Abstract
Objective
To explore biopsychosocial factors (beliefs, depression, catastrophizing cytokines) in individuals newly diagnosed with lung cancer and no pain in order to determine their relationship at diagnosis and across time and to determine whether these factors contribute to pain intensity or pain interference with function at pain onset.
Methods
A longitudinal, exploratory, pilot study was implemented in a private medical center and a VA medical center in the southeast. Twelve subjects not experiencing pain related to cancer of the lung or its treatment were recruited. A Karnofsky status of 40% and Hemoglobin of 8 grams were required. Five questionnaires were completed and 10 cc of blood was drawn at Baseline; 4 questionnaires and blood draws were repeated monthly for 5 months. One Baseline questionnaire and a pain assessment were added at Final. Demographic, clinical and questionnaire data were summarized; standardized scale scores were calculated.
Results
Biopsychosocial scores that were low at Baseline increased from T1-T4 but decreased slightly T5-T6. Individuals with higher pain intensity and higher pain interference at Final had higher psychosocial scores at Baseline than individuals with lower pain intensity and lower pain interference at Final.
Conclusions
Unrelated to disease stage, metastasis or treatment, unique, levels of biopsychosocial factors are observed in patients newly diagnosed with lung cancer who report higher levels of Pain Intensity and higher levels of Pain Interference at the time pain occurs. Replication studies are needed to validate this response pattern and determine the value of repeated individual assessments.
Introduction
Adequate control of pain related to cancer remains a challenging, unresolved problem despite extensive study and thousands of articles devoted to its measurement and management. A myriad of theoretical approaches have focused on multiple modalities for decreasing pain intensity and modifying pain behavior. Distinctive biopsychosocial factors that may be predictors of cancer pain and successful treatment have not been well explored.
Thus, determining the degree to which specific factors such as pain beliefs, depression, catastrophizing, and the inflammatory response are present at diagnosis is needed. The conceptual framework for study of these relationships is drawn from the neuromatrix theory espoused by Melzack.1 This framework suggests that cognitive-evaluative, sensory-discriminative and motivational-affective inputs to the body-self produce outputs that includes pain perception, action and stress-regulation, (Melzack, 1996) including activation of the cytokine stress-regulating system.
Classical studies demonstrate that pain beliefs 2-4 and depression 5-9 can be mediators of the body-self output. These factors may predict or contribute to individual variation in perceived control over pain and report of pain interference with activities10,11 or as a predictor for the pain response.12-15 Studies also suggest that depression may reduce pain tolerance 16, contribute to greater pain intensity, or reduce a patient's ability to cope with pain.17,18
Elsewhere, studies of catastrophizing and pain suggest that pessimistic beliefs are related to misconceptions about potentially painful stimuli and contribute to a heightened pain experience19,20 and change in the perceived effectiveness of coping strategies.21 Patients who magnify the threat value of pain may increase their focus on pain and their inability to cope, creating a more intense experience.22 More specifically, patients’ tendency to catastrophize is presumed to modify the affective and evaluative components of the pain experience and to contribute to more intense pain and increased emotional distress.22-28 In fact, lack of belief in one's ability to control one's own pain has been found to be a significant predictor of postoperative pain intensity.29
Proinflammatory cytokines such as interleukin 6, known to facilitate neuropathic pain, may serve as a link between psychological factors and the experience of pain in general.30-31 Moreover, increasing interest in the role of proinflammatory cytokines and in the development of pain syndromes in patients with cancer pain31 and cancer pain treatment32-35 reflects increased interest in the motivation-affective inputs to the neuromatrix.
The purpose of this pilot study was to begin to explore biopsychosocial factors that may contribute to pain intensity and interference with function in a small sample of individuals newly diagnosed with lung cancer and no pain and to determine whether changes in these relationships occur over time. The study questions asked:
To what degree are psychosocial and biological factors such as beliefs about pain, depression, catastrophizing, and IL-6 present at diagnosis?
What changes in beliefs, depression, catastrophizing and IL-6 occur over time?
How do beliefs about pain, depression, catastrophizing and IL-6 that occur at diagnosis and over time relate to self-report of pain intensity and interference of pain with function that occurs approximately 6 months after diagnosis?
Materials and Methods
The Biobehavioral Pain Profile (BPP)3 is a 41-item self-report, 7-point Likert response scale tested in a sample of 617 subjects with chronic recurrent pain, chronic non-malignant pain or chronic malignant pain. The six BPP scales measure environmental influences (12 items), loss of control (11 items), health care avoidance (6 items), past and current experience (5 items), physiological responsivity (3 items), and thoughts of disease progression (4 items). Coefficient alphas for the original scales ranged from .77 to .94 and test-retest ranged from .57 to .73.
The Beck Depression Inventory (BDI--II)36,37 contains 21 items, each rated on a 4-point scale from 0 to 3 (“not present” to “severe”). Items are categorized as Cognitive-Affective and Somatic. Coefficient alphas ranged from .92 with psychiatric outpatients to .93 with undergraduate students. Test-retest reliability was .93 for the original BDI-II when repeated at one week. Construct validity for the BDI-II and BDI-IA was r = .93; construct validity for the BDI-II and the Beck Hopelessness Scale and the Hamilton Rating Scale was r = .68 and r = .71, respectively.
The Coping Strategies Questionnaire (CSQ)38 contains seven subscales including the catastrophizing scale (CAT). Subjects indicate how often they use a particular strategy: 0 = never, 3 = sometimes, and 6 = always, measured on a 7-point Likert scale. Possible scores for the total scale are 0-300; possible scores for the catastrophizing scale range from 0-36. Initial study showed the CAT to be internally reliable with a coefficient alpha of .78. Test-retest reliability of the CAT was .91 for patients with chronic low back pain.39.
The Brief Pain Inventory (BPI)40 was designed to provide information about pain intensity and the degree to which pain interferes with function. The BPI pain intensity measure uses a 0-10 scale to rate “worst”, “least”, “usual” pain and “pain right now.” The Pain Interference measure uses a 0-10 scale to measure the impact of pain on activity, mood, walking, relations, sleep and life enjoyment. The BPI demonstrated reliability over a brief period of time (worst, r = .93; usual, r = .59; now, r = .22); its validity has been demonstrated in several studies.41
Interleukin 6 (IL-6) can be a measure of the inflammatory response where concentrations of IL-6 are measured by high sensitivity quantitative enzyme-linked immunosorbent assays (ELISA). To control for circadian variability study samples were drawn between 8:00-10:00 a.m. with routine blood work. Specifically,10 c.c. of blood was drawn in heparinized tubes and immediately placed on ice. Samples were centrifuged and plasma was removed, aliquoted and frozen for batched assay of IL-6. All samples were assayed in duplicate. Quality control plasma of low and high cytokine concentrations were included with every assay. Assay sensitivity in this study was 0.04 pg/ml; inter- and intra-assay variability was reliably <12%.
The design of the study was exploratory and descriptive. A convenience sample of patients being seen at a large, private medical center or VA medical center in the southeast were subjects in the study. All patients signed an informed Consent. Patients newly diagnosed with lung cancer (all cell types), a hemoglobin level of 8.0 grams or better, and NOT experiencing pain were eligible for the pilot study. The Research Assistants had IRB approval to review daily appointment sheets electronically and communicate with the social worker, nurses and physicians to identify, recruit and interview potential subjects. Individuals with chart-documented psychopathology (schizophrenia or bi-polar disorder) or confusion were not eligible.
Time periods for data collection were Baseline, interviews 2-5, and Final, periods that coincided with the subjects’ regular clinic visits. The 12 subjects in the study completed 5 questionnaires and had 10 c.c. of blood drawn at the time they were diagnosed and entered the study (T1). The RA rated the individual's ability to care for him/herself and severity of symptoms on the Karnofsky42 scale (a scale of 10-100%). A rating of 40% or better was required at Baseline and each visit. Individuals were requested to repeat 4 original questionnaires and have blood drawn approximately monthly for five visits (T2-T6). At the Final interview (T6), 1 original questionnaire (BPP) and 1 new measure of pain (BPI) were added.
The RA approached potential participants, explained the study and ascertained their willingness to participate. Potential participants were told that all patients do not develop pain secondary to their disease, but that if they do develop pain its intensity can vary and that if we understand personal characteristics and the inflammatory response, we may be better prepared to make specific recommendations that could impact treatment in the future. Individuals were able to complete all the Baseline questionnaires in 45 minutes or less. Subjects were given $25.00 gas cards after the final interview period as reimbursement for travel costs.
Demographic and clinical information and summary data on beliefs, depression, catastrophizing, IL-6, pain intensity and pain interference with function for patients newly diagnosed with cancer of the lung were evaluated. Subjects were divided into groups with Pain Intensity levels above and below the sample median and Pain Interference levels above and below the sample median. Standardized scores (Z-scores)43 were calculated on the outcome measures (beliefs, depression, catastrophizing, and cytokines) to permit normalized comparisons on these variables between Pain Intensity groups and between Pain Interference groups.
Results
The subject's age ranged from 42-78; all were able to speak and read English. Eight of the subjects were female; 4 were male. Seven individuals were Caucasian; 5 were African American. Four individuals were working full-time, 3 were retired, 3 were on disability and 2 were not employed.
One-half of the subjects (6) had metastatic disease at diagnosis; one-half were Stage 2 or 3, the remaining half were Stage 4. Karnofsky (KPS) scores ranged from 80-100 at Baseline and 60-100 thereafter. Hemoglobin levels - - required to be 8.0 grams or better for admission to or continuance in the study - - ranged from 8.1-16.3 across all time points for females; hemoglobin levels ranged from 9.3-15.4 across all times points for males. In general, average hemoglobin levels decreased from T1 to T5 for all females and three-fourths of the males, but increased at Final (T6).
Individuals reported “no pain” at Baseline and across the four subsequent interview periods. They were not taking any analgesic medications. At T5 one individual reported a Pain Intensity score of 4.8 and an Interference of Pain with function score of 4.9, which were considered this individual's Final data collection. Across all subjects the average Pain Intensity score at Final (approximately 6 months after entering the study) was 3.65 (S.D.2.8) and the average Pain Interference score was 2.46 (S.D. 2.5). Among the individuals who reported their Pain Intensity at 0-3, two had metastatic disease at diagnosis, four did not, one was Stage 1, two were Stage 3 and three were Stage 4. Among the individuals who reported Pain Intensity >3, three had metastasis at diagnosis and three did not, three were Stage 3 and three were Stage 4. Seven individuals reported Pain Interference at 0-2 and 5 reported Pain Interference scores >2 at the Final interview with variability in stage and metastasis similar to variability reported for pain intensity.
Analysis of scores on the BPP at diagnosis and scores at the final interview revealed Baseline scores on the Avoidance scale of the BPP that were generally low (range 0-21). Scores on all other beliefs and the total belief score were, in general, moderate, although they were higher for beliefs about Past and Current Experiences (range 0-26) and Loss of Control (range 0-57). At Final Individuals reported greater influence of the majority of the biobehavioral beliefs on their pain responses than at diagnosis (Baseline); only Thoughts of Disease Progression and Environmental Influences did not demonstrate an increase in scale scores at Final. The greatest increases in beliefs were on Past and Current Experiences and Loss of Control (Table 1).
Table 1.
Summary Statistics for Each Measure Over Time
| Measure n | Time Period | ||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | |||||||
| Median (Range) | Baseline | T2 | T3 | T4 | T5 | T6 | Final† |
| BPP Total | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 49.71 (55.42) | 0.00 | 30.00 | 60.60 (56.56) | 53.00 (54.53) | |||
| 32.00 (0–201) | 42.00 (0–205) | 36.00 (0–205) | |||||
| BPP Avoidance | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 2.17 (6.00) | 0.00 | 0.00 | 3.50 (7.50) | 2.92 (6.92) | |||
| 0.00 (0–21) | 0.00 (0–22) | 0.00 (0–22) | |||||
| BPP Physiological Responsivity | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 4.25 (5.33) | 0.00 | 12.00 | 5.40 (6.04) | 5.50 (6.04) | |||
| 2.00 (0–17) | 3.50 (0–18) | 3.50 (0–18) | |||||
| BPP Thoughts of Disease Progression | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 5.00 (5.46) | 0.00 | 0.00 | 4.60 (7.06) | 3.83 (6.63) | |||
| 4.00 (0–16) | 3.50 (0–24) | 2.00 (0–24) | |||||
| BPP Environmental Influences | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 16.26 (19.83) | 0.00 | 0.00 | 16.60 (16.83) | 13.83 (16.54) | |||
| 9.00 (0–64) | 12.50 (0–50) | 11.00 (0–50) | |||||
| BPP Past and Current Experiences | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 7.75 (8.47) | 0.00 | 12.00 | 10.90 (9.47) | 10.08 (9.14) | |||
| 7.00 (0–26) | 9.00 (0–31) | 9.00 (0–31) | |||||
| BPP Loss of Control | 12 | 0 | 0 | 1 | 1 | 10 | 12 |
| 14.78 (20.87) | 0.00 | 6.00 | 19.60 (19.63) | 16.83 (18.94) | |||
| 1.00 (0–57) | 13.50 (0–60) | 8.50 (0–60) | |||||
| BDI Depression | 11 | 11 | 11 | 10 | 7 | 8 | 12 |
| 9.31 (7.30) | 11.64 (6.61) | 10.00 (5.04) | 12.16 (6.05) | 10.71 (3.95) | 9.13 (4.89) | 9.50 (5.50) | |
| 8.40 (0–28) | 11.00 (0–25) | 10.00 (1–19) | 11.80 (1–23) | 11.00 (3–16) | 10.03 (3–17) | 10.50 (1–19) | |
| CSQ Catastrophizing | 12 | 11 | 11 | 10 | 7 | 10 | 12 |
| 10.00 (8.57) | 9.91 (8.19) | 13.09 (7.97) | 11.20 (8.13) | 8.14 (4.26) | 8.80 (6.81) | 9.58 (8.63) | |
| 8.50 (0–31) | 11.00 (0–27) | 10.00 (4–27) | 9.50 (0–27) | 9.00 (0–13) | 9.00 (0–21) | 9.00 (0–27) | |
| Cytokines: Il-6 | 10 | 11 | 10 | 8 | 5 | 9 | 10 |
| 5.62 (8.02) | 4.31 (3.92) | 9.47 (19.63) | 6.03 (6.35) | 5.54 (10.39) | 11.02 (14.32) | 10.02 (13.86) | |
| 2.21 (0.56–21.32) | 3.58 (0.39–14.19) | 2.76 (0.41–64.83) | 4.47 (0.43–18.62) | 1.10 (0.36–24.12) | 5.06 (0.66–39.82) | 4.89 (0.66–39.82) | |
| BPI Pain Intensity | 0 | 0 | 0 | 1 | 1 | 10 | 12 |
| 0.00 | 4.75 | 3.90 (2.87) | 3.65 (2.85) | ||||
| 3.00 (0.50–7.75) | 3.00 (0.00–7.75) | ||||||
| BPI Pain Interference | 0 | 0 | 0 | 1 | 1 | 10 | 12 |
| 0.00 | 4.86 | 2.47 (2.55) | 2.46 (2.53) | ||||
| 1.14 (0.43-7.71) | 1.14 (0.00-7.71) | ||||||
“Final” scores were compiled by last value carried forward to Time 6: one subject's last value was collected at Time 4 another's was at Time 5
Analysis of scores on the Beck Depression Scale and the Catastrophizing Scale of the CSQ at diagnosis and across time revealed Baseline depression scores ranging from 0-28. Across time depression total scores generally increased from Baseline to T4, but decreased at Final. Individual catastrophizing scores ranged from 0-31 at Baseline. Similar to the depression scores, scores on the catastrophizing scale also increased from Baseline to T4, but decreased to a score lower than Baseline at Final.
Analysis of IL-6 values at diagnosis and across time revealed the following: Average IL-6 levels varied widely from diagnosis to final, ranging from a high of 64.83 pg/ml at T3 to a low of 0.36 pg/ml at T5 (Table 1). The change in IL-6 levels did not correspond to specific treatment protocols (chemotherapy, radiation or chemo/radiation) in these 12 subjects. Except for lower values at T5, the individual IL-6 values increased to slightly higher values at each time period across all cases.
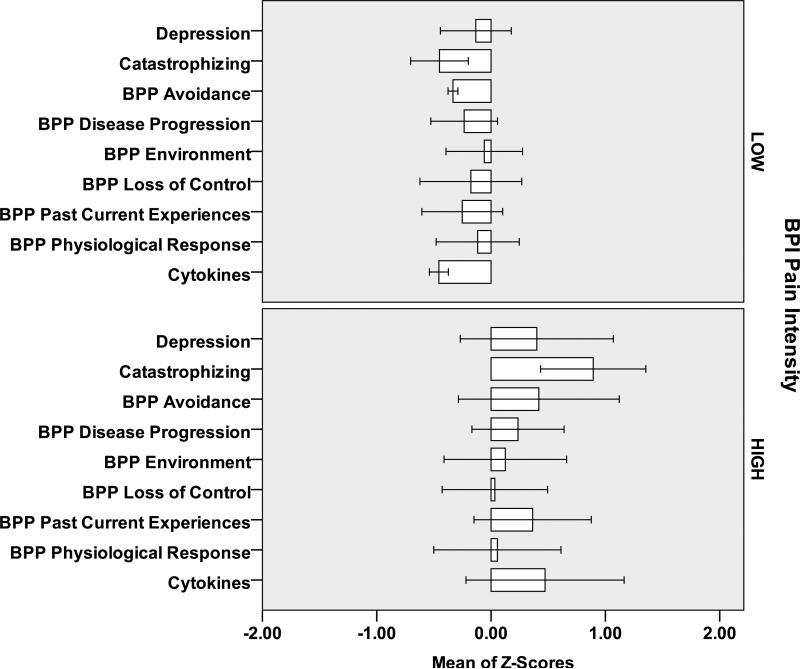
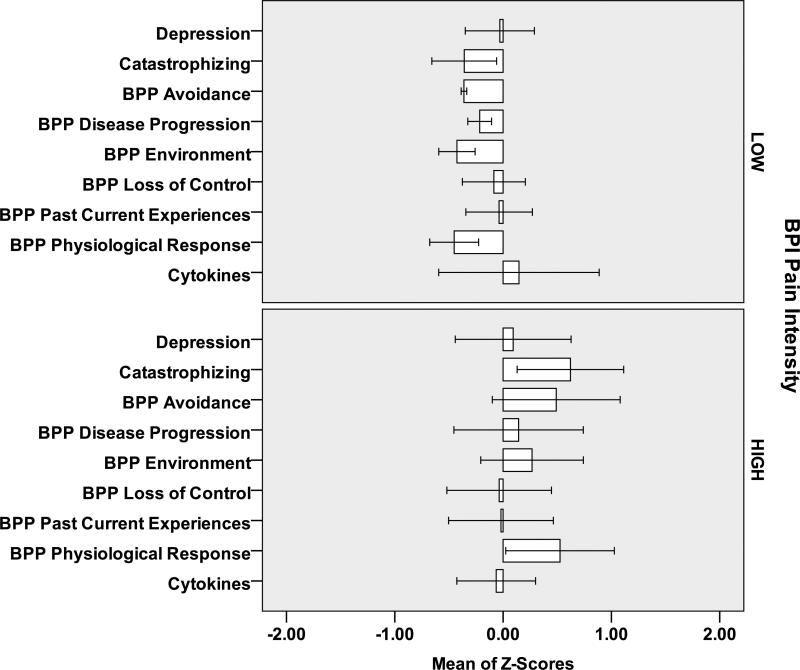
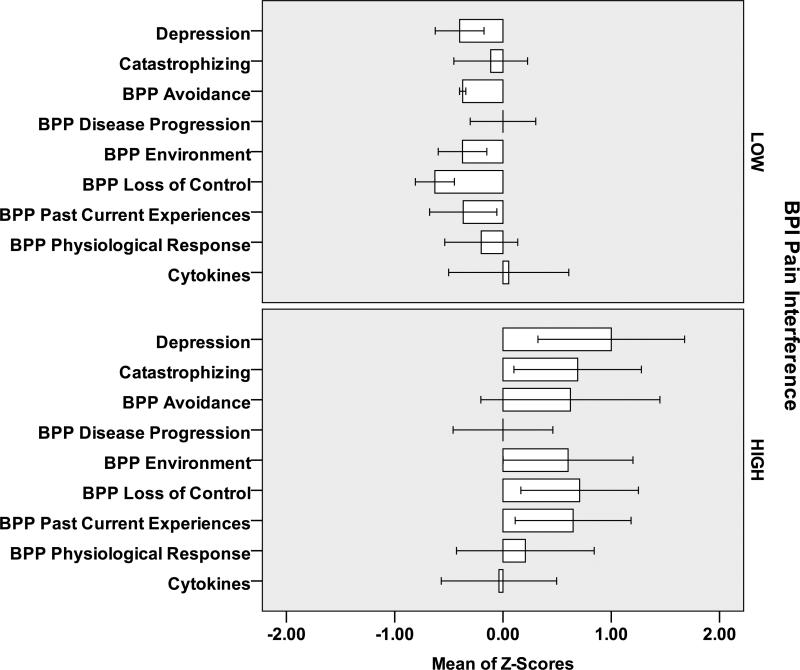
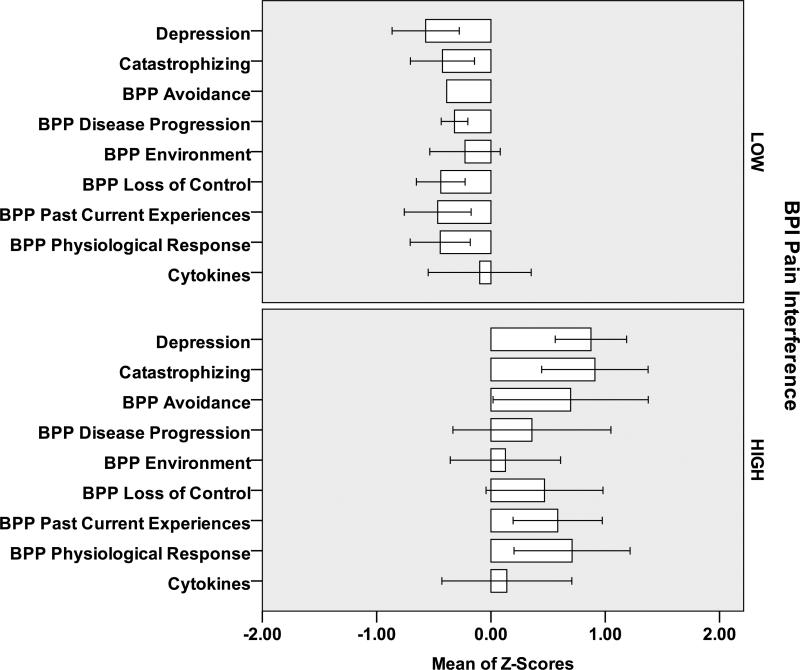
At Baseline (Figure 1) and Final (Figure 2) subjects with Pain Intensity >3 at Final had higher scores on the beliefs, depression and catastrophizing scales and higher IL-6 values than subjects with Pain Intensity 0-3. Similar relationships were observed for individuals with Pain Interference 0-2 and >2 (Figures 3,4). However, greater differential was noted for individuals with >2 versus 0-2 Pain Interference. Across all standardized scores beliefs, depression and catastrophizing demonstrate potential as multidimensional predictors for high pain interference (Figure 5).
Figure 1.
Baseline Measures (standardized Z-scores) for 12 Subjects [6 LOW Pain Intensity (<=3); 6 HIGH Pain Intensity (>3)]
Figure 2.
Final Measures (standardized Z-scores) for 12 Subjects [6 LOW Pain Intensity (<=3); 6 HIGH Pain Intensity (>3)]
Figure 3.
Baseline Measures (standardized Z-scores) for 12 Subjects [7 LOW Pain Interference (<=2); 5 HIGH Pain Interference (>2)]
Figure 4.
Final Measures (standardized Z-scores) for 12 Subjects [7 LOW Pain Interference (<=2); 5 HIGH Pain Interference (>2)]
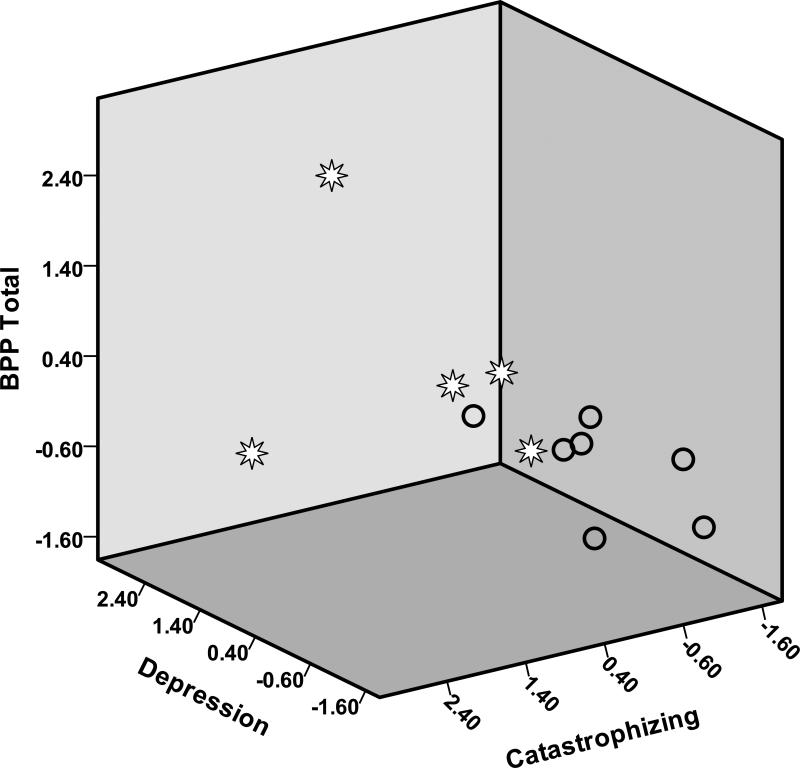
Figure 5. Scatter Plot of Final Measures by Final Pain Interference Category.
Subjects with High Pain Interference (>2) indicated by Stars; Low Pain Interference (<=2) by Circles; Vertical Axis is BPP Total with higher scores at top; Left Lower Axis is Depression with higher scores on upper left; Right Lower Axis is Catastrophizing with higher scores on lower left
Analysis of scores among specific biopsychosocial factors revealed similarities among the 12 subjects. Overall, individuals who reported higher biobehavioral belief scores regarding events such as not getting enough pain medication, past personal experience with unrelieved pain, loss of self-esteem, and uncertainty about the future also reported higher measures of depression, including fatigue and loss of appetite, and catastrophizing.
Discussion
Despite variability in presence of metastasis, stage of disease, and treatment, the 12 subjects who at diagnosis were not experiencing pain due to their cancer and whose performance status was good reported interesting combinations of biopsychosocial factors. Approximately six months later, at the onset of pain, elevation in these factors was related to higher levels of Pain Intensity and higher levels of Pain Interference with Function in one-half of the sample. These findings suggest that patterns of biopsychosocial responses may occur with the diagnosis of cancer and that the patterns may persist with the development of pain. Because this was a pilot study with a small sample, the results must be viewed as a first step in the discovery process. Nevertheless, it is a step that has demonstrated interesting responses that should be examined more rigorously in future studies.
Comparison of psychosocial factors reported at diagnosis and those reported approximately 6 months after diagnosis revealed that, in general, the 12 subjects reported greater intensity of beliefs even though a few reported new and/or different beliefs. Specifically, the subjects who reported a greater degree of beliefs about Disease Progression, i.e. “I'm terrified;” Past and Current Experiences with pain and pain treatment, i.e. “I'm not getting enough pain medication;” and Environmental Influences, i.e., stories told by family and friends, and reported greater use of catastrophizing as a coping strategy were the same subjects who reported higher levels of Pain Interference with Function at six months. The 12 subjects also reported a pattern of depression that increased across the first 4 interview periods and then decreased at the last 2 interviews. Of note, the subjects’ reports of depression peaked at approximately the same time they were concluding their first rounds of radiation therapy or chemotherapy. Their report of an increase in the use of catastrophizing across all time periods suggests a potential need for further exploration of the effect of time. It is possible that further examination of these biopsychosocial factors in a larger study would validate the responses noted in this pilot study and ultimately could lead to more individualized pain treatment protocols for co-morbid conditions.
Those individuals who reported greater influence of beliefs about Environmental Influences and Avoidant behavior and depression at Baseline were the same individuals who reported higher Interference of Pain with Function at the Final interview. The fact that as the incidence of reporting specific beliefs increased, increases in the incidence of depression and catastrophizing also occurred is important from a scientific and clinical perspective. Of note is the fact that as individuals reported greater use of catastrophizing as a coping strategy and were found to have higher IL-6 levels, they also had higher Pain Intensity levels at the Final interview period. However, wide variability in individual IL-6 values likely complicates this relationship. To better understand the role of IL-6 in this population, measurement of soluble IL-6 receptor (which binds to IL-6 to prolong its half-life) and/or downstream signaling molecules, or use of a less sensitive cytokine assay may be useful in future study. We believe that the findings that emerged at Baseline and continued across time could be seen as potential challenges for the next steps in the discovery process.
The findings of this pilot study suggest that change in multiple psychosocial factors (cognitive, affective and sensory inputs) play an important role in report of variable levels of pain intensity and function. The across-time variability in individual experiences, beliefs, and actions further emphasizes44 the need for ongoing, repeated individual assessments in research and practice. Although only 12 subjects participated in this pilot study, the use of repeated measures permitted observations across an extended time period and raised many questions. Analysis of the pattern of biopsychosocial responses in comparison to the pattern of pain interference produced striking results. A larger sample may provide evidence of a strong predictive value for beliefs, catastrophizing and depression. Additionally, the interesting changes in hemoglobin point toward more in-depth examination of its possible role in the development and treatment of pain. Finally, this pilot data produced interesting questions related to the meaning of the pattern of IL-6 response in patients with cancer and depression.
Comparison of the findings of this pilot study and a number of related studies strengthens preliminary insights that may contribute to the discovery of new information related to the trajectory of the pain response. The findings are similar to earlier discovery that beliefs about one's capabilities can be a predictor of performance45 and can affect the relationship between mood disorders and pain-related disability.46 Comparing findings from the current study to a study designed to test the hypothesis that older females who had less education, were employed less than full-time and were more depressed47 may suggest a need to test the presence of depression with depression screening in this population. These findings are similar to reports of the measurement of similar psychological factors as predictors of outcomes of muscle injury.48 Speculation that the distress of a pain event worsens when the timing or intensity of the pain is unpredictable49 may be an important consideration for predictor studies for all persons with lung cancer, but especially those with higher scores on measures of beliefs, depression, catastrophizing and IL-6. Further, some investigators50 suggest that high initial pain intensity could be a predictor of all pain outcomes. The latter study, much like this study, was limited by small sample size but consistently strengthened by the strong effect size. As in other study,51-53 we wonder if similar studies should focus on pain interference more than pain intensity.
The results of this pilot suggest that assessment of multidimensional biopsychosocial status, sometimes referred to as symptom clusters,54 is needed at the time of the patient's diagnosis of lung cancer and that the assessment needs to be repeated across the trajectory of the disease. Future research and, eventually, practice should focus on the potential benefits of identifying multidimensional patterns of biopsychosocial responses that occur at the time of diagnosis of a serious illness and across time; outcomes of this work might lead to modified treatment that could prevent or limit the degree to which pain interferes with the patient's quality of life.
Acknowledgments
Funded in part by Grant # P20 NR07798 from the National Institute of Nursing Research (NINR), NIH, to the Center for Research on Symptoms, Symptoms Interactions and Health Outcomes at Emory University School of Nursing.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melzack R. Gate control theory: On the evolution of pain concepts. Pain Forum. 1996;5:128–138. [Google Scholar]
- 2.Dalton JA, Feuerstein M. Fear, alexithymia and cancer pain. Pain. 1989;38:159–170. doi: 10.1016/0304-3959(89)90234-0. [DOI] [PubMed] [Google Scholar]
- 3.Dalton JA, Feuerstein M, Carlson J, et al. Biobehavioral pain profile: Development and psychometric properties. Pain. 1994;57:95–107. doi: 10.1016/0304-3959(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 4.Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients' attitudes toward pain. J Psychosom Res. 1987;31:393–400. doi: 10.1016/0022-3999(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 5.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psych. 2003;54:399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 6.Dobkin PL, Liu A, Abrahamowicz M, et al. Predictors of pain for patients with early inflammatory polyarthritis. Arthritis Care Res. 2012;65:992–999. doi: 10.1002/acr.21923. [DOI] [PubMed] [Google Scholar]
- 7.Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: Antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Shuman AG, Terrell JE, Light E. Predictors of pain among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138(12):1147–54. doi: 10.1001/jamaoto.2013.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaza C, Baine N. Cancer pain and psychosocial factors: A critical review of the literature. J Pain Symptom Manage. 2002;24:526–542. doi: 10.1016/s0885-3924(02)00497-9. [DOI] [PubMed] [Google Scholar]
- 10.Hanley MA, Raichle K, Jensen M. Pain catastrophizing and beliefs predict changes in pain interference and psychological functioning in persons with spinal cord injury. J Pain. 2008;9:863–871. doi: 10.1016/j.jpain.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Liew C, Brown KC, Cronan TA, et al. Predictors of pain and functioning over time in fibromyalgia syndrome: an autoregressive path analysis. Arthritis Care Res. 2012;65(2):251–6. doi: 10.1002/acr.21792. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin RH, Harstein G, Rosner HL, et al. A high-risk method for studying psychosocial antecedents of chronic pain: The prospective investigation of herpes zoster. J Abnorm Psychol. 1992;101:200–205. doi: 10.1037//0021-843x.101.1.200. [DOI] [PubMed] [Google Scholar]
- 13.Magni G, Moreschi C, Rigatti-Luchini S. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain. 1994;56:289–297. doi: 10.1016/0304-3959(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen LV, Mors O. Presentation of a new MMPI scale to predict the outcome after the first lumbar discectomy. Pain. 1988;34:191–194. doi: 10.1016/0304-3959(88)90165-0. [DOI] [PubMed] [Google Scholar]
- 15.Von Korff M, Simon G. The relationship between pain and depression. Br J Psychiatry Suppl. 1996;30:101–108. [PubMed] [Google Scholar]
- 16.Williams LF, Jacka FN, Pasco JA, et al. Depression and pain: an overview. Acta Neuropsychiatr. 2006;8:79–87. doi: 10.1111/j.1601-5215.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- 17.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biolog Psych. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 18.Schneider S, Junghaenel DU, Keefe FJ. Individual differences in the day-to-day variability of pain, fatigue and well-being in patients with rheumatic disease; associations with psychological variables. Pain. 2012;153:813–22. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keefe FJ, Brown GK, Wallston KA, et al. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 20.Keefe FJ, Lefebvre JC, Egert JR, et al. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 21.Prasertsri N, Holden J, Keefe FJ, et al. Repressive coping style: relationships with depression, pain, and pain coping strategies in lung cancer outpatients. Lung Cancer. 2011;71(2):235–40. doi: 10.1016/j.lungcan.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorn BE, Rich MA, Boothby JL, et al. Pain beliefs and coping attempts: conceptual model building. Pain Forum. 1999;8:169–171. [Google Scholar]
- 23.Lefebvre JC, Keefe FJ. Memory for pain: the relationship of pain, catastrophizing to the recall of daily rheumatoid arthritis pain. Clin J Pain. 2002;18:56–63. doi: 10.1097/00002508-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Norman SA, Lumley MA, Dooley JA, et al. For whom does it work? Moderators of the effects of written emotional disclosure in a randomized trial among women with chronic pelvic pain. Psychosom Med. 2004;66:174–183. doi: 10.1097/01.psy.0000116979.77753.74. [DOI] [PubMed] [Google Scholar]
- 25.Porter LS, Keefe FJ, Lipkus I. Ambivalence over emotional expression in patients with gastrointestinal cancer and their caregivers: associations with patient pain and quality of life. Pain. 2005;11:340–348. doi: 10.1016/j.pain.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psycholog Assessm. 1995;7:524–532. [Google Scholar]
- 27.Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Turk DC, Rudy TE. Cognitive factors and persistent pain: a glimpse into Pandora's box. Cognit Ther Res. 1992;16:99–122. [Google Scholar]
- 29.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity. Clin J Pain. 2005;21:439–445. doi: 10.1097/01.ajp.0000135236.12705.2d. [DOI] [PubMed] [Google Scholar]
- 30.Ledeboer A, Gamanos M, Lai W. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci. 2005;22:1977–1986. doi: 10.1111/j.1460-9568.2005.04379.x. [DOI] [PubMed] [Google Scholar]
- 31.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:39–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu CH, Chao PC, Borel CO, et al. Precisional intravenous pentoxifylline attenuating perioperatiave cytokine response, reducing morphine consumption and improving recover of bowel function in patients undergoing colorectal cancer surgery. Anesth Analog. 2004;99:1465–1471. doi: 10.1213/01.ANE.0000132974.32249.C8. [DOI] [PubMed] [Google Scholar]
- 33.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Rausch SM, Clark MM, Patten C, et al. Relationship between cytokine gene single nucleotide polymorphisms and symptom burden and quality of life in lung cancer survivors. Cancer. 2010;116:4103–13. doi: 10.1002/cncr.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonini G, Santini D, Vincenzi B. Cisplatin may induce cytokine-release syndrome in colorectal cancer patients. J Biol Regul Homeost Agents. 2002;16:105–109. [PubMed] [Google Scholar]
- 36.Beck A, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 37.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Genl Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 39.Main CJ, Waddell G. A comparison of cognitive measures in low back pain: statistical structure and clinical validity at initial assessment. Pain. 1991;46:187–198. doi: 10.1016/0304-3959(91)90112-B. [DOI] [PubMed] [Google Scholar]
- 40.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 41.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 42.Karnofsky DA, Agelmann W, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–56. [Google Scholar]
- 43.Springer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 44.Dalton JA, Keefe FJ, Carlson J, et al. Tailoring cognitive-behavioral treatment for cancer pain. Pain Manag Nurs. 2004;5:3–18. doi: 10.1016/s1524-9042(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 45.Riley JF, Ahern DK, Follick MJ. Chronic pain and functional impairment: Assessing beliefs about their relationship. Arch Phy Med Rehabi. 1988;69:579–582. [PubMed] [Google Scholar]
- 46.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? Pain. 2009;10:619–627. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch AT, Waxenburg LB, Atchinson JW. Evidence for sex differences in the relationship of pain, mood and disability. J Pain. 2006;7:592–601. doi: 10.1016/j.jpain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Parr JJ, Borsa PS, Fillingim RB. Pain-related fear and catastrophizing predict pain intensity and disability independently using an induced muscleinjury model. J Pain. 2012;13:370–378. doi: 10.1016/j.jpain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oka S, Chapman CR, Kim B, et al. Predictability of pain stimulation modulates subjective and physiological responses. J Pain. 2010;11:239–246. doi: 10.1016/j.jpain.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Clay FJ, Newstead SV, Watson WL, et al. Bio-Psychosocial determinants of persistent pain 6 months after non-life-threatening acute orthopedic trauma. Pain. 2010;11:420–430. doi: 10.1016/j.jpain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Drolet M, Brisson M, Schmader K, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11:1203–21. doi: 10.1016/j.jpain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Miakowski C. Outcome measures to evaluate the effectiveness of pain management in older adults with cancer. Oncol Nurs Forum, Supp. 2010;37(5):27–32. doi: 10.1188/10.ONF.S1.27-32. [DOI] [PubMed] [Google Scholar]
- 53.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 54.Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classed in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38:33–42. doi: 10.1188/11.ONF.33-42. [DOI] [PubMed] [Google Scholar]