Abstract
A growing number of studies support an important contribution of astrocytes to neurovascular coupling, i.e., the phenomenon by which variations in neuronal activity trigger localized changes in blood flow that serve to match the metabolic demands of neurons. However, since both constriction and dilations have been observed in brain parenchymal arterioles upon astrocyte stimulation, the specific influences of these cells on the vasculature remain unclear. Using acute brain slices, we present evidence showing that the specific degree of constriction of rat cortical arterioles (vascular tone) is a key determinant of the magnitude and polarity of the diameter changes elicited by signals associated with neurovascular coupling. Thus elevation of extracellular K+ concentration, stimulation of metabotropic glutamate receptors (mGluR), or 11,12-epoxyeicosatrienoic acid application all elicited vascular responses that were affected by the particular resting arteriolar tone. Interestingly, the data suggest that the extent and/or polarity of the vascular responses are influenced by a delimited set point centered between 30 and 40% tone. In addition, we report that distinct, tone-dependent effects on arteriolar diameter occur upon stimulation of mGluR during inhibition of enzymes of the arachidonic acid pathway [i.e., phospholipase A2, cytochrome P-450 (CYP) ω-hydroxylase, CYP epoxygenase, and cycloxygenase-1]. Our findings may reconcile previous evidence in which direct astrocytic stimulation elicited either vasoconstrictions or vasodilations and also suggest the novel concept that, in addition to participating in functional hyperemia, astrocyte-derived signals play a role in adjusting vascular tone to a range where dilator responses are optimal.
Keywords: potassium, glutamate, epoxyeicosatrienoic acid, glia, neurovascular coupling
although much attention has been given to the characterization of the signals that mediate “functional hyperemia,” the rapid and localized increase in blood flow that occurs after neuronal activation (41, 53), a prominent gap remains in our understanding of the vascular factors that influence the overall neurovascular response. Because vasoactive stimuli typically affect changes in two interdependent physiological parameters of vascular smooth muscle cells (VSMC), namely membrane potential (Vm) and intracellular Ca2+ concentration ([Ca2+]i), it is conceivable that the responsiveness of the vessels when presented to such stimuli is in turn affected by the resting arteriolar tone, defined by the particular status of Vm and [Ca2+]i in VSMC. Previous work in pial arterioles showed indeed that vascular tone critically influences the type of response evoked by several neurogenic stimuli (3, 52). In contrast, the influence of vascular tone in the responses of brain intracortical arterioles to signals associated with glial activation has not been evaluated.
Astrocytes establish intimate contacts with both neurons and vessels and are thus uniquely positioned to function as bridges between these cell types, contributing to neurovascular coupling (NVC) and functional hyperemia (24). A turning point in our understanding of the role of astrocytes in mediating NVC came with the observation that the dilation of cortical arterioles upon neuronal stimulation involves activation of metabotropic glutamate receptors (mGluR) leading to an increase in [Ca2+]i in astrocytes (60). More recently, rapid increases in astrocyte [Ca2+]i upon somatosensory stimulation have been shown to be temporally correlated with the onset of hemodynamic responses in the mouse cortex in vivo (59). Rises in astrocytic [Ca2+]i are known to mediate the synthesis and/or release of vasoactive agents, with vasodilator [e.g., K+, epoxyeicosatrienoic acids (EETs), and prostaglandins] or vasoconstrictor [e.g., 20-hydroxyeicosatetraenoic acid (20-HETE)] effects importantly involved in the control of the brain microcirculation (28). In addition, astrocytes may respond to signals released by GABAergic interneurons, which have also been shown to mediate constrictions (e.g., neuropeptide Y and somatostatin) or dilations [e.g., nitric oxide (NO) and vasoactive intestinal peptide] in cortical arterioles (10, 55). Noteworthy, a recent study showed that spatially defined vasodilation and vasoconstriction characterizes the vascular response to somatosensory stimulation in vivo (13). Although the involvement of astrocytes in these phenomena was not specifically addressed, the ability of glial cells to constrict and dilate blood vessels has been clearly demonstrated by studies in the rodent cortex and retina (39, 42). In the cortex, but not in the retina, evidence would suggest that basal vascular tone influences the type of response evoked by rises in glial [Ca2+]i (42). In line with previous suggestions (49), we hypothesize that, although both constricting and dilating agents can be released upon neuronal activation, the specific degree of constriction of the VSMC (vascular tone) is a key determinant of the direction and extent of the ensuing response.
We addressed this hypothesis by evaluating the influence of vascular tone in the response of brain intraparenchymal arterioles to three stimuli associated with NVC: 1) moderate elevation of extracellular K+ concentration ([K+]o), a known vasodilator stimulus, 2) mGluR activation and subsequent Ca2+-dependent activation of the arachidonic acid (AA) pathway, and 3) exposure to 11,12-EET, a purported astrocyte-derived vasodilator signal. Some of these results have been presented in preliminary form (6, 7).
METHODS
Brain slice preparation.
Cortical brain slices were prepared from 3- to 10-wks-old Sprague-Dawley rats following protocols approved by the Office of Animal Care Management at the University of Cincinnati. Following anesthesia with pentobarbital, the brain was rapidly removed, and 300-μm-thick coronal slices were cut in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM) 3 KCl, 120 NaCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 10 glucose, and 0.4 l-ascorbic acid, equilibrated with 95% O2-5% CO2. Ascorbic acid was added to reduce cell swelling associated with oxidative stress (8). An aCSF with identical composition was used for bath perfusion in all experiments, except for those assessing the effects of high external K+ concentration ([K+]), in which control aCSF contained 4.2 mM KCl, and KCl replaced NaCl to increase [K+] to 10 mM. Osmolality of aCSF was ∼290 mosm/kgH2O. Following the slicing procedure, slices were kept at room temperature in aCSF equilibrated with 95% O2-5% CO2 (pH ∼7.45) until used.
Reagents.
The thromboxane A2 receptor agonist 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U-46619), prostaglandin F2α, methyl arachidonyl fluorophosphonate (MAFP), N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET-0016), N-methylsulfonyl-6-(2-propargyloxyphenyl)-hexanamide (MS-PPOH), sc-560, and ozagrel (all purchased from Cayman Chemical, Ann Arbor, MI) were prepared as stocks in dimethyl sulfoxide (DMSO) and subsequently added to the aCSF. DMSO content in the experimental solutions was ≤0.1%. Trans-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD; Tocris, Ellisville, MO) was diluted in aCSF from a 50 mM stock in equimolar NaOH. 11,12-EET was obtained from Biomol (Plymouth Meeting, PA) and added to aCSF as supplied, from 156 μM aliquots in ethanol. Endothelin-3 (American Peptide, Sunnyvale, CA) was added to aCSF from a stock in dH2O. Tetraethylammonium (TEA; Sigma, St. Louis, MO) was dissolved in aCSF.
Video microscopy.
Diameter changes in cortical arterioles (8–19 μm internal diameter) were recorded using an upright Zeiss Axioscope 2FS microscope (Carl Zeiss, Thornwood, NY) equipped with infrared Differential Interference Contrast (IR-DIC) optics, a water-immersion objective (Zeiss 63x, numerical aperture 0.9), and an EMCCD camera (iXon+885; Andor Technology, South Windsor, CT). Images were acquired at 1 frame/s, visualized, and stored using IQ software (Andor Tech). The slices were perfused with aCSF (35 ± 2°C) gassed with 95% O2-5% CO2 and were allowed to equilibrate for ≥10 min before beginning of recording. Oxygen activity, as measured in the perfusate arriving to the recording chamber, was 54.8 ± 2% (at 35°C; n = 3; ISO2 oxygen system; World Precision Instruments, Sarasota, FL). Only one arteriole per slice was recorded.
Slices were perfused with U-46619-containing aCSF to induce vasoconstriction, and test solutions were applied in the constant presence of U-46619 after a stable preconstriction was attained. In experiments where pharmacological blockers were used, brain slices were preincubated in such blockers for at least 30 min before the experiment. HET-0016, sc-560, and ozagrel (100 nM), as well as MS-PPOH (10 μM) were also perfused throughout the experiments.
The proportion of vessels that failed to constrict upon exposure to U-46619 (50–250 nM) in control conditions or after preincubation in pharmacological inhibitors was as follows: control = 22.8% (n = 127); MAFP = 20.8% (n = 24); HET-0016 = 9.1% (n = 22); MS-PPOH = 10.5% (n = 19); sc-560 = 14.3% (n = 21); ozagrel = 20% (n = 20). These data show that U-46619-induced constriction was not affected by these pharmacological treatments. Vessels that did not respond to U-46619 were discarded for analysis.
Calcium imaging.
Ca2+ imaging was performed with a confocal spinning unit (Yokogawa CSU 10) using the imaging system and microscope objective described above. Cortical slices were incubated (∼1 h) at room temperature in aCSF containing 10 μM fluo 4-AM (Invitrogen, Eugene, OR) and pluronic acid (2.5 μg/ml) in a custom chamber gassed with 95% O2-5% CO2. Fluo 4 was excited at 488 nm using a diode-pumped solid-state laser (Melles Griot, Carlsbad, CA), and fluorescence emission was collected at >495 nm. Images were acquired at 3–6 frames/s.
Data analysis.
Data from arteriolar diameter (IR-DIC) and Ca2+ imaging experiments were analyzed with custom software created by Dr. Adrian D. Bonev (Univ. of Vermont). Changes in the internal (luminal) diameter of arterioles were determined from averaged measurements taken from multiple points across the arteriolar lumen. Baseline diameter (represented as 100%) was determined during the first ∼10 min of sampling, before any experimental stimulation. During this period, arterioles appeared dilated and showed no or little oscillatory activity. All arteriolar diameter values are expressed as percent (%) relative to baseline. Vascular tone is expressed as “degree of constriction” relative to baseline. For example, for a vessel in which diameter is 60%, the corresponding tone is 40%.
Summary data are expressed as means ± SE. Differences between two means were determined using two-tailed Student's t-test. One way ANOVA followed by Dunnett's test was used for multiple group comparisons against control data. P < 0.05 was considered significant.
RESULTS
Graded vasoconstriction induced by U-46619.
The thromboxane A2 receptor agonist U-46619 has been successfully used to induce preconstriction of parenchymal arterioles in brain slices (20, 21, 36, 37). To characterize the contractile response of the vessels, various concentrations of U-46619 (50, 100, 150, and 250 nM) were tested. The resting intraluminal diameter of nonstimulated arterioles was 12.2 ± 0.4 μm (range = 8–19 μm; n = 56). U-46619 caused a dose-dependent constriction (Fig. 1; Supplemental Video 1) (Supplemental data for this article is available online at the American Journal of Physiology: Heart and Circulatory Physiology website). The onset of constriction occurred at 3.4 ± 0.3 min after U-46619 application and reached a plateau at 12.6 ± 0.7 min (pooled data for 50–250 nM U-46619) that was maintained thereafter. In the rest of the experiments presented here, distinct U-46619 concentrations (from 50 to 250 nM) were applied to induce different levels of preconstriction.
Fig. 1.
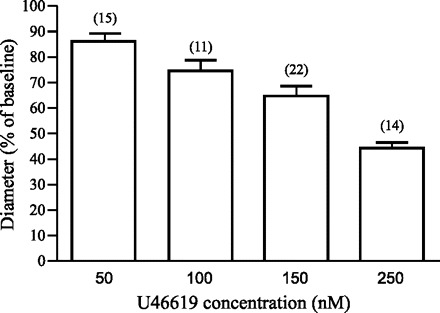
Graded vasoconstriction induced by U-46619. Summary histogram of dose-dependent vasoconstriction induced by U-46619 in parenchymal arterioles in cortical brain slices. Results represent means ± SE. Six out of 56 vessels were exposed to two U-46619 concentrations.
Increasing extracellular K+ induces tone-dependent vasodilations.
Modest elevations in [K+]o result in hyperpolarization and reduction of Ca2+ entry in VSMC and constitute a powerful vasodilator stimulus in the cerebral circulation (32, 35, 38). Recent work provided evidence that K+ signaling by astrocytes is a key mechanism in NVC (21). As a first step in addressing the influence of resting vascular tone on the response of cortical arterioles to astrocyte-derived signals, the effects of moderately elevated [K+]o were studied. To this end, we measured changes in diameter from arterioles preconstricted to various degrees using U-46619 (50–250 nM) and exposed to 10 mM K+, keeping constant the original U-46619 concentration. Raising [K+]o had a net vasodilator effect. The magnitude of the vasodilation correlated positively with the initial level of preconstriction: as vascular tone increased, proportionally larger vasodilator responses were evoked (r = 0.65). A representative trace of the observed changes is shown in Fig. 2A. In Fig. 2B the changes in diameter in response to 10 mM K+ are expressed as a function of the initial (preconstricted) diameter of the arterioles (n = 19). The onset of vasodilations occurred 0.9 ± 0.1 min following exposure to 10 mM K+, and the mean time to peak dilation was 4.5 ± 0.5 min. It is instructive to examine the tone-dependent effects of elevated [K+]o when diameters at low (control) and high [K+]o are plotted as a percent of the initial baseline for individual arterioles ordered by the level of initial preconstriction (Fig. 2C). At low preconstriction levels (>60% of baseline; n = 9), K+-induced dilation nearly restored diameter to the initial baseline dilated state, whereas for more preconstricted arterioles the final steady-state diameter, relative to baseline, was lower and more variable.
Fig. 2.
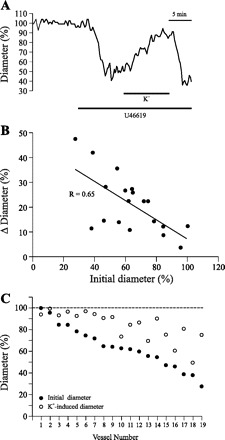
Elevation of extracellular K+ concentration ([K+]) elicits tone-dependent vasodilation. A: changes in diameter measured in an arteriole exposed to elevated extracellular [K+] ([K+]o) (10 mM). B: changes in vascular diameter plotted as a function of the initial diameter (relative to baseline) in 19 arterioles exposed to 10 mM [K+]o. C: data from B are expressed as percent diameter (relative to baseline) before and after exposure to 10 mM [K+]o, for each individual arteriole.
mGluR activation elicits tone-dependent constrictions and dilations in cortical arterioles.
In addition to K+, a number of constrictor and dilator signals can be produced and released upon increases in astrocyte [Ca2+]i following mGluR activation by glutamate released at the synapse (19). It is not clear, however, how the opposing effects of these vasoactive substances are integrated into a defined vascular response. We hypothesize that the response of brain arterioles to neuronal- and/or glial-derived signals is dictated by both the nature of the signals released and the degree of vascular tone. To assess this hypothesis, we exposed brain cortical slices to t-ACPD, a selective mGluR agonist known to elicit Ca2+ increases in astrocytes (34, 60), and measured the resulting diameter changes in arterioles presenting different levels of preconstriction. As shown in Fig. 3A, exposure to t-ACPD (100 μM; ∼10 min duration) consistently elicited constrictions in arterioles having low to moderate preconstriction (up to ∼70% of baseline), whereas dilations were observed in vessels initially showing a more pronounced tone (n = 27; see Supplemental Videos 2A and 2B). The onset of constrictions occurred at 1.9 ± 0.6 min, and peak constriction was observed at 3.9 ± 0.7 min (n = 11). Dilatory responses started 2.4 ± 0.5 min after t-ACPD application and reached a plateau at 5.5 ± 0.6 min (n = 16). Comparison of onset and peak times of constrictions vs. dilations revealed no significant differences. To further analyze these responses, data from Fig. 3A were replotted to show the t-ACPD-induced diameter (expressed as percent of maximal, baseline diameter) as a function of the initial diameter (Fig. 3B). Interestingly, the plot shows that, upon t-ACPD exposure, 16 out of 19 vessels initially displaying either low (less than ∼30%) or high (greater than ∼50%) preconstriction attained a uniform diameter (∼36.9 ± 2% constricted), which lies within the range of tone at which vascular responses reversed polarity. Two additional observations suggest that this range of tone (∼50–70% of baseline diameter) may represent a set point that defines the polarity and extent of vascular responses. First, the largest vasodilations, in absolute terms (i.e., those that were closer to reaching baseline diameter), were observed in arterioles with initial, resting diameters situated at this putative set point range (Fig. 3B). Second, upon stimulation with t-ACPD, some of the vessels with initial diameters afar from the set point transiently reached the set-point and then showed a dilation (Fig. 3, B and C).
Fig. 3.
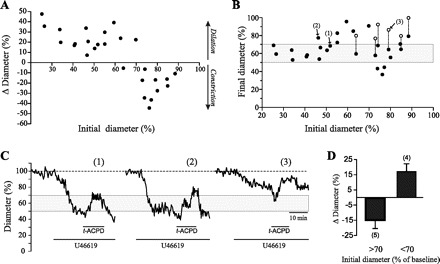
Glial activation elicits tone-dependent constrictions and dilations in brain parenchymal arterioles. A: plot of the changes in vascular diameter in response to the metabotropic glutamate receptor (mGluR) agonist trans-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) (100 μM) as a function of the initial diameter (relative to baseline) (n = 27). B: data from A are expressed to show the diameter of the arterioles after t-ACPD exposure (•) as a function of their initial diameter. Biphasic responses (○) represent secondary dilations in vessels that initially constricted in response to t-ACPD (denoted by dashed lines connecting the symbols). C: traces depicting the diameter changes measured for three arterioles as indicated by nos. in B. D: summary data of the responses elicited by t-ACPD (100 μM) on arterioles preconstricted with PGF2α (5–35 μM). Results represent means ± SE.
Because the observed responses may be conditioned by the preconstriction treatment, i.e., the specific effects of the thromboxane A2 receptor agonist U-46619 on vascular tone, we tested the effects of mGluR activation on arterioles preconstricted with a different agonist, i.e., PGF2α. As shown in Fig. 3D, tone-dependent constrictions and dilations were also observed upon exposure to t-ACPD (100 μM; ∼10 min duration) in arterioles preconstricted with PGF2α (5–35 μM). Of a total of nine arterioles recorded, four showing an initial diameter ≤70% of baseline (namely preconstricted by ≥30%) responded to t-ACPD with vasodilation. The remaining five arterioles, with resting diameters >70% of baseline, showed instead a constriction. As with U-46619, biphasic responses (constriction followed by dilation) were also observed in three out of the five vessels of the latter group. Because comparable responses were observed upon preconstriction with different agonists, these results suggest that constrictions and dilations of brain cortical arterioles in response to mGluR activation are strongly dependent on their resting tone.
AA metabolites are differentially involved in vascular responses to mGluR activation.
Following mGluR activation in astrocytes, the resulting [Ca2+]i elevation can trigger AA production, which can be further metabolized to several vasoconstrictor and vasodilator signals (33). To evaluate the participation of AA metabolites in the vasoconstrictions and vasodilations induced by mGluR activation, and the influence of vascular tone on these responses, we performed experiments similar to those described in the previous section, in the presence of selective blockers of AA production or metabolism. Control experiments in which MAFP (100 μM), HET-0016 (100 nM), MS-PPOH (20 μM), or sc-560 (100 nM) were perfused during 30 min showed that baseline diameter (measured during the first 10 min in control aCSF) was not significantly affected by these inhibitors (variation range = −2.6 to 3.5%; P = 0.106, ANOVA followed by Dunnett's test; n = 4 for each treatment). At the end of these experiments, vessel reactivity was assessed by applying endothelin-3 (100 nM), in the constant presence of the test drug. Endothelin-3 induced marked vasoconstriction (>50%) in all vessels and under all testing conditions, indicating that vascular reactivity was preserved (data not shown).
Results were compared with control data taken from Fig. 3A, and the responses were grouped into three categories encompassing discrete ranges of initial, arteriolar tone: 1) <70% of baseline, a range in which arterioles are presumably dilated beyond resting levels; 2) 70–50% of baseline, a range that corresponds to the active tone developed by pressurized intraparenchymal brain vessels and likely resembles the prevailing physiological myogenic tone (9, 12), and 3) >50% of baseline, a range that may represent constriction beyond physiological levels. First, we tested the effects of selective and irreversible inhibition of phospholipase A2 (PLA2), the enzyme that catalyzes Ca2+-dependent AA production from membrane phospholipid. A diminished sensitivity to U-46619 was apparent in vessels preincubated with MAFP (100 μM). In these experiments, the highest dose of U-46619 (250 nM) had to be applied in most cases to achieve clear preconstriction.
Preincubation of brain slices with MAFP (100 μM) significantly inhibited t-ACPD-induced constrictions and dilations (n = 19) (Fig. 4). These results are consistent with previous observations in which the absence of functional PLA2 significantly inhibited astrocyte-induced vasodilation in vivo (56), and vasoconstrictions in vitro (42), and add strong support to the view that AA, or some of its metabolites, are key determinants of the vascular response evoked by mGluR activation.
Fig. 4.
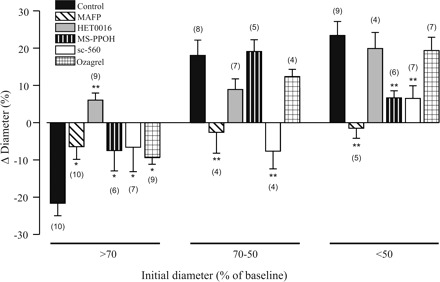
Arachidonic acid metabolites are differentially involved in the constrictions and dilations induced by mGluR activation. Summary data showing the diameter changes in cortical arterioles induced by t-ACPD (100 μM) after incubation of brain slices with pharmacological blockers of the AA pathway. Vascular responses were grouped based on the initial degree of preconstriction. Results represent means ± SE. *P < 0.05 and **P < 0.01. Dunnett's test.
Previous reports showed that inhibition of cytochrome P-450 (CYP) ω-hydroxylase, the enzyme that mediates the production of the AA-derived metabolite 20-HETE, strongly attenuated vasoconstrictions following glial activation in rodent brain cortex (42) and retina (39). To assess the involvement of 20-HETE in the vascular responses elicited by mGluR activation, brain slices were preincubated with HET-0016 (100 nM), a selective CYP ω-hydroxylase inhibitor (40). After HET-0016 treatment, all constrictions reversed to dilations (n = 9; P < 0.01), while dilations were not significantly affected in vessels with higher resting tone (Fig. 4). Thus these data support 20-HETE as a major vasoconstricting signal following mGluR activation.
A role for astrocyte-derived EETs in functional hyperemia was suggested almost ten years ago (26). Astrocytes in vitro and in situ express a CYP epoxygenase isoform (2, 48), and its activity is stimulated by glutamate (1). Experimentally, inhibition of CYP epoxygenase reduces hyperemic responses in the brain in vivo (1, 5, 47, 48) and abrogates the late, sustained phase of vasodilation induced by AMPA receptor activation in brain slices (36). To assess the involvement of EETs in t-ACPD-induced vascular responses, brain slices were preincubated with MS-PPOH (20 μM), a selective CYP epoxygenase substrate inhibitor (57). As shown in Fig. 4, MS-PPOH treatment significantly attenuated dilations in vessels with marked preconstriction (>50% of baseline) (n = 6; P < 0.01) but did not affect vascular responses in vessels initially showing intermediate levels of tone. In addition, constrictions were also significantly reduced in the group of vessels initially displaying the lowest preconstriction levels (n = 6; P < 0.05), suggesting that EETs contribute to both constrictor and dilatory responses in a manner strongly dependent on the specific arteriolar tone.
An important role for cyclooxygenase (COX) metabolites in the maintenance of resting cerebral blood flow and as mediators of the neurovascular response has been highlighted by in vivo (44, 45, 56) and in vitro (21, 60) studies. Because attenuation of COX-1, but not COX-2, effectively reduced dilator responses in vivo (56), we assessed whether COX-1 activity could influence arteriolar responses to mGluR activation. COX-1 was selectively inhibited by preincubating slices with sc-560 (100 nM). As shown in Fig. 4, COX-1 inhibition significantly reduced t-ACPD-induced constrictions and dilations. The most prominent effect, however, was observed in the group of vessels with moderate preconstriction levels (70–50% of baseline) in which vasodilations reversed into vasoconstrictions (n = 4; P < 0.01).
To evaluate whether the attenuation of vasoconstrictions observed during COX-1 blockade may represent reduced synthesis of thromboxane, t-ACPD was applied to vessels treated with ozagrel (100 nM), a thromboxane synthase inhibitor (30). As shown in Fig. 4, the extent of vasoconstriction was significantly reduced in vessels with low preconstriction (n = 9; P < 0.05).
11,12-EET induces tone-dependent arteriolar diameter changes and increases intracellular calcium in astrocytes.
Glial-derived EETs have been proposed as important mediators of NVC in the brain (26), although the underlying mechanisms are still little understood. In light of this, and to extend our findings, we explored the effects of one of the main glial CYP epoxygenase products, namely 11,12-EET (2), on arteriolar diameter as well as on [Ca2+]i dynamics in astrocytes. In a first set of experiments, cortical arterioles were preconstricted with U-46619 (50–250 nM) and exposed to 11,12-EET (100–500 nM). Notably, it was found that responses to 11,12-EET were also highly dependent on vascular tone. Thus biphasic responses (i.e., constriction followed by dilation) were observed in four out of six vessels showing relatively low preconstriction (initial diameter more than ∼70% of baseline) while, in contrast, only dilations were evoked in arterioles presenting higher resting tones (n = 10; Fig. 5, A–C). There was no relationship between the concentration of 11,12-EET applied and the type of response evoked. Note that, as it was the case for mGluR activation, the tone at which the reversal of constrictions into dilations occurred corresponded to ∼70% of baseline diameter (i.e., ∼30% constriction). This observation further supports the notion of a vascular set point that modulates the polarity of the arteriole's response.
Fig. 5.
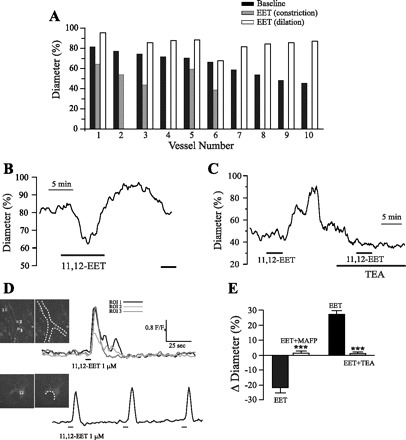
11,12-Epoxyeicosatrienoic acid (EET) induces tone-dependent constrictions and dilations in parenchymal cortical arterioles and increases astrocytic intracellular Ca2+ concentration ([Ca2+]i). A: individual responses of 10 arterioles to 11,12-EET (100–500 nM). Constrictions (gray) followed by dilations (white) were observed in vessels with baseline preconstriction (black) values less than ∼30%, whereas dilatory responses occurred in more constricted vessels. B: representative example of the biphasic response (constriction followed by dilation) elicited by 11,12-EET (500 nM) in a cortical arteriole. C: changes in luminal diameter in an arteriole exposed to 11,12-EET (200 nM) in the absence or presence of TEA (1 mM). Note that 11,12-EET-induced vasodilation is blocked by TEA. D: example of the [Ca2+]i transients elicited by focal application of 11,12-EET (1 μM) in perivascular glial endfeet. Dashed lines indicate vessels’ boundaries. ROI, region of interest. E: summary histogram of the changes in vascular diameter elicited by 11,12-EET in the absence (filled bars) or the presence of methyl arachidonyl fluorophosphonate (MAFP) (100 μM) or TEA (1 mM). MAFP data (and corresponding controls) were obtained from arterioles initially presenting a preconstriction <20% (n = 4); TEA data (and corresponding controls) were obtained from vessels initially displaying preconstriction >30% (n = 3). Results represent means ± SE. ***P < 0.001.
The question arises as to what are the mechanisms that mediate the vascular actions of 11,12-EET? EETs are known to activate large-conductance, Ca2+-dependent K+ channels (BK channels) in VSMC (23), leading to vasodilation. In addition, because some EET regioisomers have been shown to mediate increases in [Ca2+]i in vascular endothelial cells (58) and cultured astrocytes (54), it is possible that other mechanisms may also be at play. To gain insight into the mechanisms involved in 11,12-EET-induced constrictions and dilations, we investigated the effects of 11,12-EET on astrocytic [Ca2+]i. Using confocal Ca2+ imaging in brain slices loaded with the Ca2+ indicator fluo 4, we found that short application (5–30 s) of 11,12-EET (1 μM) produced rapid and robust increases in [Ca2+]i in astrocytic somata and endfeet (ΔF/Fo = 2.2 ± 0.3; n = 8 slices). A representative example of this response is shown in Fig. 5D (also see Supplemental Video 3). Thus, consistent with previous effects of 5,6-EET in cultured astrocytes (54), our data suggest that, in addition to possible paracrine effects on the vasculature, glial-derived EETs might also act in an autocrine manner, e.g., amplifying and/or sustaining glutamate-induced [Ca2+]i increases, which may in turn affect AA production as well as other Ca2+-dependent processes. To assess this possibility, brain slices were preincubated with MAFP (100 μM), and vasoconstrictor responses were evaluated in minimally constricted arterioles (<20%). As shown in Fig. 5E, MAFP treatment significantly abolished vasoconstrictor responses to 11,12-EET (300 nM) (n = 4; P < 0.001).
Finally, to test if BK channels are involved in 11,12-EET-induced vasodilations, we performed experiments in which 11,12-EET (200 nM) was applied in the presence of the BK channel inhibitor TEA (1 mM). In support of BK channel involvement, vasodilation was abrogated in the presence of TEA (Fig. 5, C and E; P < 0.01; n = 3). Further studies are needed to assess whether 11,12-EET-induced dilator effects involved BK channels expressed on VSMC, astrocytic endfeet, or both.
DISCUSSION
There is increasing evidence that activation of mGluR in astrocytes, and the subsequent increase in glial [Ca2+]i, plays an important role in functional hyperemia (28). However, both in vivo and in vitro studies have associated [Ca2+]i increases in astrocytes not only to vasodilation (20, 36, 39, 55, 60) but also to vasoconstriction (11, 39, 42). Although these responses have in some cases been related to the specific action of constrictor and dilator signals, the factors that determine whether vessels dilate or constrict upon neuronal and/or glial activation are still unclear. In this study, we attempted to shed light on the mechanisms determining the vascular response to astrocyte activation by addressing a fundamental question: do alterations in the properties of the vasculature itself account for the type of response evoked?
A possible influence of vascular tone in the response of brain arterioles to mGluR has been suggested by Mulligan and MacVicar (42). In their work, t-ACPD induced constrictions in vessels that were initially dilated and dilations in vessels preconstricted via inhibition of NO synthase. However, because NO production was blocked, it is not clear to what extent dilator responses were influenced by alterations in vascular tone per se or rather depended on NO availability. In this sense, a critical role for NO in glial-induced constrictions and dilations was demonstrated in the isolated rat retina, albeit here the initial tone of the vessels was reported to have no effect (39).
Using different kinds of stimuli associated with neurovascular signaling mechanisms (modestly elevated [K+]o, a mGluR agonist, and 11,12-EET), we show that the polarity and magnitude of the evoked diameter changes in brain cortical arterioles are in fact dictated by their particular vascular tone. Notably, all stimuli elicited a differential response pattern, defined by the presence of a set point centered at ∼30–40% tone. Thus elevating [K+]o (from 4.2 to 10 mM) induced consistent, near-maximal dilations in vessels with initial diameters up to ∼60% of baseline, whereas the dilations observed in more constricted vessels, although proportionally larger, did not reach the same final amplitude. This suggests that increases in [K+]o at the gliovascular interface, proposed to be a key mechanism of NVC in the brain (21), may be highly effective in eliciting functional hyperemia when the tone of the arterioles lies at or above the set point. Nevertheless, it must be noted that neuronal influences in the responses to elevated [K+]o cannot be ruled out, inasmuch as 10 mM K+ will decrease Vm in neurons. Although elevated [K+]o exerted a net dilator effect, both constrictions and dilations were observed upon mGluR activation with t-ACPD. Constrictions were observed in arterioles initially presenting low to moderate levels of tone (constricted up to ∼30%), whereas dilations occurred in vessels preconstricted above ∼30%. Notably, for both slightly and highly preconstricted vessels, mGluR activation tended to bring arteriolar diameter to a consistent level (∼37% constricted) close to that at which the polarity of the response to t-ACPD reversed. In addition, prominent dilations occurred in vessels in which diameters were already around the set point, as well as in some arterioles that initially constricted and reached the set point. Because the latter resembles the level of myogenic constriction measured in brain penetrating arterioles in vitro subjected to a broad range of intraluminal pressures (20–100 mmHg) (9, 12), we speculate that, under physiological conditions in vivo, vessels are likely to be stabilized around this putative set point and hence they readily dilate upon neuronal activation. In summary, our results may indicate that astrocyte-derived signals act in concert to adjust vascular tone around a predefined set point, which represents the level of vascular tone at which dilator responses are optimal. They also indicate that the sensitivity of the vasculature to dilator and constrictor stimuli depends primarily on the intrinsic properties of the VSMC, represented mainly by fluctuations in Vm and [Ca2+]i (31).
In response to mGluR activation, both dilations and constrictions were dependent on functional PLA2 activity, confirming the involvement of AA metabolites in these responses. It can be argued that, after pharmacological blockade of AA metabolism, the rise in astrocytic [Ca2+]i resulting from mGluR activation would still have led to activation of BK channels in glial endfeet and subsequent K+-induced vasodilation. We speculate that, in the absence of neuronal stimulation, K+ gradients are absent or reduced (29), and endfeet [K+] does not attain substantial levels for this mechanism to operate. We also showed that the effects of blocking specific AA metabolic pathways were highly dependent on vascular tone. Thus, and in agreement with previous reports (39, 42), selective inhibition of CYP ω-hydroxylase-mediated 20-HETE synthesis converted constrictions into dilations in vessels with low preconstriction. Because a positive correlation between 20-HETE-mediated vasoconstriction and oxygen pressure has been documented in the skeletal muscle microcirculation (27) and in preliminary form also for the brain (25), it is likely that vasoconstrictions were similarly potentiated in our experiments, in which the perfused aCSF's O2 activity was ∼55%.
In arterioles with intermediate levels of tone, but not in highly constricted arterioles, t-ACPD-induced vasodilation was clearly attenuated (∼50%) after inhibition of 20-HETE synthesis. Although this reduction did not attain statistical significance, the possibility that 20-HETE plays a permissive, or perhaps a direct role, in the vasodilation induced by mGluR activation in cortical arterioles needs to be further evaluated (16). More studies are also needed to elucidate whether 20-HETE is generated and released by astrocytes (43), produced by VSMC from glial-derived AA (22), or both of these events.
In weakly preconstricted vessels, t-ACPD-induced vasoconstrictions were significantly attenuated upon inhibition of CYP epoxygenase, COX-1, or thromboxane synthase, which suggests that EETs and COX-1-derived prostanoids (e.g., thromboxane) facilitate this response. The effect of CYP epoxygenase inhibition on t-ACPD-induced constrictions is not clear but may involve altered Ca2+ dynamics in astrocytes (see below), leading to lower production of vasoconstrictors by these cells. Of note, because CYP epoxygenase and COX-1 are present not only in astrocytes (2, 56) but also in vascular endothelial cells (18), a contribution of the latter cell type to the observed effects cannot be excluded. Inhibition of COX-1, but not of CYP epoxygenase, was effective in preventing dilations in vessels with intermediate levels of tone (30–50% preconstriction). Because we showed that maximal dilations in response to t-ACPD occurred in this range of resting tone, which likely resembles the prevailing physiological tone in vivo, our results appear in principle to be consistent with those of Takano et al. (56), which showed that COX-1 inhibition, but not CYP epoxygenase inhibition, reduces vasodilation following astrocytic activation in vivo.
In contrast, our results suggest that EETs, as well as COX-1 products (e.g., PGE2), contribute to vasodilation in substantially constricted (>50%) vessels. In this respect, a potential target of both PGE2 and EETs is the BK channel in VSMC. Thus, concurrent increases in cAMP [mediated by PGE2 acting through prostaglandins E2 receptor subtypes EP2/EP4 receptors (51)] and [Ca2+]i [via EETs (14, 17)] may lead to BK channel activation by mechanisms involving phosphorylation (4, 15) and/or by increasing the frequency of Ca2+ “sparks” (14, 46, 50). Taken together, these results strongly suggest that, although both constrictor and dilator signals are likely to be released upon mGluR activation in astrocytes, the overall vascular response is determined by the specific resting tone of the arterioles.
The final part of this work focused on the vasoactive effects of a CYP epoxygenase product, i.e., 11,12-EET. Our results showed that, similarly to the responses elicited by t-ACPD, 11,12-EET constricted and dilated cortical arterioles in a tone-dependent manner: biphasic responses (constriction followed by dilation) were predominant in weakly preconstricted vessels, whereas dilations occurred in vessels displaying higher resting tones. Notably, as with t-ACPD-induced constrictions and dilations, the polarity of the primary responses to 11,12-EET also reversed around a similar level of tone (∼30% constriction), which reinforces the presence of a putative vascular set point. It is likely that some of the effects of EETs stem from their ability to affect Ca2+ changes in astrocytes, as shown previously for 5,6-EET (54) and in this study for 11,12-EET. Because astrocytes are capable of synthesizing EETs, these evidences support a possible autocrine role for EETs in the amplification of Ca2+ transients (54), which may be functional for hyperemic mechanisms in vivo. 11,12-EET-induced vasoconstriction was prevented by MAFP, which suggests that it depends on AA synthesis and may be mediated by AA-derived vasoconstrictors (e.g., 20-HETE and thromboxane). In line with this, the attenuation of vasoconstriction to t-ACPD upon inhibition of EETs synthesis with MS-PPOH (see Fig. 4) could also be due to reduced synthesis of the above AA metabolites. In both cases, however, the cellular sources of AA and its downstream constrictor by-products remain unclear. On the other hand, exogenous 11,12-EET had clear vasodilator effects in vessels preconstricted above ∼30%, but vasodilation in response to mGluR activation during CYP epoxygenase inhibition was partially reduced only in highly constricted (>50%) vessels. This may be explained by the relatively large concentration of 11,12-EET used, which may have acted directly on VSMC, or may have stimulated the synthesis of dilator prostanoids secondarily to the Ca2+ increase in the astrocyte. Our results also show that, consistent with the role of EETs as activators of BK channels in VSMC (14, 23), 11,12-EET-induced vasodilation was sensitive to 1 mM TEA. Altogether, these data clearly show that the vasoactive effects of CYP epoxygenase products are conditioned by the status of arteriolar tone.
In conclusion, this study provides a comprehensive examination of the influence of vascular tone on the response of brain intracortical arterioles to neuron- and glial-derived signals and supports a novel role for astrocytes in the dynamic control of the cerebral microcirculation. A tentative model of the mechanisms involved is presented in Fig. 6. It must be emphasized that several differences must certainly exist in the mechanisms that operate during pressure-induced myogenic tone and those that determine constriction to U-46619 in vitro. Importantly, endothelial influences are likely to be limited by the lack of intraluminal perfusion characteristic of the brain slice model. Notwithstanding, our results open up the possibility that, in addition to contributing to functional hyperemia, astrocyte-derived signals play an important role in adjusting vascular tone toward a defined “set point” around which the hyperemic response is optimized. It is conceivable that such mechanism is not restricted to circumstances of enhanced neuronal activation but may also be operative during periods of basal brain activity.
Fig. 6.
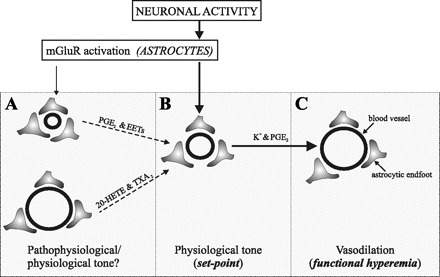
Working model. We propose that, under physiological conditions, a combination of intrinsic and extrinsic mechanisms maintains vascular tone at an optimal range, defined here as the “set point” (B). Following neuronal stimulation, released glutamate activates mGluR in astrocytes, resulting in an increase in [Ca2+]i. The rise in [Ca2+]i facilitates K+ movement in the perivascular space via large-conductance, Ca2+-dependent K+ channel (BK) activation at the endfoot, and it also initiates the metabolism of arachidonic acid (AA) via activation of phospholipase A2 (PLA2). Rapid vasodilation (C) is thus elicited by both K+ and vasoactive signals derived from AA metabolism, in particular cyclooxygenase (COX)-1 metabolites (e.g., PGE2). We propose that, when vascular tone deviates from its set point, as it might occur under pathological or perhaps physiological conditions (A), astrocyte-derived signals will restore vascular tone to its set point. Although a combination of constrictor and dilator signals may be released by astrocytes, the resultant effect on vascular diameter will be determined by the sensitivity of the vessels to such signals, dictated in turn by their specific tone.
GRANTS
This work was supported by grants from the American Heart Association [0535231N (J. A. Filosa) and 0640092N (J. E. Stern)] and the National Heart, Lung, and Blood Institute of the Institute National of Health [R01-HL089067 (J. A. Filosa)].
Supplementary Material
Acknowledgments
We thank Drs. Marilyn Cipolla and Jonathan Ledoux (University of Vermont) and Laura Gonzalez-Bosc (University of New Mexico) for discussions and helpful comments on the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.AlkayedNJAlkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997. [DOI] [PubMed] [Google Scholar]
- 2.AlkayedNJAlkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971–979, 1996. [DOI] [PubMed] [Google Scholar]
- 3.ArmsteadWMArmstead WM, Mirro R, Busija DW, Leffler CW. Vascular responses to vasopressin are tone-dependent in the cerebral circulation of the newborn pig. Circ Res 64: 136–144, 1989. [DOI] [PubMed] [Google Scholar]
- 4.BarmanSABarman SA, Zhu S, Han G, White RE. cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 284: L1004–L1011, 2003. [DOI] [PubMed] [Google Scholar]
- 5.BhardwajABhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-d-aspartate. Am J Physiol Heart Circ Physiol 279: H1616–H1624, 2000. [DOI] [PubMed] [Google Scholar]
- 6.BlancoVMBlanco VM, Filosa JA. The Influence of Resting Arteriolar Tone on Vascular Responses to Glial Activation: Neuroscience Meeting Planner. San Diego, CA: Soc Neurosci, program no. 479.12, 2007.
- 7.BlancoVMBlanco VM, Stern JE, Filosa JA. Role of Ca2+-activated K+ channels in 11,12-epoxyeicosatrienoic acid-induced vasodilation of parenchymal arterioles: possible role of astrocytes. FASEB J 21: 748–744, 2007. [Google Scholar]
- 8.BrahmaBBrahma B, Forman RE, Stewart EE, Nicholson C, Rice ME. Ascorbate inhibits edema in brain slices. J Neurochem 74: 1263–1270, 2000. [DOI] [PubMed] [Google Scholar]
- 9.BrekkeJFBrekke JF, Gokina NI, Osol G. Vascular smooth muscle cell stress as a determinant of cerebral artery myogenic tone. Am J Physiol Heart Circ Physiol 283: H2210–H2216, 2002. [DOI] [PubMed] [Google Scholar]
- 10.CauliBCauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 24: 8940–8949, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ChuquetJChuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci 27: 4036–4044, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CipollaMJCipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol 44: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 13.DevorADevor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EarleySEarley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. [DOI] [PubMed] [Google Scholar]
- 15.EsguerraMEsguerra M, Wang J, Foster CD, Adelman JP, North RA, Levitan IB. Cloned Ca(2+)-dependent K+ channel modulated by a functionally associated protein kinase. Nature 369: 563–565, 1994. [DOI] [PubMed] [Google Scholar]
- 16.FangXFang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol 291: H2301–H2307, 2006. [DOI] [PubMed] [Google Scholar]
- 17.FangXFang X, Weintraub NL, Stoll LL, Spector AA. Epoxyeicosatrienoic acids increase intracellular calcium concentration in vascular smooth muscle cells. Hypertension 34: 1242–1246, 1999. [DOI] [PubMed] [Google Scholar]
- 18.FaraciFMFaraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 78: 53–97, 1998. [DOI] [PubMed] [Google Scholar]
- 19.FilosaJAFilosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Exp Physiol 92: 641–646, 2007. [DOI] [PubMed] [Google Scholar]
- 20.FilosaJAFilosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 95: e73–e81, 2004. [DOI] [PubMed] [Google Scholar]
- 21.FilosaJAFilosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006. [DOI] [PubMed] [Google Scholar]
- 22.GebremedhinDGebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 23.GebremedhinDGebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992. [DOI] [PubMed] [Google Scholar]
- 24.GirouardHGirouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006. [DOI] [PubMed] [Google Scholar]
- 25.GordonRJGordon RJ, Choi H, MacVicar BA. O2 Determines the Polarity of Astrocyte-mediated Vasomotor Responses: Neuroscience Meeting Planner. San Diego, CA: Soc Neurosci, program no. 148.8, 2007.
- 26.HarderDRHarder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29: 229–234, 1998. [DOI] [PubMed] [Google Scholar]
- 27.HarderDRHarder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman RJ. Identification of a putative microvascular oxygen sensor. Circ Res 79: 54–61, 1996. [DOI] [PubMed] [Google Scholar]
- 28.HaydonPGHaydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86: 1009–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 29.HolthoffKHolthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia 29: 288–292, 2000. [DOI] [PubMed] [Google Scholar]
- 30.IizukaKIizuka K, Akahane K, Momose D, Nakazawa M, Tanouchi T, Kawamura M, Ohyama I, Kajiwara I, Iguchi Y, Okada T, Taniguchi K, Miyamoto T, Hayashi M. Highly selective inhibitors of thromboxane synthetase. 1. Imidazole derivatives. J Med Chem 24: 1139–1148, 1981. [DOI] [PubMed] [Google Scholar]
- 31.ImtiazMSImtiaz MS, Katnik CP, Smith DW, van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J 90: 1–23, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.KnotHJKnot HJ, Zimmermann PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol 492: 419–430, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KoehlerRCKoehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol 100: 307–317, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.KrieglerSKriegler S, Chiu SY. Calcium signaling of glial cells along mammalian axons. J Neurosci 13: 4229–4245, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KuschinskyWKuschinsky W, Wahl M, Bosse O, Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res 31: 240–247, 1972. [DOI] [PubMed] [Google Scholar]
- 36.LovickTALovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol 90: 131–140, 2005. [DOI] [PubMed] [Google Scholar]
- 37.LovickTALovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 92: 47–60, 1999. [DOI] [PubMed] [Google Scholar]
- 38.McCarronJGMcCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol Heart Circ Physiol 259: H902–H908, 1990. [DOI] [PubMed] [Google Scholar]
- 39.MeteaMRMetea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MiyataNMiyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MossoAMosso A. Sulla circolazione del cervello dell'uomo. Atti R Accad Lincei 5: 237–358, 1880. [Google Scholar]
- 42.MulliganSJMulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. [DOI] [PubMed] [Google Scholar]
- 43.NithipatikomKNithipatikom K, Grall AJ, Holmes BB, Harder DR, Falck JR, Campbell WB. Liquid chromatographic-electrospray ionization-mass spectrometric analysis of cytochrome P450 metabolites of arachidonic acid. Anal Biochem 298: 327–336, 2001. [DOI] [PubMed] [Google Scholar]
- 44.NiwaKNiwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci 20: 763–770, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.NiwaKNiwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res 88: 600–608, 2001. [DOI] [PubMed] [Google Scholar]
- 46.PaternoRPaterno R, Faraci FM, Heistad DD. Role of Ca2+-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke 27: 1603–1608, 1996. [DOI] [PubMed] [Google Scholar]
- 47.PengXPeng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002. [DOI] [PubMed] [Google Scholar]
- 48.PengXPeng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab 24: 509–517, 2004. [DOI] [PubMed] [Google Scholar]
- 49.PeppiattCPeppiatt C, Attwell D. Neurobiology: feeding the brain. Nature 431: 137–138, 2004. [DOI] [PubMed] [Google Scholar]
- 50.PorterVAPorter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol Cell Physiol 274: C1346–C1355, 1998. [DOI] [PubMed] [Google Scholar]
- 51.ReganJWRegan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci 74: 143–153, 2003. [DOI] [PubMed] [Google Scholar]
- 52.RosenblumWIRosenblum WI, Nelson GH. Tone regulates opposing endothelium-dependent and -independent forces: resistance brain vessels in vivo. Am J Physiol Heart Circ Physiol 259: H243–H247, 1990. [DOI] [PubMed] [Google Scholar]
- 53.RoyCSRoy CS, Sherrington C. On the regulation of the blood supply of the brain. J Physiol 11: 85–108, 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.RzigalinskiBARzigalinski BA, Willoughby KA, Hoffman SW, Falck JR, Ellis EF. Calcium influx factor, further evidence it is 5,6-epoxyeicosatrienoic acid. J Biol Chem 274: 175–182, 1999. [DOI] [PubMed] [Google Scholar]
- 55.StraubSVStraub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol 128: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.TakanoTTakano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- 57.WangMHWang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther 284: 966–973, 1998. [PubMed] [Google Scholar]
- 58.WatanabeHWatanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003. [DOI] [PubMed] [Google Scholar]
- 59.WinshipIRWinship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci 27: 6268–6272, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ZontaMZonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


