Abstract
Thrombin causes blood-brain barrier disruption and this study examined whether thrombin can cause brain hemorrhage through protease-activated receptor-1 (PAR-1). Male wild type and PAR-1 knockout mice had an intracerebral injection of thrombin or saline. Mice then underwent serial T2 magnetic resonance imaging and were euthanized for brain hemoglobin, iron and interleukin-1β measurements. Thrombin caused massive T2 lesions and brain hemorrhage in wild type mice. These effects were markedly reduced in PAR-1 knockout mice. Thrombin also increased brain interleukin-1β and this was absent in PAR-1 knockout mice. In conclusion, thrombin increases interleukin-1β levels and induces intracerebral hemorrhage through PAR-1 activation.
Keywords: cerebral hemorrhage, interleukin-1β, protease activated receptor-1(PAR-1), thrombin
Introduction
Thrombin is a serine protease and contributes to brain injury after both intracerebral hemorrhage and cerebral ischemia(1–4). Thrombin can be produced in the brain either immediately after a brain hemorrhage or after blood-brain barrier (BBB) disruption. It is well known that high concentrations of thrombin in the brain are harmful(5).
The effects of thrombin can be non-receptor-mediated as in, for example, cleavage of fibrinogen to fibrin, or receptor-mediated as in, for instance, activation of p44/42 mitogen activated protein kinases(5). Three protease-activated receptors (PARs), PAR-1, PAR-3 and PAR-4, are thrombin receptors and can be activated by thrombin(6). Activation of PAR-1 has been linked to many intracellular signaling pathways and is related to brain injury after hemorrhagic and ischemic stroke(7, 8).
Hemorrhagic transformation and hematoma enlargement exacerbate brain damage after ischemic and hemorrhagic stroke. There are many triggers and mediators of hemorrhagic transformation after cerebral ischemia including the inflammatory mediator, interleukin-1 (9). Our previous studies have demonstrated that thrombin activity is increased in ischemic and hemorrhagic brain and thrombin can cause BBB disruption(5). In the current study, we investigated whether thrombin can cause brain hemorrhage and whether this is through PAR-1. In addition, we also examined the role of PAR-1 in thrombin-induced upregulation of brain interleukin-1β (IL-1β), as a potential mediator of vascular disruption.
Materials and Methods
Animal Preparation and Intracerebral Infusion
The University of Michigan Committee on the Use and Care of Animals approved the protocols for these animal studies. Male PAR-1 knockout mice (PAR-1 KO, University of Michigan Breeding Core) and male wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME), aged 2–3 months, were used in the study. Mice were anesthetized with ketamine (90 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). Rectal temperature was maintained at 37.5°C. The mice received an injection of rat thrombin (0.5 U, Sigma) in 10-µl saline or 10-µl saline alone into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm ventral, and 2.5 mm lateral to the bregma). After injection, the needle was removed, and the skin incision was closed with suture.
Experimental Groups
This study was divided into 4 parts: 1) Male WT and PAR-1 KO mice had 0.5 U thrombin or saline injected into right basal ganglia. Some animals had serial magnetic resonance imaging (MRI) T2 imaging at days 1 and 3. Mice (n=5, each group) were euthanized at 1, 3 and 7 days later for brain histology; 2) WT and PAR-1 KO mice (n=5, each group) were euthanized at day 3 after thrombin injection for brain hemoglobin content determination; 3) WT mice (n=6, each group) were euthanized at 6, 12, 24, 48 and 72 hours after thrombin injection to determine the time course of IL-1β content; and 4) WT and KO mice (n=5, each group) had a thrombin injection and were euthanized at 12 hours later for IL-1β measurement.
Magnetic Resonance Imaging
MRI was performed using a 7.0-T MR scanner. A T2 fast spin-echo sequence (repetition time/echo time=4000/60 ms) was performed. Twenty-five slices of 0.5 mm thickness were performed. T2 lesion volumes were measured with Image J (10). All measurements were repeated three times and the mean value was used.
Hemoglobin Measurement
Cerebral hemorrhage was evaluated using a spectrophotometric assay to determine hemoglobin content(11). At 3 days after thrombin injection, animals were perfused transcardially with 0.1mol/L phosphate-buffered saline under deep anesthesia until the outflow fluid from the right atrium was colorless. The brain was rapidly removed and dissected into the left and right hemispheres. Total hemispheric hemoglobin content was expressed in micrograms.
Enhanced Perls’ staining
Brain sections were incubated in Perls’ solution (1:1, 5% potassium ferrocyanide and 5% HCl) for 45 minutes, and incubated again in 0.5% diamine benzidine tetrahydrochloride with nickel for 60 minutes.
Brain tissue IL-1β measurement
For IL-1β measurement, basal ganglia samples were taken and IL-1β was determined using an enzyme-linked immunosorbent assay kit for mouse IL-1β (R&D Systems, Minneapolis) (12). This kit measures the active form of IL-1β. Results were expressed as nanograms per gram (ng/g) of brain tissue.
Statistics
All data in this study are presented as mean ± S.D. Data were analyzed with Student’s t-test and analysis of variance. Statistical significance was set at p<0.05.
Results
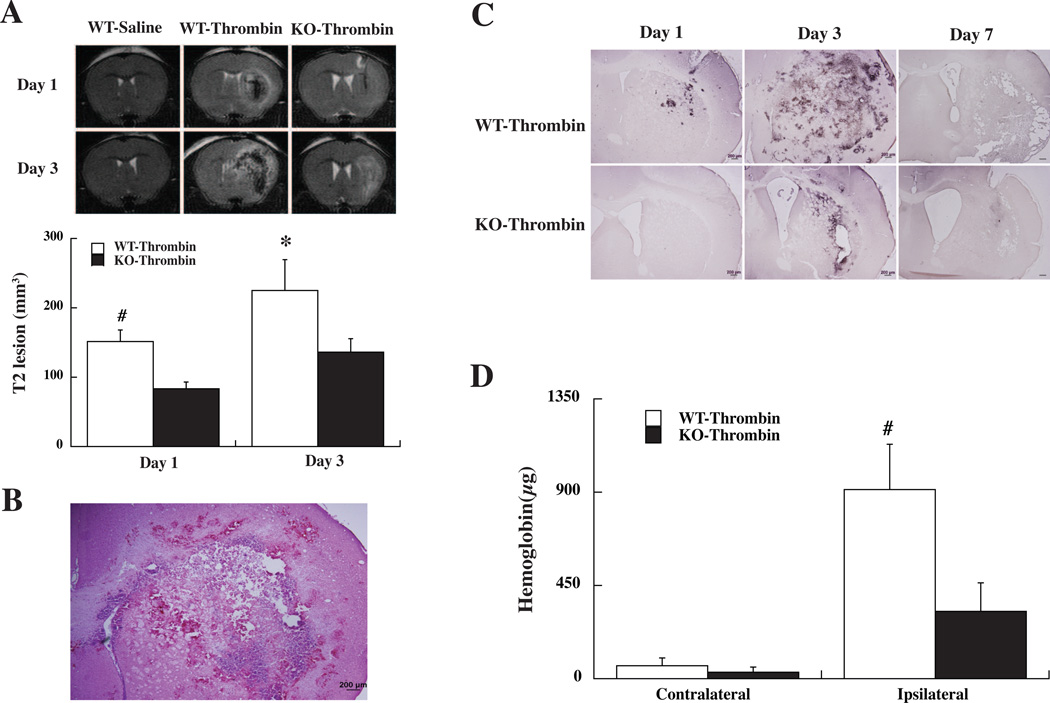
Mortality after intracerebral thrombin injection was not significantly different in WT (11%) and PAR-1 KO mice (9%). Thrombin but not saline resulted in massive T2 lesions in WT mice (Fig. 1A). MRI studies showed that the T2 lesions induced by thrombin were smaller in PAR-1 KO mice compared to those in WT mice (Fig. 1A).
Figure 1.
(A) MRI T2-weighted images showing thrombin-induced brain injury in wild type (WT) and PAR-1 knockout (KO) mice at days 1 and 3. Intracerebral injection of saline did not result in T2 lesion. Values are mean ± SD, n=5, #p<0.01, *p<0.05 versus PAR-1 KO. (B) An example of a brain section from a WT mouse 3 days after thrombin injection. Note the multiple areas of hemorrhage around the ipsilateral hemisphere. (C) Perls’ staining showing iron accumulation in the ipsilateral hemisphere of WT and KO mice after thrombin injection. Scale bar=200 µm. (D) Brain hemoglobin levels in the contralateral and ipsilateral hemispheres of WT and KO mice 3 day after thrombin injection. Values are mean ± SD, n=5, #p<0.01 versus PAR-1 KO mice and the contralateral hemisphere.
An examination of brain sections after thrombin injection showed areas of hemorrhage in the ipsilateral hemisphere (Fig. 1B). This hemorrhage was examined using Perls’ staining for iron and by measuring brain hemoglobin. Perls’ staining showed that thrombin caused significant brain hemorrhage in WT mice and that hemorrhage peaked at day-3. Perls’ positive cells were less in PAR-1 KO mice (Fig. 1C). Brain hemoglobin content was measured at day-3 after thrombin injection to assess hemorrhage volume. Thrombin-induced marked hemoglobin accumulation in the ipsilateral hemisphere in WT-type mice and this was markedly reduced in PAR-1 KO mice (hemoglobin: 327±134 vs. 913±221 µg in the WT mice, p<0.01, Fig. 1D).
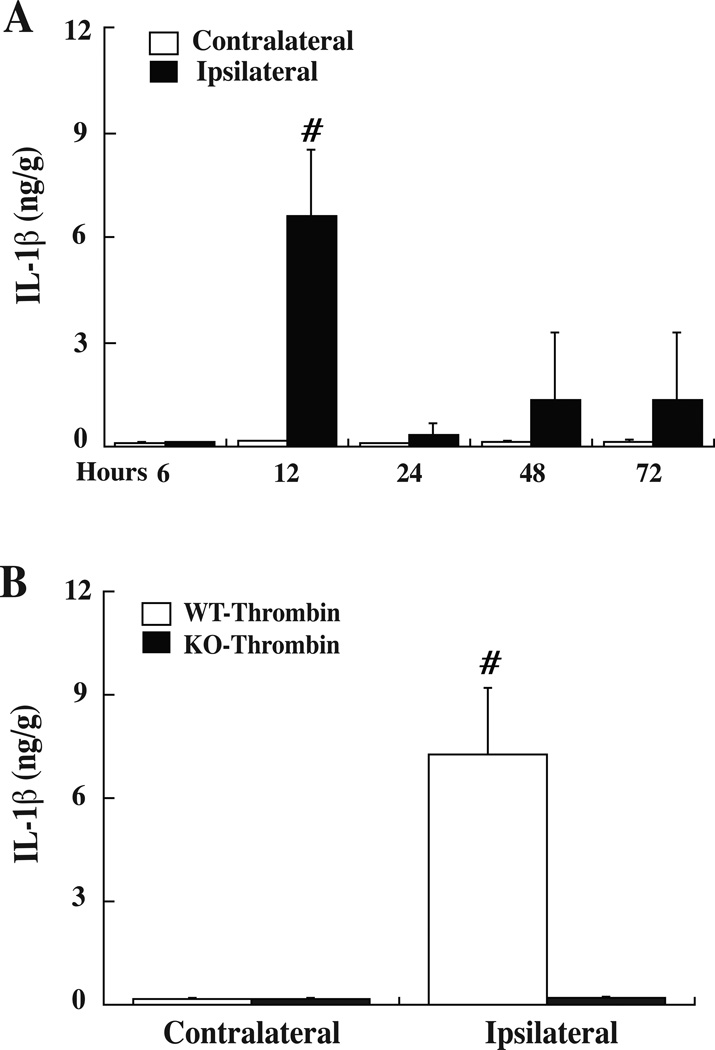
The role of PAR-1 in brain IL-1β levels after thrombin stimulation was also examined. Figure 2A shows the time course of brain IL-1β levels following thrombin injection in WT mice. Brain IL-1β levels peaked at 12 hours and returned to low levels after 24 hours. We then measured brain IL-1β levels in WT and PAR-1 KO mice and found that thrombin-induced upregulation of brain IL-1β was basically absent in PAR-1 KO mice (p<0.01, Fig. 2B).
Figure 2.
(A) IL-1β protein levels in the basal ganglia of WT mice after intracerebral injection of thrombin (0.5 U). Values are mean ± SD, n= 6, #p<0.01 versus the contralateral side and the other time points. (B) IL-1β protein levels in the basal ganglia of WT and PAR-1 KO mice at 12 hours after thrombin injection. Values are mean ± SD, n=5, # p<0.01 versus PAR-1 KO mice.
Discussion
A major finding of current study is that intracerebral thrombin can cause cerebral hemorrhage, despite the known effects of thrombin on hemostasis. Thrombin activity is increased in ischemic brain and hemorrhagic transformation is a major problem for ischemic stroke, especially with tissue plasminogen activator treatment.
Our previous studies have demonstrated that thrombin causes BBB disruption, brain edema, inflammatory cell infiltration and neuronal death. Thrombin also exacerbates ischemic brain injury (13) and intracerebral injection of hirudin, an inhibitor of thrombin, attenuates infarct volume and neurological deficits without altering local cerebral blood flow(14). Intraventricular injection of hirudin also reduces hippocampal neuronal death in a gerbil model of global cerebral ischemia(15). Another recent study found that thrombin mediates ischemia-induced BBB disruption, an effect blocked by argatroban, a thrombin inhibitor (1). These results suggest that thrombin is a target for stroke therapy. Future studies should determine whether thrombin inhibition can reduce hemorrhagic transformation after cerebral ischemia.
The generation of thrombin from plasma prothrombin is a critical for hemostasis. However, extravascular thrombin has many effects in the brain, including BBB disruption (5). The current study demonstrates that the extravascular effects of thrombin can result in intracerebral hemorrhage, i.e. it appears that those effects can outweigh the hemostatic effects of thrombin. The balance between the hemostatic and pro-hemorrhagic actions of thrombin is likely dependent upon multiple factors such as the concentration of extravascular thrombin and the expression of thrombin receptors (as shown in the current study) and inhibitors in a particular disease state.
Our data showed that PAR-1 plays a role in thrombin-induced hemorrhage. It is well known that PAR-1 activation is related to hemorrhagic and ischemic brain injury. For example, thrombin- and intracerebral hemorrhage-induced brain injury is less in PAR-1 KO mice(8). Brain infarction is also reduced in PAR-1 KO mice and intracerebroventricular injection of PAR-1 antagonist BMS-200261 reduces infarct volume in a mouse cerebral ischemia model (7). The role of PAR-1 activation in hemorrhagic transformation needs to be investigated further.
It is important to understand how thrombin causes brain hemorrhage. A previous study found that intracerebral injection of thrombin did not affect cerebral blood flow in rats(16). Our present results found that thrombin increases brain IL-1β through PAR-1, which may contribute to BBB disruption and brain hemorrhage. Investigations have shown that overexpression of interleukin-1 receptor antagonist in the brain reduces thrombin-induced brain edema (17) and inhibition of IL-1β reduces BBB permeability after subarachnoid hemorrhage (18). It should be noted that the effects of thrombin on inflammatory mediators is not limited to IL-1β. For example, we have shown that an intracerebral injection of thrombin causes a marked increase in tumor necrosis factor-α (12). It is possible that the effects of thrombin on a combination of such mediators ultimately result in hemorrhage.
It is becoming clear that thrombin has multifactorial actions in stroke dependent on site (intra- or extravascular) and mode of action (fibrinogen cleavage or activation of which type of receptor). This adds complexity to targeting thrombin in stroke. The current study suggests that targeting PAR-1, while not effecting thrombin-mediated cleavage of fibrinogen to fibrin, might be one potential approach.
In conclusion, intracerebral thrombin can result in brain hemorrhage through PAR-1 activation. IL-1β may have a role in thrombin-induced hemorrhage.
Acknowledgments
Sources of Funding
This study was supported by grants NS-057539, NS-073595, NS-079157 and NS-084049 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest:
Yingying Cheng, Guohua Xi, Hang Jin, Richard F. Keep, Jiachun Feng and Ya Hua declare that they have no conflict of interest.
References
- 1.Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010 Oct;41(10):2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 3.Bodmer D, Vaughan KA, Zacharia BE, Hickman ZL, Connolly ES. The Molecular Mechanisms that Promote Edema After Intracerebral Hemorrhage. Transl Stroke Res. 2012 Jul;3:S52–S61. doi: 10.1007/s12975-012-0162-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu DZ, Sharp FR. Excitatory and Mitogenic Signaling in Cell Death, Blood-brain Barrier Breakdown, and BBB Repair after Intracerebral Hemorrhage. Transl Stroke Res. 2012 Jul;3:S62–S69. doi: 10.1007/s12975-012-0147-z. [DOI] [PubMed] [Google Scholar]
- 5.Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? Journal of Neurochemistry. 2003;84(1):3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 6.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 7.Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci USA. 2003;100(22):13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M, Hollenberg MD, Demchuk A, Yong VW. Relative importance of proteinaseactivated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke. [Research Support, Non-U.S. Gov't] 2009 Jun;40(6):2199–2204. doi: 10.1161/STROKEAHA.108.540393. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- 10.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010 Feb;41(2):375–382. doi: 10.1161/STROKEAHA.109.569830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygeninduced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007 Apr;38(4):1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 12.Hua Y, Wu J, Keep R, Nakamura T, Hoff J, Xi G. Tumor necrosis factor-alpha increases in the brain following intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58(3):542–550. doi: 10.1227/01.NEU.0000197333.55473.AD. [DOI] [PubMed] [Google Scholar]
- 13.Hua Y, Wu J, Keep RF, Hoff JT, Xi G. Thrombin exacerbates brain edema in focal cerebral ischemia. Acta Neurochir Suppl. 2003;86:163–166. doi: 10.1007/978-3-7091-0651-8_34. [DOI] [PubMed] [Google Scholar]
- 14.Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow & Metab. 2004 Feb;24(2):159–166. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- 15.Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KR, Kawai N, Kim S, Sagher O, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of thrombin on cerebral blood flow, blood-brain barrier permeability, and cell survival in a rat model. J Neurosurg. 1997;86(2):272–278. doi: 10.3171/jns.1997.86.2.0272. [DOI] [PubMed] [Google Scholar]
- 17.Masada T, Hua Y, Xi G, Yang GY, Hoff JT, Keep RF. Attenuation of intracerebral hemorrhage and thrombin-induced brain edema by overexpression of interleukin-1 receptor antagonist. J Neurosurg. 2001;95(4):680–686. doi: 10.3171/jns.2001.95.4.0680. [DOI] [PubMed] [Google Scholar]
- 18.Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009 Jul;40(7):2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]