Abstract
In animal models of partial urethral obstruction (PUO), altered smooth muscle function/contractility may be linked to changes in molecules that regulate calcium signaling/sensitization. PUO was created in male rats, and urodynamic studies were conducted 2 and 6 wk post-PUO. Cystometric recordings were analyzed for the presence or absence of nonvoiding contractions [i.e., detrusor overactivity (DO)]. RT-PCR and Western blots were performed on a subpopulation of rats to study the relationship between the expression of RhoA, L-type Ca2+ channels, Rho kinase-1, Rho kinase-2, inositol 1,4,5-trisphosphate, ryanodine receptor, sarco(endo)plasmic reticulum Ca2+-ATPase 2 and protein kinase C (PKC)-potentiated phosphatase inhibitor of 17 kDa, and urodynamic findings in the same animal. Animals displayed DO at 2 (38%) and 6 wk (43%) post-PUO, increases were seen in in vivo pressures at 2 wk, and residual volume at 6 wk. Statistical analysis of RT-PCR and Western blot data at 2 wk, during the compensatory phase of detrusor hypertrophy, documented that expression of molecules that regulate calcium signaling and sensitization was consistently lower in obstructed rats without DO than those with DO or control rats. Among rats with DO at 2 wk, linear regression analysis revealed positive correlations between in vivo pressures and protein and mRNA expression of several regulatory molecules. At 6 wk, in the presence of overt signs of bladder decompensation, no clear or consistent alterations in expression of these same targets were observed at the protein level. These data extend prior work to suggest that molecular profiling of key regulatory molecules during the progression of PUO-mediated bladder dysfunction may shed new light on potential biomarkers and/or therapeutic targets.
Keywords: hypertrophy, dysfunction, calcium signaling
benign prostatic hyperplasia (BPH) may lead to prostatic enlargement (BPE) in turn causing partial outflow obstruction (BPO), which can eventually result in severe bladder dysfunction (2). It is estimated that ∼50% of men over the age of 40 yr develop BPH, and lower urinary tract symptoms (LUTS) develop in about one-half of those men (∼25% of the total population) (40). Given the increasing age of the population, the number of patients with LUTS suggestive of BPH is expected to increase substantially: by 2025, it has been estimated that 52 million adults in the US will have LUTS, dramatically increasing the total cost of treatment (25, 38).
While removal of obstruction via prostate resection ameliorates LUTS in the majority of BPH patients, symptoms persist in many (∼20–30%) (40). Pharmacological intervention of LUTS, both before and after resection targeting membrane receptors (i.e., α1-receptor blockers, antimuscarinics), have been utilized, but with limited success (2, 12). The efficacy of these treatments is undoubtedly dependent on many factors that are difficult to examine in humans, such as the cause, duration, and degree of obstruction.
As such, animal models have been created in several species (37), such as the rat (27, 29, 49), rabbit (9, 11, 50), pig (32–33), and mouse (6, 35, 51), which enable consistent levels of obstruction, and the use of many methodologies to characterize the response of the bladder urodynamically, pharmacologically, histologically, molecularly, etc. (46, 47). Partial urethral obstruction (PUO) in these animal models results in functional changes similar to those seen in BPH, the most striking of which is compensatory hypertrophy of the detrusor smooth muscle to overcome increased outlet resistance. The bladder enlargement seen both in clinical BPO and animal PUO models eventually leads to impaired bladder function in the form of increased residual volume (RV), increased pressure generations, detrusor overactivity (DO), and prolonged hypertrophy can lead to complete detrusor decompensation and bladder failure (26, 27, 36). Even if LUTS per se cannot be studied in these animal models, they have been effectively utilized to identify new therapeutic targets (54).
In this regard, many studies have investigated changes in different calcium-handling or calcium-sensitization proteins in response to obstruction (e.g., caldesmon, calcineurin, etc.) (16, 53). Elevation of the intracellular calcium ion concentration ultimately leads to phosphorylation of myosin light chain and is a critical step in the contraction of smooth muscle cells. It has been shown that obstruction leads to a lowered rate of calcium influx, perhaps due to the lower surface area-to-volume ratio seen in hypertrophic muscle (4, 44). However, it has also been shown that PUO alters molecular signals involved in both mechanisms that increase intracellular calcium: influx of calcium from extracellular space (10, 22), as well as release of calcium from sarcoplasmic reticulum stores (24, 46).
For example, it has been shown that decompensation of the obstructed rabbit bladder is associated with decreases in sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), which is involved in restoring the sarcoplasmic reticulum's calcium stores (46). The Rho kinase pathway has also been implicated in a rabbit model, as inhibition of Rho kinase led to slightly less myosin light chain phosphorylation (7). Additionally, Chang et al. (11), using this rabbit model, showed that protein kinase C (PKC) levels are altered in decompensation, and that PKC activity is correlated with frequency of urination. To our knowledge, this was the first study aiming to correlate bladder function with targetable signaling pathways involved in smooth muscle contraction.
However, comparison of studies from different research groups is sometimes difficult because of many factors, including the animal model, the duration of obstruction, and/or the criteria/preference for identifying decompensated or DO animals (39, 45). In the present study, we utilized the rat model, with which we have extensive experience, to examine signals critical for calcium handling and contraction in response to both short-term (2 wk) and longer term (6 wk) obstruction. Specific emphasis has been given to identifying calcium-associated molecules that are up- and/or downregulated in animals that display DO upon urodynamic investigation in vivo. The ultimate goal was to begin to establish molecular milestones associated with disease progression to identify potential predictors of obstruction-related DO (i.e., biomarkers), as well as associated compensatory mechanisms that may permit earlier and more aggressive intervention via novel and more effective therapeutic targets.
MATERIALS AND METHODS
Animals
A total of 120 12-wk old male Sprague-Dawley rats, weighing 250–275 g, were used in this study. Ten animals were used as age-matched controls at both 2 and 6 wk (n = 20), while 100 animals underwent PUO for analysis at 2 and 6 wk. All animals in the study groups underwent urodynamic testing and, based on their cystometrograms, were classified as DO or non-DO. Bladders excised from both DO and non-DO animals were randomly assigned to molecular studies (RT-PCR and Western blotting) and pharmacological testing (organ bath studies). All protocols were approved by the Animal Care and Use Committee of the Wake Forest University School of Medicine.
PUO
Animals were anesthetized with 2% isoflurane, and the abdominal wall and perineum were shaved and decontaminated with povidone-iodine. A midperineal incision was made to expose the penile shaft in between the two testicles. The whole penile shaft was dissected from surrounding tissues, and dissection of the urethra from the corpus cavernosa was performed. A 0.9-mm-diameter metal rod was inserted into the urethra, and a 3–0 proline suture was tied around the urethra and rod. After securing the proline knot, the metal rod was removed to ensure a partial rather than a total urethral obstruction.
Bladder Catheter Implantation
Rats were again anesthetized with 2% isoflurane, and povidone-iodine was again used for surgical site sanitization. A low midline incision was made, and the bladder was dissected and delivered outside of the body. A small incision was made in the bladder dome, and a PE-50 intramedic polyethylene catheter (Becton-Dickinson, Sparks, MD) with cuff was inserted and anchored with a 5–0 purse string silk suture. The catheter was then tunneled subcutaneously and brought out through the nape of the animals and held in place with cloth tape anchored to the skin via a 3–0 Vicryl suture. The abdominal wall and skin were closed in two layers with 3–0 vicryl sutures, and the free end of the catheter was thermally sealed.
Cystometric Analysis
All cystometric studies were performed 3 days after catheter implantation in conscious, freely moving rats, as previously described (8, 14, 15, 30, 35, 42, 43, 52, 55). Briefly, the bladder catheter was connected to a two-way valve that is, in turn, connected to a pressure transducer and an infusion pump. The pressure transducer is connected to an ETH 400 (CD Sciences, Dover, NH) transducer amplifier and, consequently, connected to a PowerLab/8e (Analog Digital Instruments, Castle Hill, NSW, Australia) data acquisition board. The pressure transducers and acquisition board were calibrated in centimeters of H2O before each experiment. Room temperature saline was infused at a rate of 10 or 20 ml/h. Micturition volumes (MVs) were measured with a silicone-coated funnel leading into a collection tube, which is connected to a force displacement transducer. Analysis began after a stable voiding pattern was established. The following cystometric parameters were investigated: basal pressure (BP, lowest pressure between voids), micturition pressure (MP; the maximum bladder pressure during micturition), threshold pressure (TP; pressure at which voiding is initiated), inter-MP (IMP; mean bladder pressure between voids), bladder capacity (Bcap; the amount of saline infused between voiding cycles), MV (amount of measured expelled urine), RV (Bcap-MV), and bladder compliance (Bcom; the change in pressure between voiding contractions). Nonvoiding contractions (NVCs; increases in pressure between micturitions and not associated with the expulsion of fluid) of at least 5 cmH2O were used as a surrogate for DO (3). The presence of more than three or more contractions measuring above the designated 5 cmH2O in the analysis period led to the designation of DO in those animals.
Pharmacological Studies
After cystometric analysis, animals were killed with CO2 inhalation and bilateral thoracotomy, and the bladders of a subset of randomly selected animals were harvested and immediately placed in ice-cold Krebs buffer for pharmacological studies. The bladders were cut into equally sized strips along the longitudinal axis. The strips were scraped gently to remove urothelial cells and were attached to tissue holders at one end and force transducers at the other in a double-jacketed organ bath system (Radnoti Glass Technology, Monrovia, CA) containing 15 ml of Krebs buffer aerated with 95% O2/5% CO2 at 37°C. Bladder strips were subjected to a resting tension of 2 g and allowed to stabilize for at least 60 min. They were then primed using 5 μM carbachol and subsequently 60 μM KCl. Contractions were recorded as changes in tension from baseline in response to both carbachol and electrical field stimulation. Carbachol dose-response curves were generated by adding increasing concentrations of carbachol at one-half log increments starting at 3 nM up to 100 μM. For electrical field stimulation, strips were placed between two platinum electrodes in the organ chamber, and electrical pulses (0.1-ms pulse width, 20 V in the bath) were delivered, lasting 30 s at increasing frequencies (1, 2, 4, 8, 16, and 32 Hz) using a S88 stimulator (Grass Instruments, W. Warwick, RI). All tissue responses were normalized to grams of tissue weight.
Molecular Analysis
RNA purification and real-time PCR.
A subset of bladders not subjected to pharmacological analysis was used for mRNA studies. These bladders were randomly selected from the already designated subsets of DO and non-DO animals at 2 and 6 wk, respectively. The urothelium was stripped, and the tissue placed in RNAlater (Ambion, Austin, TX). Approximately 35 mg of bladder were homogenized for isolation of total RNA using RNeasy Mini Kit (Qiagen, Valencia, CA), according to manufacturer's instructions for isolation of total RNA from animal tissues. RNA was eluted in RNase-free water and quantified by 260-nm-to-280-nm optical density ratio, and subsequently stored at −20°C. Synthesis of first-strand cDNA from 500 ng of total RNA in a 20-μl reaction mix and priming with random hexamers was done using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) in the presence of RNase inhibitor, according to manufacturer's instructions.
Real-time PCR was performed on 1 μl of cDNA template in a 20-μl reaction volume in an ABI Prism7000 Sequence Detection System (Applied Biosystems, Foster City, CA) using TaqMan Universal PCR Mastermix, No AmpErase UNG (Applied Biosystems). The primers for each of the rat genes were purchased as premade TaqMan Gene Expression Assays from Applied Biosystems and contained a FAM sequence specific probe for PCR detection. Because these primers are proprietary, sequence information is not available. The genes tested included the following: inositol 1,4,5-trisphosphate (IP3)-R3, PKC-potentiated phosphatase inhibitor of 17 kDa (CPI-17), RhoA, Rho kinase (Rock) 1, Rock 2, α1c- and α1d-subunits of L-type voltage-gated Ca2+ channel, and SERCA2. The real-time PCR was performed with the following profile: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. All samples were normalized to 18s rRNA expression, and relative gene expression levels were calculated using the 2−ΔΔCT method.
SDS-PAGE and Western blot analysis.
Another subset of surgically excised rat bladders was flash frozen in liquid nitrogen for Western blotting. Those bladders were again randomly selected from the previously designated subgroups of DO and non-DO at 2 and 6 wk time points, respectively. Clean bladder tissue (urothelial cells scraped off the inner bladder walls and cut in smaller pieces) was homogenized in RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with 1:10 protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein concentrations were determined using Bio-Rad's DC protein assay. Total protein (1–3 μg/ml) for all samples was separated on the 7.5, 10, or 12% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis (SDS-PAGE), along with a molecular ladder (Bio-Rad Laboratories, Hercules, CA) and prestained biotinylated protein ladder (Cell Signaling Technology, Danvers, MA). Gels were then transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), and probed with the following antibodies, dilutions, and incubation times (Table 1): CPI-17 (1:350 overnight, 4°C), L-type C1α (1:400 overnight, 4°C), Rho A (1:400, 1.5 h, room temp), Rock 1 (1:150 overnight, 4°C), Rock 2 (1:200 overnight, 4°C), and SERCA2 (1:400 overnight, 4°C) diluted in 5% wt/vol skim milk/1× PBS-Tween (0.5% vol/vol Tween 20), followed by the corresponding secondary antibody conjugated with horseradish peroxidase (1:2,000) for 1 h at room temperature. The specificity and sensitivity of all antibodies used were optimized in this laboratory. Interestingly, several commonly used “housekeeping” proteins (β-actin and β-tubulin) were upregulated in response to PUO, consistent with results seen in cardiac overload (48). Moreover, urethral obstruction has been shown to increase levels of β-actin in the rabbit (28) and GAPDH mRNA levels in the rat (5, 41). Therefore, we utilized α-tubulin expression for normalization of protein expression. Chemiluminescence detection was performed using Supersignal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL) or Western blotting Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA). Images were obtained using a Fujifilm LAS3000 Imager. α-Tubulin was used as a housekeeping gene, with α-tubulin and goat anti-mouse-horseradish peroxidase antibodies obtained from Novus Biologicals (Littleton, CO) and Pierce Biotechnology, (Rockford, IL), respectively. All other primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), unless otherwise noted, and expression levels were normalized to α-tubulin band densities.
Table 1.
List of antibodies used for Western blot studies
| Target | Catalog No. | Dilution | Temperature, °C | Time, h |
|---|---|---|---|---|
| CPI-17 | sc-17561 | 1:350 | 4 | O/N |
| L-type C1a | sc-16230 | 1:400 | 4 | O/N |
| RhoA | sc-418 | 1:400 | RT | 1.5 |
| Rock 1 | sc-17794 | 1:150 | 4 | O/N |
| Rock 2 | sc-1851 | 1:200 | 4 | O/N |
| SERCA2 | sc-8094 | 1:400 | 4 | O/N |
| α-Tubulin | MAB1637 | 1:1,000 | 4 | O/N |
All antibodies were purchased from Santa Cruz Biotechnologies, except for α-tubulin (Chemicon). CPI-17, PKC-potentiated phosphatase inhibitor of 17 kDa; Rock, Rho kinase; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase 2; RT, room temperature; O/N, overnight.
Statistical Analysis
For analysis of the pharmacological and cystometric parameters, statistical evaluations were performed using GraphPad Prism software. One-way ANOVA with Newman-Keuls posttesting were performed on pharmacological analyses and cytometric parameters. For the molecular studies, both parametric and nonparametric analyses were used. More specifically, for the RT-PCR studies at 2 wk, we compared the three groups (nonhyperactive, hyperactive, and control) for each of the nine outcome measures individually, using SAS 9.2 software. For each outcome we first fit a 1-way ANOVA to determine whether there was evidence of a difference in mean values among the three groups. If the overall ANOVA model indicated that there was a significant difference among the groups, then we would examine the pairwise comparisons between each of the three groups. Using this hierarchical approach, we did not perform any pairwise comparisons, unless the overall ANOVA model was first significant. In addition, close examination of the three groups revealed a consistent pattern where the nonhyperactive group mean was always lower than the other two group means. In addition, for seven of the nine molecular targets of interest, the hyperactive mean was the highest among the three groups. To determine whether this ordering was statistically significant, we fit a model that examined whether, across all nine outcomes, there was a consistent pattern of outcomes based on the mean values measured. To do this, we ranked for each outcome the mean value as 1, 2, or 3 (1 = lowest mean and 3 = highest mean). We then tested the hypothesis of whether the ranking across the nine outcomes was significantly different among the three groups (control, hyperactive, and nonhyperactive) using a Kruskal-Wallis test (a nonparametric approach to fitting a one-way ANOVA using ranked data). Using this approach, a 2 degree of freedom χ2 statistic was estimated to determine statistical significance. Next, comparisons of Western blots were made using ANOVA models. First, a two-way (time and group) ANOVA model was fit to determine whether the difference between groups was consistent across time. If it was found to be consistent (i.e., no time-by-group interaction in the two-way model), then the groups were compared adjusting for time. However, if the time-by-group interaction was found to be significant, then the models were refit stratifying by time. In all cases, P values < 0.05 were considered significant. Unless otherwise stated, results are presented as the arithmetic mean ± SE.
RESULTS
A total of 100 animals underwent PUO, and 9 animals died within the first week postoperatively due to urinary retention. All surviving PUO animals underwent catheterization at either 2 or 6 wk after the surgery, as well as 10 age-matched controls at each time point. No mortalities were seen due to the second survival surgery. The bladder drastically remodeled in response to the obstruction, as indicated by the fact that the bladder weights more than doubled by 2 wk (363.43 ± 16.48 mg) and 6 wk (369.86 ± 40.45mg) after obstruction compared with controls (155.97 ± 6.81mg) (Fig. 1).
Fig. 1.
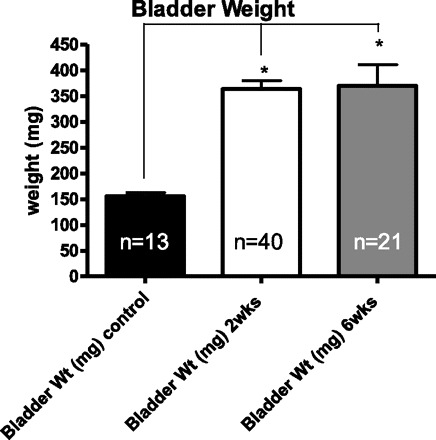
Comparison of bladders revealed obstruction caused a twofold increase in the weight of the bladder upon euthanasia. Values are means ± SE; n, no. of rats. *P < 0.0001.
Cystometric Analysis
Stable, analyzable voiding patterns were obtained in the vast majority of rats (40/46, ∼87%) at the 2-wk time point, but in a smaller proportion of animals at the 6-wk time point (21/45, ∼47%). Of note, the failure to detect definable and reproducible micturition events was coupled with the appearance of dribbling incontinence (see Fig. 2 for representative example), which is indicative of urinary retention and impending end-organ failure (i.e., completely decompensated). These animals were not included in our analysis. The remaining animals were characterized as either displaying DO (hyperactive) or not (non-DO; nonhyperactive), based on the presence of NVCs in the cystometric records, again, as illustrated by the representative examples in Fig. 2. A similar percentage of animals displayed DO at the 2- (38%) and 6-wk (43%) time points, and mean values for all cystometric parameters in the three groups are summarized in Table 2. Since age-matched control animals showed no detectable cystometric differences between the two time points, they were considered a homogeneous population and pooled into a single group for purposes of statistical analysis. Although the observed increase in bladder mass was not associated with a corresponding increase in Bcap, by 6 wk post-PUO, there was a significant decrease in MV, and a subsequent increase in RV (0.70 ± 0.30 ml), compared with age-matched control animals (0.03 ± 0.01 ml), but only in the DO animals. An apparent increase in outlet resistance was also observed, as judged by the fact that all pressures measured (BP, TP, MP, IMP) were increased 2 wk post-PUO in the DO animals. However, 6-wk post-PUO, only the mean maximum pressures generated in overactive animals were different from controls. No significant differences were detected in Bcom at either time point, and at no time were there differences in non-DO animals compared with controls.
Fig. 2.
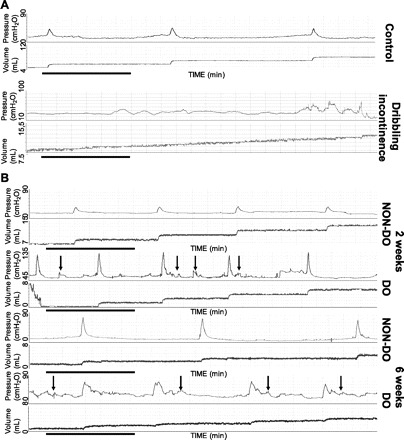
A: representative cystometric tracings for control rats and rats displaying dribbling incontinence after obstruction. Clear micturition events with measurable voided efflux are seen in the control animals, while a gradual increase in volume with no micturition-related pressure increases are seen with dribbling incontinence. Horizontal black bars represent 5 min of data collection. The pressure scale is elevated in 2-wk detrusor overactivity (DO) animals because of higher baseline pressures; however, each scale spans 90 cmH2O, and volume spans 8 ml. B: representative analyzable cystometric tracings for rats after obstruction. Arrows indicate nonvoiding contractions in overactive (DO) rats at both 2 and 6 wk post-partial urethral obstruction (PUO), which are absent in non-DO animals. Black horizontal bars represent 5 min of data collection. The pressure scale is elevated in 2-wk DO animals because of higher baseline pressures; however, each scale spans 90 cmH2O, and volume spans 8 ml.
Table 2.
Urodynamic parameters as determined by in vivo cystometry
| n | Bcap, ml | MV, ml | RV, ml | BP, cmH2O | TP, cmH2O | MP, cmH2O | IMP, cmH2O | Bcom, ml/cmH2O | |
|---|---|---|---|---|---|---|---|---|---|
| Controls | 13 | 1.38 ± 0.12 | 1.38 ± 0.13 | 0.03 ± 0.01 | 8.30 ± 0.61 | 21.26 ± 1.70 | 40.46 ± 2.37 | 12.92 ± 0.79 | 0.12 ± 0.01 |
| 2 wk DO | 15 | 1.32 ± 0.14 | 1.13 ± 0.16 | 0.21 ± 0.08 | 25.60 ± 4.06† | 51.71 ± 6.85† | 100.20 ± 8.78† | 35.35 ± 5.22† | 0.08 ± 0.02 |
| 2 wk non-DO | 25 | 1.23 ± 0.11 | 1.03 ± 0.10 | 0.22 ± 0.06 | 15.22 ± 1.60 | 31.09 ± 3.17 | 51.28 ± 4.24 | 19.54 ± 1.96 | 0.10 ± 0.02 |
| 6 wk DO | 8 | 1.39 ± 0.30 | 0.69 ± 0.11* | 0.7 ± 0.30† | 14.12 ± 1.52 | 28.00 ± 2.59 | 69.31 ± 3.71* | 21.46 ± 1.14 | 0.18 ± 0.09 |
| 6 wk non-DO | 13 | 1.08 ± 0.11 | 0.98 ± 0.14 | 0.18 ± 0.05 | 15.37 ± 1.45 | 23.60 ± 2.00 | 57.65 ± 3.53 | 18.78 ± 1.56 | 0.15 ± 0.02 |
Values are means ± SE; n, no. of rats. Age-matched controls revealed no differences and were subsequently grouped together as controls. DO, detrusor overactivity; Bcap, bladder capacity; MV, micturition volume; RV, residual volume; BP, basal pressure; TP, threshold pressure; MP, micturition pressure; IMP, intermicturition pressure; Bcom, bladder compliance.
Significance compared with † 6 wk and * controls: P < 0.05.
Pharmacological Studies
For the pharmacological assessment of the retrieved bladder tissues, cumulative dose-response curves were generated from individual bladder smooth muscle strips harvested from control animals (n = 17 strips; 9 animals), as well as animals at 2 (n = 29 strips, 18 animals) and 6 wk (n = 11 strips, 8 animals) post-PUO. Mean log (EC50) values were 5.62 ± 0.08, 5.55 ± 0.06, and 5.59 ± 0.11 for age-matched controls, 2-wk PUO, and 6-wk PUO, respectively. Mean slope factor values were 1.69 ± 0.19, 1.57 ± 0.11, and 1.69 ± 0.18 for age-matched controls, 2-wk PUO, and 6-wk PUO, respectively. As illustrated in Fig. 3A, there was a statistically significant increase in the calculated maximal steady-state contractile response to carbachol at 2 wk post-PUO, which returned to control values at the 6-wk time point. No detectable differences were observed among the three groups for either the EC50 or slope factor values. Figure 3B illustrates that, while both DO and non-DO animals had higher maximal steady-state contractile values 2 wk post-PUO, only the DO animals showed statistically higher values.
Fig. 3.
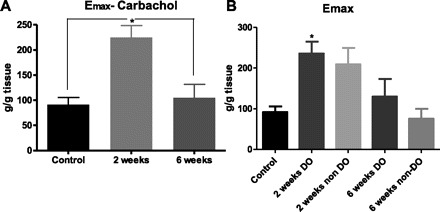
Calculated maximal steady-state contractile (Emax) values for bladder smooth muscle strips in response to the muscarinic agonist carbachol. A: significant increases in maximal steady-state contraction were observed 2 wk postobstruction (*P < 0.0001). B: when DO and non-DO animals were compared as different groups, analysis reveals that the increase in Emax is only statistically different in the DO animals 2 wk postobstruction (*P < 0.01). Values are means ± SE.
Molecular Studies
Real-Time PCR techniques, directed toward known regulators of detrusor smooth muscle calcium signaling/contraction, were utilized to identify alterations in relevant molecular targets that might be associated with the progression of PUO-related bladder dysfunction in vivo. These initial studies were performed only at the 2-wk time point and conducted on bladders harvested from a subpopulation of animals in the absence (n = 7) or presence (n = 10) of NVCs (i.e., DO; see Fig. 2 for details). Animals were classified as displaying DO (n = 10) or not (non-DO; n = 7) based on the appearance of NVCs during cystometry (see above and Fig. 2). As displayed in Fig. 4, statistical analysis of mean expression levels determined by RT-PCR revealed significantly increased mRNA expression in two out of nine transcripts studied (L-type Ca2+ channel α1c; P = 0.035, CPI-17; P = 0.038), with one additional transcript showing marginally elevated levels (SERCA; P = 0.0675) in rats that displayed DO, relative to control and non-DO rats. However, closer inspection of the three groups revealed a consistent pattern where the non-DO group mean was always lower than the other two group means. In addition, for seven out of the nine molecular targets of interest, the hyperactive mean (i.e., DO) was the highest among the three groups. To this end, we conducted nonparametric studies of this dataset (see materials and methods) and performed a Kruskal-Wallis test using ranked data. This test examined whether there was a consistent ordering of the observed means across the nine molecular targets. The χ2 statistic estimated from this test was 21.5 (2 degrees of freedom) with a P < 0.0001, indicating that this ordering of mean values was highly significant and consistent across the nine molecular targets. Western blots were also performed for targets of interest (CPI-17, L-type Ca2+ channel α1c, Rock 1, Rock 2, SERCA2, and Rho A), representative images are shown, with mean values and statistical conclusions displayed, for 2 and 6 wk (Figs. 5–8). Two weeks post-PUO, bladder tissue excised from non-DO PUO (n = 4) animals had significantly lower expression than control animals of every target except CPI-17 (Fig. 6). Additionally, SERCA2 expression 2 wk post-PUO was lower in the non-DO animals compared with the DO (n = 3) animals. At 6 wk post-PUO, CPI-17 protein expression was significantly lower in the non-DO PUO (n = 3) animals compared with controls (Fig. 8). Rock 1 expression was greater in DO (n = 3) animals compared with controls, and, furthermore, Rock 2 expression was higher in both non-DO and DO animals compared with controls. Similar to the findings with mRNA expression levels, nonparametric statistics also revealed an obvious trend toward lower protein expression at the 2-wk time point. Specifically, the Kruskal Wallis test indicated that there was a significant difference among the groups in terms of the rankings across the six measures. Here we found that the controls consistently ranked higher than the DO animals, which were consistently higher than the non-DO animals across all measures (χ2 statistic = 11.8, 2 degrees of freedom P = 0.0027). At 6 wk, the three groups were still found to be significantly different based on the Kruskal Wallis test (χ2 = 6.1, P = 0.046); however, the trend was not consistent, as had been observed at 2 wk, with the DO animals having the highest ranking across the six measures, followed by the control animals and then the non-DO animals, respectively. When we examined the three rankings, we found that the non-DO and control animals had closer values, whereas the DO animals seemed to have the largest values across the six measures.
Fig. 4.
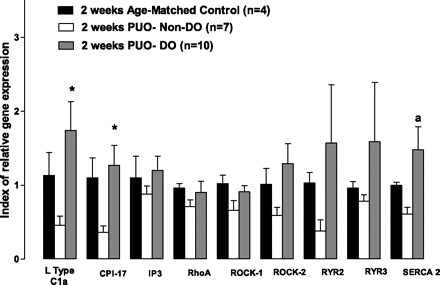
mRNA expression of calcium handling genes normalized to 18s RNA expression from the smooth muscle layer of control rats and rats 2-wk postobstruction. While all nine targets showed decreased expression in nonhyperactive animals 2 wk postsurgery, four targets were expressed more in animals displaying hyperactivity. *P < 0.05. Two additional targets exhibited a trend for increased expression in this group (a P = 0.067). All alpha values were determined via one-way ANOVA. Values are means ± SE; n, no. of rats. CPI-17, PKC-potentiated phosphatase inhibitor of 17 kDa; IP3, inositol 1,4,5-trisphosphate; Rock, Rho kinase; RYR, ryanodine receptor; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase 2.
Fig. 5.
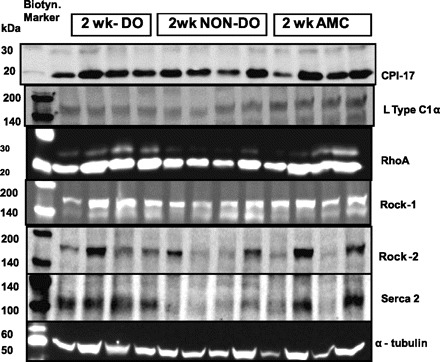
Representative Western blotting bands 2 wk postobstruction. Band shows up at the following molecular masses: CPI-17: 17 kDa; L-type Ca: 164 kDa; RhoA: 24 kDa; Rock 1: 160 kDa; Rock 2: 150 kDa; SERCA2: 100 kDa. AMC, age-matched control.
Fig. 8.
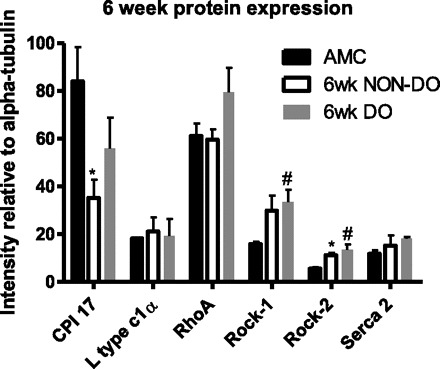
Protein expression determined by quantitative Western blotting, 6 wk after PUO, normalized to expression of α-tubulin. Values are means ± SE; n = 3 in all groups. *Non-DO group significantly different from sham controls;#DO group significantly different from sham controls (P < 0.05).
Fig. 6.
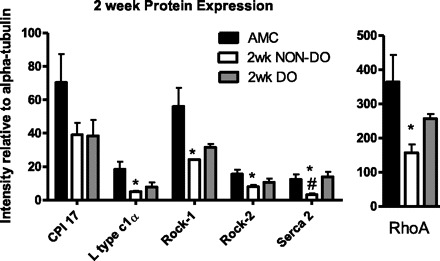
Protein expression determined by quantitative Western blotting, 2 wk after PUO, normalized to expression of α-tubulin. Values are means ± SE; n = 4 sham and non-DO groups, n = 3 in DO group. *Non-DO group significantly less than sham controls;#non-DO group significantly less than DO group (P < 0.05).
Fig. 7.
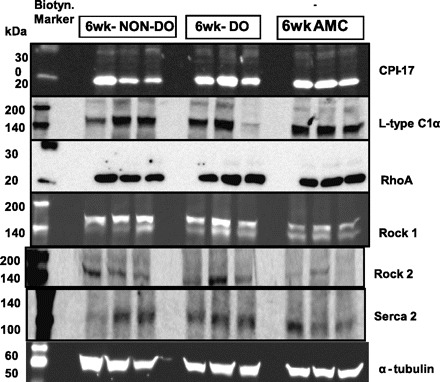
Representative Western blotting bands 6 wk postobstruction. Band show up at the following molecular masses: CPI-17: 17 kDa; L-Type Ca: 164 kDa; RhoA: 24 kDa; Rock 1: 160 kDa; Rock 2: 150 kDa; SERCA2: 100 kDa.
Functional and Molecular Correlation Analysis
Regression analysis was used to further evaluate the potential importance of the selected molecular markers to in vivo function of the bladder. Although there was no correlation between mRNA expression for any of the targets of interest and cystometric parameters on non-DO PUO rats or age-matched control rats, three of the targets were significantly correlated with pressure responses measured on DO PUO rats, and these are summarized in Fig. 9. For example, both IP3 and RhoA mRNA levels were positively correlated with not only resting BPs of the bladder, but also IMP. Additionally, TP was positively correlated with Rock 1 (P < 0.05 in all cases) (Fig. 9).
Fig. 9.
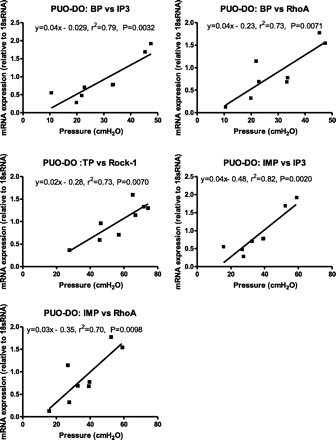
Correlation of mRNA expression to pressure parameters obtained via urodynamics 2 wk postobstruction. Nonlinear regression reveals a significant relationship of threshold pressure (TP) to Rock 1, and basal pressures (BP) and intermicurition pressures (IMP) correlate with both RhoA and IP3. P < 0.05 in all cases.
The smaller cohort studied for the Western blot analysis precluded separate analyses of the DO PUO and non-DO PUO groups at both time points. However, consistent with the nonparametric analyses, the protein expression values for the two groups were clearly differentially clustered at the 2-wk time point (Fig. 10). Specifically, Rock 1 was significantly correlated with both spontaneous activity (SA) and MP, while SERCA 2 was also positively correlated with MP. No other significant correlations were detected, and, once again, there were no correlations observed in bladders retrieved from age-matched control animals.
Fig. 10.
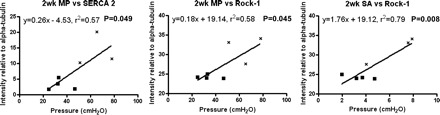
Correlation of protein expression to pressure parameters obtained via urodynamics. Nonlinear regression reveals a significant relationship of (MP) to SERCA2 and Rock-1, as well as (SA) to Rock-1. X, hyperactive animals; ■, nonhyperactive animals. MP, micturition pressure; SA, spontaneous activity. P < 0.05 in all cases.
DISCUSSION
In humans, BPO, with or without LUTS, is a common disease in aging males and may lead to compensatory hypertrophy of the bladder. PUO in animal models has been developed as a surrogate with the hope of studying factors that may be relevant for the human condition. In this study, we have created PUO in male rats and examined early (2 wk) and later (6 wk) time points to analyze the progression of bladder remodeling and to reveal potential biomarkers at an early stage, where intervention is still possible. Obstructed bladders showed just over a doubling in mass compared with controls, which is less than previous reports showing anywhere from a three- to sevenfold increase in bladder weights (4, 20, 22, 29, 34, 45). Assuming a relationship exists between the increase in bladder weight and the degree of obstruction, this indicates that we have created a mild obstruction, which may be more representative of the human condition. Additionally, there was no further increase in bladder weight from 2 to 6 wk, suggesting stability of the ensuing detrusor hypertrophy per se. Importantly, we have also shown that PUO rats displayed signs similar to those found in the clinical situation in terms of increased pressure generation at earlier time points (see Fig. 2; Table 2), relative to age-matched control animals, in the absence of increased RV (i.e., consistent with physiological compensation at 2 wk post-PUO), whereas only MP is different from controls at 6 wk post-PUO in DO animals, and, moreover, RV is significantly increased, consistent with ensuing decompensation. We define decompensation as a reduced ability to empty the bladder (i.e., the 6-wk DO animals with increased RV), and complete decompensation/end organ failure as the absence of a definable micturition event [i.e., animals at 2 and 6 wk post-PUO that display dribbling incontinence (see Fig. 2)]. More specifically, in this scenario, increased RV would be the starting point of decompensation, while dribbling incontinence would be the endpoint, that is, indicative of impending end organ failure. Consistent with this definition, urodynamic records from animals that displayed completely decompensated bladders at 6 wk showed a lack of micturition events, coupled with dribbling incontinence (again, see Fig. 2). Urodynamic studies on the remaining DO animals at 6 wk revealed analyzable cystometric records with an increased residual urine: an indication of progressive damage to detrusor function, which will eventually end in dribbling incontinence, if left untreated.
Cystometric analysis revealed that the bladders of virtually all PUO rats were apparently compensated at 2 wk post-PUO (as judged by the lack of change in RV) and the fact that fewer than 15% of the animals displayed dribbling incontinence at this time point. However, by 6 wk post-PUO, more than one-half of the animals (24 out of 45 rats) displayed a complete lack of micturition events, coupled with dribbling incontinence during cystometry, indicative of complete decompensation, thus demonstrating clear disease progression. It is worth noting that the observed rate of decompensation was higher than in earlier reports (7, 16, 46), which may be due to several factors, including the surgical method, species, criteria for decompensation, or the experimental procedure. In the present study, we have considered decompensation to mean an inability of the bladder to empty. In this regard, we still found that ≈45% of the animals were still able to void during cystometry, and, furthermore, DO animals at this time point displayed a significantly higher RV. The presence of NVCs (increases in bladder pressure ≥ 5 cmH2O without a subsequent release of fluid), along with the enhanced RV, is also a clear sign of bladder dysfunction (i.e., decompensation). It is worth noting that non-DO animals were not different in any aspect in terms of function, and only animals displaying NVCs showed abnormal bladder function.
NVCs (i.e., DO) were a focal point of these investigations, as our overall goal was to improve insight into potential mechanisms responsible for the progression of bladder dysfunction following PUO. Frequent NVCs in this animal model were regarded as a surrogate for DO in the clinical setting and correlated with accelerated disease progression. As such, we investigated the relationship between expression of critical regulators of calcium signaling and contraction in detrusor smooth muscle and physiologically relevant indexes of bladder function as determined by urodynamics. A somewhat similar approach was used by Chang et al. (11), in which they showed PKC activity and PKC-α isoform protein expression correlated with urinary frequency. The goal of our present study was to further elucidate the relationship between mRNA and protein expression for molecular targets of interest with other specific measures of bladder function, that is, NVCs and bladder pressures generated during the micturition cycle. The scientific rationale for this approach is that such information should reveal potentially important mechanistic insights into other important aspects of BPH/LUTS. These insights, in turn, might identify novel and more efficacious therapeutics targets. In addition, the longitudinal experimental design could also lead to the identification of biomarkers of disease progression (i.e., molecular antecedents of bladder pathophysiology) during a time frame in which intervention/prophylaxis may still be achievable and productive.
To this end, molecular studies were conducted on mRNA and protein expression levels of molecular targets of interest (Table 1) in bladder tissues excised from both DO and non-DO PUO rats at the 2- and 6-wk time points. Rigorous statistical analysis of the data summarized in Figs. 4–6 support a novel hypothesis. Specifically, that 2 wk post-PUO, a relatively global downregulation in mRNA expression levels (relative to age-matched control and DO PUO rats) for molecular targets of critical importance to calcium signaling and detrusor smooth muscle contraction per se is associated with prevention of DO (i.e., NVCs). A similar conclusion is reached for protein expression levels at the 2-wk time point. Consistent with this supposition, we observed a significant positive correlation between mRNA (Fig. 9) and protein (Fig. 10) levels for several molecular targets and important measures of bladder function such as BP, TP, IMP, and SA. However, these correlations were only significant among the DO PUO rats, as no such relationships were ever detected in the non-DO PUO or age-matched control rats. Such observations are consistent with previous reports, where differences in L-type Ca2+ channel expression (4) and the Rho kinase pathway has also been implicated in response to obstruction (7, 13). Furthermore, SERCA2 is involved with repletion of calcium stores in the sarcoplasmic reticulum and has already been shown to be downregulated in obstruction-induced decompensation, with marginal increases after relief of obstruction (46). Additionally, a study by Lassmann et al. (24) used a murine model of obstruction to show that deletion of a SERCA2 allele offered protection against bladder hypertrophy. Taken together, both prior work in the field, as well as the data provided in the present report, further strengthen the argument that modulation of calcium signaling and sensitization pathways post-PUO provide an attractive therapeutic target.
One interpretation of this observation is that the sensitivity of the smooth muscle to initiation of contraction may need to be reset, in this case downregulated, to inhibit the appearance of DO. Perhaps 2 wk post-PUO, there is an increased stimulus to contract (e.g., an increased afferent sensitivity or increased release of relevant cytokines/spasmogens). In this scenario, a decrease in important modulators of the calcium signaling pathway would assist with buffering the level of contraction in the smooth muscle cell in the presence of this “heightened” contractile stimulus, and thereby preventing the appearance of DO, at least temporarily, at this time point, and in this model.
In summary, these are the first data we are aware of that indicate that putting a “break” on transcription and/or translation of key molecular targets may reflect a compensatory adaption to outlet obstruction, such that normal bladder emptying can be achieved in the absence of DO (i.e., NVCs). That is, the apparent decreased expression of these targets did not result in any decrease in bladder function and, therefore, may well be “protective”. Such findings suggest that downregulation of these targets or their relevant effectors, either at the pharmacological or molecular level, may provide an attractive strategy for abatement of DO related to outlet obstruction. Moreover, the observed downregulation may be a compensatory harbinger of future DO, thereby also providing both a biomarker of future pathology.
It is also worth noting that, in fact, the overall trends for increases in marker expression in hyperactive bladders at the 2-wk time point may be masked/understated due to a potentially heterogeneous response to PUO of different sites in the bladder wall. For example, SA associated with Ca2+ transients is a localized phenomenon, and the organization of these transients (and the subsequent pressure increases) varies with age and distension (21). Furthermore, there are areas of the bladder wall that are more susceptible to nerve damage (i.e., patchy denervation) in overactive bladders from both mice (23, 35) and humans (17, 31). In this scenario, localized contractions have been suggested to lead to afferent nerve activation and autonomous contractile activity (1, 19). Moreover, PUO in rats has been used to show that micromotions during the filling phase are more organized and can lead to transiently increased intravesical pressures (18). DO due to these micromotions may be associated with heterogenous expression of calcium sensitization/mobilization proteins, which raises the possibility that localized changes in gene expression are not reflected when performing biochemical analyses on the whole bladder.
In stark contrast to our observations at the 2-wk time point, at 6 wk, in the presence of overt signs of bladder decompensation in the DO-PUO animals, no clear or consistent alterations in the expression of these same targets were observed at the protein level. Presumably, this is a reflection of the fact that the advent of bladder decompensation can be associated with a plethora of molecular and physiological derangements, obfuscating attempts to obtain clear mechanistic insights into pathophysiological changes, and further emphasizing the importance of investigating molecular events/alterations that may modulate the compensatory phase of bladder hypertrophy.
In conclusion, to our knowledge, this is the first study that suggests that the overall molecular profile of the calcium signaling and sensitization pathways that regulate contraction of detrusor smooth muscle may be differentially altered, depending on the duration of obstruction and, therefore, the corresponding degree of bladder compensation or dysfunction. Taken together, these data confirm and extend prior work in the field to suggest that molecular profiling of key regulatory molecules during the progression of PUO-mediated bladder hypertrophy and dysfunction may shed new light on potential biomarkers of, and/or therapeutic targets for, PUO-related bladder dysfunction. As such, this approach to the study of obstruction-related bladder hypertrophy and ensuing dysfunction has potentially exciting implications for the development of novel and more efficacious diagnostics and therapeutics.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.B. and T.A. performed experiments; D.B. and R.D.J. analyzed data; D.B., T.A., K.-E.A., and G.J.C. interpreted results of experiments; D.B. and T.A. prepared figures; D.B. and G.J.C. drafted manuscript; D.B., R.D.J., K.-E.A., and G.J.C. edited and revised manuscript; D.B., T.A., R.D.J., K.-E.A., and G.J.C. approved final version of manuscript; T.A., K.-E.A., and G.J.C. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Bimjhana Bishworkarma, Yagna Jarajapu, and Chanda Turner for the technical assistance.
REFERENCES
- 1.Andersson KE. Muscarinic acetylcholine receptors in the urinary tract. Handb Exp Pharmacol 202: 319–344, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62: 3–10, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE, Soler R, Fullhase C. Rodent models for urodynamic investigation. Neurourol Urodyn 30: 636–646, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Arner A, Sjuve Scott R, Haase H, Morano I, Uvelius B. Intracellular calcium in hypertrophic smooth muscle from rat urinary bladder. Scand J Urol Nephrol 41: 270–277, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aydin M, Downing K, Villegas G, Zhang X, Chua R, Melman A, Disanto ME. The sphingosine-1-phosphate pathway is upregulated in response to partial urethral obstruction in male rats and activates RhoA/Rho-kinase signalling. BJU Int 106: 562–571, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Beamon CR, Mazar C, Salkini MW, Phull HS, Comiter CV. The effect of sildenafil citrate on bladder outlet obstruction: a mouse model. BJU Int 104: 252–256, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, Wein AJ, Chacko S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol 285: F990–F997, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Boczko J, Tar M, Melman A, Jelicks LA, Wittner M, Factor SM, Zhao D, Hafron J, Weiss LM, Tanowitz HB, Christ GJ. Trypanosoma cruzi infection induced changes in the innervation, structure and function of the murine bladder. J Urol 173: 1784–1788, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chacko S, DiSanto M, Wang Z, Zderic SA, Wein AJ. Contractile protein changes in urinary bladder smooth muscle during obstruction-induced hypertrophy. Scand J Urol Nephrol Suppl 184: 67–76, 1997 [PubMed] [Google Scholar]
- 10.Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang S, Hypolite JA, Mohanan S, Zderic SA, Wein AJ, Chacko S. Alteration of the PKC-mediated signaling pathway for smooth muscle contraction in obstruction-induced hypertrophy of the urinary bladder. Lab Invest 89: 823–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapple CR. Alpha adrenoceptor antagonists in the year 2000: is there anything new? Curr Opin Urol 11: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Christ GJ, Andersson KE. Rho-kinase and effects of Rho-kinase inhibition on the lower urinary tract. Neurourol Urodyn 26: 948–954, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, Melman A. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281: R1699–R1709, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Christ GJ, Venkateswarlu K, Day NS, Valcic M, Santizo C, Zhao W, Wang HZ, Persson K, Andersson KE. Intercellular communication and bladder function. Adv Exp Med Biol 539: 239–254, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Clement MR, Delaney DP, Austin JC, Sliwoski J, Hii GC, Canning DA, DiSanto ME, Chacko SK, Zderic SA. Activation of the calcineurin pathway is associated with detrusor decompensation: a potential therapeutic target. J Urol 176: 1225–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Drake MJ, Gardner BP, Brading AF. Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU Int 91: 702–710, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JI. Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol 170: 276–279, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93: 478–483, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka O, Persson K, Mattiasson A, Naylor A, Wyllie M, Andersson K. Micturition in conscious rats with and without bladder outlet obstruction: role of spinal alpha 1-adrenoceptors. Br J Pharmacol 117: 962–966, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H, Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder. Am J Physiol Regul Integr Comp Physiol 298: R1310–R1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int 97: 1332–1337, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lassmann J, Sliwoski J, Chang A, Canning DA, Zderic SA. Deletion of one SERCA2 allele confers protection against bladder wall hypertrophy in a murine model of partial bladder outlet obstruction. Am J Physiol Regul Integr Comp Physiol 294: R58–R65, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int 100: 820–825, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lluel P, Duquenne C, Martin D. Experimental bladder instability following bladder outlet obstruction in the female rat. J Urol 160: 2253–2257, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Malmgren A, Sjogren C, Uvelius B, Mattiasson A, Andersson KE, Andersson PO. Cystometrical evaluation of bladder instability in rats with infravesical outflow obstruction. J Urol 137: 1291–1294, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Mannikarottu AS, Disanto ME, Zderic SA, Wein AJ, Chacko S. Altered expression of thin filament-associated proteins in hypertrophied urinary bladder smooth muscle. Neurourol Urodyn 25: 78–88, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mattiasson A, Uvelius B. Changes in contractile properties in hypertrophic rat urinary bladder. J Urol 128: 1340–1342, 1982 [DOI] [PubMed] [Google Scholar]
- 30.Melman A, Tar M, Boczko J, Christ G, Leung AC, Zhao W, Russell RG. Evaluation of two techniques of partial urethral obstruction in the male rat model of bladder outlet obstruction. Urology 66: 1127–1133, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Mills IW, Greenland JE, McMurray G, McCoy R, Ho KM, Noble JG, Brading AF. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J Urol 163: 646–651, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Moore JA, Brading AF. A porcine model of bladder outlet obstruction incorporating radio-telemetered cystometry. BJU Int 100: 1192–1193, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Nielsen KK. Changes in morphology, function and blood flow in mini-pig urinary bladder after chronic outflow obstruction and recovery from obstruction. Scand J Urol Nephrol Suppl 195: 1–39, 1997 [PubMed] [Google Scholar]
- 34.Oka M, Fukui T, Ueda M, Tagaya M, Oyama T, Tanaka M. Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol 182: 382–390, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Pandita RK, Fujiwara M, Alm P, Andersson KE. Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J Urol 164: 1385–1389, 2000 [PubMed] [Google Scholar]
- 36.Park MG, Park HS, Lee JG, Kim HJ. Changes in awake cystometry and expression of bladder beta-adrenoceptors after partial bladder outlet obstruction in male rats. Int Neurourol J 14: 157–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons BA, Drake MJ. Animal models in overactive bladder research. Handb Exp Pharmacol 202: 15–43, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 59: 877–883, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Polyak E, Boopathi E, Mohanan S, Deng M, Zderic SA, Wein AJ, Chacko S. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J Urol 182: 2497–2503, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am 95: 87–100, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Sawada N, Yao J, Hiramatsu N, Hayakawa K, Araki I, Takeda M, Kitamura M. Involvement of hypoxia-triggered endoplasmic reticulum stress in outlet obstruction-induced apoptosis in the urinary bladder. Lab Invest 88: 553–563, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Schroder A, Tajimi M, Matsumoto H, Schroder C, Brands M, Andersson KE. Protective effect of an oral endothelin converting enzyme inhibitor on rat detrusor function after outlet obstruction. J Urol 172: 1171–1174, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schroder A, Uvelius B, Newgreen D, Andersson KE. Bladder overactivity in mice after 1 week of outlet obstruction. Mainly afferent dysfunction? J Urol 170: 1017–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Scott RS, Uvelius B, Arner A. Changes in intracellular calcium concentration and P2X1 receptor expression in hypertrophic rat urinary bladder smooth muscle. Neurourol Urodyn 23: 361–366, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Shakirova Y, Sward K, Uvelius B, Ekman M. Biochemical and functional correlates of an increased membrane density of caveolae in hypertrophic rat urinary bladder. Eur J Pharmacol 649: 362–368, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Stein R, Gong C, Hutcheson JC, Canning DA, Zderic SA. The decompensated detrusor III: impact of bladder outlet obstruction on sarcoplasmic endoplasmic reticulum protein and gene expression. J Urol 164: 1026–1030, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Stein R, Hutcheson JC, Krasnopolsky L, Canning DA, Carr MC, Zderic SA. The decompensated detrusor V: molecular correlates of bladder function after reversal of experimental outlet obstruction. J Urol 166: 651–657, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Tian B, Carlyle WC, Weigold WG, McDonald KM, Judd DL, Toher CA, Homans DC, Cohn JN. Localization of changes in beta-actin expression in remodeled canine myocardium. J Mol Cell Cardiol 31: 751–760, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Uvelius B, Arner A. Changed metabolism of detrusor muscle cells from obstructed rat urinary bladder. Scand J Urol Nephrol Suppl 184: 59–65, 1997 [PubMed] [Google Scholar]
- 50.Wang ZE, Gopalakurup SK, Levin RM, Chacko S. Expression of smooth muscle myosin isoforms in urinary bladder smooth muscle during hypertrophy and regression. Lab Invest 73: 244–251, 1995 [PubMed] [Google Scholar]
- 51.Woo LL, Tanaka ST, Anumanthan G, Pope JCt Thomas JC, Adams MC, Brock JW, 3rd, Bhowmick NA. Mesenchymal stem cell recruitment and improved bladder function after bladder outlet obstruction: preliminary data. J Urol 185: 1132–1138, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Woodman SE, Cheung MW, Tarr M, North AC, Schubert W, Lagaud G, Marks CB, Russell RG, Hassan GS, Factor SM, Christ GJ, Lisanti MP. Urogenital alterations in aged male caveolin-1 knockout mice. J Urol 171: 950–957, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Zhang EY, Stein R, Chang S, Zheng Y, Zderic SA, Wein AJ, Chacko S. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of non-muscle caldesmon. Am J Pathol 164: 601–612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Seftel A, DiSanto ME. Blebbistain, a myosin II inhibitor, as a novel strategy to regulate detrusor contractility in a rat model of partial bladder outlet obstruction. PLos One 6: e25958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zotova EG, Christ GJ, Zhao W, Tar M, Kuppam SD, Arezzo JC. Effects of fidarestat, an aldose reductase inhibitor, on nerve conduction velocity and bladder function in streptozotocin-treated female rats. J Diabetes Complications 21: 187–195, 2007 [DOI] [PubMed] [Google Scholar]


