Abstract
The cellular epigenetic landscape changes as pluripotent stem cells differentiate to somatic cells or when differentiated cells transform to a cancerous state. These epigenetic changes are commonly correlated with differences in gene expression. Whether active DNA replication is also associated with distinct chromatin environments in these developmentally and phenotypically diverse cell types has not been known. Here, we used BrdU-seq to map active DNA replication loci in human embryonic stem cells (hESCs), normal primary fibroblasts and a cancer cell line, and correlated these maps to the epigenome. In all cell lines, the majority of BrdU peaks were enriched in euchromatin and at DNA repetitive elements, especially at microsatellite repeats, and coincided with previously determined replication origins. The most prominent BrdU peaks were shared between all cells but a sizable fraction of the peaks were specific to each cell type and associated with cell type-specific genes. Surprisingly, the BrdU peaks that were common to all cell lines were associated with H3K18ac, H3K56ac, and H4K20me1 histone marks only in hESCs but not in normal fibroblasts or cancer cells. Depletion of the histone acetyltransferases for H3K18 and H3K56 dramatically decreased the number and intensity of BrdU peaks in hESCs. Our data reveal a unique epigenetic signature that distinguishes active replication loci in hESCs from normal somatic or malignant cells.
Keywords: epigenetics, histone modifications, chromatin, DNA replication, embryonic stem cells, histone acetyltransferase, P300, CBP
Introduction
Patterns of histone modifications and DNA methylation create epigenetic signatures on chromatin that are associated with and may regulate many molecular activities including gene expression and DNA replication. DNA replication initiates at specific regions of the genome known as replication origins.1-3 In S. cerevisiae, replication origins have sequence specificity but additional genetic or chromatin features are required to define functional origins.4 In multicellular organisms, however, no clear sequence specificity is found for replication origins but certain genetic elements, such as CpG islands and G-quadruplex DNA motifs, are preferentially associated with origins.5 It has been proposed that replication origins may also be associated with specific epigenetic states.6 Early activated origins tend to localize in actively transcribed, hyperacetylated chromatin regions while late activated origins are found mainly in heterochromatin with hypo-acetylated histones. In mammalian cells, histone acetyltransferase (HAT) HBO1 mediates H4 acetylation of histones surrounding origins in a cell cycle-dependent manner and promotes loading of MCM complex onto chromatin.7 Histone H3 lysine 56 acetylation (H3K56ac) also plays roles in DNA replication in both yeast and multicellular organisms. In yeast, H3K56ac is catalyzed by Rtt109, which has genetic interactions with multiple replication machinery proteins including ORC2, CDC45, PCNA, and DNA polymerase α.8-10 In Drosophila and human cells, H3K56ac may promote the packaging of newly synthesized DNA into chromatin in DNA repair and replication.11 In yeast, the Rpd3 histone deacetylase delays replication origin firing by deacetylating histones around replication origins. Targeting of H3K18ac and H2Bac to a late activated origin promotes loading of replication factor CDC45 and leads to its earlier activation, while deletion of RPD3 promotes H3K18 and H4 acetylation and activation or increased firing efficiency at origins.12 In humans, H4K20me2/3 recruit ORC to chromatin through direct binding with ORC1 and ORCA.13,14 Certain histone modifications also correlate with replication timing and ORC binding in Drosophila.15
In human cells, the genome-wide relationship between histone modifications and DNA replication has been less understood. It has been unclear whether the DNA replication profile and the associated histone modifications are comparable in cells that are phenotypically different. In this study, we generated DNA replication profile in asynchronously growing H1 hESCs, normal human fetal IMR90 lung fibroblasts and Saos-2 osteosarcoma cells by 5-bromo-2'-deoxyuridine (BrdU) DNA immunoprecipitation followed by massively-parallel sequencing (BrdU-seq).16 We found that the BrdU peaks overlap significantly with replication origins that have high activity as defined by nascent strand (NS) DNA sequencing,5 indicating that BrdU peaks represent active DNA replication loci including a subset of active origins. The distributions of BrdU peaks in all 3 cell types were by and large similar, with majority of the peaks enriched in DNA repetitive elements within introns and intergenic regions in early replicating regions. But a considerable number of replication loci were unique to each cell type and associated with cell type-specific genes. In hESCs the histone marks H3K18ac, H3K56ac, and H4K20me1 were preferentially present at regions that had highest levels of BrdU incorporation, were highly conserved in vertebrates and were also sites of replication in differentiated cells. While BrdU was still incorporated in the same regions in fibroblasts and cancer cells, the histone modifications were absent. Finally, knockdown (KD) of EP300 and CREBBP HATs in hESCs led to a global decrease in H3K18 and H3K56 acetylation and in number and intensity of BrdU peaks, indicating a role for these HATs and their acetylated substrates in DNA replication in hESCs. The association of specific histone marks with active DNA replication loci in hESCs reveals an epigenetic signature that uniquely distinguishes pluripotent DNA replication from that of differentiated or cancerous cells.
Results
Global BrdU incorporation pattern delineates active replication loci in human cells and overlaps with a subset of known replication origins
To determine whether the chromatin environment associated with DNA replication changes during cellular differentiation, we first mapped the locations of BrdU incorporation across the human genome by BrdU-seq in control and EP300 and CREBBP KD H1 hESCs as well as normal human lung fibroblast IMR90 cells and osteosarcoma Saos-2 cancer cells which have a high degree of aneuploidy. To enable a direct comparison of BrdU incorporation and epigenetic modifications that were generated from asynchronously growing cells,17-19 we performed BrdU-seq also in asynchronous cell populations. We verified the specificity of the anti-BrdU antibody by flow cytometry and BrdU dot blot and its utility in immunoprecipitation by showing that significant amounts of DNA were immunoprecipitated from cells in S phase but not in G1 phase of the cell cycle (Figs. S1A and B). Input and immunoprecipitated DNA from each experiment was sequenced, and the obtained reads were aligned uniquely to the human genome (hg19) allowing for up to two mismatches (Table S1). We applied a stringent computational criterion to define BrdU enriched regions with high confidence. We divided the human genome into 100 bp windows and calculated a P value for Poisson distribution of enriched immunoprecipitated DNA relative to input for each window. Significant peaks were defined as those windows with a P value < 10−4 and with 2 neighboring windows at the same significance. Based on these criteria, we identified 5086, 5743, and 6272 high confident BrdU peaks in hESCs, IMR90 and Saos-2 cells respectively (Table 1) with false discovery rates (FDR) < 3%. A less restrictive P value < 10−3 identified approximately twice as many peaks for each cell line with similar distributions but the FDR was >5% (Table S2). We therefore performed subsequent analyses with the peaks defined at P value < 10−4.
Table 1. Summary of BrdU peaks and blocks.
| Cells | Total peaks | FDR | Peaks overlapping with NS DNA | Peaks mapped to repeat sequences | Total blocks |
Coverage (Mbp) | Peaks in blocks |
|---|---|---|---|---|---|---|---|
| H1 | 5086 | 2.04% | 3417 (67.18%) | 3850 (75.7%) | 296 | 321 (10.38%) | 3062 (60.2%) |
| IMR90 | 5743 | 2.54% | 4774 (83.13%) | 4063 (70.75%) | 282 | 358 (11.56%) | 4294 (74.77%) |
| Saos-2 | 6272 | 2.87% | N/A | 5250 (83.71%) | 265 | 373 (12.03%) | 4533 (72.27%) |
NS, nascent strand
While NS DNA analysis is the method of choice for identification of replication origins,5 the BrdU peaks identified in our study showed high concordance with published NS DNA distribution in H9 hESCs and IMR90 cells. Genome-wide, 67.2% and 83.1% of BrdU peaks in H1 hESCs and IMR90 cells respectively overlapped with NS DNA peaks (Table 1; no NS data are available for Saos-2 cells). Figure 1A shows the pattern of BrdU incorporation (red track) at a representative region of the genome for hESCs, IMR90 fibroblasts and Saos-2 cells. The peaks of BrdU coincided with elevated sequence tag counts in the input genomic DNA (black track) and with peaks of NS DNA (purple track). Furthermore, BrdU-seq peaks identified the highly active origins. This was evident when we centered and sorted NS DNA peaks based on their abundance and calculated the NS DNA value for the corresponding BrdU-seq peaks. The identified BrdU peaks coincided with the upper portion of NS DNA distribution (Fig. 1B) and had higher NS DNA values in both H1 hESCs and IMR90 cells (Fig. 1C). This is because for BrdU peaks to meet the significance criterion, the number of sequence reads from immunoprecipitated DNA has to be significantly enriched relative to input DNA which, at sites of DNA replication, contains higher sequence reads compared with the genome average (Fig. S1C, left panel). This constraint does not apply to NS DNA analysis. Thus, the locations where NS DNA is detected but BrdU is not preferentially incorporated are not considered as BrdU peaks (Fig. S1C, right panel). But when no statistical cutoff is applied, the total number of sequence reads in BrdU immunoprecipitated sample parallels those measured by NS DNA (Fig. S1D). Taken together, these data indicate that the BrdU peaks significantly overlap with highly active replications origins. However, since BrdU is incorporated in regions of the genome that are being replicated at the time of the BrdU pulse, our BrdU-seq data identifies regions of active DNA replication regardless of whether these regions function as replication origins.
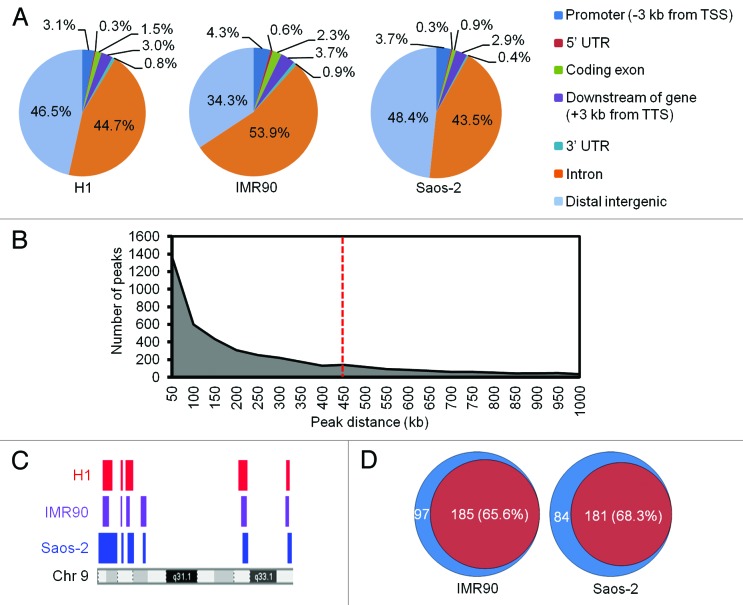
Figure 1. BrdU peaks correspond to actively replicating origins in human cells. (A) Genome browser views of NS DNA, BrdU peaks and input DNA tracks along a representative region of chromosome 9. No NS DNA data were available for Saos-2 cells. Red arrows indicate peaks that occurred in all cell lines and also mapped to microsatellite repeats. Black arrows indicate direction of transcription of the indicated genes. (B) Heat maps of nascent strand DNA abundance at replication origins in H9 hESCs and IMR90 cells and the corresponding NS DNA value for the BrdU peaks identified (NS-Br). (C) Box plots show the distributions of nascent strand DNA values at replication origins in H9 hESCs and IMR90 cells and the corresponding values for BrdU peaks in this study. The thick horizontal line in each box represents the median value which is indicated. P values for 2-sample Kolmogorov–Smirnov test are shown.
Genome-wide distribution of BrdU peaks
Analysis of BrdU peak distribution by the cis-regulatory element annotation system (CEAS) software20 revealed similar genomic distributions in the 3 cell types with most enrichment in intron and distal intergenic regions. BrdU peak distribution in hESCs was more similar to Saos-2 cancer cells than IMR90 fibroblasts (Fig. 2A). Greater than 70% of BrdU peaks in all cell lines occurred at DNA repetitive elements as defined by repeat masker (rmsk) (Table 1).21 Of the numerous types of repetitive elements, microsatellite repeats especially (TG)n repeats contained the majority of BrdU peaks (Table S3). The sequenced reads were uniquely aligned to these loci due to the polymorphisms present in microsatellites.22 DNA motif analysis also revealed that BrdU peaks were enriched at TG- and G-rich sequences in all cell types (Fig. S2A).
Figure 2. The majority of BrdU peaks occur in proximity of each other. (A) Genomic distribution of BrdU peaks in each cell line is shown as pie charts. Distal intergenic regions are defined as being at least 3 kb away from the start and end of genes. (B) The chart shows the distribution of distances between consecutive BrdU peaks. Red dashed line indicates the 450 kb cutoff which includes >70% of the BrdU peaks. (C) Locations of BrdU blocks as colored bars along a region of chromosome 9 are shown for each of the three cell types. (D) The Venn diagrams show the extent of overlap of BrdU blocks between IMR90 or Saos-2 cells and H1 hESCs. Red, common blocks; blue, unique blocks.
The BrdU peaks were not randomly distributed throughout the genome but clustered mostly in vicinity of each other. Plotting the distribution of distances between consecutive BrdU peaks indicated that more than 70% of peaks have distances smaller than 450 kb (Fig. 2B). This is consistent with the fact that the origins of replication occur in DNA replicons ranging from 50–450 kb in length.23 We therefore scanned the genome using a moving 450-kb window to define BrdU blocks of enrichment. We identified >250 BrdU blocks in each cell line, covering over 320 Mb of the genome and accounting for the majority of BrdU peaks (Table 1). The blocks showed a high degree of overlap with 65.6% and 68.3% of blocks in IMR90 and Saos-2 cells respectively overlapping with those in hESCs. Thus a large subset of BrdU blocks were conserved between hESCs and differentiated normal and cancer cells (Fig. 2C and D).
The BrdU blocks were located in early replicating euchromatin (Fig. S2B–D) and depleted from lamina-associated domains (LADs), heterochromatic regions that replicate late in S phase (Fig. S2E and F).24 The blocks also showed differential methylation status in ESCs vs. IMR90 fibroblasts. The DNA methylation level of BrdU blocks in hESCs was not significantly different from randomly selected regions while the BrdU blocks contained significantly more DNA methylation than might be expected based on background levels in IMR90 cells (binomial P value < 1e-300; Fig. S2G).17 We found a similar relationship between DNA methylation and the published early replicating regions in BG02 hESCs and BJ fibroblasts (data not shown).25 Thus, the early replicating regions in differentiated fibroblasts are preferentially enriched for DNA methylation which is not the case in hESCs.
BrdU peaks are associated with cell type-specific genes
The majority of BrdU peaks was found ± 5 kb or further away from transcription start sites (TSS) in all three cell lines (Fig. 3A). We therefore used the Genomic Regions Enrichment of Annotations Tool (GREAT)26 to determine the genes associated with distant peaks of BrdU. In hESCs, BrdU peaks occurred near genes that are involved in early development and maintenance of pluripotency such as OCT4, NODAL, and BMPR1A (Fig. 3B; Fig. S3). In contrast, BrdU peaks associated with genes that function in differentiation and morphogenesis in IMR90 fibroblasts such as HES1 and ACTB. In Saos-2 cells, BrdU peaks were linked to ion channels and transporters (Fig. 3C and D), which are involved in cancer progression.27,28 Therefore, BrdU peaks tend to associate with genes that are functionally relevant in each cell type.
Figure 3. BrdU peaks are associated with cell type-specific genes. (A) The bar chart shows the distribution of the distances of BrdU peaks from TSS. (B–D) GO analysis of BrdU peaks in each cell line as indicated. Bars represent -log10 of the binomial raw P values.
Co-occupancy of histone H3K18ac, H3K56ac and H4K20me1 with BrdU peaks in hESCs
Since histone acetylation plays a role in DNA replication,7,12,15 we compared the distribution of histone acetylation marks to BrdU peaks. As shown in Figure 4A and B for a representative region on chromosome 18 and genome-wide, respectively, there were significant overlaps between H3K18ac and H3K56ac with BrdU peaks only in hESCs. Distribution of H3K56ac in H9 hESCs also overlapped with BrdU peaks in H1 hESCs, indicating this co-occupancy may be a general feature of hESCs (Fig. 4A). We also found strong occupancy of H4K20me1 at BrdU peaks in hESCs, consistent with a role for H4K20 methyltransferase, PR-SET7, in regulation of certain replication origins.29 Globally, 23.4%, 28%, and 50.5% of BrdU peaks in hESCs had H3K18ac, H3K56ac, and H4K20me1 nearby while only 9%, 6.55%, and 13.2% of BrdU peaks in IMR90 cells were associated with these marks, respectively. Other histone acetylation sites such as H3K9ac and H3K27ac showed somewhat opposite patterns with no enrichment in hESCs and slight enrichment in fibroblasts’ BrdU incorporating regions, indicating the high specificity of H3K18ac and H3K56ac co-occupancy with BrdU peaks in hESCs (Fig. 4B; Fig. S4A).
Figure 4. Co-occupancy of BrdU peaks with histone modifications is ESCs specific. (A) Shown are genome browser views of the indicated features along a representative region of chromosome 18. Red arrows indicate BrdU peaks that overlap with histone modifications. Arrowheads indicate the histone modification peaks at the TSS. Black arrows indicate direction of transcription. (B) The percentage of BrdU peaks that overlap with histone modifications. (C) The average levels of the indicated histone modifications (significant reads counts) relative to the center of BrdU incorporating regions are shown for the top and bottom 20% according to the BrdU signal intensity in comparison to all BrdU incorporating regions. (D–E) Levels of H3K56ac at BrdU incorporating regions in H1, BMP4-induced differentiated H1 cells and adipocytes are shown for (D) a representative locus on chromosome 18 and (E) genome-wide.
To further characterize the relationship between BrdU peaks and H3K18ac, H3K56ac, and H4K20me1, we grouped genomic regions that have BrdU peaks based on peak intensity and centered all regions. We then plotted the average levels of histone modifications in hESCs and IMR90 cells within ± 2 kb of each BrdU region center. As shown in Figure 4C and Figure S4A, the regions with highest levels of BrdU incorporation (top 20%) also had higher levels of H3K18ac, H3K56ac, and H4K20me1, but not H3K9ac, H3K27ac, or H4K5ac in hESCs. In IMR90 cells, there was no preferential enrichment of H3K18ac, H3K56ac, and H4K20me1 within BrdU labeled regions. When we analyzed published H3K56ac data sets in adipocytes and BMP4-induced mesendoderm cells differentiated from H1 hESCs, we also observed loss of H3K56ac at replication origins upon differentiation. This was not due to complete absence of H3K56ac as new peaks of H3K56ac appeared at similar places in mesendoderm as in IMR90 cells (Fig. 4D; Fig. S4B). Globally, the number of BrdU peaks that overlapped with H3K56ac was dramatically reduced upon differentiation (binomial P value < 1e-300, Fig. 4E). Similarly, we did not detect enrichment of H4K20me1 levels in HeLa S3 and K562 leukemia cells at the genomic regions that in H1 hESCs the BrdU peaks overlapped with H4K20me1 (Fig. S4B). Taken together, our analysis shows that H3K18ac, H3K56ac, and H4K20me1 are enriched at the regions of active DNA replication mainly in hESCs and correlate positively with the intensity of BrdU peaks.
The H1-IMR90-Saos-2 shared peaks of BrdU are associated with high level of histone marks in hESCs and conserved DNA sequences across vertebrates
To rule out that the association of histone modifications with DNA replication occur at H1-specific BrdU peaks, we divided the BrdU peaks into those that are shared between H1, IMR90, and Saos-2 cells and those that are unique to H1 (1167 vs 2813 BrdU peaks, respectively). The average levels of BrdU intensity was much higher in shared regions than in H1 specific regions. The shared regions also had higher levels of H3K18ac, H3K56ac, and H4K20me1 while H1 specific regions showed low levels of histone modifications (Fig. 5A). Gene ontology analysis indicated that the shared peaks were associated with differentiation genes while H1 unique peaks were linked to embryonic development genes (Fig. 5B). Furthermore, the shared BrdU regions had higher degree of DNA sequence conservation among vertebrates compared with H1-specific BrdU peaks (Fig. 5C). Thus the shared BrdU regions account for most of the enrichment observed for the associated histone modifications, indicating that patterns of histone modifications are indeed different at the same replicating regions between hESCs and differentiated cells.
Figure 5. The BrdU peaks shared by the three cell types are preferentially associated with histone marks in hESCs and conserved DNA sequences. (A) Average levels of BrdU incorporation and histone modifications in hESCs at the center of BrdU peaks that are shared between H1, IMR90 and Saos-2 cells or that are unique to H1 cells. (B) GO analysis of shared (left panel) and H1-specific (right panel) BrdU peaks. (C) Conservation plots of shared and H1-specific BrdU peaks.
EP300/CREBBP depletion decreases global BrdU incorporation in hESCs
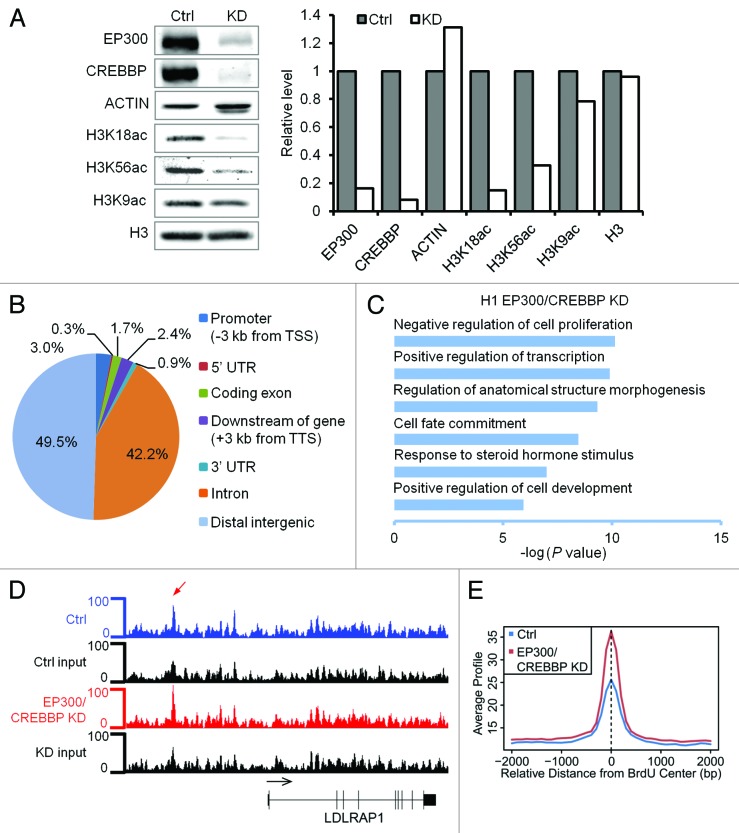
EP300 and CREBBP are the main HATs for H3K18 and H3K56.11,30 We therefore tested whether EP300 and CREBBP were required for DNA replication in hESCs. Since EP300 and CREBBP are largely redundant in acetylation of H3K18,30 we co-transfected siRNAs against both genes into hESCs and performed BrdU-seq (Table S1). As shown in Figure 6A, EP300 and CREBBP protein levels decreased ~85–90% upon knockdown. Levels of H3K18ac and H3K56ac also decreased dramatically after knockdown, indicating that EP300/CREBBP were responsible for the bulk of H3K18ac and H3K56ac in hESCs.
Figure 6. EP300 and CREBBP HATs are required for normal pattern of BrdU incorporation in hESCs. (A) Western blots and signal quantifications of the indicated factors are shown for control (ctrl) and KD of EP300/CREBBP in H1 hESCs. (B) Distribution of BrdU peaks in KD cells is shown as a pie chart. (C) GO analysis of BrdU peaks in KD cells. (D) Enhanced BrdU incorporation upstream of LDLRAP1 gene on chromosome 1 is detected in KD relative to ctrl H1 hESCs. Red arrow points to the BrdU peak. (E) Genome-wide average of BrdU incorporation (all tag counts) at regions with newly detected BrdU peaks in EP300/CREBBP KD relative to ctrl H1 cells.
The number of BrdU peaks decreased from 5086 in control siRNA to 1755 in EP300/CREBBP KD hESCs. Depletion of EP300/CREBBP also significantly decreased BrdU blocks from 296 to 33. The remaining BrdU peaks after KD were similarly distributed across the genome with 42.2% and 49.5% of peaks in introns and distal intergenic regions, respectively (Fig. 6B). The majority (64%) of the BrdU peaks in EP300/CREBBP KD cells still occurred within DNA repeats (Table S3) and were associated with negative regulators of cell proliferation such as cyclin-dependent kinase inhibitors, genes involved in cell morphogenesis and cell fate commitment (Fig. 6C). Among the 1755 BrdU peaks in KD cells, we detected 1489 new peaks compared with control. However, a closer examination of these 1489 regions revealed smaller BrdU peaks in the control sample, which had not reached our statistical threshold for significance to be included in the analysis (Fig. 6D). Globally, the level of BrdU incorporation over the newly detected regions was much lower in control KD cells than in EP300/CREBBP KD cells (Fig. 6E), suggesting that these regions were being replicated by a minority of hESCs prior to EP300/CREBBP KD. These data reveal that depletion of EP300/CREBBP significantly decreases BrdU peaks and block formation and changes genome-wide replication pattern, revealing an essential role for these HATs in regulation of DNA replication in hESCs.
Discussion
We have compared active DNA replication patterns in pluripotent stem cells, the fully differentiated IMR90 primary normal fibroblasts and the Saos-2 cancer cell line. Our genome-wide BrdU maps overlapped partially with the early origins of DNA replication as determined previously by nascent strand detection and early timing of replication.5,25 The genomic regions with higher levels of NS DNA also showed preferential BrdU incorporation demonstrating that BrdU peaks overlap with highly active replication origins. Overall the distribution of BrdU peaks were similar between all cell types indicating that DNA replication is a highly conserved process even in cells with vastly different phenotypes or with significant chromosomal abnormalities such as Saos-2 cells.31 In all cell types, the bulk of BrdU peaks were at (TG)n dinucleotide microsatellite repeats and poly G sequences. A similar trend for significantly overrepresented sequences at replication origins in Drosophila and mouse cells has also been observed.32
Despite the similarity of BrdU incorporation patterns in the 3 cell types, the epigenetic marks at or around the BrdU peaks were quite distinct. We observed significant enrichment of H3K18ac, H3K56ac, and H4K20me1 at BrdU peaks specifically in hESCs but not at the same replicating regions in fibroblasts or cancer cells, suggesting that active DNA replication is associated with distinct epigenetic marks in hESCs. While certain histone marks have been previously associated with DNA replication origins,12,15,29 the cell type specificity of such association is unexpected. Several possibilities may underlie these observations. First, the same histone marks may still associate with DNA replication in fibroblasts but only transiently in a narrower window of cell cycle which would be missed when examining asynchronously growing cells. Second, these histone marks may have been erased at some point in the differentiation lineage from hESCs to fibroblasts or replaced by other epigenetic modifications. It was shown recently that H3K56me1 regulates DNA replication in HeLa cells through direct interaction with PCNA.33 Since methylation and acetylation of lysines are mutually exclusive, H3K56ac in hESCs may be replaced by H3K56me1 in more differentiated cells. Third, hESCs and fibroblasts may represent classes of cell types with different epigenetic requirements for active replication. Examination of additional cell types may reveal categories of cells that have similar replication-associated epigenomic profiles as hESCs. For instance, in U2OS osteosarcoma cell line, PR-Set7 mediates H4K20 monomethylation of histones at a limited number of examined replication origins at the onset of replication origin licensing.29 This may also be the case in Saos-2 osteosarcoma cells but no genome-wide data set on H4K20me1 is available in either cancer cell line. Further genome-wide studies of replication origins and epigenetic marks will be required to resolve these possibilities.
Whether the histone marks play a direct role in DNA replication in hESCs remain to be established. Knockdown of EP300/CREBBP significantly decreased H3K18ac, H3K56ac, and BrdU incorporation but since EP300 and CREBBP have many cellular activities34 and interact with and acetylate other replication machinery proteins,35,36 our data cannot unequivocally link histone acetylation to regulation of DNA replication in hESCs. Knockdown of these enzymes may also result in loss of hESCs pluripotency with subsequent effects on DNA replication. Indeed, the KD cells showed morphological changes associated with differentiation (data not shown). Nonetheless, ectopic targeting of histone acetyltransferases to a replication origin stimulates the origin’s activity12,37 and H4 methylation can help recruit ORC to chromatin.13,14 Whatever the case may be, our data reveal that the global association of DNA replication with epigenetic marks is dependent on the cell type. It will be of interest to determine whether the epigenetic profiles of replication origins in iPS cells are similar to ESCs or to the cell types from which iPS cells were derived.
Materials and Methods
Cell culture
H1 hESCs were obtained from UCLA embryonic stem cell bank and maintained with mTeSR1 Basal Medium (STEMCELL Technologies) at 37 °C in 5% CO2. IMR90 and Saos-2 cells were purchased from ATCC. Cells were maintained at 37 °C in 5% CO2 with DMEM containing 10% FBS and penicillin-streptomycin.
BrdU labeling and genomic DNA purification
Cells were pulse labeled with 30 µM BrdU for 30 min before being harvested. Genomic DNA was purified as previously described with some modification.38 Briefly, BrdU labeled cells were suspended in lysis Buffer (10 mM Tris-HCl, pH 8.0, 200 mM NaCl, 25 mM EDTA, 1% SDS and 600 µg/ml proteinase K) and incubated at 37 °C overnight. Each sample was then extracted twice with phenol:chloroform:isoamyl alcohol (25:24:1) and once with chloroform. After extraction, DNA was precipitated with isopropanol and centrifuged for 30 min at 4 °C. The pellet was resuspended in TE buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA) and treated with 10 µg/ml RNase A at 37 °C for 30 min. The DNA was further extracted with phenol:chloroform:isoamyl alcohol and precipitated with ethanol following standard procedures. Purified DNA was re-suspended in TE buffer.
Immunoprecipitation and sequencing library preparation
Chromatin immunoprecipitation was performed as previously described using rabbit anti-H3K18ac antibody.39 The immunoprecipitation of BrdU labeled DNA was adapted from Azuara et al. with modifications.40 Four micrograms of BrdU labeled DNA was heated in 0.1 M NaOH at 95 °C for 5 min to remove RNA primers from nascent strand DNA. HCl was then added to neutralize the pH. TE buffer (10 mM Tris-HCl pH 7.4, 1 mM EDTA) was added to bring the total volume 500 µl. DNA was fragmented by sonication and denatured by heating at 95 °C for 5 min to generate single-stranded DNA that can be identified by anti-BrdU antibody. An amount of 0.1 volume of adjusting buffer (110 mM sodium phosphate buffer, pH7.0, 1.54 M NaCl, 0.55% Triton X-100) was then added to DNA. DNA was subsequently incubated with 2 µg of anti-BrdU antibody and Dynabeads Protein G (Life Technologies). After immunoprecipitation, DNA was purified using phenol:chloroform:isoamyl alcohol extraction and ethanol precipitation. Single-stranded DNA was quantified by Qubit Fluorometer (Life Technologies). Double-stranded DNA was generated from input and immunoprecipitated DNA by brief random priming with the Bioprime DNA Labeling System (Life Technologies) at 37 °C for 20 min. Sequencing libraries were prepared with ChIP-seq DNA Sample Prep Kit (Illumina) according to the manufacturer's instructions and sequenced using Illumina Genome Analyzer IIx or HiSeq 2000 sequencing systems at the High Throughput Sequencing Core of the UCLA Broad Stem Cell Research Center.
Gene ontology analysis
The analysis was performed with GREAT.26 The gene regulatory domain was defined as the region that extends from TSS in both directions to the nearest gene's TSS but no more than 100 kb extension in one direction. The BrdU peaks were then overlapped with gene regulatory domains to identify associated genes.
Data access
Sequencing data have been submitted to GEO under accession number GSE43152.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Maria Vogelauer for critical reading of the manuscript and Matteo Pellegrini for help on bioinformatics analysis. We acknowledge the support of the UCLA Broad Stem Cell Research Center High-Throughput Sequencing Core Resource. Li JY was supported by a training grant of California Institute for Regenerative Medicine (CIRM) in stem cell research at UCLA. This work was funded by a CIRM grant to Kurdistani SK.
Glossary
Abbreviations:
- BrdU
5-bromo-2'-deoxyuridine
- CEAS
cis-regulatory element annotation system
- FDR
false discovery rate
- GEO
Gene Expression Omnibus
- GREAT
Genomic Regions Enrichment of Annotations Tool
- HAT
histone acetyltransferase
- hESCs
human embryonic stem cells
- iPS cells
induced pluripotent stem cells
- KD
knockdown
- LADs
lamina-associated domains
- MCM
mini chromosome maintenance
- NS
nascent strand
- rmsk
repeat masker
- ORC
origin recognition complex
- ORCA
origin recognition complex associated
- TSS
transcription start sites
Footnotes
Previously published online: www.landesbioscience.com/journals/epigenetics/article/26870
References
- 1.Gilbert DM. Evaluating genome-scale approaches to eukaryotic DNA replication. Nat Rev Genet. 2010;11:673–84. doi: 10.1038/nrg2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stillman B. Origin recognition and the chromosome cycle. FEBS Lett. 2005;579:877–84. doi: 10.1016/j.febslet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Remus D, Diffley JF. Eukaryotic DNA replication control: lock and load, then fire. Curr Opin Cell Biol. 2009;21:771–7. doi: 10.1016/j.ceb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Eaton ML, Galani K, Kang S, Bell SP, MacAlpine DM. Conserved nucleosome positioning defines replication origins. Genes Dev. 2010;24:748–53. doi: 10.1101/gad.1913210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnard E, Babled A, Lapasset L, Milhavet O, Parrinello H, Dantec C, Marin JM, Lemaitre JM. Unraveling cell type-specific and reprogrammable human replication origin signatures associated with G-quadruplex consensus motifs. Nat Struct Mol Biol. 2012;19:837–44. doi: 10.1038/nsmb.2339. [DOI] [PubMed] [Google Scholar]
- 6.Méchali M. DNA replication origins: from sequence specificity to epigenetics. Nat Rev Genet. 2001;2:640–5. doi: 10.1038/35084598. [DOI] [PubMed] [Google Scholar]
- 7.Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell. 2010;37:57–66. doi: 10.1016/j.molcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suter B, Tong A, Chang M, Yu L, Brown GW, Boone C, Rine J. The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae. Genetics. 2004;167:579–91. doi: 10.1534/genetics.103.024851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–13. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Zhou H, Horazdovsky B, Zhang K, Xu RM, Zhang Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–5. doi: 10.1126/science.1133234. [DOI] [PubMed] [Google Scholar]
- 11.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–7. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelauer M, Rubbi L, Lucas I, Brewer BJ, Grunstein M. Histone acetylation regulates the time of replication origin firing. Mol Cell. 2002;10:1223–33. doi: 10.1016/S1097-2765(02)00702-5. [DOI] [PubMed] [Google Scholar]
- 13.Beck DB, Burton A, Oda H, Ziegler-Birling C, Torres-Padilla ME, Reinberg D. The role of PR-Set7 in replication licensing depends on Suv4-20h. Genes Dev. 2012;26:2580–9. doi: 10.1101/gad.195636.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo AJ, Song J, Cheung P, Ishibe-Murakami S, Yamazoe S, Chen JK, Patel DJ, Gozani O. The BAH domain of ORC1 links H4K20me2 to DNA replication licensing and Meier-Gorlin syndrome. Nature. 2012;484:115–9. doi: 10.1038/nature10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton ML, Prinz JA, MacAlpine HK, Tretyakov G, Kharchenko PV, MacAlpine DM. Chromatin signatures of the Drosophila replication program. Genome Res. 2011;21:164–74. doi: 10.1101/gr.116038.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CL, Rappailles A, Duquenne L, Huvet M, Guilbaud G, Farinelli L, Audit B, d’Aubenton-Carafa Y, Arneodo A, Hyrien O, et al. Impact of replication timing on non-CpG and CpG substitution rates in mammalian genomes. Genome Res. 2010;20:447–57. doi: 10.1101/gr.098947.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, et al. Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell. 2010;6:479–91. doi: 10.1016/j.stem.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie W, Schultz MD, Lister R, Hou Z, Rajagopal N, Ray P, Whitaker JW, Tian S, Hawkins RD, Leung D, et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell. 2013;153:1134–48. doi: 10.1016/j.cell.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin H, Liu T, Manrai AK, Liu XS. CEAS: cis-regulatory element annotation system. Bioinformatics. 2009;25:2605–6. doi: 10.1093/bioinformatics/btp479. [DOI] [PubMed] [Google Scholar]
- 21.Jurka J. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 2000;16:418–20. doi: 10.1016/S0168-9525(00)02093-X. [DOI] [PubMed] [Google Scholar]
- 22.Eckert KA, Hile SE. Every microsatellite is different: Intrinsic DNA features dictate mutagenesis of common microsatellites present in the human genome. Mol Carcinog. 2009;48:379–88. doi: 10.1002/mc.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe Y, Fujiyama A, Ichiba Y, Hattori M, Yada T, Sakaki Y, Ikemura T. Chromosome-wide assessment of replication timing for human chromosomes 11q and 21q: disease-related genes in timing-switch regions. Hum Mol Genet. 2002;11:13–21. doi: 10.1093/hmg/11.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 25.Hansen RS, Thomas S, Sandstrom R, Canfield TK, Thurman RE, Weaver M, Dorschner MO, Gartler SM, Stamatoyannopoulos JA. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc Natl Acad Sci U S A. 2010;107:139–44. doi: 10.1073/pnas.0912402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol. 2011;301:C255–65. doi: 10.1152/ajpcell.00047.2011. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–61. doi: 10.1158/0008-5472.CAN-12-4188. [DOI] [PubMed] [Google Scholar]
- 29.Tardat M, Brustel J, Kirsh O, Lefevbre C, Callanan M, Sardet C, Julien E. The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat Cell Biol. 2010;12:1086–93. doi: 10.1038/ncb2113. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz GA, Zhang K, McBrian MA, Grunstein M, Kurdistani SK, Berk AJ. Adenovirus small e1a alters global patterns of histone modification. Science. 2008;321:1084–5. doi: 10.1126/science.1155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheel C, Schaefer KL, Jauch A, Keller M, Wai D, Brinkschmidt C, van Valen F, Boecker W, Dockhorn-Dworniczak B, Poremba C. Alternative lengthening of telomeres is associated with chromosomal instability in osteosarcomas. Oncogene. 2001;20:3835–44. doi: 10.1038/sj.onc.1204493. [DOI] [PubMed] [Google Scholar]
- 32.Cayrou C, Coulombe P, Vigneron A, Stanojcic S, Ganier O, Peiffer I, Rivals E, Puy A, Laurent-Chabalier S, Desprat R, et al. Genome-scale analysis of metazoan replication origins reveals their organization in specific but flexible sites defined by conserved features. Genome Res. 2011;21:1438–49. doi: 10.1101/gr.121830.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Y, Song C, Zhang Q, DiMaggio PA, Garcia BA, York A, Carey MF, Grunstein M. Histone H3 lysine 56 methylation regulates DNA replication through its interaction with PCNA. Mol Cell. 2012;46:7–17. doi: 10.1016/j.molcel.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–77. [PubMed] [Google Scholar]
- 35.Glozak MA, Seto E. Acetylation/deacetylation modulates the stability of DNA replication licensing factor Cdt1. J Biol Chem. 2009;284:11446–53. doi: 10.1074/jbc.M809394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem. 2004;279:20194–9. doi: 10.1074/jbc.M312850200. [DOI] [PubMed] [Google Scholar]
- 37.Goren A, Tabib A, Hecht M, Cedar H. DNA replication timing of the human beta-globin domain is controlled by histone modification at the origin. Genes Dev. 2008;22:1319–24. doi: 10.1101/gad.468308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdurashidova G, Deganuto M, Klima R, Riva S, Biamonti G, Giacca M, Falaschi A. Start sites of bidirectional DNA synthesis at the human lamin B2 origin. Science. 2000;287:2023–6. doi: 10.1126/science.287.5460.2023. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari R, Su T, Li B, Bonora G, Oberai A, Chan Y, Sasidharan R, Berk AJ, Pellegrini M, Kurdistani SK. Reorganization of the host epigenome by a viral oncogene. Genome Res. 2012;22:1212–21. doi: 10.1101/gr.132308.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azuara V. Profiling of DNA replication timing in unsynchronized cell populations. Nat Protoc. 2006;1:2171–7. doi: 10.1038/nprot.2006.353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.