in the gastrointestinal tract. Localized Tregs have been described to be essential to maintain peripheral tolerance in the gut of mice, and their suppressive function is dependent upon CTLA4.27,28 In humans afflicted with inflammatory bowel disease, increased levels of circulating, and potentially autoimmune, T helper 17 cells are accompanied by a corresponding reduction in Tregs, suggesting a critical role for Tregs in the maintenance of gut T cell homeostasis.29
In our study, systemic administration of anti-CTLA4 mAb resulted in elevated therapeutic antibody concentrations in the serum of treated animals, but there was some leakage of antibodies into the circulation upon localized treatment as well, albeit at lower levels. A recent Phase II study by Wolchok et al. concluded that the best overall therapeutic responses were dose-dependent, but unfortunately, so were associated immune-related adverse events.30 We concede that our mouse model likely has limitations with respect to the evaluation of skin rash and colitis, the 2 most common anti-CTLA4-induced toxicities in humans. However, as these complications correlate with systemic drug levels in the clinical setting, it is highly likely that a localized treatment approach would be less toxic in patients. If systemic drug levels can be reduced while retaining the essential antitumor efficacy, it would be beneficial to switch patients with dose-limiting toxicity to a local administration regimen. In the case of pancreatic cancer, with a known resistance to drug penetration due to dense fibrotic tumor stroma, local injections directly into the tumor mass may be efficacious in eliminating tumor-infiltrating Tregs and our findings provide rationale to investigate this possibility further in a clinical setting. The high pressure present within a tumor may lead to initial drug escape subsequent to the local injection. However, dissemination of the drug to local TDLNs may also target antigen-experienced T-cells residing there. The TDLN is commonly known to be an important site for the T-cell mediated priming of dendritic cells (DCs) and we recently demonstrated that locally delivered agonistic CD40 antibodies accumulate in the TDLN. Specifically, we found that as a result of repeated local CD40 injections, antigen-presenting cells in the TDLN increased in number and upregulated their surface CD40 expression.20
Earlier experimental studies have reported elevated frequencies of extratumoral Tregs in mice without impairment of their regulatory capacity after prolonged systemic anti-CTLA4 antibody therapy.6 Moreover, Kavanagh et al. detected 26 that systemic anti-CTLA4 mAb administration increased the levels of circulating Tregs in humans in a dose-dependent manner.23 In this study, we also observed higher levels of Tregs in the spleen and TDLN of animals following systemic high-dose therapy but not in response to localized low-dose treatment. Prior studies have shown that untreated murine Panc02 tumors are heavily infiltrated with Tregs exhibiting an effector/memory phenotype, suggesting an enhanced immunosuppressive activity and proliferative capacity.31 Here, we report reduced levels of FoxP3-expressing cells in the tumor-infiltrating CD4+ T-cell pool after both local low-dose and high-dose therapy and occurring in conjunction with increased percentage of tumor-infiltrating CD4+ and CD8+ Teff cells. It has been reported that CTLA4 blockade concurrent with Treg depletion results in synergistic22,32 or enhanced33 antitumor effects. Peggs et al. elegantly concluded that anti-CTLA4-mediated action on both Tregs and Teff cells are necessary for full therapeutic effect.34 Thus, local Treg depletion may boost the immunological ability of Teff cells to eradicate a primary tumor whereas systemic induction of Tregs may have an impact on how well Teff cells target disseminated disease. With this caveat in mind, optimal dosing of anti-CTLA4 mAb appears crucial to enhance effector T-cell function and selectively deplete Tregs specifically within the tumor while avoiding systemic suppressor Treg cell expansion.
To summarize, our data convincingly demonstrate that it is possible to administer lower doses of CTLA4 blocking antibody into the tumor area and still achieve antitumor effects, without accompanying expansion of systemic Tregs, in experimental pancreatic adenocarcinoma. In the clinical setting, intratumoral injections of anti-CTLA4 mAb, or other immunomodulatory agents, could be performed by ultrasound-guided needle injection.
Materials and Methods
Cell lines and reagents
The Panc02 cell line (murine ductal adenocarcinoma derived from C57BL/6) was kindly provided by Dr R Heuchel (Karolinska University Hospital). Cells were cultured in DMEM+ GlutaMax supplemented with 10% fetal bovine serum (FBS), 1mM sodium pyruvate, 100 U/mL penicillin/streptomycin (all from Invitrogen) and maintained at 37 °C and 5% CO2. Antagonistic mouse-anti-mouse CTLA4 mAb (clone: 9D9, Bio X Cell) was diluted in phosphate buffered saline (PBS) prior to injection, as indicated. To quantify Tregs, tumor, splenic, and lymph node tissues were disrupted and cells were immunofluorescently stained using fluorophore-conjugated antibodies against the surface receptors CD4 (clone GK1.5, BioLegend), CD8 (clone 53-6.7, BioLegend) and CD25 (clone PC61, BD Bioscience), and for intracellular FoxP3 (clone 150D, BioLegend), according to the manufacturer’s protocol. Samples were analyzed using a FACSCalibur, LSRII or Canto II (BD Biosciences) flow cytometer. Data analysis was performed with FlowJo software (Tree Star).
Animals and in vivo experimental design
C57BL/6 mice were obtained from Scanbur B&K (Sollentuna). Animals were housed at the Rudbeck animal facility and cared for by the staff according to European and national regulatory standards. All animal experiments were approved by Uppsala Animal Ethics Committee (Dnr: C21/10, C91/8, and C163/8). Allograft tumor cell transplantation was performed using 2.5 × 105 Panc02 cells inoculated subcutaneously in the right flank of C57BL/6 mice on day 0 with CTLA4-blocking antibody treatment starting on day 5 and administered either 3 or 6 times with 3-d intervals between injections. Antibody solution was administered in 100 μL and injected either peritumorally, subcutaneously¸ intraperitoneally, or intravenously. The dose and administration route for each experiment are indicated in the figure legends. Tumor growth and survival were monitored throughout the experiment using a caliper and tumor size was calculated by the ellipsoid volume formula: 4/3π*a(radius of length)*b(radius of width)*c(radius of depth). For rechallenge experiments, mice were s.c. injected with 2.5 × 105 Panc02 cells in the contralateral side. Animals were sacrificed in the event that the tumor volume exceeded 1cm3 or if ulcers developed.
Anti-CTLA4 antibody ELISA
Blood was collected from the experimental animals by tail vein incision and the separated serum was stored at −80 °C. Serum quantification of circulating CTLA4 mAb was performed by enzyme-linked immunosorbant assay (ELISA). Briefly, wells were coated with 0.3 μg/mL recombinant murine CTLA4 (R&D Systems) and blocked with 3% milk powder before serum samples (1:100–1:500 dilution in PBS) were added. Horse radish peroxidase-conjugated goat-α-mouse IgG (Dako) was used to detect the mouse-anti-mouse CTLA4 therapeutic antibody. A positive signal was developed by adding the substrate (SuperSignal Pico Chemoluminescent Substrate, Pierce) before luminescence was measured with an ELISA plate reader (Fluostar optimal, Labvision). Samples were run in triplicate.
Statistical analyses
Statistical analyses for the Kaplan–Meier plotted survival curves of animals treated with different regimens were performed by log-rank test. When applicable, D´Agostino and Pearson omnibus normality test or Kolmogorov–Smirnov test were applied before selection of statistical test. Where indicated, the difference between groups was evaluated using unpaired Student’s t test, Mann–Whitney or 1-way ANOVA with the Bonferroni post hoc test. In all cases, P values < 0.05 were considered statistically significant and stars indicate the degree of significance (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc.).
Disclosure of Potential Conflicts of Interest
LS, PE, AL, TT, and SM have a royalty agreement with Alligator Biosciences AB. TT is a consultant for Alligator Bioscience AB and Immuvent Inc. AL is the CEO of Lokon Pharma AB and a scientific advisor at NEXTTOBE AB. FE declares no conflict of interest.
Acknowledgments
We thank Ann-Charlotte Hellström and Anna Rosén (Alligator Bioscience, Inc.) for excellent technical assistance. This study was supported by The Swedish Cancer Society (T.H.T Tötterman), the Swedish Research Council (T.H.T Tötterman), and FP7 MCA-ITN 317445 (S.M. Mangsbo). P. Ellmark was sponsored by the Swedish Research Council (VR-IFA hosted by Lund University).
Glossary
Abbreviations:
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADP
antibody-dependent phagocytosis
- CTLA4
cytotoxic T-lymphocyte antigen-4
- FcγR
Fc gamma receptor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- mAb
monoclonal antibody
- p.t.
peritumoral
- TDLN
tumor-draining lymph node
- Teff
effector T
- Treg
regulatory T cell
Citation: Sandin LC, Eriksson F, Ellmark P, Loskog AS, Tötterman TH, Mangsbo SM. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. OncoImmunology 2014; 3:e27614; 10.4161/onci.27614
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27614
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer. Gut. 2013;62:317–26. doi: 10.1136/gutjnl-2012-303588. [DOI] [PubMed] [Google Scholar]
- 3.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinar P, Tempero MA. Monoclonal antibodies and other targeted therapies for pancreatic cancer. Cancer J. 2012;18:653–64. doi: 10.1097/PPO.0b013e3182758985. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 6.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron F, Whiteside G, Perry C. Ipilimumab: first global approval. Drugs. 2011;71:1093–104. doi: 10.2165/11594010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr., Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fransen MF, van der Sluis TC, Ossendorp F, Arens R, Melief CJ. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res. 2013;19:5381–9. doi: 10.1158/1078-0432.CCR-12-0781. [DOI] [PubMed] [Google Scholar]
- 14.Tuve S, Chen BM, Liu Y, Cheng TL, Touré P, Sow PS, Feng Q, Kiviat N, Strauss R, Ni S, et al. Combination of tumor site-located CTL-associated antigen-4 blockade and systemic regulatory T-cell depletion induces tumor-destructive immune responses. Cancer Res. 2007;67:5929–39. doi: 10.1158/0008-5472.CAN-06-4296. [DOI] [PubMed] [Google Scholar]
- 15.Simmons AD, Moskalenko M, Creson J, Fang J, Yi S, VanRoey MJ, Allison JP, Jooss K. Local secretion of anti-CTLA-4 enhances the therapeutic efficacy of a cancer immunotherapy with reduced evidence of systemic autoimmunity. Cancer Immunol Immunother. 2008;57:1263–70. doi: 10.1007/s00262-008-0451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–93. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby MJ. E.J.J., Quigley M., Henning K.A., Chen T., Srinivasan M., Korman A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunology Research. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 19.Marabelle A, Kohrt H, Levy R. Intratumoral anti-CTLA-4 therapy: enhancing efficacy while avoiding toxicity. Clin Cancer Res. 2013;19:5261–3. doi: 10.1158/1078-0432.CCR-13-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Tötterman TH, Mangsbo SM. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res. 2014;2:80. doi: 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]
- 21.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33:225–35. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 22.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavanagh B, O’Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 27.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 29.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–9. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 30.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Jr., et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 31.Shevchenko I, Karakhanova S, Soltek S, Link J, Bayry J, Werner J, Umansky V, Bazhin AV. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. 2013;133:98–107. doi: 10.1002/ijc.27990. [DOI] [PubMed] [Google Scholar]
- 32.Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, Sutmuller RP, Adema GJ. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 33.Saha A, Chatterjee SK. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scand J Immunol. 2010;71:70–82. doi: 10.1111/j.1365-3083.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 34.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]