Abstract
The MOZ/MORF complexes represent an example of a chromatin-binding assembly whose recruitment to specific genomic regions and activity can be fine-tuned by posttranslational modifications of histones. Here we detail the structures and biological functions of epigenetic readers present in the four core subunits of the MOZ/MORF complexes, highlight the imperative role of combinatorial readout by the multiple readers, and discuss new research directions to advance our understanding of histone acetylation.
Keywords: MOZ, MORF, KAT6A, KAT6B, BRPF, ING5, histone, acetylation, PHD, reader
Introduction
Acetylation of lysine residues in histone proteins provides a fundamental mechanism for the regulation of chromatin structure and gene transcription. Acetylation is the most frequently occurring posttranslational modification (PTM) that neutralizes the positive charge of the epsilon amino group of lysine residues, thereby weakening the interaction of histones with negatively charged DNA and relaxing chromatin. It also increases the accessibility of DNA to gene-processing machinery, forms docking sites for acetyllysine-specific modules, and is generally associated with transcriptionally active genes. The acetylation reaction is catalyzed by lysine acetyltransferase (KAT) enzymes, whereas histone deacetylases (HDACs) reverse it, removing the acetyl group. One of the major sets of human KATs, the MYST (Moz, Ybf2/Sas3, Sas2, Tip60) family, is comprised of five members: KAT6A, traditionally called MOZ (monocytic leukemic zinc-finger protein) or MYST3; KAT6B, also known as MORF (MOZ-related factor) or MYST4; KAT7, named HBO1 or MYST2; KAT8, referred to as hMOF or MYST1; and KAT5 (or Tip60).
In eukaryotic cells, KAT6A and KAT6B form stable multisubunit complexes, MOZ and MORF, respectively, which are responsible for acetylation of a substantial portion of histone H3.1-4 The histone acetyltransferase (HAT) activity of the MOZ/MORF complexes is required for normal developmental programs, including hematopoiesis and skeletogeneis, and for the regulation of various genes, especially the Hox family.2,5-12 Dysregulation of the KAT6A/B functions caused by chromosomal translocations is associated with aggressive forms of leukemia, myelodysplastic syndromes, leiomyomata, and other human blood malignancies and solid tumors. Both KAT6A and KAT6B fuse with multiple genes including CBP (CREB-binding protein) and p300 HATs.13-17 The resulting transformed proteins possess 2 catalytic HAT domains, one from the KAT6A/B fragment and another from the CBP/p300 fragment. Formation of such super-HAT chimeras is thought to trigger aberrant histone acetylation and rapid transcriptional activation and overexpression of oncogenes.2,18,19 Acute leukemias involving KAT6A/B fusions are associated with poor prognosis and the survival rate of 5 mo, rendering the MOZ/MORF complexes as attractive targets for therapeutic intervention.20,21
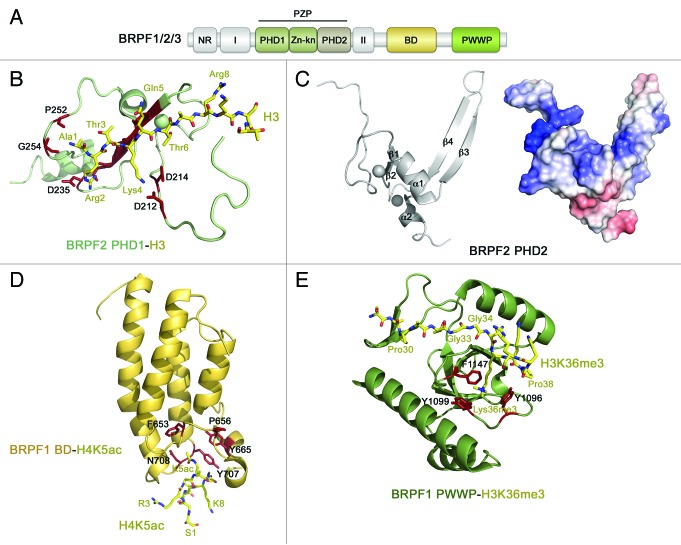
Along with the catalytic subunits KAT6A and KAT6B, the MOZ/MORF complexes contain adaptor proteins BRPF1/2/3 (bromodomain PHD finger proteins 1, 2, or 3), ING5 (inhibitor of growth 5), and hEAF6 (homolog of Esa1-associated factor 6)1,2,5 (Fig. 1A). Of the four subunits, the three KAT6A/B, BRPF1/2/3, and ING5 contain PTM readers, including a bromodomain (BD), a PWWP domain, and a number of PHD fingers, organization and functions of which differ substantially (Fig. 1B). The tandem PHD fingers in the catalytic KAT6A/B subunit are coupled to form a distinct module-the double PHD finger (DPF). The BRPF1/2/3 subunit has an assembly of two PHD fingers closely linked by a zinc knuckle, named a PZP module, and a typical single PHD finger is seen in ING5.
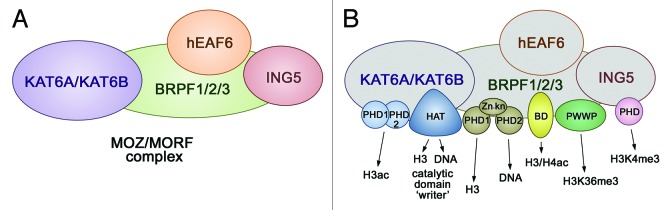
Figure 1. The MOZ/MORF composition. (A) The 4 core subunits of the MOZ/MORF complexes are shown. (B) The readers, a writer, and DNA-binding domains identified in each subunit of the MOZ/MORF complexes are colored and labeled.
Why do the MOZ/MORF complexes contain so many epigenetic readers? Clearly, the coordinated binding of multiple readers should enhance apparent affinity and specificity of the complexes. Such combinatorial readout of the epigenetic environment can provide a lock and key-type mechanism to recruit or stabilize the MOZ/MORF complexes at particular genomic sites, which in turn is necessary to produce the desired functional outcome.22 A number of studies in the past several years reveal distinctive roles of readers present in the individual subunits of the MOZ/MORF complexes as well as structural bases of their interactions with chromatin. In this review, we compare the chromatin-binding mechanisms of the MOZ/MORF-related readers and discuss implications of crosstalk between these readers for activity of the MOZ/MORF complexes in normal signaling and oncogenesis.
KAT6A/B
The human KAT6A gene was first identified in t(8;16)(p11;p13) chromosome translocations in 1996, and KAT6B was discovered 3 y later through a BLAST search against the KAT6A sequence.13,23 The KAT6A/B proteins encompass 2004/2073 residues and have similar domain organization, which includes an NEMM (N-terminal part of Enok, MOZ or MORF) domain, a double PHD finger (DPF), and a catalytic MYST module, followed by the ED (glutamate/aspartate-rich) and SM (serine/methionine-rich) regions (Fig. 2A). Although the biological role of the NEMM domain remains unclear, some sequence similarity to histones H1 and H5 suggests a regulatory function for this region.4 The only reader domain present in KAT6A/B, the DPF module, recognizes histone H3 tails, selecting for acetylated species.24,25 The following MYST domain functions as a PTM writer, catalyzing transfer of the acetyl group from acetyl-CoA to lysine residues in histone H3 and non-histone substrates, including KAT6A itself, and interacts with BRPF1/2/3.1,2,4,5,8,26 The ED and SM regions likely play a role in transcriptional activity of the complex.27
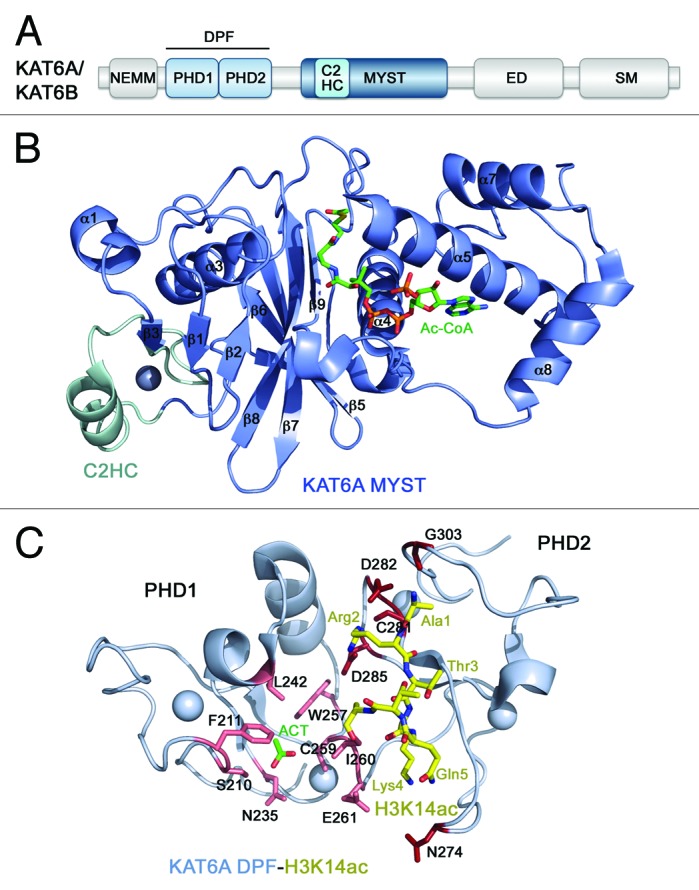
Figure 2. The structural basis for ligand binding by KAT6A/B. (A) The KAT6A/B domain architecture. (B) The crystal structure of the KAT6A MYST domain in complex with Ac-CoA (PDB: 2RC4). (C) The crystal structure of the double PHD finger (DPF) of KAT6A in complex with H3K14ac peptide (PDB: 3V43).
The catalytic MYST domain of KAT6A consists of eight α-helices and nine β-strands folded into two β-sheets surrounded by α-helical bundles (PDB: 2RC4)28 (Fig. 2B). The central core region is involved in the Ac-CoA cofactor binding, harboring the key enzymatic E680 and C646 residues, and is conserved in other KATs. However, unlike other acetyltransferase domains, it contains a C2HC-type zinc finger at the N-terminus (colored cyan in Fig. 2B) and the α6-helix-turn-α7-helix motif at the C-terminus, and both these additional fragments interact tightly with DNA. The KAT6A MYST domain represents a unique module that combines enzymatic and DNA-binding activities, and these activities are not mutually exclusive28.
The DPF module of KAT6A and KAT6B shows preference for acetylated histone H3 tails.24,25 Binding affinities measured by fluorescence spectroscopy and ITC and analysis of NMR chemical shift perturbations reveal that although DPF interacts with unmodified H3 peptide as robustly as a typical single PHD finger interacts with H3 (Kd = ~4 µM), acetylation of H3K14 or H3K9 enhances the binding 3-fold. This at first glance only a slight increase in binding affinity has significant implication for the function of KAT6A and KAT6B and is conserved in DPF3, where it was initially discovered.29
The 1.47 Å-resolution crystal structure of the KAT6A DPF module in complex with histone H3K14ac peptide provides insight into the selectivity of this reader toward acetyllysine substrates24 (PDB: 3V43) (Fig. 2C). The structure reveals that each PHD finger in DPF adopts a typical zinc-finger topology, but the 2 domains have an extensive interface, which results in the overall unique globular scaffold. The first four N-terminal residues of H3K14ac peptide are bound in the acidic groove of the second PHD2 finger. Notably, the side chain of R2 of the histone peptide is restrained by 5 hydrogen bonds involving D282, D285 and C281 of DPF, whereas E261 and N274 form water-mediated hydrogen bonds with the amino group of H3K4. As a result of such stringent constrain of R2 and K4, methylation of either histone residue disrupts binding of H3.
The first PHD1 module of DPF is a unique zinc finger that possesses an acetyllysine-binding pocket.24 Although in the KAT6A-H3K14ac complex this pocket is occupied by a solvent acetate group, the overall structure of the KAT6A complex superimposes well with the structure of homologous DPF3-H3K14ac complex, suggesting a conserved mode of interaction with H3K14ac. Like DPF3, KAT6A contains hydrophobic F211, L242, W257, and I260 residues, which can accommodate the methyl group of K14ac, whereas S210 and N235 at the rim of this binding pocket can form hydrogen bonds with the acetylamide group of H3K14ac.
The DPF-H3K14ac interaction contributes to localization of KAT6A at the HOXA9 promoter and coincides with the increase in levels of H3K14ac and HOX9A transcripts.24 Likewise, interaction of KAT6B DPF with H3K14ac is essential for the recruitment of KAT6B to specific genomic regions.25 Furthermore, HAT assays using native chromatin and H3K14ac peptide suggest that binding of DPF to H3K14ac promotes spreading of this epigenetic mark.25 Once the H3K14ac mark is established and bound by DPF, the MYST domain can target unmodified K14 of nearby H3 histone tails or act in trans. This mechanism could be critical for the formation of hyperacetylated chromatin domains through propagating the same histone mark from one nucleosome to another, a concept proposed to be at the heart of epigenetic marking and memory.30
BRPF1/2/3
BRPF1 (also known as BR140 or Peregrin) is essential in the maintenance of expression levels of Hox genes and the development of multiple tissues, axial skeleton and the hematopoietic system.31-33 BRPF1 is a scaffolding subunit of the MOZ/MORF complexes that links together KAT6A/B, ING5 and hEAF6 in the tetrameric assembly.4 The native MOZ/MORF complexes include either BRPF1 or one of its paralogs, BRPF2 (BRD1) or BRPF3, which differ slightly in size (1214-, 1058-, and 1205-residue, respectively, proteins) but share ~50% sequence identity. BRPF1/2/3 contains a short, 20-residue N-terminal region (NR) that directs specificity of the complex toward a particular histone tail,34 the MOZ/MORF-binding (I) domain,4 and a cysteine-rich sequence encompassing 2 PHD fingers connected by a zinc knuckle (Zn-kn) (Fig. 3A). This conserved arrangement of zinc-coordinating modules, termed a PHD1-Zn-kn-PHD2 or PZP domain, is found in various eukaryotic proteins, including Jade1/2/3 and AF10/17.35,36 Recent studies have demonstrated that while the first PHD1 finger of BRPF1/2 binds unmodified H3 tail, the second PHD2 finger associates with DNA.34,37,38 The PZP domain is followed by a short sequence (II) responsible for the association with both ING5 and hEAF6, the acetyllysine-binding bromodomain (BD), and the C-terminal PWWP module that recognizes trimethylated K36 of histone H3 (H3K36me3).4,32,39,40
Figure 3. The structural basis for ligand binding by BRPF1/2. (A) The BRPF1/2/3 domain architecture. (B) The solution structure of the unmodified H3-bound PHD1 finger of BRPF2 (PDB: 2L43). (C) The solution structure and electrostatic potential surface of the PHD2 finger of BRPF2 in the apo-state (PDB: 2LQ6). (D) The NMR structure of the H4K5ac-bound BD of BRPF1 (PDB: 2RS9). (E) The crystal structure of the PWWP domain of BRPF1 in complex with H3K36me3 peptide (PDB: 2X4W).
The first PHD1 finger of PZP in BRPF1 and BRPF2 is highly specific for unmodified H3 tail, with trimethylation of H3K4, dimethylation of H3R2, and phosphorylation of T3 and T6 abolishing or considerably reducing this interaction.34,37 The PHD1 finger folds into a small globular domain consisting of several loops stabilized by 2 zinc-coordinating clusters, 2 short β-strands and 2 small α-helices (Fig. 3B). The solution structure of the BRPF2 PHD1-H3 complex shows that the unmodified H3 tail adopts an extended conformation and pairs with the β-sheet of the PHD1 finger, forming an additional anti-parallel β-strand37 (PDB: 2L43). An extended network of intermolecular hydrogen bonds and ionic interactions tightly locks almost every residue in the A1-R2-T3-K4-Q5-T6 sequence of H3, most likely being responsible for the high specificity of BRPF2 PHD1 toward unmodified H3. The N-amino group of A1 of the H3K4 peptide is hydrogen bonded to the backbone carbonyls of P252 and G254, and the guanidino moiety of R2 is fixed through hydrogen bonding and ionic interactions with C234 and D235. The side chain ammonium group of K4 contacts D212 and D214, and T6 forms hydrogen bonds with N229.
Unlike other so far characterized PHD fingers,41 the second PHD2 finger of BRPF1/2 does not bind histones and instead interacts with DNA.34,38 It is an atypical PHD finger, as it carries a histidine in place of a cysteine at the eighth zinc-coordinating position and contains an additional long β3-β4 hairpin, which is usually found in another subfamily of RING fingers, FYVE domains.42 Interestingly, like the phosphoinositide-binding FYVE domain, the BRPF2 PHD2 finger has a saddle-like structure with a large basic patch spread throughout the concave surface38 (PDB: 2LQ6) (Fig. 3C). This patch, formed by lysine and arginine residues of the β1-β2 loop and the β3-β4 hairpin, is centrally involved in the interaction with double- and single-stranded DNA though no apparent nucleotide sequence preference is seen. As expected, substitution of these basic residues disrupts binding of DNA.
Histone peptide pulldown arrays indicate that bromodomain (BD) of BRPF1 binds acetylated lysine residues in a non-specific manner.39 The NMR structure of the H4K5ac-bound BD of BRPF1 shows a 4-helix bundle scaffold, which is strictly conserved within the BD family (helices αA, αB, αC, αZ) (PDB: 2RS9, unpublished) (Fig. 3D). The inter-helical ZA and BC loops of BRPF1 BD create a deep, primarily hydrophobic cavity for acetylated K5 that makes direct contacts with highly conserved Y707 and N708.
The PWWP domain of BRPF1 interacts weakly with H3K36me3, a PTM associated with the elongation phase of transcription (Kd = 2.7 mM).40 This domain is composed of a 5-stranded antiparallel β-barrel flanked by 3 α-helices (PDB: 2X4W) (Fig. 3E). Three aromatic residues, Y1096, Y1099, and F1147 at one of the open ends of the β-barrel form a cage for trimethylated K36. The aromatic side chains of these residues, positioned perpendicularly to each other, are involved in cation-π, hydrophobic and van der Waals interactions with the trimethylammonium group of K36me3. Mutation of either of these aromatic residues disrupts binding of PWWP to H3K36me3. The specificity of the PWWP domain relies on the unique recognition of G33, G34, and V35 of the H3 peptide. The engineered H3 peptide, in which both G33 and G34 are replaced with an alanine, does not interact with PWWP.
The functional importance of the histone binding activities of the 3 readers, PZP, BD and PWWP, for targeting of BRPF2 to the HoxA9 promoter was tested by ChIP assays.37 In comparison with occupancy of the wild type protein, occupancy of BRPF2 harboring loss-of-function mutations in the PHD1 finger of PZP is fairly compromised, and a similar albeit less pronounced effect is seen for the respective PHD2, BD or PWWP mutants. Likewise, loss-of-function mutations in PHD1 and PWWP partially disrupt speckled localization of GFP-fusion proteins, whereas mutations in PHD2 and BD impact the nuclear localization to a lesser extent.37 Together, these results suggest that histone binding of each BRPF2 reader is essential, however not sufficient, for targeting of BRPF2 to specific genomic sites. The PZP-chromatin interaction is also necessary for the acetyltransferase activity of MOZ,34 and the PWWP domain plays a role in the association of BRPF1 with mitotic chromosomes.32
ING5
ING5 belongs to the ING family of tumor suppressors that is implicated in transcriptional regulation, DNA repair, cell cycle control and apoptosis and is found misregulated or mutated in various human malignancies.43-46 In addition to being a core component of the MOZ/MORF complexes, ING5 co-purifies with another major HAT complex, HBO1, which is responsible for acetylation of histone H4K5/K8/K12 and H3K14.3,35,36 ING5 is a short, 240-residue protein that has an N-terminal (ING) region involved in the interactions with other proteins including BRPF1/2/3, and a single reader, the PHD finger specific for H3K4me3 (Fig. 4A).4,47,48 The presence of ING5 in the MOZ/MORF complexes has been shown to result in preferential acetylation of methylated H3K4 peptides, suggesting that ING5 acts as an adaptor subunit that tethers or stabilizes the acetyltransferase complex at genomic sites enriched in H3K4me3.3,34-36,47
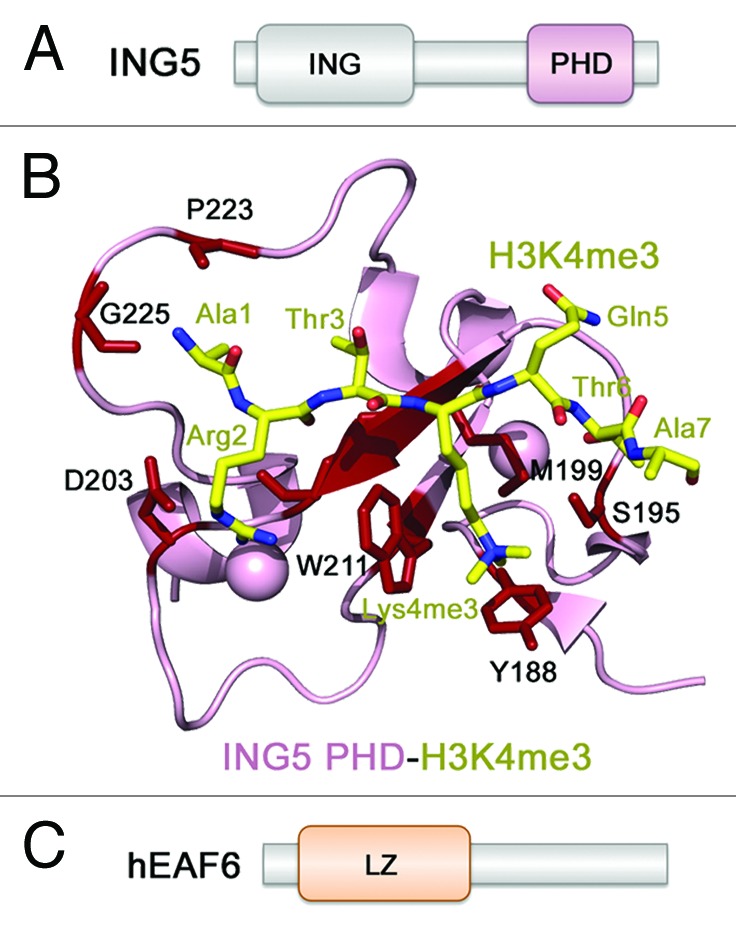
Figure 4. The structural basis for ligand binding by ING5. (A) The ING5 domain architecture. (B) The crystal structure of the H3K4me3-ING5 PHD finger complex (PDB: 3C6W). (C) The hEAF6 domain architecture.
The ING5 PHD finger associates with H3K4me3 through the mechanism, which is highly conserved within the ING1–5 family49 and is also reminiscent to the mechanism of the H3 recognition by BRPF1 PHD1. The H3K4me3 peptide occupies a large binding pocket pairing with the protein’s β1 strand47 (PDB: 3C6W) (Fig. 4B). Numerous intermolecular hydrogen bonds, including signature backbone β-sheet-like contacts with R2-T6 residues of H3, stabilize the protein-peptide complex. The fully extended side chain of K4me3 is bound in the well-defined semiaromatic cage formed by Y188, S196, M199, and W211. The bulky side chain of W211 separates this cage from the adjacent acidic R2-binding groove, where the guanidino moiety of R2 is hydrogen bonded to C202 and D203. The characteristic hydrogen bonds are also seen between the amino group of A1 and the carbonyls of G225 and P223, and between T6 and the side chain hydroxyl group of S195. The ING5 PHD finger exhibits 2.4 μM binding affinity for H3K4me3, however, binds much weaker to lower methylated states of H3K4.48
hEAF6
hEAF6 is the smallest and the least characterized subunit of the MOZ/MORF complexes, which is also found in human HBO1 and Tip60 and yeast NuA4 and NuA3 HAT complexes.3 There is no structural or biochemical information available regarding hEAF6. This protein contains 191 amino acids with the only recognizable motif, a leucine zipper (LZ), being found in the N-terminal region. hEAF6 co-purifies with the MOZ/MORF complexes when both BRPF1 and ING5 are present and promotes nuclear localization of BRPF1, supporting the idea that it associates with KAT6A/B through BRPF1.4 Careful complex reconstitution experiments and mapping intersubunit interfaces suggest that hEAF6 and ING5 interact with the II domain of BRPF1/2/3, whereas a small region within the MYST domain of KAT6A/B binds to the I domain of BRPF1/2/3.4
Crosstalk Between Readers in the MOZ/MORF Complexes
The 4 core subunits of the MOZ/MORF complexes contain a single writer module, the MYST domain of KAT6A/B, and 5 epigenetic readers with diverse preferences toward posttranslationally modified or unmodified histone tails: KAT6A/B DPF, selective for acetylated H3, H3K14ac in particular; BRPF1/2/3 PZP, highly specific for unmodified H3; acetyllysine-binding BD of BRPF1/2/3; BRPF1/2/3 PWWP that associates with H3K36me3; and H3K4me3-recognizing ING5 PHD. It is plausible to propose that the combination of unmodified H3, H3K14ac, H3/H4ac, H3K4me3, and H3K36me3 constitutes a “histone code” that is targeted by the MOZ/MORF complexes. Another possibility is that the reader-PTM interactions occur sequentially. For example, ING5 PHD can bind to the H3K4me3-enriched promoters of the MOZ/MORF target genes. Binding of the BRPF1/2/3 PZP to a neighboring unmodified H3 tail and DNA would then tether the catalytic KAT6A/B subunit. The KAT6A/B MYST-writer generates H3K14ac, which in turn is recognized by the DPF reader. The inability of the MOZ/MORF complexes to acetylate the histone tail bound by DPF would promote spreading of acetylation onto neighboring nucleosomes. Additional interactions of the BD and PWWP readers of BRPF1/2/3 should further stabilize the MOZ/MORF complexes at the regions of active transcription.
The combination of readers in the MOZ/MORF complexes represents an intriguing example of fine-tuning multivalent contacts by PTMs, affording positive and negative feedback mechanisms. The ING5 PHD finger binds exclusively to H3K4me3/2 and does not recognize unmodified H3, whereas H3K4me3 inhibits binding of DPF and PZP to unmodified H3. Conversely, acetylation of H3 enhances the interaction of DPF with unmodified histone tails. Both, BD and PWWP of BRPF1/2/3 do not recognize unmodified histones.
Aberrant Activities of the MOZ/MORF Complexes in Disease
Genetic analyses of Kat6a and Kat6b in animal models reveal that these genes are essential regulators of vertebrate development. Homozygous Kat6a knockout mice die at birth and have defective hematopoiesis and skeletal abnormalities due to reduced Hox expression.10,11 Neonatal pups show craniofacial and heart defects found in patients with DiGeorge syndrome.50 Inactivation of Kat6a in zebrafish reduces Hox gene expression and affects pharyngeal segmental identity.10,11,51 Mice lacking the HAT activity of Kat6a exhibit significant defects in hematopoietic stem cells and hematopoietic committed precursors as well as a defect in B-cell development.9 These animals also have shorter lifespan (40% of the mice die at 6 mo of age) and display lower body weight and reduced thymus and spleen. Similarly, Kat6b knockdown mouse suffer from dwarfism, craniofacial abnormalities, and cerebral cortex and neural stem cells defects.52-54 Overall, the phenotypes observed with mutant mice and fish indicate that although KAT6A and KAT6B are almost interchangeable in various molecular and cell-based studies in vitro, KAT6A has functions that cannot be fulfilled by KAT6B in vivo, or vice versa, possibly due to their unique expression patterns.
Both KAT6A and KAT6B are mutated in cancer and developmental disorders. The KAT6A gene forms fusion chimeras with multiple partners, including CBP in the recurrent translocation t(8;16)(p11;p16) associated with acute myeloid leukemia (AML).13,55 KAT6A is also rearranged in hematological malignancies with inv(8)(p11;q13) or t(8;22)(p11;q13), which result in KAT6A-TIF2 (transcription intermediary factor 2) or KAT6A-p300 protein chimeras (reviewed in refs. 2 and 55). Notably, the MOZ-TIF2 fusion promotes self-renewal of leukemic stem cells, and this process is dependent on the intact MYST domain rather than on its enzymatic activity.56-58 These findings suggest an important role of other subunits of the MOZ/MORF complexes, as the MYST domain interacts directly with BRPF1, which in turn links ING5 and hEAF6 in a tetrameric complex.4
The human KAT6B gene at 10q22 fuses with CBP in the t(10;16)(q22;p13) translocation, strongly linked to AML (reviewed in refs. 2 and 55). The KAT6B translocation t(10;17)(p11;q21) is found in uterine leiomyoma.59 Furthermore, KAT6B is mutated in the three genetic diseases: Noonan syndrome-like disorder,60 Ohdo syndrome61,62 and Genitopatellar syndrome.63,64 The Noonan syndrome-like disorder is manifested by reduced postnatal growth, cardiac defects, distinctive facial dysmorphism, and variable cognitive abnormalities.60 Patients with Ohdo syndrome exhibit blepharophimosis (eyelid dysplasia), mask-like facial appearance, and severe intellectual disability.61 Genitopatellar syndrome is associated with patellar aplasia or hypoplasia, external genital abnormalities and severe intellectual disability.63,64 Diverse defects in patients of these genetic diseases are consistent with the notion that KAT6B functions as a pleiotropic transcriptional coregulator in a variety of biological processes.
Much like the catalytic KAT6A/B subunit, the scaffolding subunit BRPF1/2 has been shown to play a critical role in normal developmental processes and erythropoiesis.31-33 BRPF2 is implicated in brain development and is linked to schizophrenia and bipolar disorder.65,66 Electroconvulsive seizures trigger expression of BRPF2 in the frontal cortex and hippocampus.67 BRPF2 fuses with PAX5, a master regulator of B-cell development, in childhood acute lymphoblastic leukemia.68 Zebrafish BRPF1 is required for craniofacial development.32 Another subunit of the MOZ/MORF complexes, ING5 is downregulated or mutated in various human malignancies, including head and neck squamous cell, gastric, colorectal, and oral squamous cell carcinomas.43-46,69-71 Thus, the core components of the MOZ/MORF complexes represent attractive drug targets as translocations, mutations, and dysregulation of these components are linked to cancer and developmental diseases. The structural insight into the reader-ligand interactions and the interplay between the readers within the MOZ/MORF complexes offer new tools in the development of therapeutics to treat diseases caused by aberrant acetylation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Research in Kutateladze TG laboratory is supported by grants GM096863 and GM101664 from the National Institutes of Health. Research in Yang XJ laboratory is supported by the grant MOP-97057 from the Canadian Institutes of Health Research (CIHR). Research in Côté J laboratory is supported by the grant MOP-64289 from CIHR.
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/26792
References
- 1.Sapountzi V, Côté J. MYST-family histone acetyltransferases: beyond chromatin. Cell Mol Life Sci. 2011;68:1147–56. doi: 10.1007/s00018-010-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007;26:5408–19. doi: 10.1038/sj.onc.1210609. [DOI] [PubMed] [Google Scholar]
- 3.Doyon Y, Cayrou C, Ullah M, Landry AJ, Côté V, Selleck W, Lane WS, Tan S, Yang XJ, Côté J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Ullah M, Pelletier N, Xiao L, Zhao SP, Wang K, Degerny C, Tahmasebi S, Cayrou C, Doyon Y, Goh SL, et al. Molecular architecture of quartet MOZ/MORF histone acetyltransferase complexes. Mol Cell Biol. 2008;28:6828–43. doi: 10.1128/MCB.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avvakumov N, Côté J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–407. doi: 10.1038/sj.onc.1210608. [DOI] [PubMed] [Google Scholar]
- 6.Thomas T, Voss AK. The diverse biological roles of MYST histone acetyltransferase family proteins. Cell Cycle. 2007;6:696–704. doi: 10.4161/cc.6.6.4013. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Campo FM, Costa G, Lie-a-Ling M, Kouskoff V, Lacaud G. The MYSTerious MOZ, a histone acetyltransferase with a key role in haematopoiesis. Immunology. 2013;139:161–5. doi: 10.1111/imm.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voss AK, Collin C, Dixon MP, Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev Cell. 2009;17:674–86. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Campo FM, Borrow J, Kouskoff V, Lacaud G. The histone acetyl transferase activity of monocytic leukemia zinc finger is critical for the proliferation of hematopoietic precursors. Blood. 2009;113:4866–74. doi: 10.1182/blood-2008-04-152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas T, Corcoran LM, Gugasyan R, Dixon MP, Brodnicki T, Nutt SL, Metcalf D, Voss AK. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 2006;20:1175–86. doi: 10.1101/gad.1382606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsumoto T, Aikawa Y, Iwama A, Ueda S, Ichikawa H, Ochiya T, Kitabayashi I. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20:1321–30. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump JG, Swartz ME, Eberhart JK, Kimmel CB. Moz-dependent Hox expression controls segment-specific fate maps of skeletal precursors in the face. Development. 2006;133:2661–9. doi: 10.1242/dev.02435. [DOI] [PubMed] [Google Scholar]
- 13.Borrow J, Stanton VP, Jr., Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dubé I, Frischauf AM, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 14.Panagopoulos I, Fioretos T, Isaksson M, Samuelsson U, Billström R, Strömbeck B, Mitelman F, Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13) Hum Mol Genet. 2001;10:395–404. doi: 10.1093/hmg/10.4.395. [DOI] [PubMed] [Google Scholar]
- 15.Terui K, Sato T, Sasaki S, Kudo K, Kamio T, Ito E. Two novel variants of MOZ-CBP fusion transcripts in spontaneously remitted infant leukemia with t(1;16;8)(p13;p13;p11), a new variant of t(8;16)(p11;p13) Haematologica. 2008;93:1591–3. doi: 10.3324/haematol.13020. [DOI] [PubMed] [Google Scholar]
- 16.Boyd EM, Bench AJ, Vaghela KJ, Campbell GN, Chowdhury FB, Gudgin EJ, Scott MA, Erber WN. Therapy-related acute myeloid leukaemia with t(8;16)(p11;p13);MOZ-CBP and polymorphisms in detoxifying and DNA repair genes. Leukemia. 2009;23:1164–7. doi: 10.1038/leu.2008.383. [DOI] [PubMed] [Google Scholar]
- 17.Chaffanet M, Gressin L, Preudhomme C, Soenen-Cornu V, Birnbaum D, Pébusque MJ. MOZ is fused to p300 in an acute monocytic leukemia with t(8;22) Genes Chromosomes Cancer. 2000;28:138–44. doi: 10.1002/(SICI)1098-2264(200006)28:2<138::AID-GCC2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Champagne N, Pelletier N, Yang XJ. The monocytic leukemia zinc finger protein MOZ is a histone acetyltransferase. Oncogene. 2001;20:404–9. doi: 10.1038/sj.onc.1204114. [DOI] [PubMed] [Google Scholar]
- 19.Camós M, Esteve J, Jares P, Colomer D, Rozman M, Villamor N, Costa D, Carrió A, Nomdedéu J, Montserrat E, et al. Gene expression profiling of acute myeloid leukemia with translocation t(8;16)(p11;p13) and MYST3-CREBBP rearrangement reveals a distinctive signature with a specific pattern of HOX gene expression. Cancer Res. 2006;66:6947–54. doi: 10.1158/0008-5472.CAN-05-4601. [DOI] [PubMed] [Google Scholar]
- 20.Falk H, Connor T, Yang H, Loft KJ, Alcindor JL, Nikolakopoulos G, Surjadi RN, Bentley JD, Hattarki MK, Dolezal O, et al. An efficient high-throughput screening method for MYST family acetyltransferases, a new class of epigenetic drug targets. J Biomol Screen. 2011;16:1196–205. doi: 10.1177/1087057111421631. [DOI] [PubMed] [Google Scholar]
- 21.Kindle KB, Collins HM, Heery DM. MOZ-TIF2-mediated destruction of CBP/p300 is blocked by calpain inhibitor 2. Leukemia. 2010;24:1359–61. doi: 10.1038/leu.2010.92. [DOI] [PubMed] [Google Scholar]
- 22.Musselman CA, Lalonde ME, Côté J, Kutateladze TG. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 2012;19:1218–27. doi: 10.1038/nsmb.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champagne N, Bertos NR, Pelletier N, Wang AH, Vezmar M, Yang Y, Heng HH, Yang XJ. Identification of a human histone acetyltransferase related to monocytic leukemia zinc finger protein. J Biol Chem. 1999;274:28528–36. doi: 10.1074/jbc.274.40.28528. [DOI] [PubMed] [Google Scholar]
- 24.Qiu Y, Liu L, Zhao C, Han C, Li F, Zhang J, Wang Y, Li G, Mei Y, Wu M, et al. Combinatorial readout of unmodified H3R2 and acetylated H3K14 by the tandem PHD finger of MOZ reveals a regulatory mechanism for HOXA9 transcription. Genes Dev. 2012;26:1376–91. doi: 10.1101/gad.188359.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali M, Yan K, Lalonde ME, Degerny C, Rothbart SB, Strahl BD, Côté J, Yang XJ, Kutateladze TG. Tandem PHD fingers of MORF/MOZ acetyltransferases display selectivity for acetylated histone H3 and are required for the association with chromatin. J Mol Biol. 2012;424:328–38. doi: 10.1016/j.jmb.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci U S A. 2013;110:3895–900. doi: 10.1073/pnas.1300490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelletier N, Champagne N, Stifani S, Yang XJ. MOZ and MORF histone acetyltransferases interact with the Runt-domain transcription factor Runx2. Oncogene. 2002;21:2729–40. doi: 10.1038/sj.onc.1205367. [DOI] [PubMed] [Google Scholar]
- 28.Holbert MA, Sikorski T, Carten J, Snowflack D, Hodawadekar S, Marmorstein R. The human monocytic leukemia zinc finger histone acetyltransferase domain contains DNA-binding activity implicated in chromatin targeting. J Biol Chem. 2007;282:36603–13. doi: 10.1074/jbc.M705812200. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L, Zhang Q, Li S, Plotnikov AN, Walsh MJ, Zhou MM. Mechanism and regulation of acetylated histone binding by the tandem PHD finger of DPF3b. Nature. 2010;466:258–62. doi: 10.1038/nature09139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–96. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hibiya K, Katsumoto T, Kondo T, Kitabayashi I, Kudo A. Brpf1, a subunit of the MOZ histone acetyl transferase complex, maintains expression of anterior and posterior Hox genes for proper patterning of craniofacial and caudal skeletons. Dev Biol. 2009;329:176–90. doi: 10.1016/j.ydbio.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Laue K, Daujat S, Crump JG, Plaster N, Roehl HH, Kimmel CB, Schneider R, Hammerschmidt M, Tübingen 2000 Screen Consortium The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development. 2008;135:1935–46. doi: 10.1242/dev.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishima Y, Miyagi S, Saraya A, Negishi M, Endoh M, Endo TA, Toyoda T, Shinga J, Katsumoto T, Chiba T, et al. The Hbo1-Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood. 2011;118:2443–53. doi: 10.1182/blood-2011-01-331892. [DOI] [PubMed] [Google Scholar]
- 34.Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M, Paquet E, Yan K, Tong Q, Klein BJ, et al. Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity. Genes Dev. 2013;27:2009–24. doi: 10.1101/gad.223396.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avvakumov N, Lalonde ME, Saksouk N, Paquet E, Glass KC, Landry AJ, Doyon Y, Cayrou C, Robitaille GA, Richard DE, et al. Conserved molecular interactions within the HBO1 acetyltransferase complexes regulate cell proliferation. Mol Cell Biol. 2012;32:689–703. doi: 10.1128/MCB.06455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C, Paquet E, Ullah M, Landry AJ, Côté V, et al. HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail. Mol Cell. 2009;33:257–65. doi: 10.1016/j.molcel.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin S, Jin L, Zhang J, Liu L, Ji P, Wu M, Wu J, Shi Y. Recognition of unmodified histone H3 by the first PHD finger of bromodomain-PHD finger protein 2 provides insights into the regulation of histone acetyltransferases monocytic leukemic zinc-finger protein (MOZ) and MOZ-related factor (MORF) J Biol Chem. 2011;286:36944–55. doi: 10.1074/jbc.M111.244400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Qin S, Zhang J, Ji P, Shi Y, Wu J. Solution structure of an atypical PHD finger in BRPF2 and its interaction with DNA. J Struct Biol. 2012;180:165–73. doi: 10.1016/j.jsb.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–31. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vezzoli A, Bonadies N, Allen MD, Freund SM, Santiveri CM, Kvinlaug BT, Huntly BJ, Göttgens B, Bycroft M. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat Struct Mol Biol. 2010;17:617–9. doi: 10.1038/nsmb.1797. [DOI] [PubMed] [Google Scholar]
- 41.Musselman CA, Kutateladze TG. Handpicking epigenetic marks with PHD fingers. Nucleic Acids Res. 2011;39:9061–71. doi: 10.1093/nar/gkr613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6:507–13. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soliman MA, Riabowol K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 2007;32:509–19. doi: 10.1016/j.tibs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Unoki M, Kumamoto K, Harris CC. ING proteins as potential anticancer drug targets. Curr Drug Targets. 2009;10:442–54. doi: 10.2174/138945009788185059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher SK, Helbing CC. Modulators of inhibitor of growth (ING) family expression in development and disease. Curr Drug Targets. 2009;10:392–405. doi: 10.2174/138945009788185095. [DOI] [PubMed] [Google Scholar]
- 46.Piche B, Li G. Inhibitor of growth tumor suppressors in cancer progression. Cell Mol Life Sci. 2010;67:1987–99. doi: 10.1007/s00018-010-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champagne KS, Saksouk N, Peña PV, Johnson K, Ullah M, Yang XJ, Côté J, Kutateladze TG. The crystal structure of the ING5 PHD finger in complex with an H3K4me3 histone peptide. Proteins. 2008;72:1371–6. doi: 10.1002/prot.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peña PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, Gozani O, Zhao R, Kutateladze TG. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature. 2006;442:100–3. doi: 10.1038/nature04814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champagne KS, Kutateladze TG. Structural insight into histone recognition by the ING PHD fingers. Curr Drug Targets. 2009;10:432–41. doi: 10.2174/138945009788185040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voss AK, Vanyai HK, Collin C, Dixon MP, McLennan TJ, Sheikh BN, Scambler P, Thomas T. MOZ regulates the Tbx1 locus, and Moz mutation partially phenocopies DiGeorge syndrome. Dev Cell. 2012;23:652–63. doi: 10.1016/j.devcel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller CT, Maves L, Kimmel CB. moz regulates Hox expression and pharyngeal segmental identity in zebrafish. Development. 2004;131:2443–61. doi: 10.1242/dev.01134. [DOI] [PubMed] [Google Scholar]
- 52.Thomas T, Voss AK, Chowdhury K, Gruss P. Querkopf, a MYST family histone acetyltransferase, is required for normal cerebral cortex development. Development. 2000;127:2537–48. doi: 10.1242/dev.127.12.2537. [DOI] [PubMed] [Google Scholar]
- 53.Merson TD, Dixon MP, Collin C, Rietze RL, Bartlett PF, Thomas T, Voss AK. The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci. 2006;26:11359–70. doi: 10.1523/JNEUROSCI.2247-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheikh BN, Dixon MP, Thomas T, Voss AK. Querkopf is a key marker of self-renewal and multipotency of adult neural stem cells. J Cell Sci. 2012;125:295–309. doi: 10.1242/jcs.077271. [DOI] [PubMed] [Google Scholar]
- 55.Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–76. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deguchi K, Ayton PM, Carapeti M, Kutok JL, Snyder CS, Williams IR, Cross NC, Glass CK, Cleary ML, Gilliland DG. MOZ-TIF2-induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2-mediated recruitment of CBP. Cancer Cell. 2003;3:259–71. doi: 10.1016/S1535-6108(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 57.Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M, Terui K, Ito E, Ohno H, Stanley ER, Singh H, et al. PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med. 2010;16:580–5, 1p, 585. doi: 10.1038/nm.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, Rowan R, Amaral S, Curley D, Williams IR, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–96. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 59.Moore SD, Herrick SR, Ince TA, Kleinman MS, Dal Cin P, Morton CC, Quade BJ. Uterine leiomyomata with t(10;17) disrupt the histone acetyltransferase MORF. Cancer Res. 2004;64:5570–7. doi: 10.1158/0008-5472.CAN-04-0050. [DOI] [PubMed] [Google Scholar]
- 60.Kraft M, Cirstea IC, Voss AK, Thomas T, Goehring I, Sheikh BN, Gordon L, Scott H, Smyth GK, Ahmadian MR, et al. Disruption of the histone acetyltransferase MYST4 leads to a Noonan syndrome-like phenotype and hyperactivated MAPK signaling in humans and mice. J Clin Invest. 2011;121:3479–91. doi: 10.1172/JCI43428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clayton-Smith J, O’Sullivan J, Daly S, Bhaskar S, Day R, Anderson B, Voss AK, Thomas T, Biesecker LG, Smith P, et al. Whole-exome-sequencing identifies mutations in histone acetyltransferase gene KAT6B in individuals with the Say-Barber-Biesecker variant of Ohdo syndrome. Am J Hum Genet. 2011;89:675–81. doi: 10.1016/j.ajhg.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szakszon K, Salpietro C, Kakar N, Knegt AC, Oláh E, Dallapiccola B, Borck G. De novo mutations of the gene encoding the histone acetyltransferase KAT6B in two patients with Say-Barber/Biesecker/Young-Simpson syndrome. Am J Med Genet A. 2013;161A:884–8. doi: 10.1002/ajmg.a.35848. [DOI] [PubMed] [Google Scholar]
- 63.Simpson MA, Deshpande C, Dafou D, Vissers LE, Woollard WJ, Holder SE, Gillessen-Kaesbach G, Derks R, White SM, Cohen-Snuijf R, et al. De novo mutations of the gene encoding the histone acetyltransferase KAT6B cause Genitopatellar syndrome. Am J Hum Genet. 2012;90:290–4. doi: 10.1016/j.ajhg.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campeau PM, Kim JC, Lu JT, Schwartzentruber JA, Abdul-Rahman OA, Schlaubitz S, Murdock DM, Jiang MM, Lammer EJ, Enns GM, et al. Mutations in KAT6B, encoding a histone acetyltransferase, cause Genitopatellar syndrome. Am J Hum Genet. 2012;90:282–9. doi: 10.1016/j.ajhg.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christensen JH, Elfving B, Müller HK, Fryland T, Nyegaard M, Corydon TJ, Nielsen AL, Mors O, Wegener G, Børglum AD. The Schizophrenia and Bipolar Disorder associated BRD1 gene is regulated upon chronic restraint stress. Eur Neuropsychopharmacol. 2012;22:651–6. doi: 10.1016/j.euroneuro.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Nyegaard M, Severinsen JE, Als TD, Hedemand A, Straarup S, Nordentoft M, McQuillin A, Bass N, Lawrence J, Thirumalai S, et al. Support of association between BRD1 and both schizophrenia and bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:582–91. doi: 10.1002/ajmg.b.31023. [DOI] [PubMed] [Google Scholar]
- 67.Fryland T, Elfving B, Christensen JH, Mors O, Wegener G, Børglum AD. Electroconvulsive seizures regulates the Brd1 gene in the frontal cortex and hippocampus of the adult rat. Neurosci Lett. 2012;516:110–3. doi: 10.1016/j.neulet.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 68.Nebral K, Denk D, Attarbaschi A, König M, Mann G, Haas OA, Strehl S. Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia. Leukemia. 2009;23:134–43. doi: 10.1038/leu.2008.306. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Kikuchi K, Takano Y. ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma. J Oncol. 2011;2011:963614. doi: 10.1155/2011/963614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cengiz B, Gunduz E, Gunduz M, Beder LB, Tamamura R, Bagci C, Yamanaka N, Shimizu K, Nagatsuka H. Tumor-specific mutation and downregulation of ING5 detected in oral squamous cell carcinoma. Int J Cancer. 2010;127:2088–94. doi: 10.1002/ijc.25224. [DOI] [PubMed] [Google Scholar]
- 71.Xing YN, Yang X, Xu XY, Zheng Y, Xu HM, Takano Y, Zheng HC. The altered expression of ING5 protein is involved in gastric carcinogenesis and subsequent progression. Hum Pathol. 2011;42:25–35. doi: 10.1016/j.humpath.2010.05.024. [DOI] [PubMed] [Google Scholar]