Abstract
Sepsis represents the host's systemic inflammatory response to a severe infection. It causes substantial human morbidity resulting in hundreds of thousands of deaths each year. Despite decades of intense research, the basic mechanisms still remain elusive. In either experimental animal models of sepsis or human patients, there are substantial physiological changes, many of which may result in subsequent organ injury. Variations in age, gender, and medical comorbidities including diabetes and renal failure create additional complexity that influence the outcomes in septic patients. Specific system-based alterations, such as the coagulopathy observed in sepsis, offer both potential insight and possible therapeutic targets. Intracellular stress induces changes in the endoplasmic reticulum yielding misfolded proteins that contribute to the underlying pathophysiological changes. With these multiple changes it is difficult to precisely classify an individual's response in sepsis as proinflammatory or immunosuppressed. This heterogeneity also may explain why most therapeutic interventions have not improved survival. Given the complexity of sepsis, biomarkers and mathematical models offer potential guidance once they have been carefully validated. This review discusses each of these important factors to provide a framework for understanding the complex and current challenges of managing the septic patient. Clinical trial failures and the therapeutic interventions that have proven successful are also discussed.
I. INTRODUCTION
Undeniably, sepsis is still a profoundly damaging and life-threatening condition for many individuals. The incidence of sepsis is increasing with a consequent rise in hospitalizations and resource utilization in providing care to septic patients (90, 199). The annual cost of providing care to septic patients is approximately $24 billion in the United States, representing a 57% increase in expenditures from 2003 to 2007 (204). It is expected that the worldwide incidence will continue to grow in a milieu of antimicrobial resistance, a greater proportion of elderly people, wider use of immunosuppressive therapies, and more accessible medical technology and interventions. To place the problem of sepsis in the appropriate context, sepsis survival studies typically follow patients for 28 days, while most cancer studies evaluate 5-yr survival (1,825 days). Despite an overall modest decline in the proportional mortality from sepsis, the total number of patients dying from sepsis is greater than in the past (233). Those patients who initially survive sepsis experience functional deficits and diminished quality of life, in addition to being at risk for increased long-term mortality (157, 300).
Implementation of more timely, aggressive initial supportive care has improved survival outcomes in early sepsis but leaves patients susceptible to later onset morbidity and death. Medical advancements that support failing organs such as better Intensive Care Unit (ICU) mechanical ventilation practices and continuous veno-venous hemofiltration allow patients to live longer, but they have impaired, incomplete recovery. As Gentile et al. (129) describe, septic patients often develop nutritional deficiency, repeated infections, increased energy requirements, and significant but sustainable organ injury before leaving the ICU setting in a debilitated functional state or eventually succumbing to a secondary infection.
Our understanding of the pathophysiology of sepsis has evolved over time, impacting the ability to conceive and conduct effective clinical trials. For many years, the inflammatory dynamics of sepsis have been incompletely understood. Early septic deaths were originally presumed to be due to an unrestrained, overzealous spike in a host's proinflammatory immune response (43). Vigorous release of systemic cytokines such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6) were well-documented in the septic human response and relevant animal models. These findings fueled the dominant concept of sepsis as a hyperinflammatory state and instigated many unsuccessful anti-inflammatory studies (12, 317). Then a multimodal hypothesis of sepsis was proposed in which an initial systemic inflammatory response syndrome (SIRS) in sepsis was believed to be followed temporally by a compensatory anti-inflammatory response syndrome (CARS) (162, 276, 327) often increasing the risk of nosocomial infections and other adverse events. Subsequently, concomitant production of circulating proinflammatory and anti-inflammatory cytokines has been demonstrated in a model of polymicrobial sepsis, supporting that a continuously, highly mixed anti-inflammatory response syndrome (MARS) is present (285). Human studies show similar results and underscore that both classes of cytokines have an integral role in sepsis from the onset and onward (271, 374). Inadequate understanding of the pathophysiology of sepsis has created fundamental problems in the design of clinical trials to address a better approach the problem of sepsis.
Currently, there is no pharmacological therapeutic intervention directed against a specific mediator of sepsis which is currently approved by the Food and Drug Administration (FDA) or the European Medicines Evaluation Agency. In the past 30 years, there has only been one FDA approved intervention, but it was withdrawn in 2011 by the manufacturer since follow-up studies failed to show substantial improvement in the survival of the septic shock patient (314). While there are no current therapies directed against a specific target, there are recommended guidelines for early goal-directed medical care to improve resuscitation which have demonstrated effectiveness.
Why do sepsis therapies fail to cure the disease or improve outcome? The complexity of the disease presents substantial challenges to our understanding of what is aberrant and why the alteration is deleterious. Simultaneous derangement of multiple pathways likely drives sepsis mortality rather than a single mediator. This review will explore many of these detrimental changes in humans and a clinically applicable animal model in an attempt to increase understanding of the basic pathophysiological aspects of the disease and provide insight into future directions of sepsis investigation. Inherent variability in the human response to sepsis appears to necessitate a more individualized approach to developing improved therapeutic response.
II. PHYSIOLOGICAL ALTERATIONS
A. Physiological Alterations in Animal Models of Sepsis
Sepsis is a highly lethal disease seen in intensive care patients and trauma victims. It is the clinical syndrome of a systemic inflammatory response that complicates severe infection. Diagnostic criteria include a documented or suspected pathogen plus two or more SIRS abnormalities listed in Table 1 (2). The host response to eliminate pathogens results in physiological and immune system dysregulation of normal processes. Sepsis occurs when the response to infection becomes systemic and affects initially uninvolved tissues and organ systems at distant sites.
Table 1.
Diagnostic criteria for sepsis
| A documented or suspected pathogen plus two or more of the following SIRS abnormalities: |
| 1. Temperature >38.5°C or <35°C |
| 2. Heart rate >90 beats/min |
| 3. Respiratory rate >20 breaths/min or PaC02 <32 mmHg |
| 4. WBC >12,000 cells/mm3, or <4000 cells/mm3, or >10% immature (band) forms |
SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
Understanding the pathogenesis of sepsis is an important first step in improving outcomes. Several animal models of sepsis mimic the signs and laboratory findings present in septic patients. Such models include the intravenous administration of endotoxin\lipopolysaccharide (LPS) or live bacteria, pneumonia induction, colon ascendens stent peritonitis, and the creation of a nidus of infection with cecal ligation and puncture (CLP) (222, 293, 429). The CLP model closely replicates the clinical picture of sepsis encountered in human patients and has become the most frequently used model of sepsis (52, 76, 321, 328). A specific focus of infection in the abdomen produces fecal peritonitis, imitating the human diseases of perforated appendicitis or diverticulitis, to induce polymicrobial sepsis often in murine studies. CLP is often used in combination with fluid, analgesia, and antibiotic administration to strengthen the clinical relevance. The genetic variability of humans can be partially simulated by utilizing outbred mice to increase applicability of the model to a more diverse clinical population. The CLP model is reproducible, allows production of varying injury severity, enables release of multiple types of infectious organisms from the host's specific flora, and originates as a nidus of infection before spreading systemically (171). Metabolic, cardiovascular, immunological, and other responses in sepsis can be reproduced to examine underlying mechanisms and investigate therapeutic interventions.
The CLP model of sepsis has important limitations associated with using studies in small rodents. The circulating blood volume of rodents is limited, making it difficult to obtain serial blood samples for multiple assays or those requiring larger quantities to perform. Due to the availability and relatively low cost of small rodents, they can be killed serially at specific intervals for sampling, but newer, more refined methods have been described. For example, multiplex immunoassays have made it possible to repeatedly draw tiny amounts of blood (∼20 μl) from live mice to determine their cytokine profile without detrimental effects (70, 422). The diminutive anatomy of the murine blood vessels makes design and use of physiological monitoring tools more challenging. Discrepancies in rodent physiology and lack of comorbidities, compared with humans, clearly limit translational applicability (349). However, careful selection of a model most closely representative of a target clinical study population (i.e. fecal peritonitis versus pneumonia), varying the severity of sepsis to investigate therapy efficacy, and using animals with comorbidities like diabetes and kidney disease will improve the utility of animal models (52, 105).
No perfect animal model exists, but CLP and other models have added greatly to our understanding of the host response to sepsis. It is important to continually improve upon and better implement them to study sepsis more effectively. As drug development usually initiates in an animal model and advances to human studies, we will discuss animal alterations in sepsis followed by humans. A review of the physiological changes in murine sepsis provides insight into alterations in human sepsis, even though there are important caveats when extropolating murine models to study human disease.
1. Body weight
Murine weight gain in the first 24 h after CLP predicts death within the next 3 days (263). This finding is likely due to sepsis-induced vascular leakage causing fluid retention. Autopsies of CLP mice show generalized tissue edema, including abundant subcutaneous fluid accumulation and ascites (444). Mice that live have a significant decrease in their body weight from baseline during the first 5 days after CLP (288, 318). Diminished body weight is a result of the metabolic stress placed on the animal by the initial surgical procedure followed by the subsequent bacterial infection. In the chronic phase of sepsis, gradual recovery of body weight is seen over time. Body weight differences become less apparent in survivors compared with nonsurvivors as sepsis progresses.
2. Temperature
Alterations in body temperature are frequently observed in experimental animals. Humans typically increase their body temperature during sepsis while most rodents have a decrease in their temperature in response to a significant infection such as CLP (339). The postoperative decrease in body temperature during the first 5 h is likely due to effects of the anesthesia, but after 5 h it signifies part of the physiological response to sepsis (323). In animal models of sepsis, postoperative hypothermia is associated with worse outcomes and alterations in the inflammatory response (323, 444).
3. IL-6 Levels
IL-6 is a signaling protein that modulates immune function. It is produced primarily by macrophages and T cells in the setting of inflammation that occurs after injury or infection. Unrelenting and recurring inflammatory insults in sepsis can cause sufficient damage so that dysregulation occurs, creating a situation of metabolic disorder in which the host can no longer control its own inflammatory response (41). IL-6 has a number of in vivo activities that may play a role in the septic response. The cytokine functions to stimulate hepatic synthesis of acute phase proteins as well as class specific differentiation of immunoglobulins. IL-6 also has a role in temperature regulation (188, 210), and IL-6 levels in mice with normothermia and hypothermia correlate with survival status. In both the alive and dead mice, hypothermia is associated with higher levels of IL-6 (444).
Several clinical studies have demonstrated that septic patients who die have increased plasma levels of IL-6 (275, 355), and elevated plasma IL-6 accurately predicts mortality in CLP mice. Specifically, IL-6 levels at 6 h after the onset of sepsis accurately forecast survival over the first 3 days of injury (320). Stratification of septic mice into groups predicted to live or die based on plasma IL-6 has been successfully used to selectively target corticosteroid or cytokine inhibitor treatment to improve survival of mice predicted to die (284, 319). Another group employed the plasma measurement to show that early antibiotic treatment also saves a portion of the mice that would be predicted to die (413).
A recently described dual cut-off system eliminates the reduction in sensitivity and specificity of a single cut-off and offers even better outcome prediction (70). In this study, an IL-6 concentration over 12 ng/ml 24 h post-CLP predicted death by 5 days with 100% specificity and a value under 1.5 ng/ml predicted survival by day 5 with 100% sensitivity. This approach allows for robust experiments in which mice that have been subjected to a similar septic insult can be differentiated early and quickly based on predicted survival so that end organ damage can be assessed (178) and targeted therapy may be given only to those at high risk of dying. This will allow the therapies sufficient time to be effective before death occurs. However, while IL-6 levels provide useful prognostic information, no single mediator in the complex picture of sepsis is able to offer definitive diagnostic or treatment guidance in human patients.
4. White blood cell, lymphocyte, and neutrophil counts
The total white blood cell (WBC) count is diminished after CLP. Murine peripheral blood cells are mostly lymphocytes, although neutrophils are also present. There is often a significant decrease in lymphocytes induced by the sepsis secondary to massive lymphocyte apoptosis (24, 167, 168). The number of neutrophils in the peripheral blood is an important prognostic sign in sepsis, and mice with a higher neutrophil count have better survival (320). Overall, mice that die in the early phase of sepsis have significant peripheral blood alterations compared with survivors.
5. Blood glucose
In contrast to human patients, metabolic derangements in rodents lead to hypoglycemia in response to a septic insult and are associated with worse outcomes. CLP-induced sepsis in rodents also causes a decrease in blood glucose. Hypoglycemia persists and worsens in nonsurviving mice prior to their death, while resolving over 96 h in surviving mice (286). Similarly, rats dying early after post-CLP sepsis exhibit hypoglycemia compared with survivors (156). While septic animals manifest the characteristic signs of increased heart rate and respiratory rate, their regulation of temperature and glucose is distinct compared with human patients in sepsis.
B. Physiological Alterations in Septic Human Patients
The human response to systemic infection and inflammation depends on the severity of disease, ranging from sepsis to severe sepsis to septic shock with multiple organ dysfunction syndrome (MODS). Clinical features vary depending on where a patient falls in that continuum. Fever is typically present in sepsis with hypothalamic control of body temperature readjusting to a higher set-point due to a shift in heat equilibrium. Rigors may accompany a fever as skeletal muscle contractions function to produce additional heat. Tachypnea in septic patients may be attributable to increased activation of the respiratory center in the medulla by inflammatory mediators or as response to offset metabolic acidosis. Tachycardia is almost universally present and represents an important compensatory mechanism to maintain perfusion in response to intravascular volume deficits, reduced cardiac contractility, and vasodilation. Severe sepsis occurs in the setting of hypoperfusion or organ dysfunction which may manifest physiologically as a sudden alteration in mental status or oliguria. In the progression to septic shock, hypotension (systolic blood pressure <90 mmHg) occurs in spite of appropriate fluid resuscitation. These individual symptoms are not pathognomonic for sepsis and may be present in a variety of other conditions. Conversely, classic symptoms of systemic inflammation may be absent in severe sepsis, especially in elderly and immunosuppressed patients.
Specific alterations in the human inflammatory response may signal an increased susceptibility to severe disease and mortality. For instance, some people do not develop typical signs of sepsis such as fever and leukocytosis. Failure to develop a fever or the presence of hypothermia (temperature <35.5°C) is more common among nonsurvivors of sepsis than survivors (17 vs. 5%) in a study of 519 patients with sepsis (190). Hypothermia is also associated with adverse outcomes including increased surgical wound infections. A prospective, randomized, controlled study shows that patients with hypothermia have a 19% incidence of wound infection compared with 6% of normothermic patients (202). Leukopenia (WBC <4,000/mm3) is similarly more frequent among nonsurvivors than survivors (15 vs. 7%) in a study of 612 patients with Gram-negative sepsis (196). Overall, these features are associated with a higher severity of illness in the sepsis spectrum.
III. END ORGAN DAMAGE IN SEPSIS
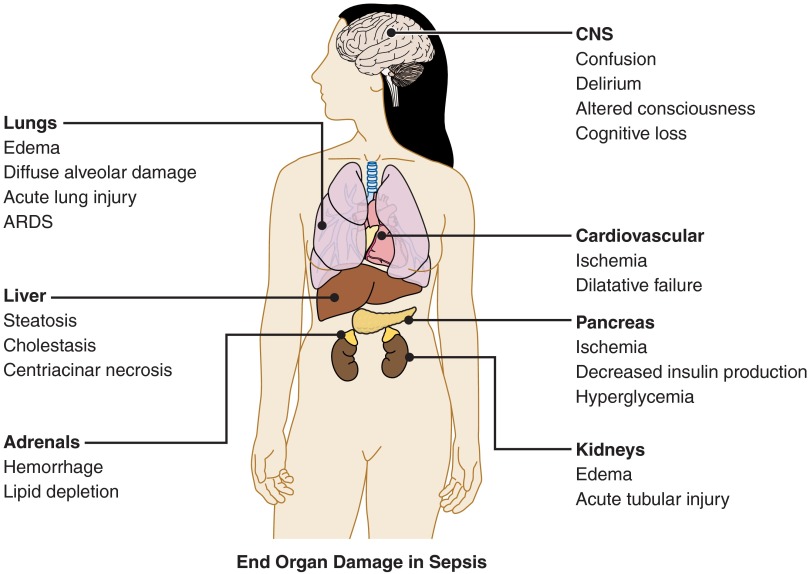
Virtually every organ in the body may be damaged in the septic response, and one of the recognized complications of sepsis is MODS. Figure 1 briefly lists the major organs that may suffer injury in septic patients. Discrete organ injury also occurs, although it may be difficult to separate direct, primary injury from damage secondary to poor perfusion.
Figure 1.
End organ damage in sepsis. Multiple organs may be damaged during the septic response. There may be dysfunction and failure of an individual organ, or multi-system organ failure may occur.
A. Kidney
Acute kidney injury (AKI) is defined as an absolute increase in serum creatinine =1.5-fold from baseline or a urine output of <0.5 ml·kg−1·h−1 for 6 h (294). One study showed that in 64% of septic patients, the development of septic AKI is associated with a significant increase in 90-day mortality compared with septic patients without AKI (58 vs. 35%) (26). The severity of AKI is classified using the Risk, Injury, Failure, Loss, and End-stage renal disease (RIFLE) criteria listed in Table 2. Kim et al. (187) found that RIFLE class on admission was not a predictor of 28-day mortality in septic patients, but the maximum RIFLE class reached during their stay in the ICU was associated with increased mortality (risk: 17.2%, injury: 30.9%, failure: 37.1%) (187).
Table 2.
Criteria for kidney injury
| Risk (R) | Serum creatinine increased ≥1.5X over baseline |
| Injury (I) | Serum creatinine increased ≥2X over baseline |
| Failure (F) | Serum creatinine increased ≥3X over baseline |
| Loss (L) | Loss of renal function >4 wk |
| End-stage renal disease (E) | Loss of renal function >3 mo |
The primary pathological process behind septic AKI is acute tubular injury (ATI). ATI is characterized by renal tubular cell death throughout the nephron (221). Histologically, the septic kidney shows interstitial edema, detachment of tubular epithelium, and shedding of necrotic cells into the tubular lumen. In sepsis, the kidney is subjected to ischemia, inflammatory cytokines, and both local and circulating damage-associated molecular pattern molecules (DAMPs) resulting in widespread tubular cell apoptosis (153, 221, 334). Increased TNF-α signaling, GSK3β activation, and TLR-4 activation in tubular cells have been found to be the main mediators of ATI in sepsis (153, 334).
B. Cardiovascular
Cardiovascular changes commonly seen in sepsis are tachycardia, hypotension, and reduced cardiac ejection fractions (216, 221). Pathologically, the septic myocardium shows some apoptotic myocytes and mononuclear cells in the interstitium (221). These changes, however, are usually mild and do not persist after recovery. Studies in rodent models have shown that, during sepsis, the myocardium enters a state of hibernation characterized by low oxygen demand and contractility (216). This hibernation may prevent more serious damage to the myocardium while also accounting for the observed physiological dysfunction in septic patients.
C. Pulmonary
Sepsis is the second most common cause of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (299). ALI is defined as the presence of bilateral pulmonary infiltrates on chest radiograph and arterial hypoxemia (PaO2/FiO2 =300). If the PaO2/FiO2 ratio drops below 200, the condition is classified as ARDS (235, 299). The pathological process underlying this sequence is diffuse alveolar damage (235). Diffuse alveolar damage begins with increased lung vascular permeability due to neutrophil accumulation and degranulation in the pulmonary capillaries (299). This allows fibrin-rich edema fluid, red blood cells, and neutrophils to enter the alveoli. In sepsis, bacterial products and inflammatory cytokines in the circulation activate the neutrophils (235). Inflammatory mediators and proteases damage the alveolar epithelium and denude the alveolar basement membrane (221). The dead cells and exposed collagen result in fibrin deposition forming hyaline membranes within the alveoli that further impede gas exchange and serve to activate alveolar macrophage production of proinflammatory cytokines and chemokines including IL-1, IL-6, TNF-α, and IL-8 (235, 299). Coupled with inactivation of surfactant, these alterations collapse the alveoli and thus compromise pulmonary function. This pathology can either resolve through regeneration of type II alveolar epithelial cells and endothelial remodeling or through interstitial fibrosis (221). Of these outcomes, interstitial fibrosis may result in long-term impairment.
D. Hepatic
In sepsis, hepatic injury is the result of both extrahepatic (primary dysfunction) and intrahepatic (secondary dysfunction) factors. Primary dysfunction results from decreased hepatic arterial blood flow from septic shock, and it is termed primary because it is the first event to occur (80). Poor hepatic microcirculation can induce acute cellular and mitochondrial injury with associated decreases in enzymatic function and protein synthesis (80, 366). This is reflected in acutely elevated transaminases, severe hypoglycemia, and decreased production of coagulation factors which is discussed later in more detail. When perfusion is restored, these issues usually improve. Secondary dysfunction occurs at the cellular level within the liver. Kupffer cells, responsible for the clearance of circulating bacterial products and inflammatory cytokines, are damaged during the primary dysfunction stage and are unable to sufficiently detoxify the inflammatory molecules (80). This results in a spillover of bacterial products and cytokines into the liver parenchyma which may damage hepatocytes and sinusoidal endothelium. Increased neutrophil infiltration into damaged endothelium causes widespread centriacinar necrosis (221, 366). These changes reduce hepatobiliary transport resulting in cholestasis (353a). Some bacterial products can also defenestrate the sinusoidal endothelium decreasing lipid uptake from the circulation manifested clinically by hyperlipidemia and hepatosteatosis (62).
E. Adrenal
Septicemia induces the release of corticotropin releasing hormone (CRH) and subsequently adrenocorticotropic hormone (ACTH) resulting in high-level production and release of cortisol from the adrenal cortex (228). Sustained cortisol production can deplete the adrenal cortices of lipids compensated by adrenal hyperplasia (221, 228). In some cases, high ACTH levels increase adrenal blood flow, but simultaneously high catecholamine levels can constrict venous drainage of the cortices (214, 228). This imbalance in supply and outflow can result in bilateral adrenal hemorrhage with an associated severe adrenal insufficiency known as Waterhouse Friderichsen syndrome (214). Pathologically, these adrenals show arteriolar thrombosis and frank parenchymal hemorrhage (221). Impaired adrenal function in sepsis significantly decreases cortisol production and release. Cortisol works in combination with catecholamines to mediate systemic vascular resistance and cortisol deficiency can serve to perpetuate hypotension in septic shock.
F. Neurological
Sepsis-associated encephalopathy occurs in up to 87% of septic patients. This consists of alterations in mental status ranging from mild confusion to delirium or coma, motor rigidity, and polyneuropathy with cognitive deficits continuing up to years after recovery (205, 436). Reductions in cerebral blood flow, cytokines crossing the blood-brain barrier, and glutamate excitotoxicity all contribute to these manifestations (142, 436). Increased abnormality of electroencephalogram readings has been shown to be directly related to mortality in septic patients (449).
IV. EFFECTS OF AGE, GENDER, AND COMORBIDITIES
A. Effects of Age
The incidence of sepsis is disproportionately higher in elderly patients, and age is an independent predictor of mortality. An observational study of more than 10.4 million adult septic patients in the United States (U.S.) examined the effects of age in sepsis over a 24-yr time period. Elderly patients, age 65 yr and older, comprise only 12% of the U.S. population but represent ∼65% of sepsis cases, i.e., a relative risk of 13 compared with younger individuals (234). Age was found to independently predict mortality in an adjusted multivariable regression with an odds ratio of 2.26. Elderly patients are also more likely to have comorbid medical conditions. Compared with younger patients, elderly sepsis patients die at an earlier time point during hospitalization, and elderly survivors more often need skilled nursing or rehabilitation after discharge (234). A 2012 multicenter study confirmed that increased age is a significant risk factor for sepsis mortality (90). As the U.S. population ages, the incidence of sepsis is expected to rise, further increasing costs to the healthcare system. Adult sepsis is the focus of this discussion, but neonatal sepsis and associated immune system deficiencies have been previously reviewed by Wynn et al. (443).
1. Immune dysregulation and other etiologies for worse prognosis in the elderly
Older age is likely a risk factor for mortality due to its association with immune system dysregulation, potential malnutrition, increased comorbidities, nursing home exposure to resistant pathogens, and increased dependency on invasive medical devices. Elderly patients are often immunologically impaired (243), making them more susceptible to infection and its subsequent complications. This increased susceptibility occurs even though fundamental features of the innate immune response, including neutrophil and natural killer cell activity, remain essentially intact even in those individuals 100 years and older (132). The infectious response also appears to trigger normal expression of proinflammatory cytokines such as IL-6, TNF-α, interferon (IFN)-γ, as well as chemokines (140, 229). Yet, the adaptive immune system is significantly weakened (141) with evidence of abnormal B- and T-cell function in older patients (243, 423). The total T-cell repertoire decreases with age, and there is a relative paucity of helper T-cell function, particularly with CD8+, which is a significant problem for effective B-cell functions (87, 122). Costimulatory molecule expression, which is crucial to B- and T-cell communication, is also impaired in the elderly, causing a weakened antibody reaction to invasive pathogens (118).
Malnutrition is frequently seen in the elderly as a result of factors such as inactivity, poor mobility, reduced or restricted diets, chronic disease, dementia, depression, poor dentition, and polypharmacy (181). Comorbidities in elderly patients were increased twofold as illustrated by one study (233). Another sepsis trial showed that patients >75 years of age had higher rates of comorbidities compared with younger patients (96). Elderly patients in long-term care facilities are exposed to bacterial flora that has a higher level of antibiotic resistance than that seen in the community (267, 312). These individuals have higher rates of respiratory infections due to oropharyngeal colonization with gram-negative organisms, as well as a depressed cough reflex and mucociliary clearance (398). Invasive medical devices such as indwelling urinary catheters, feeding tubes, and venous catheters are commonly required and increase the risk of infection in elderly patients. The intrinsic barriers of innate immunity are interrupted, providing increased access for pathogens (181, 224, 312).
Older patients may also have an uncharacteristic response to sepsis and present with a change in mental status thereby delaying treatment that may influence their outcome. An age-associated decline in renal function, less lean body mass, and poor hepatic blood flow from shock can substantially alter the pharmacokinetics of antimicrobial agents in older patients with sepsis (236). Closer dose adjustments and monitoring of serum drug levels may be necessary for certain medications in this older septic patient population to mitigate adverse outcomes.
B. Gender Differences
Experimental animal data show females have an inherent survival advantage in polymicrobial sepsis (89, 452), but gender outcomes in human clinical studies are more varied and lack consensus (68, 92, 170). Higher, lower, and equal mortality rates are all reported in gender comparisons. In a prospective, observational clinical trial, Nachtigall et al. (259) demonstrate that if women develop sepsis, they have an increased mortality (23%) compared with men (14%). Vincent et al. (411) also showed, in their European multicenter cohort, higher mortality for women with sepsis than for men. In contrast, a prospective study by Schroder et al. (342) demonstrated a significantly better prognosis for women, which was associated with increased levels of anti-inflammatory mediators. The hospital mortality rate was 70% in male versus 26% in female patients. In a 2007 study of patients greater than 50 years old with severe sepsis, women had a lower risk of hospital mortality than men with an adjusted odds ratio 0.75 (7). Differences in gender-based findings in septic humans may be attributed to various mechanisms including the effects of sex hormones (103), sex-related gene polymorphisms (170), and disparities in healthcare intensity with men receiving more invasive interventions in the ICU setting (399). Although debated for over a decade, it remains controversial in the literature as to whether female or male gender is a predisposing factor in human sepsis mortality.
C. Comorbidities
A patient's comorbidities are also important determinants of outcome in sepsis (190). Risk factors for mortality include coexisting conditions such as immune suppression, cancer, HIV/AIDS, hepatic failure, and alcohol dependence (14, 74, 191, 233, 273). Conditions that suppress the host immune response such as cancer and HIV/AIDS, as well as immunosuppressant medications are common among patients with sepsis. A global registry of over 12,000 individuals revealed the characteristics of severely septic patients from 37 nations (232). A large proportion of the patients had comorbidities, particularly diabetes (23%), chronic lung disease (17%), active cancer (16%), congestive heart failure (14%), renal insufficiency (11%), and liver disease (7%). Septic elderly patients are seen to have an increased incidence of preexisting medical problems, compared with the nonelderly adult population, with cancer, liver disease, renal insufficiency, and chronic obstructive pulmonary disease (COPD) being more prevalent (14). Table 3 provides a list of important comorbidities that impact the pathophysiology in sepsis.
Table 3.
Effects of comorbid conditions in sepsis and their relative incidences among septic patients
| Co-Morbid Condition | Effect in Sepsis | Incidence Among Sepsis Patients, % (232, 254) |
|---|---|---|
| Diabetes mellitus | Increased risk of acute renal failure but decreased risk of acute respiratory failure (102) | 23 |
| Cancer | Chemotherapy-induced neutropenia (296) | 16 |
| Cirrhosis | Complement deficiency, impaired neutrophil function, and spontaneous bacterial peritonitis (145) | 7 |
| Chronic obstructive pulmonary disease | Significant impairment of phagocytosis in pulmonary macrophages (340) | 17 |
| End-stage renal disease | Uremia impairs innate and adaptive immune function (152) | 11 |
| HIV/AIDS | Enhanced susceptibility to bacterial pneumonia (333) | 10.3 |
Reference numbers are given in parentheses.
The mechanisms and effects of each comorbidity are incompletely understood, but some insight is available. Diabetic patients have a variety of immune defects such as reduced cell-mediated immunity and phagocytosis. The disease predisposes individuals to serious bloodstream infections (364) and the risk of sepsis-related organ dysfunction. Preclinical studies demonstrated that diabetic mice dying from acute sepsis failed to generate a systemic cytokine response (286). Compared with severe sepsis patients with euglycemia, diabetic patients are less likely to develop respiratory failure, but more likely to suffer from renal failure (101). Cancer patients are at increased risk for developing sepsis as a consequence of multiple mechanisms of immunosuppression due to the disease process itself and aggressive treatments. Included in these therapies are combined regimens of chemotherapy and radiation therapy, high-dose glucocorticoids, and stem cell transplantation (74, 378). Additionally, critically ill patients with cancer who become septic have a longer duration of both ICU and hospital stays than other septic patients, requiring greater utilization of healthcare resources (434).
Hepatic impairment also increases susceptibility to sepsis. In alcoholic cirrhosis, patients have impaired neutrophil phagocytosis and intracellular killing (313). Multiple organ failure is common in patients with cirrhosis and severe sepsis with excessive proinflammatory cytokine production likely playing an important role. Sepsis in cirrhotic patients may rapidly worsen baseline liver function, causing acute-on-chronic hepatic failure; increase the risk of ARDS and hyperdynamic circulatory failure; and interfere with the coagulation cascade with compromised hemostasis (145).
V. COAGULOPATHY IN SEPSIS
Disseminated intravascular coagulation (DIC) is an independent predictor of mortality in patients with sepsis and severe sepsis with shock. Multiple studies have shown that the severity of DIC is directly related to increased mortality (117, 211, 412). DIC is not a specific disease, but its presence underlies many diseases of grave consequence including sepsis, malignancy, autoimmune disorders, and liver diseases. DIC may also arise as a result of trauma, surgery, complicated pregnancy, or snake bites. DIC in sepsis is characterized by systemic activation of procoagulant mechanisms, loss of anticoagulant capability, and impaired fibrinolysis. Conditions that injure endothelium, activate platelets and monocytes, and compromise fibrinolysis result in inappropriate microthrombi formation within the microvasculature, contributing to ischemic injury and development of multiple organ failure. It is a dynamic process with consumption of platelets and coagulation factors that may paradoxically result in high risk of clinical bleeding.
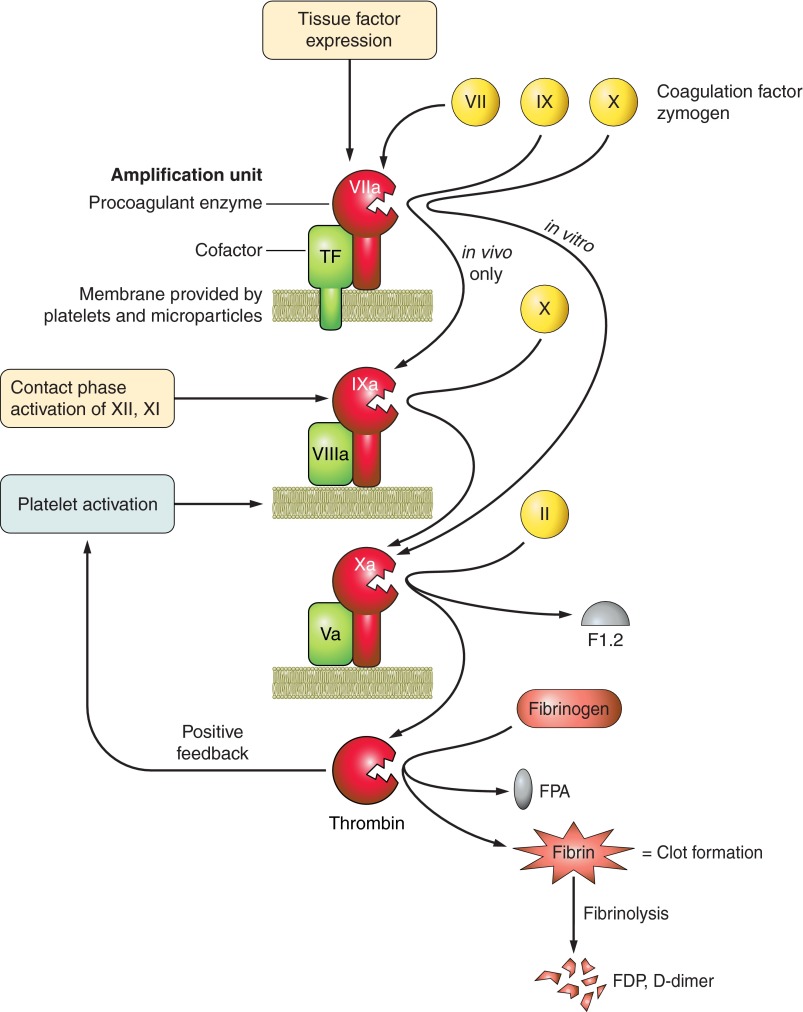
Exposure of extravascular tissue factor to blood components or via circulating microparticles from apoptotic cells (268) triggers the extrinsic and physiological coagulation pathway (Figure 2) that supports activation of factor X and assembly into the prothrombinase complex (factors Xa and Va, calcium) on anionic phospholipid cell surfaces. Bacterial surfaces can support the intrinsic coagulation pathway for factor XII activation and has been observed in children with meningococcemia and septic shock (441). Both initiating pathways result in factor Xa generation, and the prothrombinase complex proteolytically activates prothrombin to α-thrombin, the serine proteinase that selectively removes two fibrinopeptides from fibrinogen to create the aggregative fibrin. Platelets are incorporated into the developing clot, and the mesh is cross-linked and stabilized by factor XIIIa. Adhesive platelet-neutrophil interactions also promote local neutrophil disgorgement of DNA fibers and antimicrobial, proinflammatory histones, the neutrophil extracellular traps (NETs) that further stabilize the mesh (123). A robust fibrinolytic response, as measured indirectly by elevated fibrin degradation products (FDP) or d-dimer levels, is necessary to lyse the clots, and insufficient fibrinolysis is associated with multiple organ failure in patients (21).
Figure 2.
Clot formation. Coagulation is an amplified cascade that begins with exposure of a few initiating molecules and ends with many thousands of thrombin molecules. The initiators are tissue factor (extrinsic pathway; primary in vivo initiator of coagulation) or contact activation of factors XII and XI (intrinsic pathway). Subsequent reactions repeat a simple amplification unit: a cofactor (green) binds an enzyme (red) on a phospholipid surface (lime green). This assembled trimeric complex binds a circulating zymogen precursor (yellow) so that the complexed enzyme can cleave and activate it. A new enzyme is created, and this is incorporated into the next amplification unit for activation of the next zymogen precursor. Each step requires calcium (factor IV), and activated platelets provide the majority of the phospholipid surfaces needed. By this mechanism, the prothrombinase complex (factors Xa, Va, phospholipid) cleaves prothrombin (factor II), releasing fragment 1.2 and creating thrombin. Thrombin removes fibrinopeptides A and B from fibrinogen, which creates fibrin. The fibrin monomers aggregate and are cross-linked into an insoluble fibrin mesh. This provides surfaces necessary for the fibrinolytic pathway, another set of activators and enzymes that cleave the clot and release clot fragments (fibrin degradation products, FDP; d-dimer) into the circulation. Thrombin is pluripotent, acting as a coagulation enzyme, a mitogenic growth factor, and a proinflammatory mediator. Thrombin activates protease-activated receptors (PARs) on platelets and endothelial cells to propagate inflammation signaling. Many feedback loops in the coagulation cascade fine-tune clot formation to serve the needs of the immediate environment. In a patient with severe sepsis and DIC, uncontrolled coagulation results in consumption of coagulation factors and platelets, which paradoxically create a bleeding risk and considerable therapeutic challenges.
A. Diagnosing DIC
Not all sepsis patients develop coagulation abnormalities, but many do, and the severity varies widely from mildly prolonged clotting times to fulminant overt DIC with consumption of coagulation factors, fibrinogen, and platelets. Although a diagnosis of DIC might appear straightforward, it is in fact deceptively complicated, and it has only been in the past decade that a standardized definition and simple clinical scoring system for DIC was introduced (376) and validated (28, 383, 415). A Subcommittee on DIC organized within the International Society of Thrombosis and Haemostasis (ISTH) proposed diagnostic criteria for a DIC diagnosis and presented an algorithm for scoring DIC severity based on commonly used clinical tests (Figure 3) (376). It was based on an earlier scoring system developed by the Japanese Ministry of Health and Welfare (JMHW), whose efforts were among the first to standardize the diagnosis of DIC, and this scoring system is still used in Japan (192). The ISTH algorithm sought to distinguish nonovert DIC (where anticoagulant therapy would be beneficial) from overt DIC (bleeding risk) using a 1–5 severity score and universal clinical parameters. The ISTH DIC score has been validated and added significant prognostic value to the Acute Physiology and Chronic Health Evaluation (APACHE II) system (11). Identifying patients at risk for increasing coagulation abnormalities who would benefit from early intervention is a clinical priority and was addressed by the ISTH approach. A newer scoring algorithm has been proposed by the Japanese Association of Acute Medicine (JAAM), which appears to be sensitive for diagnosis of early DIC (127).
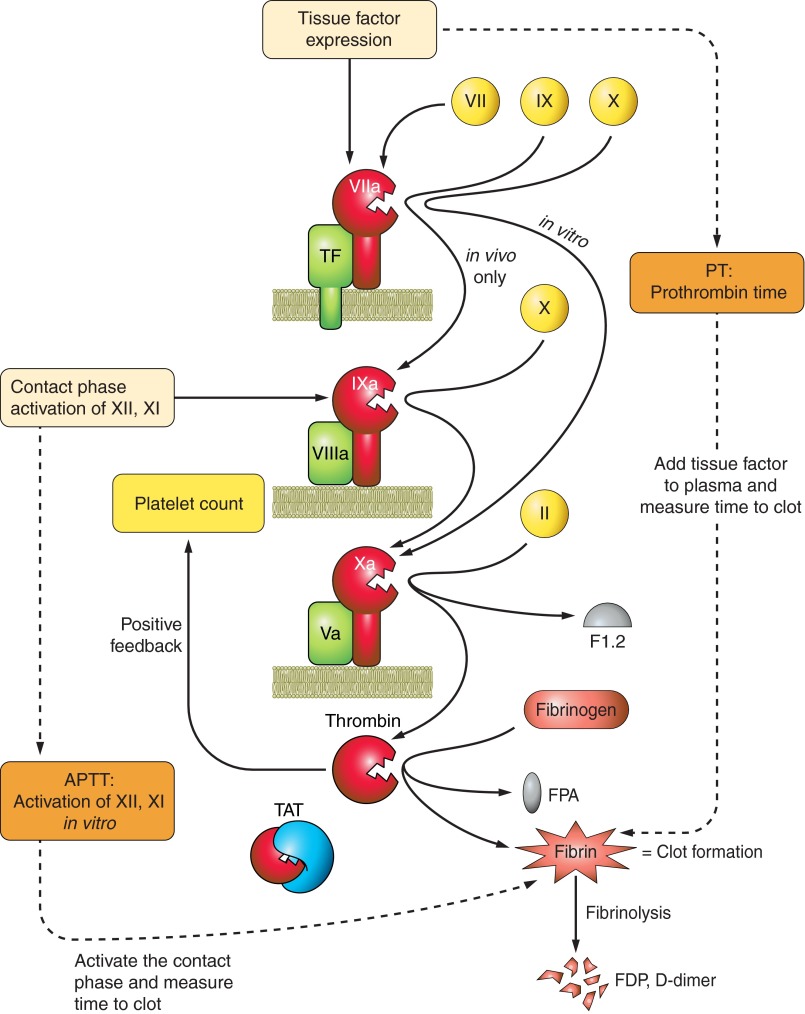
Figure 3.
Common coagulation tests. The most common clinical tests of coagulation measure the time for citrated plasma to clot. The prothrombin time (PT, orange box) is the number of seconds required for a clot to form, starting from the extrinsic pathway's amplification unit of tissue factor + VIIa + phospholipid. To obtain a PT, exogenous thromboplastin is added to citrated plasma as a source of tissue factor and phospholipid, calcium is added, and the time to clot is measured. PT results are usually reported as an international normalized ratio (INR) value, which permits comparison of values from different laboratories and different thromboplastin preparations. The activated partial thromboplastin time (APTT) is the number of seconds to make a clot starting from the intrinsic contact activation pathway. It is a “partial” clotting time because tissue factor is not present. A variety of immunoassays are available that measure products of coagulation or fibrinolysis. These are less commonly used clinically but still have great value. These assays include quantification of prothrombin fragment 1.2 (F1.2) generated during limited proteolysis of prothrombin to thrombin, the inactive thrombin-antithrombin complex (TAT), fibrinopeptide A (FPA) released during formation of fibrin, and pieces of cross-linked fibrin released during fibrinolysis of the clots (fibrin degradation products, FDP; d-dimer).
A recent 3-yr prospective study of 413 DIC patients compared the three scoring systems at admission (JMWH, ISTH, JAAM; Table 4) which use the same tests but with differing cut-off values (373). There were individual diagnostic strengths (JAAM, highest sensitivity overall and in septic patients; ISTH highest specificity for DIC) and collective weaknesses. None of the three systems was particularly good for diagnosis of DIC in trauma/burn patients, and all of them failed to diagnose late-onset DIC that occurred within 1 wk of admission. Despite these weaknesses, the three systems represent enormous progress over the last decade in forming internationally recognized consensus criteria for DIC diagnosis, without which anticoagulant clinical studies with sepsis patients were difficult and chaotic. Refinements to the scoring systems have considered evaluation of coagulation biomarkers such as antithrombin (93), or soluble thrombomodulin and protein C (416), to improve patient stratification and identification of those with non-overt DIC.
Table 4.
Different DIC scoring systems
| JMHW | ISTH | JAAM | ||||||
|---|---|---|---|---|---|---|---|---|
| Underlying disease | Absent | 0 | Underlying disease | Required | Underlying disease | Required | ||
| Present | 1 | |||||||
| Clinical presentation | No bleeding | 0 | Clinical presentation | No SIRS | 0 | |||
| Bleeding | 1* | SIRS | 1 | |||||
| No organ failure | 0 | |||||||
| Organ failure | 1 | |||||||
| Platelet counts | >120 | 0 | Platelet counts | >100 | 0 | Platelet counts | ≥120 | 0 |
| 120≥ and >80 | 1* | 100> and >50 | 1 | 120> and ≥80, or 30% reduction in 24 h | 1 | |||
| 80≥ and >50 | 2* | <50 | 2 | 80>, or 50% reduction in 24 h | 2 | |||
| ≤50 | 3* | |||||||
| FDP | <10 | 0 | Fibrin-related marker | Normal | 0 | FDP | < 10 | 0 |
| 10≤ and <20 | 1 | (e.g., soluble fibrin monomer, FDP) | 10≤ and <25 | 1 | ||||
| 20≤ and <40 | 2 | Moderate increase | 2 | 25≤ | 2 | |||
| >40 | 3 | Strong increase | 3 | |||||
| Fibrinogen | >1.5 | 0 | Fibrinogen | > 1.0 | 0 | |||
| 1.5≥ and >1.0 | 1 | < 1.0 | 1 | |||||
| ≤ 1.0 | 2 | |||||||
| PT ratio | <1.25 | 0 | Prolonged prothrombin time | < 3.0 s | 0 | PT ratio | <1.20 | 0 |
| 1.25≤ and <1.67 | 1 | 3.0< and <6.0 | 1 | ≥1.2 | 1 | |||
| ≥1.67 | 2 | > 6.0 | 2 | |||||
| Diagnosis | ≥ 7 | Diagnosis | ≥ 5 | Diagnosis | ≥ 4 |
Development of antithrombotics for sepsis patients with DIC was significantly hindered due to lack of consensus for diagnostic criteria for DIC. The three scoring systems shown are from the Japanese Ministry of Health and Welfare (JMHW), the subcommittee on DIC with the International Society of Thrombosis and Haemostasis (ISTH), and the Japanese Association of Acute Medicine (JAAM). The JMHW scoring system was the first to standardize the diagnosis of DIC. The ISTH system has the greatest specificity, is simplest to use, and has been validated in multiple clinical trials. The JAAM system is most sensitive for diagnosis of early DIC.
If hematologic malignancy, 0 point for Bleeding and Platelet and add 3 points to the total score
B. Targeting Coagulopathy in Sepsis
The paucity of evidence from well powered, randomized, placebo-controlled clinical trials to evaluate treatments for DIC gives rise to the cornerstone for treatment of DIC in sepsis patients, which is to treat the underlying disease, not the DIC (81, 213). This is due in part to the ongoing, but hopefully resolving, difficulties with internationally accepted diagnostic criteria as discussed above. Administration of fresh frozen plasma, platelet concentrates, or heparin is appropriate in some situations, but the risk of bleeding complications requires careful monitoring (213).
Theoretically, blocking thrombin or tissue factor activity is a sensible approach to the problem of rampant coagulation in severe sepsis, but to date, all efforts have been unsuccessful. There was some indication that antithrombin may be effective in subgroup analyses (KyberSept trial), but subsequent study of 20 antithrombin trials shows no survival benefit of antithrombin (8). Similarly, tissue factor pathway inhibitor (TFPI) was ineffective in a phase III double-blind, placebo-controlled trial of 2,138 patients with severe community acquired pneumonia (442). While TFPI anticoagulant activity was reflected in reduced F1.2 and TAT levels (Figure 3), there was no survival benefit.
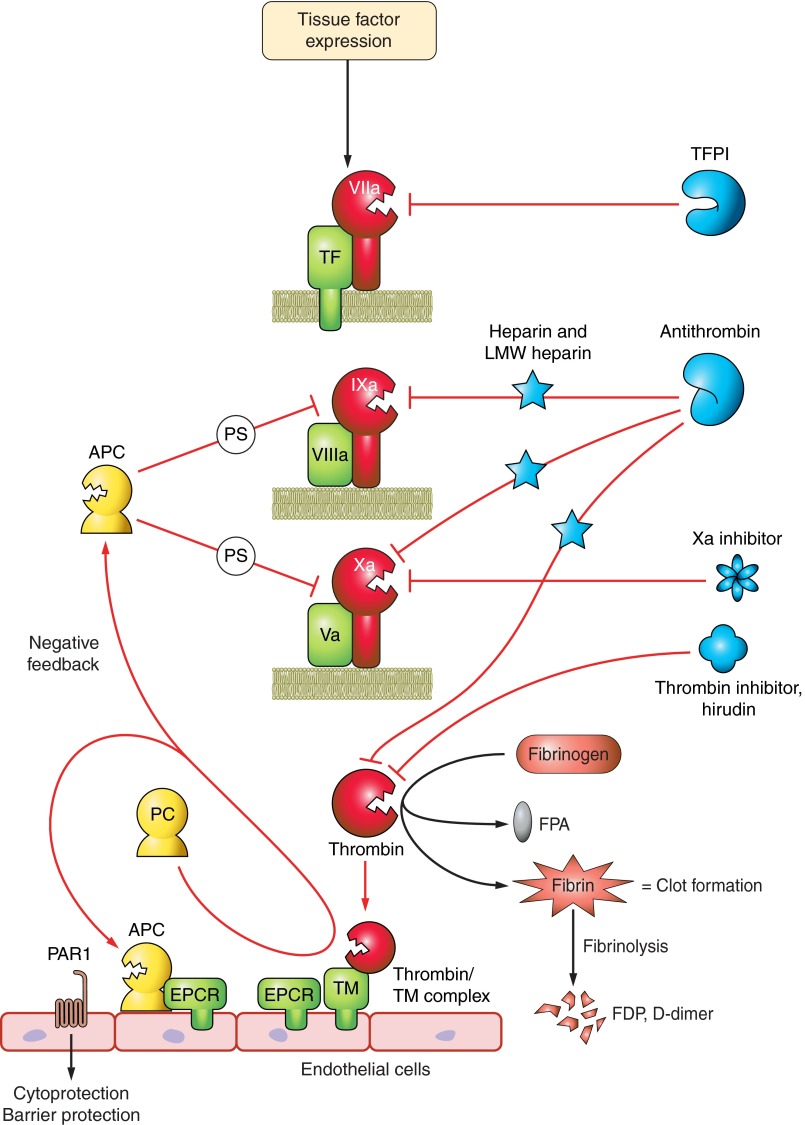
Another sensible approach is to target the coagulation cofactors factors Va and VIIIa, since these are the molecules primarily responsible for amplified production of thrombin. This is precisely the responsibility of the protein C pathway members. Circulating protein C zymogen binds to the endothelial protein C receptor (EPCR), which then presents it to a heterocomplex of thrombin bound to thrombomodulin (CD141), a transmembrane receptor and cofactor (Figure 4). When thrombin is in this complex, it cleaves protein C to activated protein C (aPC; Refs. 124, 361), the serine proteinase that degrades factors Va and VIIIa by limited proteolysis to effectively stop thrombin production (230). Until recently, recombinant activated protein C (drotrecogin alfa, activated; Xigris) was approved for use in patients with severe sepsis and multiple-organ failure. However, a limited survival benefit with increased bleeding risk (420), lack of effect in septic children (260), and lack of benefit in patients with severe sepsis but low risk of death (4) tempered enthusiasm. A recent industry-sponsored (Eli Lilly) study of 1,696 patients with septic shock (PROWESS-SHOCK) showed no difference in 28-day all-cause mortality with Xigris treatment compared with placebo-treated patients (26.4% in the drug arm vs, 24.2% in the placebo arm). Even though there was no increased bleeding risk (1.2 vs. 1.0%), the company withdrew the drug from the market (October 2011) and suspended all associated clinical trials. The results of a recent clinical trial in adults with septic shock showed that drotrecogin alfa did not improve survival at either 28 or 90 days (314).
Figure 4.
Controlling clot formation. Many natural inhibitors of coagulation target the amplification units that include tissue factor (initiator), factors VIIIa and Va (amplifying cofactors), and factor Xa (shared by intrinsic and extrinisic pathways). Tissue factor pathway inhibitor (TFPI) blocks the tissue factor pathway. The protein C pathway minimizes factors VIIIa and Va activities. This pathway on endothelial cells is responsible for making the activated protein C (APC) enzyme by the combined efforts of the endothelial protein C receptor (EPCR), thrombin, and thrombomodulin (TM). In concert with protein S (PS), activated protein C cleaves cofactors VIIIa and Va which slows clotting by orders of magnitude. APC also activates PAR-1, initiating signaling pathways that contribute to endothelial barrier protection. Antithrombin is the primary natural inhibitor of thrombin, but it also has broad specificity for several coagulation enzymes including factors IXa and Xa. Antithrombin activity is accelerated orders of magnitude by heparin and becomes a specific of factor Xa in the presence of low-molecular-weight heparin. Hirudin, originally derived from leech salivary glands and now available as a recombinant peptide, is a specific inhibitor of thrombin. The new chemical anticoagulants target factor Xa, at the junction of both arms of the coagulation cascade.
Manipulation of the protein C pathway remains an attractive target to alter coagulation because many studies have shown that protein C levels decline during the septic response, presumably due to consumption, and this correlates with poor outcome. To reduce the problem of increased bleeding risk with activated protein C, soluble thrombomodulin is being developed as adjunctive therapy. Recomodulin (ART-123; Asahi Kasei, Japan) is a recombinant human-soluble thrombomodulin-α, which retains its ability to bind thrombin and activate protein C, albeit at a slower rate than its endogenous membrane-bound counterpart (262). It was successful in an industry sponsored multicenter, randomized phase III trial of 231 DIC adult patients (JMHW criteria) with malignancy or infection (338). Compared with low-dose unfractionated heparin, Recomodulin was superior for resolving DIC symptoms (66.1 vs. 49.9%). Although the patient population with infection or sepsis was not well defined in this study, there was a trend in this population towards a lower mortality rate (6.6%) in the Recomodulin group compared with heparin (reduction in relative risk of death = 19.1%) with no differences in adverse events. A small independent study of 65 patients with severe sepsis and overt DIC (ISTH criteria) who required ventilator support showed that 28-day mortality was lower in patients treated with standard therapy and recombinant human soluble thrombomodulin (rhTM; n = 20) compared with patients receiving only standard therapy (n = 45) (adjusted hazard ratio, 0.303; 95% confidence interval, 0.106 to 0.871; P = 0.027) (447). Patients received 0.06 mg·kg−1·day−1 rhTM for 6 days with resolving SOFA scores by day 1 compared with controls. There were no differences in C reactive protein or recovery of platelet counts from controls, but possibly a faster decline in the fibrinolytic marker FDP.
A slightly different approach uses recombinant soluble thrombomodulin engineered with point mutations to enhance resistance against proteases and oxidation, Solulin (sothrombomodulin alpha; Paion AG). At low doses, circulating thrombin-soluble TM complex slows fibrinolysis by activating thrombin activatable fibrinolysis inhibitor (TAFI) and permitting clot stabilization (114), whereas at higher concentrations it preferentially generates activated protein C which is anticoagulant, profibrinolytic, and cytoprotective (251, 252, 446, 448). Consequently, low-dose Solulin is in phase Ib trials for cotreatment with factor VIII in hemophilia patients to prevent premature breakdown of clots needed to support hemostasis. Solulin was shown to be safe in healthy adult volunteers (intravenous bolus, 0.6–30 mg) without adverse events (402). While protective in rat venous or arterial thrombosis and stroke models (357, 368), Solulin has not been studied in DIC or sepsis models.
Reduced fibrinolytic capacity contributes to the severity of DIC and multiple-organ failure leading to continuing efforts to find ways to lyse clots more efficiently. TAFI is a procarboxypeptidase activated by plasmin, the thrombin-TM complex, and trypsin-like enzymes to remove exposed terminal lysines from fibrin which are needed for plasmin formation. A recent study from Buelens et al. (51) provides a novel approach to inhibiting TAFIa and promoting fibrinolysis using nanobodies. A nanobody is a single-chain monomeric antigen-binding immunoglobulin, naturally devoid of light chains, found uniquely in camelids (llama, alpaca, and Bactrian camels) (150). Nanobodies from an alpaca immunized with TAFIa showed broad specificity and sensitivity for functional inhibition of TAFI activation and good penetration into the clot (51). The small size of nanobodies (15 kDa) with relatively low immunogenicity has made them of interest in imaging applications as targeted probes for difficult to reach locations, such as tumors (404, 405). A humanized therapeutic nanobody against von Willebrand factor appears to be a safe and effective antiplatelet antithrombotic in coronary patients undergoing percutaneous coronary intervention (394, 403). Whether nanobodies will be pursued as therapeutics in sepsis or DIC remains to be seen, but there is great potential in this immune approach to manipulating inflammatory and prothrombotic mediators.
VI. ENDOPLASMIC RETICULUM STRESS RESPONSES IN SEPSIS
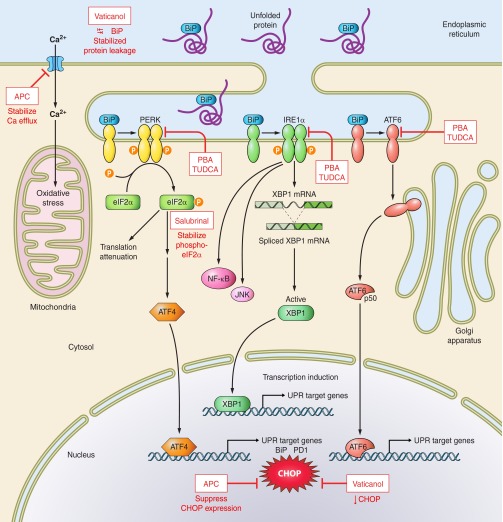
There are an estimated 1010-1014 proteins in existence with ∼4 × 104 distinct domains that contribute to functional diversity and cell survival (53). In eukaryotes, synthesis, maturation, and folding of these proteins occur in the endoplasmic reticulum (ER) using regulated pathways responsible for proper folding, posttranslational modifications, and distribution. The ER also has the highest cellular content of calcium, it contributes to lipid biosynthesis, and its oxidative environment is essential for disulfide bond formation. Cellular stresses due to hypoxia, infections, chronic or acute inflammation, or even aging will lead to accumulation of misfolded or aggregated proteins in the ER which will activate the unfolded protein response (UPR). Activation of the UPR was confirmed in hemorrhagic shock (91), endotoxic shock (195), hypoxia (379), and in septic mice (223). The UPR is an evolutionarily conserved response that provides resources for the cell to clear out misfolded proteins by shuttling them toward ubiquitin-proteosome degradation (ERAD), or in the face of overwhelming stress, to direct the cell to undergo apoptosis (371). This adaptive response stops new translation while upregulating transcription of molecular chaperones needed to assist in removing and redirecting the misfolded proteins to resolve the defect (440).
Under healthy nonstressed conditions, abundant binding immunoglobulin protein/78 kDa glucose-regulated protein (BiP/GRP78) binds to the luminal domains of three key ER sensor proteins and keeps them in an inactive state (Figure 5). When unfolded proteins are sensed in the ER lumen, the BiP chaperone dissociates (possibly via interactions with the misfolded proteins) and the sensor proteins initiate a series of phosphorylation and translocation events along their respective pathways that result in the UPR and the cell's path toward protein homeostasis or apoptosis. The molecular and regulatory mechanisms involved are described in comprehensive detail elsewhere in reviews specific to ER stress responses and the UPR signaling cascades (244, 418, 454), and are discussed here only to provide context for sepsis. The three sensor proteins held inactive by BiP are protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor-6 (ATF-6), and inositol-requiring protein-1 (IRE1). When PERK is released, it homodimerizes and phosphorylates the α-subunit of eukaryotic translation initiation factor-2α (eIF2α) which proceeds to inhibit assembly of the 80S ribosome and stop protein synthesis. Since there are already excess misfolded proteins, this pathway effectively halts additional production. In the early stages of infection, Mycobacterium tuberculosis takes advantage of this pathway by limiting eIF2α phosphorylation to promote intracellular survival (218). Yet there is still need for selective upregulation of certain mRNAs, such as heat shock proteins and other transcription factors, to provide the machinery necessary to repair the cell. For this purpose, ATF4 (CREB-2), a member of the AP-1 family and a key transcription factor, is upregulated as a result of eIF2α phosphorylation and targets genes necessary for amino acid metabolism, redox control, and apoptosis (337). A second key ER sensor is ATF6, a basic leucine zipper transcription factor, released from the ER membrane to translocate to the Golgi where it undergoes limited proteolysis and activation, and further migration to the nucleus to initiate transcription of genes need for the UPR response, including more BiP. Finally, when BiP dissociates at the luminal surface, IRE1 homodimerizes and autophosphorylates which releases its RNase activity. It removes an intronic sequence from the mRNA of X-box binding protein, creating a frame shift and a highly active transcription factor (spliced XBP1). The parallel pathways of these three key sensors are reasonably distinct, but they do overlap and coordinate functionally (208). The combined efforts of ATF4, activated ATF6, and spliced XBP1 induce transcription of the genes for chaperones, folding enzymes, and signaling molecules needed by the cell to clean out the defective proteins. However, the insult to the cell may be sufficiently severe or chronic such that none of these attempts to restore balance is successful. In this case, the UPR directs the cell to undergo apoptosis, mediated largely by upregulation of CHOP (CCAAT/enhancer-binding protein; GADD153) expression, a transcription factor which is well downstream of the three key ER stress sensors. In this way, the cell is either restored to homeostasis or selectively removed to maintain overall integrity.
Figure 5.
Endoplasmic reticulum stress responses. Under conditions of homeostasis and equilibrium, abundant BiP/GRP78 chaperone complex (shown in blue) is in contact with the three major membrane-associated sensors of the unfolded protein response: PERK, IRE1α, and ATF6. During sepsis, when a cell is injured and stressed, unfolded proteins accumulate in the endoplasmic reticulum (ER) lumen and bind to BiP, preventing BiP interactions with the membrane sensors. Once released from negative regulation, the three modulators initiate individual pathways that result in generation of transcription factors. Phosphorylated PERK activates and phosphorylates eIF2α which inhibits assembly of the 80S ribosome and generates ATF4 for gene transcription. IRE1α homodimerizes and autophosphorylates, releasing its RNAse activity to generate spliced XBP1 for nuclear translocation and transcription. ATF6 undergoes limited proteolysis and activation in the Golgi, eventually moving to the nucleus to stimulate target genes. The combined and interacting sensor pathways provide the necessary molecules to repair the cell or, in the face of overwhelming stress, to initiate apoptosis by upregulating CHOP. Inhibitors of ER stress (red boxes) including activated protein C (APC), an anticoagulant, and cytoprotective enzyme target the sensors or block CHOP expression.
A. CHOP Activities: Friend or Foe?
When CHOP translocates to the nucleus, it upregulates expression of proapoptotic molecules (e.g., TRB3, DR5, BIM, GADD34), downregulates anti-apoptotic Bcl-2, and promotes mitochondrial damage, cytochrome c release, and caspase activation (371). Apoptosis, particularly of immune cells, is a prominent pathological alteration observed during the septic response, and anti-apoptotic therapies can rescue mice from peritoneal and respiratory sepsis induced by bacteria (25, 164, 177, 179). While mechanisms responsible for caspase activation and mitochondrial dysfunction leading to cell death are well studied in sepsis models, much less is known about contributions of ER stress pathways. In the murine (CLP) sepsis model, splenic lymphocytes undergo extensive apoptosis, and the activation of UPR pathways was observed with upregulated BiP and CHOP expression, and activation of IRE1 as monitored by formation of spliced XBP1 (223).
CHOP also has activities other than promoting apoptosis, such as supporting inflammation. CHOP −/− mice have lower plasma TNF-α and IL-1β, reduced caspase-11 activation, and preserved lung integrity after intratracheal challenge with LPS (endotoxin), although responses to a septic challenge were not examined (97). CHOP expression also may be suppressed as a protective adaptive response under chronic ER stress conditions. Pretreatment of mice with low-dose endotoxin reduces apoptosis of splenic macrophages and suppresses CHOP expression via TLR4 signaling pathways that reduce transcription of ATF4 and its downstream target gene CHOP, despite normal functioning of the three key ER stress sensors (438). Endotoxemia is not sepsis, however, so whether this crosstalk between TLR and UPR pathways contributes to defense of the host during infection with a pathogen is not known.
Unfortunately, manipulating ER stress responses to ease the septic condition may not be straightforward. Under hyperoxic conditions, CHOP may be a necessary protector because CHOP null (Ddit3 −/−) mice are more susceptible to oxygen-induced lung edema, with loss of barrier function and injury (219), the clinical corollary being patients with respiratory failure requiring supplemental O2. This protection apparently is independent of the UPR pathway with no change in BiP, no XBP1 splicing, and no PERK phosphorylation and may be mediated by oxidative stress and a PRK kinase. Aging is a well-known contributor to sepsis severity (see section on comorbidities), and an age-related decline in proteasome activity leads to accumulation of misfolded proteins and ER stress in mice. Boosting the UPR and reducing negative UPR regulation in aged septic CLP mice with salubrinal 2 h after surgery enhanced eIF2α phosphorylation, restored UPR balance, and significantly increased the presence of peritoneal immune cells to clear bacteria and reduced plasma IL-6 levels at 24 h after challenge (39). The observation that the very young mice (3 wk) had a better balanced UPR with lower NFκB activation provides important insight into direct relationships between the UPR and inflammatory processes in the context of sepsis.
It is noteworthy that the IRE1 branch of the UPR is evolutionarily the oldest, and mammals have evolved two IRE1 isoforms: IRE1α is ubiquitously expressed, whereas IRE1β is restricted to the gastrointestinal and respiratory tracts (396). The vast majority of work has been done with IRE1α, so relative contributions of IRE1β are not well described. It is known that IRE1β contributes to regulation of lipoprotein assembly in intestinal CaCo-2 epithelial cells by suppressing mRNA of an ER chaperon (73). IRE1β also selectively degrades the mRNA of secretory pathway proteins in intestinal epithelial cells and has slightly different substrate specificity from IREα (261). The intestines and lungs have evolved sophisticated defensive immune mechanisms to deal with repeated exposure to pathogens, environmental insults, and toxins, and the restricted tissue expression of IRE1β to intestinal and respiratory tracts may contribute to ER stress adaptations in these microenvironments. In this regard, it is interesting that restricted protection of the intestinal environment with epidermal growth factor protects mice from Pseudomonas-induced pneumonia (83).
B. Manipulators of ER Stress for Potential Clinical Benefit
Considerable effort has gone into restoring cellular proteostasis in lysosomal storage diseases (e.g., Gaucher, Tay Sachs) by manipulating the UPR so that proper protein folding is encouraged and providing pharmacological chaperones to stabilize folded proteins, even mutant proteins (29, 255). Salubrinal, celastrol, indomethacin, and sodium salicylate affect the UPR pathways (46, 278, 425) and, as noted above, salubrinal stabilization of eIF2α phosphorylation was beneficial in the murine CLP sepsis model. These sites of stabilization are shown in Figure 5.
Given the anti-apoptotic and cytoprotective activities of activated protein C (see coagulation section), the recent report that activated protein C dampens ER stress responses in monocytes and protects them from oxidative stress and calcium efflux is interesting (384). In the presence of ER stressors, activated protein C modulated responses from the key stress sensor pathways by reducing BiP/GRP78, eIF2α phosphorylation, and CHOP expression. It is not known whether the enzymatic activity of activated protein C was required or if the site of action was surface or cytosolic, but the effects were not dependent on known cell surface-expressed receptors (EPCR, PAR-1). Since ER stress responses participate in many diseases yet activated protein C has significant clinical drawbacks due to high bleeding risk, one could surmise that recombinant activated protein C with reduced anticoagulant but preserved cytoprotective activities (250, 253) might be beneficial for relieving ER stress in a variety of disease contexts, including sepsis.
The cardioprotective benefits of resveratrol from red wine (32) has a substantial following, both scientific and in the general media, and structurally related compounds appear to alleviate cellular ER stress and inflammation. F9 embryonic carcinoma cells sensitive to ER stress (Herp null) are rescued from tunicamycin, a potent ER stressor, with vaticanol B which is a resveratrol tetramer isolated from the stem bark of Vatica rassak (372). This was accompanied by increased BiP/GRP78 expression, reduced CHOP expression, and stabilization of protein leakage from the ER, which collectively relieves ER stress. It also had anti-inflammatory effects as judged by reduced TNF-α, nitrite, and prostaglandin E2 production from LPS-stimulated RAW 264.7 cells.
Two chemical chaperones, 4-phenylbutyric acid (PBA) and tauroursodeoxycholic acid (TUDCA) (98), are United States Food and Drug Administration (FDA)-approved drugs for humans that may be beneficial in a sepsis environment with ongoing apoptosis, tissue ischemia, and energy derangement. PBA is a small anti-apoptotic fatty acid that can cross the blood-brain barrier to exert chaperone activities in the central nervous system (176, 432), and it protects against liver ischemia/reperfusion injury by downregulating ER stress and apoptosis (409). TUDCA is a hydrophilic endogenous bile acid approved for treatment of primary biliary cirrhosis. TUDCA has anti-apoptotic activity by inhibiting Bax translocation and limiting mitochondrial-mediated caspase activation. Metabolic energy dysfunction is a prominent feature of the septic response, so it is notable that both PBA and TUDCA can reduce expression of ER stress markers and restore glucose uptake and insulin receptor signaling in peripheral tissues of the ob/ob murine model of type 2 diabetes (290). While there are good reasons to exercise caution when modulating pathways fundamental to all cells, the fact that manipulation of ER stress responses and the UPR in complex, multidimensional diseases such as diabetes, atherosclerosis, and neurodegenerative diseases provides positive outcomes suggests that the field of sepsis might benefit as well. Understanding these pathways in chronic situations has particular application in the study of sepsis, which has a complicated transition from acute to chronic phases.
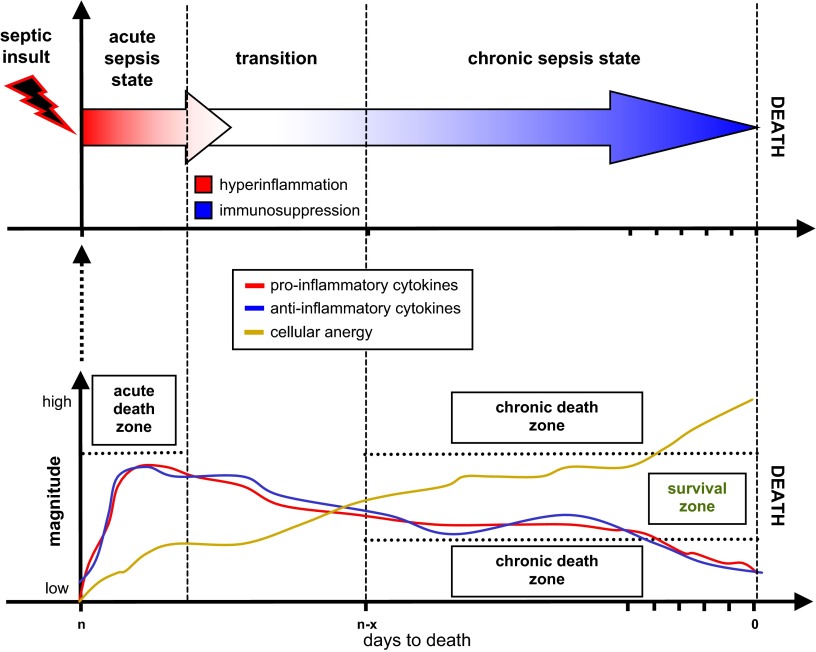
VII. ACUTE VERSUS CHRONIC SEPSIS
Dividing sepsis into the acute and chronic stages solely by specific time points may be misleading and does not substantially contribute to our understanding of the septic response. In the clinical setting, this is primarily because the time of the onset of sepsis is often unknown. In patients diagnosed in the emergency department (ED), sepsis may have been progressing for a number of hours or days, and in those already hospitalized, it may have also have been initially undetected. The latter is especially common in patients with comorbidities and those recovering after major trauma/surgery. Given their already weakened immune system, patients with sepsis, even if diagnosed very early, may present with evidence of immunosuppression without generating a typical, exaggerated acute phase inflammatory response. In preclinical models, the time of sepsis onset is known, making it easier to identify the stage of sepsis.
Demographically, septic patients are typically over 50 years of age (14), and the consensus is that a larger number of septic patients die in the late (chronic) stages of sepsis (3, 110, 111, 189, 292). The latter is the consequence of the growing sophistication of the ICU care that typically keeps septic patients alive during the early (acute) stage of the disease. Thus, from the clinical perspective, one would expect that in a majority of septic patients late deaths will be heralded by distinct immunosuppression rather than overwhelming inflammation (161). This creates a marked disparity between early phase-oriented studies and clinical reality of protracted sepsis.
A. Humoral Component
Historically, the prevailing belief has been that an initial bacterial (or an alternative microorganism) stimulus provokes an early SIRS, a spectacular inflammatory reaction, often referred to as “cytokine storm.” This storm is characterized by a systemic release of inflammatory cytokines including IL-1, IL-6, TNF-α, and IFN-γ (55, 137). This is indeed true in meningococcal sepsis (148), and the magnitude of the cytokine release appears proportional to the preseptic immunoinflammatory status of an affected patient (the healthier the patient the more robust the response) (286, 401).
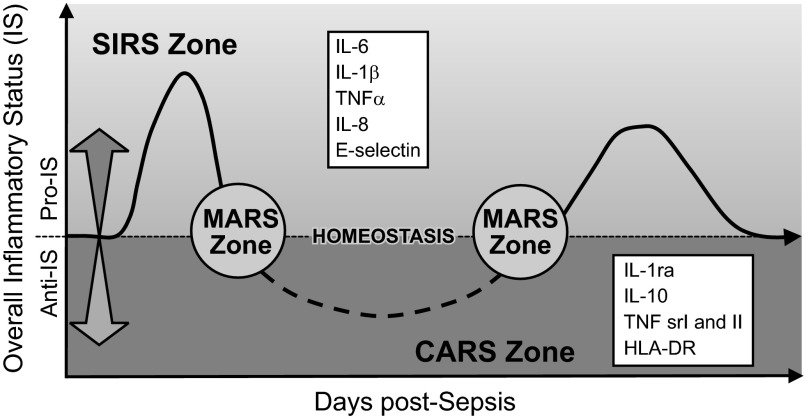
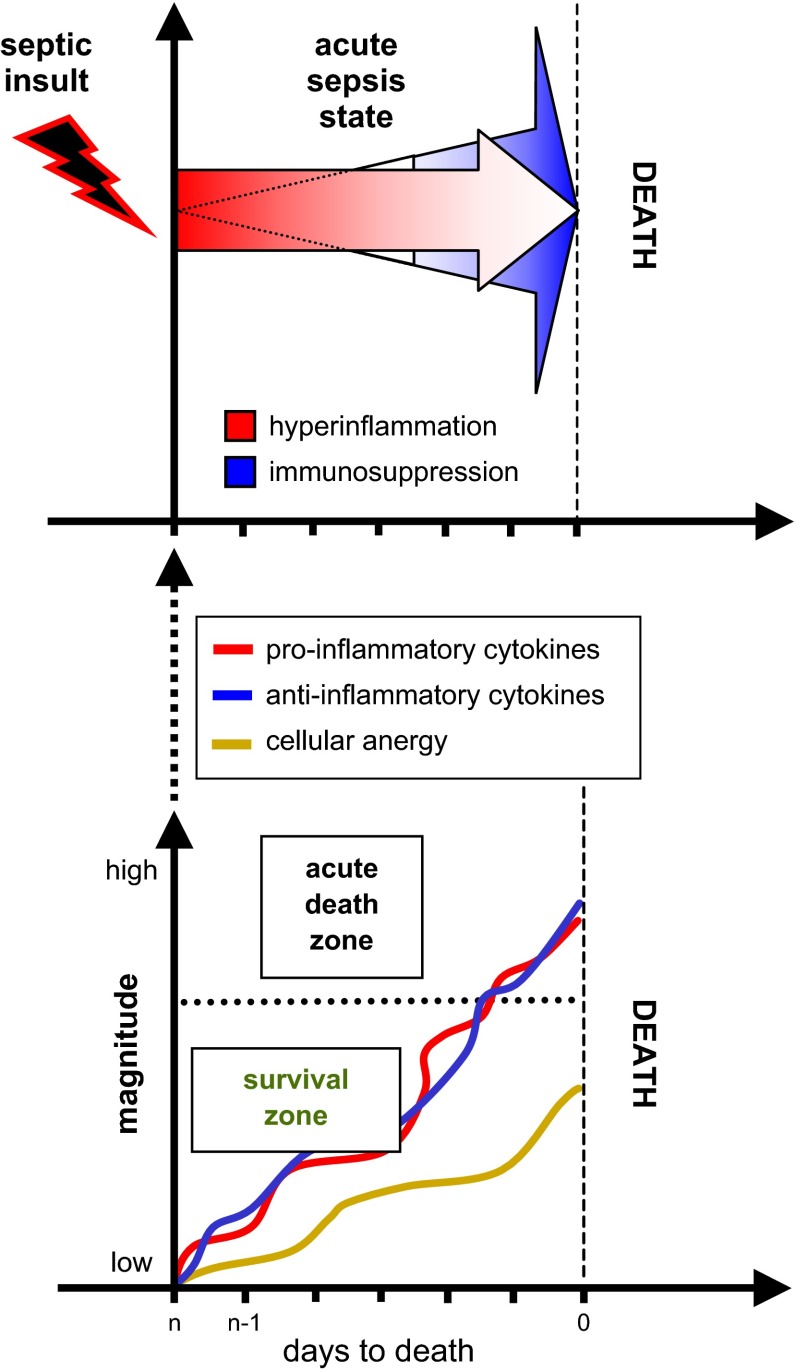
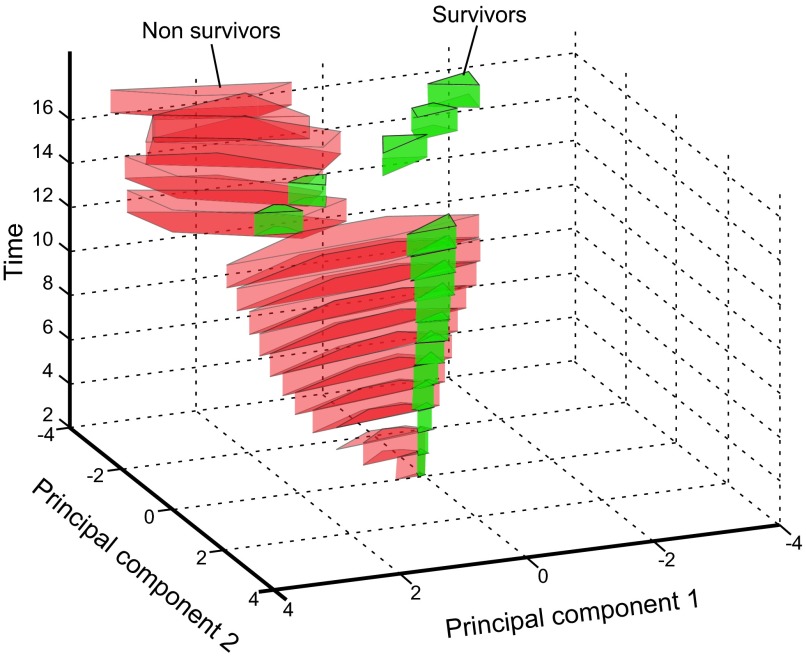
Despite these papers, close examination of the sepsis milieu reveals a far more complex process. It was quickly noted that the acute SIRS concept is too narrow given that the “cytokine storm” is not a typical occurrence, especially in late sepsis as well as in those acutely septic patients with an already weakened immunoinflammatory system. In both these situations, the systemic release of inflammatory cytokines is either markedly lower (49, 326), features a different marker composition (23, 77), or shows the absence of selective cytokines (310, 414). To classify those phenomena, in his landmark paper Bone (42) subjectively divided sepsis into two phases: the acute-phase SIRS versus the CARS in the chronic phase. The first phase of SIRS was classified by the presence of circulating TNF-α, IL-1β, and IL-6. The second phase of CARS was synonymous with a hypoinflammatory and immunosuppressive state that was classified by detectable concentration of IL-10 and increase in IL-1ra and TNF-soluble receptors. Additionally, a MARS was coined to reflect a temporary balance between SIRS and CARS when an acute (hyperinflammatory) SIRS gives way to chronic (hypoinflammatory) CARS. Oberholzer et al. (276) elegantly portrayed this concept as a linear transition of an early SIRS into the chronic CARS with possible alternating reoccurrences of both phases (via transitory MARS) during the protracted course of the disease (Figure 6). That same year Cavaillon (59) also published a graphical representation of patients in SIRS and CARS. The Oberholzer concept has been reproduced in numerous adaptations, yet it misinterprets key aspects of acute versus chronic responses. Relevant animal models (286, 287) demonstrated that regardless of outcome, acute (CLP) sepsis provokes a simultaneous release of both pro- and anti-inflammatory cytokines into the blood from the onset of the disease (Figure 7). The mixed response pattern in CLP mice has previously been observed in some septic patients (120, 148) and was corroborated by the most recent clinical evidence (271, 374). Thus classification of SIRS and/or CARS contingent on the mere presence of either classical pro- or anti-inflammatory mediators in the blood appears incorrect. Consequently, the concept of defining the patient's inflammatory status as either hyper- or hypoinflammatory by relying on the selection of circulating cytokines is also false: a septic subject with a high blood concentration of anti-inflammatory IL-10 and IL-1ra (thus meeting classical requirements for CARS), yet with a similarly robust release of IL-6 and TNF-α (fulfilling SIRS criteria) can be hardly defined as hypoinflammatory (and vice versa).
Figure 6.
Concept of the bimodal evolution of the systemic immunoinflammatory response in sepsis. After septic stimulus, there is an immediate and strong shift towards hyperinflammation (SIRS, overall proinflammatory status) defined by an excessive release of the classical proinflammatory cytokines (top box) into the blood. Over time, predominating SIRS gradually subsides and the septic host enters the hypoinflammatory phase (CARS, overall anti-inflammatory status) characterized by a robust release of anti-inflammatory cytokines (bottom box). The temporary transition period between SIRS and CARS zones is defined as MARS and features an approximate balance between the circulating pro- and anti-inflammatory mediators. A septic subject can undergo alternating shifts toward either SIRS or CARS. [Modified from Oberholzer et al. (276), with permission from Lippincott Williams & Wilkens.]
Figure 7.
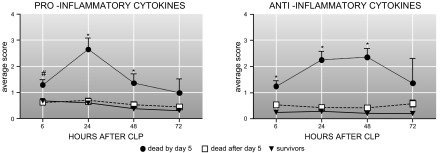
Release of pro- and anti-inflammatory cytokines in animal dying and surviving the acute phase of polymicrobial sepsis is simultaneous and of similar magnitude. Average inflammatory scores for each block were computed by retrospectively combining selected proinflammatory (A; IL-1β, IL-6, TNF-α, KC, MIP-2, MCP-1) and anti-inflammatory (B; IL-1ra, IL-10, TNF srI, and II) mediators and dividing them according to outcome. Data are presented as means + SE. *P < 0.0001, #P < 0.005. Data were generated in the mouse model of CLP sepsis. [From Osuchowski et al. (287). Copyright 2006. The American Association of Immunologists, Inc.]
If the blood of an acutely septic patient is replete with both pro- and anti-inflammatory mediators, what are the humoral blueprints of the chronic phase? It has been suggested that as sepsis progresses, the concentration of anti-inflammatory mediators tends to predominate over the still present but markedly less pronounced proinflammatory component (75, 161). This concept has been difficult to verify, especially in the clinical setting. Apart from the aforesaid difficulties with the temporal classification and confounding comorbidities, the multisource origins of sepsis further complicate any categorization attempt as they may produce divergent pattern/intensity of mediator release (133). Interestingly, murine studies investigating late sepsis showed an outcome-independent presence of a relatively steady proinflammatory and anti-inflammatory equilibrium in the chronic phase (albeit at much lower magnitude compared with acute sepsis) (285, 287, 288). Due to limited evidence, a clear characterization of humoral fluctuations in chronic sepsis is pending.
From the humoral standpoint (diagnostically most relevant) however, the mixed cytokine response pattern better matches the original Bone's criteria of the transitory MARS (42) rather than SIRS and/or CARS. This was subsequently formulated in the “Sepsis: Always in MARS” paradigm (283) that implies a constant equilibrium of pro- and anti-inflammatory mediators in the blood over the course of sepsis. Thus a hyperinflammatory SIRS, typically present on the onset of sepsis, should be defined as a general ability of the host to generate a response with proinflammatory and anti-inflammatory mediators.
B. Cellular Components
The above is further complicated by yet another key variable: immunocompetent cells. The state of immunosuppression or immunoparalysis, closely dependent on the cellular functionality, is often associated with later and more chronic disease stages. In sepsis, however, numerous preclinical and clinical studies demonstrated that during the acute phase, manifestations typical of immunosuppression may be seen. In several studies cellular indicators of immunosuppression appear to coincide with the release of inflammatory cytokines of the acute sepsis period. This phenomenon has been attribute to the “compartmentalization” of the inflammatory response (59, 60). Clinically, T-cell proliferation and production of IL-2 and TNF-α were most severely diminished in dying patients with postoperative intra-abdominal (154) and posttraumatic sepsis (304), and these markers of immune dysfunction were observed at the onset of sepsis. In severe sepsis, decreased monocytic HLA-DR expression and reduced macrophage antigen presentation (82, 390) were simultaneous with systemic hyperinflammation and also were found at the very onset of the disease.
The process of immunosuppression should be viewed as concurrent with the hyperreactive release of proinflammatory cytokines. Prior to early deaths (Figure 8), cellular exhaustion develops rapidly and coincides with the pronounced (MARS-like) cytokine production and release. In turn, chronic deaths (Figure 9) are preceded by progressively severe dysfunction of cellular compartments and with the presence of MARS-like cytokine response. It is important to note that sepsis is acknowledged to be an extremely dynamic process, and there is considerable heterogeneity in the human response to infection. Therefore, it would not be surprising that in studies of the host response during sepsis, day 1 measurements of immune markers in one patient may not correspond to measurements taken in another patient resulting in a heterogeneity of data, thus making it potentially misleading to stratify patients into distinct groups based on the time of clinical presentation. From the perspective of the patient's immunoinflammatory status, allocation of sepsis as either hyper- and hypoinflammation strictly along the axis of time (i.e., into either acute or chronic phases) appears artificial. Given that all the components described above are virtually superimposable, septic hyper- and hypoinflammation are better defined as function-dependent entities than the time-independent entities. Finally, their classification should be perhaps based on an overall capacity of a diseased host to respond rather than by the sheer presence of individual mediators/cells in the patient's plasma and/or exudative fluids.
Figure 8.
The immunoinflammatory trajectory in a subject dying from the acute-type septic response. The scheme delineates immunoinflammatory fluctuations using the time of death as the reference point given that the time of the sepsis onset in patients is typically unknown. As the severity of sepsis progresses, the magnitude of the systemic pro- and anti-inflammatory cytokine response and cellular anergy increases. A prelethal immunoinflammatory status of a subject dying from acute sepsis is characterized by both a MARS-like cytokine profile (concurrent presence of both pro- and anti-inflammatory mediators in the blood) and distinct signs of anergy in the cellular compartment. An acute inflammatory response (hyperinflammation), although typically associated with early septic deaths, may occur at any chronological phase of the disease if a septic organism is sufficiently immunologically responsive. The scheme is largely based on data generated in the mouse model of CLP sepsis.
Figure 9.
The immunoinflammatory trajectory in a subject dying from the chronic septic response. The scheme delineates immunoinflammatory fluctuations using the time of death as the reference point given that the time of the sepsis onset in patients is typically unknown. As the severity of sepsis progresses, the magnitude of the systemic pro- and anti-inflammatory cytokine response wanes and becomes erratic while the degree of cellular anergy increases. A prelethal immunoinflammatory status of a subject dying from the chronic-type septic response is characterized by a deteriorating but MARS-like cytokine profile (concurrent presence of both pro- and anti-inflammatory mediators in the blood) and robust signs of anergy in the cellular compartment. A chronic inflammatory response (hypoinflammation), although typically associated with late septic deaths, may also occur at an early chronological phase of the disease if a septic host is immunologically unresponsive. The scheme is largely based on data generated in the mouse model of CLP sepsis.
1. Antigen presentation and the cellular response
Macrophage and dendritic cells are key participants in the evolution of the immune response through early detection of invading microbes and subsequent activation of other cells. However, studies indicate that many of these functions are compromised during sepsis. Monocytes isolated from animal models and patients with sepsis release lower amounts of proinflammatory cytokines when stimulated with Toll-like receptor ligands, suggesting that their capacity to sense and respond to new infections is impaired (6, 112, 220, 257). In septic humans, the expression of HLA-DR is markedly decreased (174, 257); however, survivors demonstrate a significant recovery of HLA-DR expression compared with nonsurvivors (247, 248, 439). It has also been found that peripheral monocytes isolated from septic humans and mice produce less IL-12 in response to stimulation ex vivo with TLR ligands than those from healthy controls, yet cells from patients that survive sepsis produce greater amounts of IL-12 than those from nonsurvivors. In contrast, levels of IL-10 are elevated in nonsurvivors relative to survivors (113, 359, 439). In both experimental models and patients with sepsis, APCs demonstrate decreased capacity to stimulate the T-cell proliferative response compared with healthy controls (113, 227, 246). These findings indicate that during sepsis there is a marked loss in the immune system's ability to detect microbes and appropriately activate other cells involved in the host response, and that reversal of these abnormalities may contribute to survival.
2. Role of CSMs in sepsis
There had been increasing interest in the role of costimulatory molecules (CSMs) in cell-cell communication during the host response in sepsis. CSMs expressed on APCs such as dendritic cells and macrophages participate in the regulation of T-cell activation through providing the essential “second signal” resulting in T-cell activation and proliferation, or anergy and apoptosis (136).
The potential significance of costimulatory signaling pathways in the immune response during sepsis was highlighted after a clinical trial of a monoclonal agonist to CD28 (a CSM receptor on lymphocytes), during which humans that received the antibody developed a severe inflammatory response and clinical signs and symptoms typical of sepsis (369). The B7 family is so far the best-studied group of CSM in sepsis. It includes CD80 and CD86 which induce T-cell activation via the CD28 receptor on lymphocytes, but are also able to provide a negative-feedback signal to limit proliferation via the CTLA-4 receptor. The expression of CD80 and CD86 differs during sepsis in humans. Initially CD80 on circulating monocytes is elevated relative to healthy controls, with higher levels associated with shock, suggesting a negative benefit of CD80, although there is no clear association with survival. Comparatively, CD86 is initially decreased in patients with septic shock relative to healthy controls and patients with infection alone; however, recovery of CD86 expression is observed in survivors versus nonsurvivors (269, 270, 331). Expression of the CSM CD40 on macrophages has been found to be necessary for effective phagocytosis of bacteria (346). When T cells are activated, CD40L expression is increased, which upon binding to CD40 on APCs further upregulates expression of CD80 and CD86, as well as release of IL-12 promoting continued T-cell activation and differentiation. CD40 expression is increased on PBMCs in humans with sepsis with higher levels associated with shock (134). However, higher levels of expression have also been reported to correlate with survival, and mice who receive an agonistic anti-CD40 antibody have decreased lymphocyte apoptosis and improved survival in a double injury CLP/pneumonia (345). PL-L1 and PD-L2 are CSMs that deliver inhibitory signals via the receptor PD-1, resulting in T-cell anergy and apoptosis. PD-L1-PD1 signaling has become a focus of research interest as a potential mechanism causing the prominent degree of lymphocyte apoptosis and dysfunction observed during sepsis (165). PL-L1 expression on monocytes and PD-1 expression on lymphocytes are increased in both animal models and patients with sepsis and are associated with increased occurrence of secondary nosocomial infections (143, 456, 457). PD-1−/− mice subjected to CLP show a lower mortality and decreased inflammatory cytokine production and bacterial load, both systemically and locally in the peritoneum. (169). In addition, administration of anti-PD-1 antibody to mice after onset of CLP-induced sepsis decreased lymphocyte apoptosis and improved survival (47).
3. Lymphocytes
Patients with sepsis undergo marked apoptosis of the CD4+ and CD8+ T and B lymphocyte populations. This has been found in all lymphoid organs including the spleen, thymus, lymph nodes, and gastrointestinal-associated lymphoid tissue (335, 382, 424). The loss of immune cells is thought to be a significant contributor to the pathophysiology as reversal of apoptosis has been associated with improved survival in animal models of sepsis. Although it is not yet clear what are the specific triggers that cause this cell death, decreased antiapoptotic gene BCL2 expression and altered signaling by the costimulatory molecules CD40 and PD-1 are being investigated as possible etiologies (166, 345, 457). Recent work suggests that B lymphocytes may also contribute to survival during sepsis through producing GM-CSF and/or type I interferon early during the innate immune response. In a CLP mouse model of sepsis involving depletion of mature B cells via anti CD20 or using μMT−/− mice (in which B-cell maturation is arrested), a decreased production of IFN-I induced inflammatory cytokines and survival was observed. Survival and cytokine production were partially restored with adoptive transfer of wild-type B cells, serum from wild-type mice, or treatment with CXCL10, the IFN-I-inducible chemokine. Interestingly, mice in which B cells were unable to produce GM-CSF had a lower survival rate post CLP but conversely were found to have higher levels of IL-1β, IL-6, and TNF-α. Factors that may account for the divergent cytokine findings include differences in the strains of mice used, severity of CLP model, and timing of sampling.
VIII. BIOMARKERS
In the area of sepsis research, the use of biomarkers are as exciting as they are frustrating; close to 200 biomarker candidates in nearly 4,000 studies have been evaluated to date (301). Given that several recent review articles have exhaustingly characterized multiple biomarkers (61, 185, 231, 316), this section aims to summarize the current status using the most relevant examples.
In sepsis, interest in biomarkers was triggered by the early realization that routine physiological and laboratory parameters, the main building blocks of various ICU scoring systems (e.g., APACHE and SOFA), lack diagnostic precision. Given the delay (typically 48–72 h) (249) and frequently false negative blood cultures (69), the potential use of circulating biomarkers for rapid detection of sepsis and predicting outcome was actively explored. Historically, procalcitonin (PC) showed the most promise for accurately separating septic from noninfectious SIRS subjects (397, 421), especially after CRP failed to adequately discriminate patients (231, 391, 410). The initial enthusiasm for PCT was justified by consistent and strong increases of circulating PCT in adult (50, 348) and pediatric (20, 354) septic patients. After this initial enthusiasm, several reports revealed irregularities of PCT's performance (128, 370, 375, 388), reducing the initial diagnostic allure. At present, PCT holds only a single official recommendation for use in clinical practice, i.e., as an adjunctive tool for discrimination between the presence or absence of infection in critically ill adults developing new fever (274). Several other biomarkers were tested clinically, but the data do not support any of these as a diagnostic bedside test (Table 5).
Table 5.
Selected circulating biomarkers assessed in clinical studies for diagnosis of sepsis. At least 49 patients enrolled in any of the studies. Studies listed in the reversed chronological order starting with the most recent, maximally 4 studies per biomarker listed. Selected multiple-biomarker studies listed more than once for separate biomarkers
| Biomarker* | Number/Type+ of Patients | Comment |
|---|---|---|
| aPTT | All adult | |
| Study 1: 200 patients | Study 1: combination with PCT increased specificity (450) | |
| Study 2: 49 patients | Study 2: neutropenic hemato-oncology patients (172) | |
| CD64 | All adult | |
| S1: 293 patients | S1: sepsis versus SIRS (138) | |
| S2: 156 patients | S2: bacterial versus viral versus SIRS; CD35 also assessed (180) | |
| S3: 112 patients | S3: septic versus healthy controls; PCT also assessed (57) | |
| S4: 135 patients | S4: bacterial versus viral infection (272) | |
| EA Complex | Pediatric: 74 patients | Diagnosis of neonatal sepsis; combination with immature to total neutrophil count ratio improved accuracy; CRP also assessed (377) |
| G-CSF | All pediatric | |
| S1:156 patients | S1: diagnosis of bacterial and fungal sepsis; high negative predictive value (184) | |
| S2:190 patients | S2: confirmed versus suspected sepsis versus local infections; combination with IL-8 improved accuracy; IL-1ra also assessed (108) | |
| IL-1ra | All pediatric | |
| S1: 182 patients | S1: an increase 2 days before the sepsis onset; IL-6 and ICAM-1 also assessed (203) | |
| S2:190 patients | S2: confirmed versus suspected sepsis versus local infections poor performance, G-CSF and IL-8 also assessed (108) | |
| IL-12 | Pediatric: 112 patients | Diagnosis of neonatal sepsis, high specificity, low sensitivity; IL-10 also assessed (353) |
| IL-8 | All pediatric | |
| S1: 123 patients | S1: confirmed versus suspected sepsis; IL-6, IL-10, IL-18, INF-γ, TNF-α, PCT, CRP also assessed (35) | |
| S2: 92 patients | S2: confirmed versus suspected sepsis; IL-6, PCT, CRP also assessed (408) | |
| S3: 190 patients | S3: confirmed versus suspected sepsis versus local infections; IL-1ra, G-CSF also assessed (108) | |
| IP-10 | Pediatric: 155 patients | Recognition of bacterial sepsis and necrotizing enterocolitis; TNF-α, IL-1β, 6, 8,10, 12 and MCP-1, GROα, RANTES also assessed (265) |
| LBP | S1: Adult/Pediatric: 57 patients | S1: cancer patients with febrile neutropenia; CRP also assessed (289) |
| S2: Pediatric: 140 patients | S2: bacteremia versus no bacteremia; IL-6, CRP, HMGB-1 also assessed (295) | |
| S3: Pediatric: 92 patients | S3: patients with versus without bacterial sepsis; CD46 and PCT also assessed (139) | |
| S4: Adult: 185 patients | S4: bacteraemia versus no bacteremia; PCT and HMGB-1 also assessed (125) | |
| MCP-1 | Pediatric: 76 patients | Patients with hematologic malignancies; CRP and IL-8 also assessed (95) |
| neopterin | S1: Adult: 208 patients | S1: SIRS versus septic shock in ICU patients; PCT also assessed (336) |
| S2: Adult: 56 patients | S2: postoperative SIRS versus sepsis; EA also assessed (291) | |
| suPAR | Adult: 151 patients | SIRS patients with versus without bacterial infection; TREM-1, CRP, PCT, MIF also assessed (193) |
| TREM-1 | S1: adult: 631 patients | S1: emergency department patients; HMGB-1 and CD64 also assessed (126) |
| S2: adult: 114 patients | S2: sepsis versus SIRS; PCT also assessed (207) | |
| S3: pediatric: 52 patients | S3: SIRS versus late onset neonatal sepsis; IL-6 also assessed (341) | |
| S4: adult: 95 patients | S4: sepsis versus SIRS; CRP also assessed (30) |
Reference numbers are given in parentheses.
Modified from Pierrakos and Vincent (301). +Pediatric population includes newborns, neonates, and children up to the age of 18. S, study; aPTT, activated partial thromboplastin time; EA, elastase alpha 1-proteinase inhibitor; G-CSF, granulocyte colony-stimulating factor; IL, inteleukin; IP, interferon-induced protein; LBP, lipopolysaccharide binding protein; MCP, monocyte chemotactic protein; suPAR, soluble urokinase-type plasminogen activator receptor; TREM, triggering receptor expressed on myeloid cells.
In the quest for an infallible separation of infectious from noninfectious SIRS patients, two imminent diagnostic drawbacks appear to be somewhat overlooked. Upon a hypothetical discovery of the perfect marker(s), 1) the causative pathogen still will not be identified, and 2) the patient's immunoinflammatory status will likely remain unclear. The clear benefits of an earlier decision-making for initiation or withholding of a broad-range antimicrobial treatment (88, 198) could turn into a lethal flaw if an ineffective antibiotic is given (197). Similarly, an aggressive, life-saving immunomodulatory intervention might actually increase mortality when applied indiscriminately (109). Both flaws could be perhaps remedied, at least partially, by a rapid detection of bacterial/fungal DNA in blood or tissues (160, 225, 226) or employment of gene array-based immunoinflammatory profiling (437).
Two additional biomarker-related areas of interest lie in identifying septic patients along the spectrum of disease severity and risk of death to accurately define their underlying immunoinflammatory pattern. Both topics have attracted attention only recently as the awareness on the complexity of septic responses (161, 317) and the need for more homogeneous treatment cohorts (15, 149) had emerged as described in the above sections concerning acute versus chronic sepsis. A plethora of novel and old biomarkers including cytokines (302, 351, 356), PCT (131, 182), CRP (158, 308), neopterin (315, 367), pentraxin (173, 256, 406), suPAR (84), TREM-1 (453), NT-proBNP (407), and NT-proCNP (27) have been considered hopeful for predicting sepsis survival. These and other examples are covered in the excellent reviews by Pierrakos and Vincent (301) and Lichtenstern et al. (217). Overall, there is a consensus that simultaneous evaluation of multiple biomarkers and their serial measurements should be favored over the single time-point and single target approach. This applies both to identification and monitoring of the immunoinflammatory status (175, 186, 240) as well as prediction of the ICU outcomes (56, 131, 315, 389).
Yet another promising philosophy of harnessing biomarkers into effective treatments against sepsis can be directly adapted from oncology, namely, to identify patients who will benefit from specific therapy based not only on the disease severity (i.e., likelihood of dying or surviving) but rather on his/her own unique metabolic and physiological make up (once unequivocally identified). For example, in breast cancer, the absence of the estrogen receptor is used as an indicator to not treat with anti-estrogen drugs like tamoxifen (38), whereas presence of the epidermal growth factor receptor 2 (HER-2) is likely to result in a positive response to therapy with trastuzumab (anti-HER-2 monoclonal antibody) (298). Thus linking biomarkers responsible for specific processes to various anti-sepsis medications should improve the accuracy of sepsis interventions. The successful use of early goal-directed therapy protocols (329) and the corticotrophin-directed hydrocortisone therapy (358) can be viewed as harbingers of such an approach. In addition to targeting patients with similar sepsis severity, in both cases treatment decision making rests on specific “physiological function biomarkers” (i.e., cardiac load and short corticotropin test) that aim to identify patients with higher likelihood of positively responding to those therapies.
At present, however, the only realistic biomarker application in sepsis that has crystallized is guidance for antimicrobial therapy (343). Specifically, the routine use of a PCT-based algorithm for antibiotic management safely reduces patients' exposure to antibiotics (45, 130, 344, 363) and improves cost effectiveness of ICU care (433). Reconfirmation of this potential should be provided by data from several ongoing PCT antibiotic management trials (ClinicalTrials.gov identifier: NCT00472667, NCT 01494675, NCT 00250666, NCT 00692848, NCT 00854932). Positive findings will likely sanction a widespread use of PCT for the purpose of antibiotic management.
Besides appropriate antibiotic usage, the unfortunate reality is that nearly two decades of research have failed to produce even a single, stand-alone, and uniformly applicable biomarker based test for sepsis. This, and the dismal clinical trial record, underscores the complexity of the immunoinflammatory fingerprints of sepsis and the need for a fresh diagnostic outlook (12, 121). If successfully harnessed, biomarkers might still become the master key to an effective and rapid sepsis diagnosis, defining the individual immunoinflammatory patterns, identifying the high risk cohorts, and predicting the likelihood of treatment responses, the core components of the coveted personalized treatment dogma. Yet, the perplexing heterogeneity of septic patients, the disease itself, and a highly transient appearance of biomarkers remain insurmountable obstacles in achieving those goals at the present time.
IX. THERAPEUTIC INTERVENTIONS
A. Therapeutic Interventions: Animal Models
Animal models have been utilized in an effort to develop reproducible systems for studying the pathogenesis of sepsis and initial testing of potentially effective therapies. Despite nearly three decades of intense research, both goals have been proven very difficult to achieve. Interpretation of data from early animal models, particularly those based on injection of lipopolysaccharide (LPS) or Escherichia coli (E. coli), led to an oversimplified understanding of septic responses portraying them as uniformly hyperinflammatory (54, 322). This belief was fueled by numerous successful preclinical studies where inhibition of circulating endotoxin, TNF-α, IL-1β, or IL-6 cytokines or their receptors in mice (9, 22, 119, 451), rats (135, 201, 305), rabbits (277, 417), and non-human primates (107, 115, 386) dramatically improved survival in animals. All of these models infused high-dose boluses of LPS or E. coli. Despite these positive results, application of the same strategies in the clinical realm was not only completely futile but in some cases actually harmful (109).
This startling disparity between animal and human trials provoked careful reexamination of seemingly logical therapeutic strategies. Subsequent studies demonstrated that LPS-based models were unrealistic (321, 322) and established that the exuberant inflammatory burst was not the only (and dominating) component of the multilayer immunoinflammatory sepsis response. As a consequence, the majority of animal models used to study sepsis were deemed too simplistic and one dimensional (306, 328).
As reviewed in section IIA, several new and more sophisticated experimental models such as colon ascendens stent peritonitis (CASP), fibrin clot peritonitis, cecal ligation and puncture (CLP), second hit bacterial pneumonias, as well as protracted and low-dose infusions of live E. coli have been introduced. They all appear to more adequately recapitulate various vital aspects of clinical sepsis (171, 306). For example, newer preclinical studies reproduced failures of clinical trials (100, 413), showed clinical-like sensitivity to antibiotics (264, 392, 393), and produced late mortality (36, 288, 445) that was accompanied by distinct immunosuppression (33, 36). It needs to be stressed, however, that none of the experimental models recapitulates the spectrum of existing clinical scenarios resulting in sepsis. Treatment-related findings from each of those specific models should only be extrapolated to the specific clinical scenario it attempts to recapitulate (e.g., CLP representing polymicrobial peritonitis). Unfortunately, despite markedly improved models, preclinical testing of therapeutic substances continues to be plagued by inadequacies that hinder extrapolation of animal data to the patient population. The most prominent difficulties include 1) excessive severity of the septic insult, 2) virtually exclusive focus on early (acute) events, 3) age mismatch and lack of comorbidities in tested animals, 4) pretreatment versus posttreatment, 5) lack of broad-spectrum antibiotic coverage, and 6) relatively poor reproduction of the technical intensive care unit capabilities. This is far from an exhaustive list. These shortcomings should be rectified to allow development of better quality preclinical evidence.
Apart from a relatively straightforward assessment of dozens of potential antisepsis medications, contemporary sepsis models constitute an excellent tool for testing novel therapeutic concepts within the area of personalized medicine. One of the strongest common denominators between animal and human sepsis studies is that effectiveness of many (mostly anti-inflammatory) therapies was shown to be highly dependent on risk of death due to sepsis (94). Although activated protein C (drotrecogin alpha, activated) therapy targeting septic patients ultimately failed (314), identification and characterization of more homogeneous and narrower patient cohorts appears as a promising therapeutic avenue (94, 279, 303). This is precisely a niche where animal modeling may bring the most enlightenment and suggest new approaches without actually identifying specific, new therapeutic targets. IL-6 guided stratification is one of the early steps in this direction, since treatment with either monoclonal antitumor necrosis factor (292) in patients or dexamethasone (284) in mice only benefited those subjects with high risk of death. In contrast, inhibition of plasma PAI-1 in murine sepsis was lethal only in mice predicted to live, while the treatment revealed no effect in an unstratified (all-inclusive) population (311). Compared with labor intensive and relatively inflexible clinical trials, fine-tuning animal models and matching them with specific medications is relatively easy and fast. If the above scenarios prove effective in relevant preclinical models, they will likely encourage a relaunch of the new, much smaller, and focused clinical trials. It would not be entirely surprising if the latter approach resulted in the resurrection of the “old” therapeutics that had failed in the previous, all-inclusive trials.
B. Therapeutic Interventions: Human Septic Patients
Although the new millennium promised many improvements in survival from sepsis and septic shock, most therapeutic interventions have failed to be efficacious when investigated clinically. Similarly, the notion of the pathogenesis of sepsis as primarily due to an overzealous inflammatory response has been appropriately questioned. As of April 2013, there were over 1,000 trials registered with clinicaltrials.gov dealing with sepsis. Review of many of these studies shows that they are closed with no results posted. This information reinforces that many years of clinical trials have demonstrated that treating the disease with reasonably applied anti-inflammatory agents has not been successful. It has been suggested that septic patients possess heterogeneous immune responses which account for mortality in sepsis, i.e., not all deaths are directly attributable to overwhelming inflammation as previously discussed. Manipulation of pathways that modulate inflammation by targeting the complex interactions of early and late inflammatory mediators may offer a novel opportunity to markedly improve the mechanistic understanding of sepsis and the development of clinical therapies.
1. Current treatments
Sepsis continues to be a significant cause of morbidity and death in critically ill patients, despite optimal treatment. A patient admitted with severe sepsis has a risk of mortality approximately six times greater than if a patient was admitted with an acute myocardial infarction, and four to five times greater than if a patient had an acute stroke (72). With the exception of antibiotics and low-dose steroids in certain instances, the care of septic patients remains supportive. However, it should be noted that bathing with chlorhexidine has emerged as a preventative measure to reduce the development of hospital acquired bloodstream infections that could lead to sepsis (64).
Frequently measuring physiological and laboratory parameters is important for guiding resuscitation in the critically ill septic patient. Endotracheal intubation and mechanical ventilation may be necessary to provide respiratory support for the increased work of breathing or airway protection needed in septic patients. Pulse oximetry provides continuous noninvasive monitoring of oxygen saturation. In addition, arterial blood gas (ABG) sampling is frequently indicated, often every 4–6 h initially, to directly assess oxygenation and ventilation. It can be used together with other clinical criteria to identify the common sepsis complications of ALI or ARDS.
After the patient's airway is secure and breathing stabilized, the adequacy of perfusion is evaluated. Hypotension is a common sign of inadequate perfusion, and it is often accompanied by tachycardia. Early, frequent blood pressure measurements should be obtained, but a sphygmomanometer may be unreliable in hypotensive patients (159). Therefore, placement of an invasive arterial catheter is valuable particularly if the blood pressure is labile, a prolonged resuscitation is anticipated, or frequent arterial blood sampling is necessary. Indicators of hypoperfusion include systolic blood pressure (SBP) <90 mmHg, mean arterial pressure (MAP) <65 mmHg, change in mental status, decreased urine output, increased lactate or base deficit, and decreased mixed venous oxyhemoglobin saturation (SvO2). A central venous catheter (CVC) may also be useful in septic patients requiring resuscitation. The line may be used to infuse intravenous fluids, medications, and blood products, as well as draw blood. Serial laboratory measurements such as complete blood count (CBC) with differential, metabolic panel, and coagulation studies help guide management of the septic patient.
Scoring systems like APACHE (Acute Physiological and Chronic Health Evaluation), SAPS (Simplified Acute Physiological Score), MPM (Mortality Prediction Model), and SOFA (Sequential Organ Failure Assessment) may assign similar physiological scores to individuals but do not fully assess the inflammatory status of septic patients (362). Multiple clinical trials have shown that broadly-applied anti-inflammatory therapies are ineffective or even harmful (317). No targeted therapy has yet been developed that specifically modulates the dysfunctional immune response that occurs during sepsis. However, the standard use of early goal-directed therapy (EGDT) has been shown to improve survival rates, emphasizing the need for early identification and treatment of patients developing sepsis.
EGDT serves as a valuable treatment algorithm in the critical care setting, and this therapy has been shown to increase survival in a human clinical trial of severe sepsis and septic shock (329). It focuses on early and active supportive care of septic patients, but does not directly address the underlying infectious and inflammatory pathogenesis of sepsis. Since its inception in 2001, specific components of EGDT have been individually studied and validated. Hypotension, defined as a systolic blood pressure <90 mmHg, and a lactate level ≥4 mM have been shown in several studies to be high risk predictors of illness and mortality (242, 350, 387). The aggressive, timely intravenous fluid goals of EGDT have been validated in subsequent studies showing improved microcirculation perfusion in the early phase (282), a shift to a more anti-inflammatory state (86), and reduced mortality (258). Rapidly decreasing lactate levels within 6 h of sepsis presentation also serve as a proxy measure for resolution of global tissue hypoxia, leading to reduced organ dysfunction and improved survival (266). In a multicenter study, Pope et al. (307) show the benefit of central venous oxygen saturation (ScvO2) as a resuscitation end point since failure to reach an ScvO2 >70% within the first 6 h is associated with significantly increased mortality (307).
EGDT has developed into the Surviving Sepsis guidelines, revised in 2008 (78) and updated in 2013 (79). These international guidelines aim to diminish barotrauma due to mechanical ventilation, control infection, preserve organ perfusion, and avoid hyperglycemia. Additional measures such as the administration of low-dose steroids are recommended if indicated. Significantly, the overall benefit of the original EGDT trial has been validated in at least 19 studies of adult septic patients in the past 10 years (330). Whether EGDT is implemented in the emergency department or the ICU, these studies show that the outcome benefit is equivalent or surpasses the 2001 EGDT study (58, 194). Coba et al. (65) demonstrate that EGDT is even effective up to 18 h after a patient presents with sepsis, suggesting that delayed implementation of the resuscitation guidelines is still beneficial. Overall, EGDT outcomes are reproducible and generalizable to tertiary-care and community hospitals with the potential to prevent death in one out of every six lives affected by sepsis. It should be noted that the surviving sepsis campaign, with the bundled care protocols, decreased sepsis mortality (215).
2. Modifications to existing regimens
a) adrenal insufficiency. Septic patients are at risk for developing adrenal insufficiency of critical illness (AICI). This condition can present as hypotension and ventilator dependency such that patients may benefit from administration of exogenous steroids to restore their hemodynamic stability. A random serum cortisol level obtained in the presence of a severe endogenous stress such as sepsis evaluates the entire hypothalamic-pituitary-adrenal (HPA) axis, and evidence suggests it can be considered superior to traditional ACTH stimulation testing. Random total serum cortisol levels <10 μg/dl in the presence of hemodynamic instability, as well as a change in cortisol (after 250 μg cosyntropin) <9 μg/dl, are sufficient to diagnose AICI in such patients (17), and glucocorticoid replacement therapy should be initiated.
Previous efforts to use high-dose steroids in septic patients have not improved survival and may even cause harm (2, 44, 71, 209). However, lower dose steroids such as hydrocortisone 200–300 mg/day may have a potential benefit. A number of smaller randomized control trials support the use of low-dose steroids in sepsis (40, 48). In contrast, the largest randomized controlled trial, CORTICUS, showed no significant difference in 28-day mortality between patients treated with corticosteroids and those receiving placebo. However, it did decrease time to reversal of shock (358) and is often used as an adjunct therapy in sepsis. Conflicting results remain between the findings of the largest randomized clinical trial and multiple smaller ones (16, 31). Subsequently, the 2008 Surviving Sepsis Campaign updated the guidelines for use of corticosteroids to patients in septic shock who are poorly responsive to both fluid resuscitation and vasopressors (78). These were further refined in the third edition published in 2013 with recommendations that intravenous hydrocortisone not be used if hemodynamic stability has been achieved (79).
b) glycemic control. Stress-induced hyperglycemia frequently occurs in septic patients. Elevated blood glucose levels are associated with an increase in nosocomial infections and mortality in critically ill patients (106). The optimal blood glucose (BG) range in sepsis remains a subject of debate, and current recommendations are changing. There are multiple studies comparing intensive (often BG 80–110 mg/dl) versus conventional (usually BG 180–200 or <200 mg/dl) insulin therapy groups. Several recent trials found no difference in survival between the groups (19, 309, 400, 431). The largest randomized, controlled trial, NICE-SUGAR, even demonstrated increased mortality in the intensive insulin group (104).
Of concern, these studies also show that intensive insulin therapy also causes a significant increase in hypoglycemia (BG <40 or <50 mg/dl) compared with the conventional group. Hypoglycemia in intensive insulin groups (BG 80–110 mg/dl) is associated with deleterious effects and is independently linked to increased ICU or hospital mortality (19, 155, 309). Based on the evidence, maintaining strict glycemic control (BG 80–100 mg/dl) is not warranted, but the optimal upper limit of the target range remains unclear. Currently, the American Association of Clinical Endocrinologists and American Diabetes Association (ADA/AACE) recommends a BS range of 140–180 mg/dl for critically ill patients with an understanding that targeting the lower end of this range may be beneficial (245). The 2013 surviving sepsis guidelines target maintaining glucose <180 mg/dl (79).
Even with optimal current therapy, septic patients still experience unacceptably high morbidity and mortality, suggesting variability in the response of individuals towards treatment. It remains to be seen if current modifications in treatment guidelines for septic patients will maintain their beneficial effects with widespread implementation. However, the recent failure of promising drugs to further reduce mortality in patients underscores the need for new therapeutic approaches to combat sepsis.
3. Recent failed therapies
Many potential treatments for sepsis have been examined but have either failed to improve clinical outcomes or are harmful. Based on the results of the PROWESS study, Xigris (recombinant human activated protein C) was FDA approved in 2001 for treatment of high-risk, severely septic patients as the trial showed an improvement in 28-day all-cause mortality (37). Subsequently, the PROWESS-SHOCK study was conducted to further evaluate the benefit-risk profile of the medication after conflicting reports. These subsequent studies demonstrated that Xigris therapy did not improve survival for patients with septic shock, which is a slightly different target from severe sepsis, the original PROWESS target. The clinical studies were reviewed in greater detail in section VB.
Developing new therapies for sepsis has been particularly challenging, with more than 25 unsuccessful drug trials (276, 327, 419, 426). Efforts to suppress the host's excessively active immune response by administration of monoclonal antibodies against TNF (5, 67, 109), blocking IL-1 activity with receptor antagonists (110, 280), and antibodies to endotoxin (13) have been unsuccessful. Use of granulocyte colony-stimulating factor (filgrastim) (332) to elevate white blood cell counts also failed to show any survival benefit. Other therapies such as nitric oxide inhibitors, antithrombin, and N-acetylcysteine failed to demonstrate efficacy. The multifaceted interplay and dynamic response of the innate immune system's cytokine production during sepsis represents an ongoing difficulty to developing appropriate therapeutics. Cortisol replacement was pursued as a possible therapy in sepsis that initially showed promise but in large multi-center studies was shown to have no role in the treatment of sepsis (40, 41, 44). Hydroxyethyl starch (HES) is a newer form of fluid resuscitation, and patient outcomes were compared with standard crystalloid or albumin resuscitation. No improvement in survival was shown, and there was an increase in acute kidney injury and serious adverse events (147). It has been strongly suggested by some experts that the use of HES in the ICU should be immediately discontinued as the initial evidence favoring HES might have been biased (151).
Toll-like receptors (TLRs), which are a subfamily of pattern-recognition receptors (PRRs), have emerged as critical receptors for the recognition of damage-associated molecular patterns (DAMPs) and initiation of the inflammatory response. Recent potential therapies, such as the Toll-like receptor 4 antagonist, are novel in that they target the inflammatory cascade further upstream. A 2010 phase II clinical trial of this drug did not demonstrate improvement in survival, although the higher dose group trended toward lower mortality (380). In an international phase III randomized trial, Rice et al. (325) used the anti-TLR 4 compound eritoran tetrasodium in a cohort of patients with severe sepsis. Unfortunately, there was no cytokine suppression or improvement in 28-day all-cause mortality (325). Subsequently, the larger phase III ACCESS study of eritoran also failed to demonstrate a decrease in all-cause death by day 28 or up to 1 yr (281). One possible reason is that anti-TLR 4 therapy may only be effective in patients with Gram-negative sepsis, and it is likely that better patient stratification based on infectious source (i.e., abdominal cavity vs. lungs) would have yielded a better outcome. Overall, the results of TLR 4 antagonists in sepsis appear much less promising than originally expected.
4. New experimental interventions
Modulating the interactions between the innate immune system and T cells may represent an innovative approach to developing therapies for combating sepsis. IL-7 is essential for lymphocyte survival, and deficient IL-7 gene expression in peripheral blood leukocytes exists in septic patients (428). Kasten et al. (183) showed that treating mice with the potent, anti-apoptotic IL-7 increased Bcl-2 expression in T cells isolated from septic mice as early as 3 h and produced local and systemic increases in IL-17. The results demonstrate that IL-7 can mediate the interactions between Th1 and Th17 lymphocytes during sepsis, improving neutrophil recruitment and bacterial clearance while avoiding early end-organ tissue injury (183). Unsinger et al. (395) also report that rhIL-7 blocks apoptosis of helper T cells, restores IFN-γ levels, improves immune effector cell recruitment to the infected site, and reduces mortality. Future clinical trials with IL-7 in sepsis hold potential if current phase II clinical studies in other patient populations confirm the safety of the drug.
Blood filtration devices such as polymyxin B fiber columns act to absorb circulating endotoxins in an effort to reduce harmful interactions between potent bacterial components and the host in gram negative sepsis. Early studies of polymyxin B hemoperfusion suggested beneficial effects, but unblinded designs and publication bias emphasized the need for further rigorous trials (298). A prospective, randomized controlled clinical trial with polymyxin B hemoperfusion in Italian ICUs demonstrated improved clinical outcomes and mortality in patients with severe sepsis or septic shock undergoing emergent operations due to an intra-abdominal source (38). Cardiovascular and respiratory parameters were enhanced, organ dysfunction improved as measured by SOFA scores, and mortality was 32% in the experimental group versus 53% with standard therapy in the phase II trial. Some limitations of the study were that treating clinicians were not blinded, and the sample size was modest as a result of halting the trial early after favorable interim analysis. However, selection of a highly targeted patient population with a single source of sepsis, higher sepsis severity, and definitive source control created a trial better positioned to establish efficacy in a specific cohort. Ongoing testing of polymyxin B absorption columns such as toraymyxin which is produced by Spectral Diagnostics is being conducted (435) and could prove efficacious in targeted patients with gram-negative sepsis.
Talactoferrin, a human recombinant lactoferrin, is believed to be a compound that helps maintain the integrity of the gut mucosal barrier and can be administered enterally. A 2013 phase II clinical trial with talactoferrin for the treatment of patients with severe sepsis was recently reported. The placebo control group had a mortality of 26.9% at 28 days, consistent with most studies. The talactoferrin group had a 28-day all-cause mortality of 14.4%, and the better survival was maintained up to 6 mo. The lactoferrin was given enterally, and it is believed that the compound helps maintain the integrity of the gut mucosal barrier (144). Yet, a subsequent phase II/III trial of talactoferrin in severe sepsis was discontinued by Agennix AG after the treatment group had worse outcomes than placebo (237). Further investigation of this drug is indicated to understand its possible use in sepsis.
Other immunomodulatory therapies in sepsis are being explored such as granulocyte macrophage colony stimulating factor (GM-CSF), programmed cell-death ligand-1 (PD-L1), and IL-15 (163). Bolstering host immunity is a therapeutic approach with increasing promise as significant immunosuppression is recognized in sepsis, especially with prolonged disease recovery and an older patient population with significant comorbidities. After selecting for immunosuppressed septic patients by evidence of monocyte HLA-DR downregulation, GM-CSF administration improved HLA-DR expression, in-patient length of stay, and mechanical ventilation time (239). A multicenter phase III trial is needed. Interfering with PD-1, an inhibitory costimulatory molecule, signaling may can improve T-cell function and reduce microbial burden (352). A 2012 study demonstrated success of an anti-PD-1 antibody in cancer patients (385), and this type of immunomodulation could be effective in sepsis, as abnormally high levels of PD-1 expression on T cells was associated with immune dysregulation, nosocomial infections, and reduced survival (143). Additional studies are required to determine if anti-PD-1 treatment should be investigated clinically in septic patients. Various other immunomodulators like IL-15 and adenosine receptor antagonists are in preclinical testing and represent possible targets for future sepsis therapy (34, 177).
Even with optimal current therapy, septic patients still experience unacceptably high morbidity and mortality, suggesting variability in the response of individuals towards treatment. Sepsis can occur in a range of individuals from a 21-yr-old healthy man with traumatic brain injury and a ventilator-associated pneumonia to an 85-yr-old diabetic, end-stage renal disease woman with perforated diverticulitis. Since septic patients are not a uniform population, better stratification is needed in clinical trials to target therapies to a narrower group of individuals who are more likely to benefit. Therapies are also unlikely to be effective in sepsis from all infectious sources. Whether the invasive pathogen originates in the lungs, abdomen, urinary tract, skin, or other location may impact the type of therapy and subpopulation in which it is implemented. As the hypoinflammatory component of sepsis is more understood and septic patients are surviving initial insults with prolonged recovery, new therapies targeting immunosuppression may emerge. It remains to be seen if current modifications in treatment guidelines for septic patients will maintain their beneficial effects with widespread implementation. However, the recent failure of promising drugs to further reduce mortality in patients underscores the need for new therapeutic approaches to combat sepsis.
X. DYNAMIC MATHEMATICAL VIEW OF SEPSIS
One of the most difficult problems in sepsis is patient heterogeneity due to a multitude of infecting pathogens eliciting a diverse range of host responses in an exceedingly outbred population. These complicate every aspect of sepsis clinically and scientifically, from diagnosis to prognosis, to design and interpretation of clinical trials. A diagnosis of sepsis is based on clinical symptoms and suspicion: altered temperature, heart rate, respiration rate and white cell counts, and possible infection (see Table 1). Since infection is identified in only about half of sepsis patients (233), clinical suspicion of infection remains a large part of the diagnostic equation. While considerable effort is being invested in biosensor development for clinical pathogen detection and identification to eliminate this uncertainty, technical obstacles remain (116). Promising biomarkers are being evaluated to distinguish patients with septic SIRS and sterile SIRS (455), but have yet to be validated in large populations. Please refer to section VIII on biomarkers for additional information. Even when the pathogen is identified, most patients present with comorbidities, and every patient differs in their genetic make-up and in their response to intervention. Differentiation of sepsis patient groups according to disease severity (shock, multiple organ failure) has obvious clinical relevance, but has limited use for design of prospective interventional clinical trials because there is no means to predict whether a patient will have a relatively uncomplicated septic course, or decline into shock with respiratory failure and DIC.
To eliminate these ambiguities, the emerging field of personalized medicine seeks to combine genetic, proteomic, and metabolomic data from each patient to guide clinical diagnosis and treatment decisions which will be most effective for that individual. This data approach is proactive, differing from the largely reactive method of traditional clinical practice which relies on clinical symptoms, laboratory and imaging data, family history, and personal health history. Broad-platform precision technologies, bioinformatics, and the evolving field of systems biology provide tools for acquisition and analysis of vast datasets to develop comprehensive networked maps of healthy and diseased cellular activities. For sepsis patients, predictions from genomic microarray data will likely be less about risk of disease, such as risk of breast cancer, because they already have sepsis, and more about risk of complications. A sepsis patient who was previously otherwise healthy, but identified upon admission to have a risk of pulmonary fibrosis due to α1-antitrypsin deficiency (365) or coagulopathy due to a prothrombotic antithrombin polymorphism (18) could be monitored closely and treated prophylactically. One could envision a panel of genes and SNPs that report risk of sepsis severity and morbidity, but these will need careful validation in patients. The presence of factor V Leiden is predicted by animal models to be prothrombotic, but in reality makes little contribution to development of DIC or outcome in sepsis patients (212).
Genomic data describe a static environment, whereas sepsis is an acute, rapidly dynamic illness. In this context, strategies are developing to apply mathematical modeling tools to datasets acquired from cell or tissue mRNA profiles and biomarkers in fluids (blood, urine) for descriptive profiling patterns. With the use of DNA microarray for almost 7,400 genes, the multiorgan response to mixed microbial peritonitis (surgical CLP model) in a rat model was shown in tissues from 6, 12, 18, and 24 h postchallenge (63). While a time profile was not clear from this study, it did provide a multiorgan view to distinguish responses within and between organs. In a similar experiment in C57BL/6 male mice, the differential gene expression (588 genes) profiles in liver and spleen were compared 24 h after CLP challenge (66). Distinct changes in cDNA profiles were observed in the two organs, most of which were linked to regulation of inflammation. This static view of mRNA expression has since been developed by the same group toward time-based profiling using leukocyte mRNA from patients with ventilator-associated pneumonia (VAP; n = 11) (238). The day of VAP diagnosis was designated day 0, and samples from day −3 to day +3 were analyzed. mRNA species with abundance changes were clustered and the dynamics projected using a method similar to principal components analysis (Karhunen-Loeve Decomposition). Changes in the PC2-PC3 space of 85 human genes showed that a trajectory toward illness could be identified several days before the VAP diagnosis. As patients recovered, their individual variance in the PC2 space decreased, providing a “riboleukogram” of each patient's clinical course. However, mRNA abundance frequently does not correlate with translation to protein or biological outcomes.
With the use of a different approach, datasets of common clinical physiology and experimental cytokine parameters obtained over time in a nonhuman primate model of aseptic SIRS (360) were grouped, normalized, and analyzed without knowledge of survival outcome as a function of time using principal component analysis (H. Hardway, D. J. Stearns-Kurosawa, and S. Kurosawa, unpublished data). It was found that while physiology data (e.g., liver and renal profiles, blood pressure, respiration rates, etc.) differentiated between survivors and nonsurvivors, the distinction came too late and had no predictive value. In contrast, cytokine time-map paths (PC1 vs. PC2) separated survivors and nonsurvivors by 10 h postchallenge giving confident prognosis by day 2–3 postchallenge, well before death (Figure 10). This is another encouraging glimpse into a beneficial future of biomarkers for patient stratification approaches.
Figure 10.
Modeling responses to septic challenge. Mathematical modeling of responses to sepsis can include equations and analyses that consider the host hemodynamic, physiological and inflammation parameters, as well as pathogen distribution or growth. Principal component analysis (PCA) is a technique that analyzes the variance between observations. It is used to identify patterns in data and to express the data in such a way as to highlight their similarities and differences. In the example shown here, PCA analysis of normalized and grouped cytokine data from animals (n = 24) challenged with a proinflammatory toxin reveals gradual separation of survivors and nonsurvivors over time when viewed in the PC1-PC2 space. Genomic and microarray data provide a static view of a physiological condition, but patients with sepsis, septic shock, and comorbidities change rapidly so consideration of time in modeling methods is necessary for relevance to patients.
Several equation-based modeling approaches have been used to model inflammatory responses. A model using deterministic equations of circulation, infection, organ damage, and recovery provide a different approach to modeling sepsis and septic outcomes (458). These equations incorporate parameters such as rate of blood circulation, body size (BMI), pathogen rate of reproduction, age, end-organ damage, and energy consumption. The blood circulation component (by echocardiography and cardiac stroke volume) was included to model white blood cell migration through the vasculature, dissemination of pathogen particles, and interactions between blood cells and pathogens (particle interactions based on systemic and Brownian motion). The combined equations describe a parameter H derived from easily measured variables (e.g., body mass, height, fasting glucose, lung capacity) as a measure of health monitoring, and as H deviates from unity, it predicts increased risk of disease with unfavorable outcome. To date, this is a theoretical model and has not been tested with data from sepsis patients.
A reduced model of acute inflammation also used a differential equations approach to distinguish a healthy response to infection, where a pathogen is cleared without undue complications, from recurrent and persistent infections and immunodeficiency with a low inflammatory response (200). These equations rely on rates of change in pathogen concentration as well as early (TNF-α, IL-1β) and late inflammatory mediators (IL-6, HMGB1). Perhaps not surprisingly, the model predicts patient group complexity and that a therapeutic strategy to treat all sepsis patients as a single population is unlikely to work because of the distinct physiological subsets of inflammatory responses. This is consistent with failed sepsis clinical trials for adjunctive therapies where subsequent meta-analysis identified subgroups that responded positively to the intervention (206, 347, 430).
Agent-based modeling approaches have also been used to model inflammatory responses. The nonlinear dynamics of acute self-limiting or unresolved endotoxin-induced inflammatory responses was modeled using agent-based rules based on leukocyte transcriptional data, signaling, and physiological components with a focus on NFκB signaling (85). The model predicted that resolution of the endotoxin-mediated inflammatory response depended on the balance between pro- and anti-inflammatory mediators and was regulated by the energetic state of immune cells. It further predicted the endotoxin hyporesponsiveness observed in biological models (427).
Translational systems biology is a nascent field of applied mathematics intended to bridge the needs of preclinical investigations, clinical trials, rational drug design, and hospital care using evidence-based modeling approaches (241). A model of inflammatory processes needs to be detailed only to where the patient can be assigned within a multidimensional space that describes and predicts the course of the clinical behavior and response to adjunctive therapy, hopefully with resolution of the process and return to equilibrium. Acute inflammation modeled using four differential equations incorporates estimates of pathogen level, activated innate immune cells (e.g., circulation time of neutrophils), tissue damage, and adjunctive anti-inflammatory mediators (e.g., cortisol, IL-10) (324). Simulated time-dependent modulation of an anti-inflammatory mediator initiated at 10 h postchallenge showed that a transient and relatively small increase in anti-inflammatory mediators would positively alter the balance from nonseptic death to health, but larger increases would not ensure sufficient control over the more robust inflammation. This recapitulates the current consensus based on hard experience that some inflammation is needed and beneficial. Ideally a multidimensional model will describe a patient responding within the context of sepsis, trauma, burns, hemorrhage, or shock by incorporating networked biologic systems (381), genetic profiling (297), and epigenetic mechanisms (99).
XI. CONCLUSIONS
Clearly, sepsis causes substantial alterations which culminate in subsequent organ injury and high lethality. No single mediator or pathway drives the septic response which increases the complexity of the disease. All previous attempts to target a single molecule failed to improve outcome, reinforcing that the complex and heterogeneous nature of sepsis which challenges physicians and basic scientists. However, given the substantial morbidity and hundreds of thousands of septic deaths each year, there is an imperative to further define and understand the basic alterations in pathophysiology to elucidate targeted sepsis therapies. This understanding is essential to reducing morbidity and healthcare costs and, most importantly, may help save lives.
GRANTS
This work was supported by National Institutes of Health Grants R01 GM82962, R01 GM97320, and T32 GM86308 (to D. G. Remick); WWTF LS07-065 and EU Marie Curie IRG FP7-203685 (to M. F. Osuchowski); and UO1 AI075386, U19 AI062629, and RO1 AI058107 (to S. Kurosawa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: D. G. Remick, 670 Albany St., Rm. 405, Boston, MA 02118 (e-mail: remickd@bu.edu).
REFERENCES
- 1.American College of Chest Physicians/Society of Crit Care Med. College of Chest Physicians/Society of Crit Care Med Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Med 20: 864–874, 1992 [PubMed] [Google Scholar]
- 2.A Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 317: 659–665, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, Kudsk K, Bruining HA, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A. p55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial Ro 45-2081 Study Group. JAMA 277: 1531–1538, 1997 [PubMed] [Google Scholar]
- 4. Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, Rossaint R, Perrotin D, Sablotzki A, Arkins N, Utterback BG, Macias WL, and the Administration of Drotrecogin Alfa in Early Stage Severe Sepsis Study Group Drotrecogin Alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med 353: 1332–1341, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome A randomized, controlled, double-blind, multicenter clinical trial TNF-α MAb Sepsis Study Group. JAMA 273: 934–941, 1995 [PubMed] [Google Scholar]
- 6. Adib-Conquy M, Adrie C, Fitting C, Gattolliat O, Beyaert R, Cavaillon JM. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Critical Care Med 34: 2377–2385, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Adrie C, Azoulay E, Francais A, Clec'h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Orgeas M, Timsit JF. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest 132: 1786–1793, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Afshari A, Wetterslev J, Brok J, Moller A. Antithrombin III in critically ill patients: systematic review with meta-analysis and trial sequential analysis. BMJ 335: 1248–1251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander HR, Doherty GM, Fraker DL, Block MI, Swedenborg JE, Norton JA. Human recombinant interleukin-1 alpha protection against the lethality of endotoxin and experimental sepsis in mice. J Surg Res 50: 421–424, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: a useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction scores. Crit Care Med 34: 314–320, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA 306: 2614–2615, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, Graham DR, Dedhia HV, Homann S, MacIntyre N. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 283: 1723–1730, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Annane D. Improving clinical trials in the critically ill: unique challenge–sepsis. Crit Care Med 37: S117–128, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 301: 2362–2375, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med 174: 1319–1326, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Anton AI, Teruel R, Corral J, Minano A, Martinez-Martinez I, Ordonez A, Vicente V, Sanchez-Vega B. Functional consequences of the prothrombotic SERPINC1 rs2227589 polymorphism on antithrombin levels. Haematologica 94: 589–592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 36: 3190–3197, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Arkader R, Troster EJ, Lopes MR, Junior RR, Carcillo JA, Leone C, Okay TS. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Childhood 91: 117–120, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, Morishita E, Yamazaki M, Aoshima K, Nakao S. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med 29: 1164–1168, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ashkenazi A, Marsters SA, Capon DJ, Chamow SM, Figari IS, Pennica D, Goeddel DV, Palladino MA, Smith DH. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc Natl Acad Sci USA 88: 10535–10539, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-gamma release while increasing IL-4/IL-10 production. J Surg Res 56: 579–585, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood 87: 4261–4275, 1996 [PubMed] [Google Scholar]
- 25. Ayala A, Perl M, Venet F, Lomas-Neira J, Swan R, Chung CS. Apoptosis in sepsis: mechanisms, clinical impact and potential therapeutic targets. Curr Pharm Des 14: 1853–1859, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871–881, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Bahrami S, Pelinka L, Khadem A, Maitzen S, Hawa G, van Griensven M, Redl H. Circulating NT-proCNP predicts sepsis in multiple-traumatized patients without traumatic brain injury. Crit Care Med 38: 161–166, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med 32: 2416–2421, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Barati M, Bashar FR, Shahrami R, Zadeh MH, Taher MT, Nojomi M. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J Crit Care 25: 362 e361–366, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Batzofin BM, Sprung CL, Weiss YG. The use of steroids in the treatment of severe sepsis and septic shock. Best Pract Res Clin Endocrinol Metab 25: 735–743 [DOI] [PubMed] [Google Scholar]
- 32. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Belikoff B, Hatfield S, Sitkovsky M, Remick DG. Adenosine negative feedback on A2A adenosine receptors mediates hyporesponsiveness in chronically septic mice. Shock 35: 382–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol 186: 2444–2453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bender L, Thaarup J, Varming K, Krarup H, Ellermann-Eriksen S, Ebbesen F. Early and late markers for the detection of early-onset neonatal sepsis. Danish Med Bull 55: 219–223, 2008 [PubMed] [Google Scholar]
- 36. Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukocyte Biol 75: 408–412, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699–709, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Bliss JM, Kilburn LS, Coleman RE, Forbes JF, Coates AS, Jones SE, Jassem J, Delozier T, Andersen J, Paridaens R, van de Velde CJ, Lonning PE, Morden J, Reise J, Cisar L, Menschik T, Coombes RC. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol 30: 709–717, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS ONE 5: e15480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26: 645–650, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Bone RC. The pathogenesis of sepsis. Ann Intern Med 115: 457–469, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 24: 1125–1128, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 268: 3452–3455, 1992 [PubMed] [Google Scholar]
- 44. Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. New Engl J Med 317: 653–658, 1987 [DOI] [PubMed] [Google Scholar]
- 45. Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Regnier B, Brun-Buisson C, Chastre J, Wolff M. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375: 463–474, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukocyte Biol 88: 233–240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 27: 723–732, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Brunialti MK, Martins PS, Barbosa de Carvalho H, Machado FR, Barbosa LM, Salomao R. TLR2, TLR4, CD14, CD11B, and CD11C expressions on monocytes surface and cytokine production in patients with sepsis, severe sepsis, and septic shock. Shock 25: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med 26 Suppl 2: S148–S152, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Buelens K, Hassanzadeh-Ghassabeh G, Muyldermans S, Gils A, Declerck PJ. Generation and characterization of inhibitory nanobodies towards thrombin activatable fibrinolysis inhibitor. J Thromb Haemost 8: 1302–1312, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Caetano-Anolles G, Wang M, Caetano-anolles D, Mittenthal JE. The origin, evolution and structure of the protein world. Biochem J 417: 621–637, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Calandra T, Baumgartner JD, Glauser MP. Anti-lipopolysaccharide and anti-tumor necrosis factor/cachectin antibodies for the treatment of gram-negative bacteremia and septic shock. Prog Clin Biol Res 367: 141–159, 1991 [PubMed] [Google Scholar]
- 55. Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J, Verhoef J, Glauser MP. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis 161: 982–987, 1990 [DOI] [PubMed] [Google Scholar]
- 56. Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, Matthay MA. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med 39: 711–717, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cardelli P, Ferraironi M, Amodeo R, Tabacco F, De Blasi RA, Nicoletti M, Sessa R, Petrucca A, Costante A, Cipriani P. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol 21: 43–49, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Holanda MS, Ortiz F, Llorca J, Delgado-Rodriguez M. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 38: 1036–1043 [DOI] [PubMed] [Google Scholar]
- 59. Cavaillon JM, Adib-Conquy M, Cloez-Tayarani I, Fitting C. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J Endotoxin Res 7: 85–93, 2001 [PubMed] [Google Scholar]
- 60. Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 12: 151–170, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Chan T, Gu F. Early diagnosis of sepsis using serum biomarkers. Expert Rev Mol Diagnostics 11: 487–496, 2011 [DOI] [PubMed] [Google Scholar]
- 62. Cheluvappa R, Denning GM, Lau GW, Grimm MC, Hilmer SN, Le Couteur DG. Pseudomonas aeruginosa and the hyperlipidaemia of sepsis. Pathology 41: 615–621, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Chinnaiyan AM, Huber-Lang M, Kumar-Sinha C, Barrette TR, Shankar-Sinha S, Sarma VJ, Padgaonkar VA, Ward PA. Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am J Pathol 159: 1199–1209, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, Weinstein RA, Sepkowitz KA, Jernigan JA, Sanogo K, Wong ES. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 368: 533–542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coba V, Whitmill M, Mooney R, Horst HM, Brandt MM, Digiovine B, Mlynarek M, McLellan B, Boleski G, Yang J, Conway W, Jordon J. Resuscitation bundle compliance in severe sepsis and septic shock: improves survival, is better late than never. J Intensive Care Med [DOI] [PubMed] [Google Scholar]
- 66. Cobb JP, Laramie JM, Stormo GD, Morrissey JJ, Shannon WD, Qiu Y, Karl IE, Buchman TG, Hotchkiss RS. Sepsis gene expression profiling: murine splenic compared with hepatic responses determined by using complementary DNA microarrays. Crit Care Med 30: 2711–2721, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Cohen J, Carlet J. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Int Sepsis Trial Study Group Crit Care Med 24: 1431–1440, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Combes A, Luyt CE, Trouillet JL, Nieszkowska A, Chastre J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med 37: 2506–2511, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children's hospital. Pediatrics 119: 891–896, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol 185: 6930–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ., Jr Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med 23: 1430–1439, 1995 [DOI] [PubMed] [Google Scholar]
- 72. Cronshaw HL, Daniels R, Bleetman A, Joynes E, Sheils M. Impact of the Surviving Sepsis Campaign on the recognition and management of severe sepsis in the emergency department: are we failing? Emerg Med J 28: 670–675 [DOI] [PubMed] [Google Scholar]
- 73. Dai K, Khatun I, Hussain MM. NR2F1 and IRE1beta suppress microsomal triglyceride transfer protein expression and lipoprotein assembly in undifferentiated intestinal epithelial cells. Arteriosclerosis Thrombosis Vasc Biol 30: 568–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest 129: 1432–1440, 2006 [DOI] [PubMed] [Google Scholar]
- 75. de Pablo R, Monserrat J, Reyes E, Diaz-Martin D, Rodriguez Zapata M, Carballo F, de la Hera A, Prieto A, Alvarez-Mon M. Mortality in patients with septic shock correlates with anti-inflammatory but not proinflammatory immunomodulatory molecules. J Intensive Care Med 26: 125–132, 2011 [DOI] [PubMed] [Google Scholar]
- 76. Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204: 1463–1474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36: 296–327, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39: 165–228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 29: S42–S47, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Di Nisio M, Baudo F, Cosmi B, D'Angelo A, De Gasperi A, Malato A, Schiavoni M, Squizzato A. Diagnosis and treatment of disseminated intravascular coagulation: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2011 [DOI] [PubMed] [Google Scholar]
- 82. Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature Med 3: 678–681, 1997 [DOI] [PubMed] [Google Scholar]
- 83. Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, Leathersich AM, Dunne WM, Coopersmith CM. Epidermal growth factor improves survival and prevents intestinal injury in a murine model of Pseudomonas aeruginosa pneumonia. Shock 36: 381–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med 10: 2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dong X, Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Agent-based modeling of endotoxin-induced acute inflammatory response in human blood leukocytes. PLoS ONE 5: e9249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dorresteijn MJ, van Eijk LT, Netea MG, Smits P, van der Hoeven JG, Pickkers P. Iso-osmolar prehydration shifts the cytokine response towards a more anti-inflammatory balance in human endotoxemia. J Endotoxin Res 11: 287–293, 2005 [DOI] [PubMed] [Google Scholar]
- 87. Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine 18: 1638–1641, 2000 [DOI] [PubMed] [Google Scholar]
- 88. Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious use of antibiotics. Am Family Physician 55: 1647–1654, 1997 [PubMed] [Google Scholar]
- 89. Drechsler S, Weixelbaumer K, Raeven P, Jafarmadar M, Khadem A, van Griensven M, Bahrami S, Osuchowski MF. Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post-traumatic sepsis. PloS One 7: e51457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dreiher J, Almog Y, Sprung CL, Codish S, Klein M, Einav S, Bar-Lavie Y, Singer PP, Nimrod A, Sachs J, Talmor D, Friger M, Greenberg D, Olsfanger D, Hersch M, Novack V. Temporal trends in patient characteristics and survival of intensive care admissions with sepsis: a multicenter analysis. Crit Care Med 40: 855–860, 2012 [DOI] [PubMed] [Google Scholar]
- 91. Duvigneau JC, Kozlov AV, Zifko C, Postl A, Hartl RT, Miller I, Gille L, Staniek K, Moldzio R, Gregor W, Haindl S, Behling T, Redl H, Bahrami S. Reperfusion does not induce oxidative stress but sustained endoplasmic reticulum stress in livers of rats subjected to traumatic-hemorrhagic shock. Shock 33: 289–298, 2010 [DOI] [PubMed] [Google Scholar]
- 92. Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg 134: 1342–1347, 1999 [DOI] [PubMed] [Google Scholar]
- 93. Egi M, Morimatsu H, Wiedermann CJ, Tani M, Kanazawa T, Suzuki S, Matsusaki T, Shimizu K, Toda Y, Iwasaki T, Morita K. Non-overt disseminated intravascular coagulation scoring for critically ill patients: the impact of antithrombin levels. Thromb Haemost 101: 696–705, 2009 [PubMed] [Google Scholar]
- 94. Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 166: 1197–1205, 2002 [DOI] [PubMed] [Google Scholar]
- 95. El-Maghraby SM, Moneer MM, Ismail MM, Shalaby LM, El-Mahallawy HA. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. J Pediatr Hematol Oncol 29: 131–136, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Ely EW, Angus DC, Williams MD, Bates B, Qualy R, Bernard GR. Drotrecogin alfa (activated) treatment of older patients with severe sepsis. Clin Infect Dis 37: 187–195, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176: 6245–6253, 2006 [DOI] [PubMed] [Google Scholar]
- 98. Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obesity Metab 12: 108–115, 2010 [DOI] [PubMed] [Google Scholar]
- 99. Erce MA, Pang CN, Hart-Smith G, Wilkins MR. The methylproteome and the intracellular methylation network. Proteomics 2012 [DOI] [PubMed] [Google Scholar]
- 100. Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 148: 2724–2730, 1992 [PubMed] [Google Scholar]
- 101. Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care 13: R18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care 13: R18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Federman DD. The biology of human sex differences. N Engl J Med 354: 1507–1514, 2006 [DOI] [PubMed] [Google Scholar]
- 104. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360: 1283–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 105. Fink MP. Animal models of sepsis and its complications. Kidney Int 74: 991–993, 2008 [DOI] [PubMed] [Google Scholar]
- 106. Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA 290: 2041–2047, 2003 [DOI] [PubMed] [Google Scholar]
- 107. Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, DeForge L, Kenney JS, Remick DG, Bloedow DC. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest 89: 1551–1557, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fischer JE, Benn A, Harbarth S, Nadal D, Fanconi S. Diagnostic accuracy of G-CSF, IL-8, and IL-1ra in critically ill children with suspected infection. Intensive Care Med 28: 1324–1331, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Fisher CJ, Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med 334: 1697–1702, 1996 [DOI] [PubMed] [Google Scholar]
- 110. Fisher CJ, Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271: 1836–1843, 1994 [PubMed] [Google Scholar]
- 111. Fisher CJ, Jr, Opal SM, Dhainaut JF, Stephens S, Zimmerman JL, Nightingale P, Harris SJ, Schein RM, Panacek EA, Vincent JL. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis The CB0006 Sepsis Syndrome Study Group. Crit Care Med 21: 318–327, 1993 [DOI] [PubMed] [Google Scholar]
- 112. Flohe SB, Agrawal H, Flohe S, Rani M, Bangen JM, Schade FU. Diversity of interferon gamma and granulocyte-macrophage colony-stimulating factor in restoring immune dysfunction of dendritic cells and macrophages during polymicrobial sepsis. Mol Med 14: 247–256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J Leukocyte Biol 79: 473–481, 2006 [DOI] [PubMed] [Google Scholar]
- 114. Foley JH, Petersen KU, Rea CJ, Harpell L, Powell S, Lillicrap D, Nesheim ME, Sorensen B. Solulin increases clot stability in whole blood from individuals and dogs with hemophilia. Blood 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fong Y, Tracey KJ, Moldawer LL, Hesse DG, Manogue KB, Kenney JS, Lee AT, Kuo GC, Allison AC, Lowry SF. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J Exp Med 170: 1627–1633, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fournier-Wirth C, Coste J. Nanotechnologies for pathogen detection: future alternatives? Biologicals 38: 9–13, 2010 [DOI] [PubMed] [Google Scholar]
- 117. Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, Marey A, Lestavel P. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest 101: 816–823, 1992 [DOI] [PubMed] [Google Scholar]
- 118. Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine 18: 1717–1720, 2000 [DOI] [PubMed] [Google Scholar]
- 119. Frasa H, Benaissa-Trouw B, Tavares L, van Kessel K, Poppelier M, Kraaijeveld K, Verhoef J. Enhanced protection by use of a combination of anticapsule and antilipopolysaccharide monoclonal antibodies against lethal Escherichia coli O18K5 infection of mice. Infection Immunity 64: 775–781, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Friedman G, Jankowski S, Marchant A, Goldman M, Kahn RJ, Vincent JL. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care 12: 183–187, 1997 [DOI] [PubMed] [Google Scholar]
- 121. Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. The American Surgeon 78: 1–8, 2012 [PubMed] [Google Scholar]
- 122. Fry TJ, Mackall CL. Current concepts of thymic aging. Springer Semin Immunopathol 24: 7–22, 2002 [DOI] [PubMed] [Google Scholar]
- 123. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 107: 15880–15885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fukudome K, Ye X, Tsuneyoshi N, Tokunaga O, Sugawara K, Mizokami H, Kimoto M. Activation mechanism of anticoagulant protein C in large blood vessels involving the endothelial cell protein C receptor. J Exp Med 187: 1029–1035, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gaini S, Koldkjaer OG, Moller HJ, Pedersen C, Pedersen SS. A comparison of high-mobility group-box 1 protein, lipopolysaccharide-binding protein and procalcitonin in severe community-acquired infections and bacteraemia: a prospective study. Crit Care 11: R76, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gamez-Diaz LY, Enriquez LE, Matute JD, Velasquez S, Gomez ID, Toro F, Ospina S, Bedoya V, Arango CM, Valencia ML, De La Rosa G, Gomez CI, Garcia A, Patino PJ, Jaimes FA. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Academic Emergency Med 18: 807–815, 2011 [DOI] [PubMed] [Google Scholar]
- 127. Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, Mayumi T, Murata A, Ikeda T, Ishikura H, Ueyama M, Ogura H, Kushimoto S, Saitoh D, Endo S, Shimazaki S. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 34: 625–631, 2006 [DOI] [PubMed] [Google Scholar]
- 128. Gattas DJ, Cook DJ. Procalcitonin as a diagnostic test for sepsis: health technology assessment in the ICU. J Crit Care 18: 52–58, 2003 [DOI] [PubMed] [Google Scholar]
- 129. Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72: 1491–1501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Georgopoulou AP, Savva A, Giamarellos-Bourboulis EJ, Georgitsi M, Raftogiannis M, Antonakos N, Apostolidou E, Carrer DP, Dimopoulos G, Economou A, Efthymiou G, Galanakis N, Galani L, Gargalianos P, Karaiskos I, Katsenos C, Kavatha D, Koratzanis E, Labropoulos P, Lada M, Nakos G, Paggalou E, Panoutsopoulos G, Paraschos M, Pavleas I, Pontikis K, Poulakou G, Prekates A, Sybardi S, Theodorakopoulou M, Trakatelli C, Tsiaoussis P, Gogos C, Giamarellou H, Armaganidis A, Meisner M. Early changes of procalcitonin may advise about prognosis and appropriateness of antimicrobial therapy in sepsis. J Crit Care 26: 331 e331–337, 2011 [DOI] [PubMed] [Google Scholar]
- 131. Giamarellos-Bourboulis EJ, Mega A, Grecka P, Scarpa N, Koratzanis G, Thomopoulos G, Giamarellou H. Procalcitonin: a marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med 28: 1351–1356, 2002 [DOI] [PubMed] [Google Scholar]
- 132. Girard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clin Infectious Dis 40: 719–727, 2005 [DOI] [PubMed] [Google Scholar]
- 133. Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K, Lymberopoulou K, Mainas E, Marioli A, Massouras C, Mavrou I, Mpalla M, Michalia M, Mylona H, Mytas V, Papanikolaou I, Papanikolaou K, Patrani M, Perdios I, Plachouras D, Pistiki A, Protopapas K, Rigaki K, Sakka V, Sartzi M, Skouras V, Souli M, Spyridaki A, Strouvalis I, Tsaganos T, Zografos G, Mandragos K, Klouva-Molyvdas P, Maggina N, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14: R96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gold JA, Parsey M, Hoshino Y, Hoshino S, Nolan A, Yee H, Tse DB, Weiden MD. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infection Immunity 71: 3521–3528, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Goto M, Zeller WP, Hurley RM, Jong JS, Lee CH. Prophylaxis and treatment of newborn endotoxic shock with anti-lipid A monoclonal antibodies. Circ Shock 35: 60–64, 1991 [PubMed] [Google Scholar]
- 136. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 23: 515–548, 2005 [DOI] [PubMed] [Google Scholar]
- 137. Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol 106: 106–115, 2003 [DOI] [PubMed] [Google Scholar]
- 138. Gros A, Roussel M, Sauvadet E, Gacouin A, Marque S, Chimot L, Lavoue S, Camus C, Fest T, Le Tulzo Y. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med 38: 445–452, 2012 [DOI] [PubMed] [Google Scholar]
- 139. Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med 35: 1950–1958, 2009 [DOI] [PubMed] [Google Scholar]
- 140. Grubeck-Loebenstein B, Berger P, Saurwein-Teissl M, Zisterer K, Wick G. No immunity for the elderly. Nature Med 4: 870, 1998 [DOI] [PubMed] [Google Scholar]
- 141. Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol 80: 243–284, 2002 [DOI] [PubMed] [Google Scholar]
- 142. Guerra-Romero L, Tureen JH, Fournier MA, Makrides V, Tauber MG. Amino acids in cerebrospinal and brain interstitial fluid in experimental pneumococcal meningitis. Pediatr Res 33: 510–513, 1993 [DOI] [PubMed] [Google Scholar]
- 143. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Cheron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, Venet F. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 15: R99, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guntupalli K, Dean N, Morris PE, Bandi V, Margolis B, Rivers E, Levy M, Lodato RF, Ismail PM, Reese A, Schaumberg JP, Malik R, Dellinger RP. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit Care Med 41: 706–716, 2013 [DOI] [PubMed] [Google Scholar]
- 145. Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology 50: 2022–2033, 2009 [DOI] [PubMed] [Google Scholar]
- 147. Haase N, Perner A, Hennings LI, Siegemund M, Lauridsen B, Wetterslev M, Wetterslev J. Hydroxyethyl starch 130/0.38–0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ 346: f839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hackett SJ, Thomson AP, Hart CA. Cytokines, chemokines and other effector molecules involved in meningococcal disease. J Med Microbiol 50: 847–859, 2001 [DOI] [PubMed] [Google Scholar]
- 149. Hall TC, Bilku DK, Al-Leswas D, Horst C, Dennison AR. The difficulties of clinical trials evaluating therapeutic agents in patients with severe sepsis. Irish J Med Sci 181: 1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 150. Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature 363: 446–448, 1993 [DOI] [PubMed] [Google Scholar]
- 151. Hartog CS, Skupin H, Natanson C, Sun J, Reinhart K. Systematic analysis of hydroxyethyl starch (HES) reviews: proliferation of low-quality reviews overwhelms the results of well-performed meta-analyses. Intensive Care Med 38: 1258–1271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hauser AB, Stinghen AE, Kato S, Bucharles S, Aita C, Yuzawa Y, Pecoits-Filho R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit Dial Int 28 Suppl 3: S183–187, 2008 [PubMed] [Google Scholar]
- 153. Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg 178: 288–292, 1999 [DOI] [PubMed] [Google Scholar]
- 155. Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, Hoekstra JB, DeVries JH. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 38: 1430–1434 [DOI] [PubMed] [Google Scholar]
- 156. Heuer JG, Sharma GR, Gerlitz B, Zhang T, Bailey DL, Ding C, Berg DT, Perkins D, Stephens EJ, Holmes KC, Grubbs RL, Fynboe KA, Chen YF, Grinnell B, Jakubowski JA. Evaluation of protein C and other biomarkers as predictors of mortality in a rat cecal ligation and puncture model of sepsis. Crit Care Med 32: 1570–1578, 2004 [DOI] [PubMed] [Google Scholar]
- 157. Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med 28: 3599–3605, 2000 [DOI] [PubMed] [Google Scholar]
- 158. Ho KM, Lee KY, Dobb GJ, Webb SA. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a prospective cohort study. Intensive Care Med 34: 481–487, 2008 [DOI] [PubMed] [Google Scholar]
- 159. Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med 32: 1928–1948, 2004 [DOI] [PubMed] [Google Scholar]
- 160. Horz HP, Scheer S, Vianna ME, Conrads G. New methods for selective isolation of bacterial DNA from human clinical specimens. Anaerobe 16: 47–53, 2010 [DOI] [PubMed] [Google Scholar]
- 161. Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nature Med 15: 496–497, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003 [DOI] [PubMed] [Google Scholar]
- 163. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet 13: 260–268, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822, 2006 [DOI] [PubMed] [Google Scholar]
- 165. Hotchkiss RS, Opal S. Immunotherapy for sepsis–a new approach against an ancient foe. N Engl J Med 363: 87–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol 174: 5110–5118, 2005 [DOI] [PubMed] [Google Scholar]
- 167. Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med 25: 1298–1307, 1997 [DOI] [PubMed] [Google Scholar]
- 168. Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 162: 4148–4156, 1999 [PubMed] [Google Scholar]
- 169. Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA 106: 6303–6308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hubacek JA, Stuber F, Frohlich D, Book M, Wetegrove S, Ritter M, Rothe G, Schmitz G. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med 29: 557–561, 2001 [DOI] [PubMed] [Google Scholar]
- 171. Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock 24 Suppl 1: 52–57, 2005 [DOI] [PubMed] [Google Scholar]
- 172. Hussain N, Hodson D, Marcus R, Baglin T, Luddington R. The biphasic transmittance waveform: an early marker of sepsis in patients with neutropenia. Thrombosis Haemostasis 100: 146–148, 2008 [DOI] [PubMed] [Google Scholar]
- 173. Huttunen R, Hurme M, Aittoniemi J, Huhtala H, Vuento R, Laine J, Jylhava J, Syrjanen J. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PloS One 6: e17653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Hynninen M, Pettila V, Takkunen O, Orko R, Jansson SE, Kuusela P, Renkonen R, Valtonen M. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock 20: 1–4, 2003 [DOI] [PubMed] [Google Scholar]
- 175. Iapichino G, Marzorati S, Umbrello M, Baccalini R, Barassi A, Cainarca M, Colombo Pavini F, Mantovani E, Mauri A, Moroni B, Noto A, Melzi D'Eril GV, Langer M. Daily monitoring of biomarkers of sepsis in complicated long-term ICU-patients: can it support treatment decisions? Minerva Anestesiol 76: 814–823, 2010 [PubMed] [Google Scholar]
- 176. Inden M, Kitamura Y, Takeuchi H, Yanagida T, Takata K, Kobayashi Y, Taniguchi T, Yoshimoto K, Kaneko M, Okuma Y, Taira T, Ariga H, Shimohama S. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem 101: 1491–1504, 2007 [DOI] [PubMed] [Google Scholar]
- 177. Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol 184: 1401–1409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med 41: 159–170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Iwata A, de Claro RA, Morgan-Stevenson VL, Tupper JC, Schwartz BR, Liu L, Zhu X, Jordan KC, Winn RK, Harlan JM. Extracellular administration of BCL2 protein reduces apoptosis and improves survival in a murine model of sepsis. PLoS One 6: e14729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Jalava-Karvinen P, Hohenthal U, Laitinen I, Kotilainen P, Rajamaki A, Nikoskelainen J, Lilius EM, Nuutila J. Simultaneous quantitative analysis of Fc gamma RI (CD64) and CR1 (CD35) on neutrophils in distinguishing between bacterial infections, viral infections, and inflammatory diseases. Clin Immunol 133: 314–323, 2009 [DOI] [PubMed] [Google Scholar]
- 181. Jensen GL, McGee M, Binkley J. Nutrition in the elderly. Gastroenterol Clin N Am 30: 313–334, 2001 [DOI] [PubMed] [Google Scholar]
- 182. Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med 34: 2596–2602, 2006 [DOI] [PubMed] [Google Scholar]
- 183. Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infection Immunity 78: 4714–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kennon C, Overturf G, Bessman S, Sierra E, Smith KJ, Brann B. Granulocyte colony-stimulating factor as a marker for bacterial infection in neonates. J Pediatr 128: 765–769, 1996 [DOI] [PubMed] [Google Scholar]
- 185. Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrobial Chemotherapy 66 Suppl 2: ii33–40, 2011 [DOI] [PubMed] [Google Scholar]
- 186. Kiehntopf M, Schmerler D, Brunkhorst FM, Winkler R, Ludewig K, Osterloh D, Bloos F, Reinhart K, Deufel T. Mass spectometry-based protein patterns in the diagnosis of sepsis/systemic inflammatory response syndrome. Shock 36: 560–569, 2011 [DOI] [PubMed] [Google Scholar]
- 187. Kim WY, Huh JW, Lim CM, Koh Y, Hong SB. Analysis of progression in risk, injury, failure, loss, and end-stage renal disease classification on outcome in patients with severe sepsis and septic shock. J Crit Care 27: 104 e101–107, 2012 [DOI] [PubMed] [Google Scholar]
- 188. Kluger MJ, Kozak W, Leon LR, Conn CA. The use of knockout mice to understand the role of cytokines in fever. Clin Exp Pharmacol Physiol 25: 141–144, 1998 [DOI] [PubMed] [Google Scholar]
- 189. Knaus WA, Harrell FE, Jr, LaBrecque JF, Wagner DP, Pribble JP, Draper EA, Fisher CJ, Jr, Soll L. Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. The rhIL-1ra Phase III Sepsis Syndrome Study Group. Crit Care Med 24: 46–56, 1996 [DOI] [PubMed] [Google Scholar]
- 190. Knaus WA, Sun X, Nystrom O, Wagner DP. Evaluation of definitions for sepsis. Chest 101: 1656–1662, 1992 [DOI] [PubMed] [Google Scholar]
- 191. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100: 1619–1636, 1991 [DOI] [PubMed] [Google Scholar]
- 192. Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol 265–275, 1983 [DOI] [PubMed] [Google Scholar]
- 193. Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, Larsen K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care 11: R38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Kortgen A, Niederprum P, Bauer M. Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med 34: 943–949, 2006 [DOI] [PubMed] [Google Scholar]
- 195. Kozlov AV, Duvigneau JC, Miller I, Nurnberger S, Gesslbauer B, Kungl A, Ohlinger W, Hartl RT, Gille L, Staniek K, Gregor W, Haindl S, Redl H. Endotoxin causes functional endoplasmic reticulum failure, possibly mediated by mitochondria. Biochim Biophys Acta 1792: 521–530, 2009 [DOI] [PubMed] [Google Scholar]
- 196. Kreger BE, Craven DE, McCabe WR. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med 68: 344–355, 1980 [DOI] [PubMed] [Google Scholar]
- 197. Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Simon D, Peters C, Ahsan M, Chateau D. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136: 1237–1248, 2009 [DOI] [PubMed] [Google Scholar]
- 198. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34: 1589–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 199. Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 140: 1223–1231, 2011 [DOI] [PubMed] [Google Scholar]
- 200. Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theoret Biol 230: 145–155, 2004 [DOI] [PubMed] [Google Scholar]
- 201. Kuppermann N, Nelson DS, Saladino RA, Thompson CM, Sattler F, Novitsky TJ, Fleisher GR, Siber GR. Comparison of a recombinant endotoxin-neutralizing protein with a human monoclonal antibody to endotoxin for the treatment of Escherichia coli sepsis in rats. J Infectious Dis 170: 630–635, 1994 [DOI] [PubMed] [Google Scholar]
- 202. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 334: 1209–1215, 1996 [DOI] [PubMed] [Google Scholar]
- 203. Kuster H, Weiss M, Willeitner AE, Detlefsen S, Jeremias I, Zbojan J, Geiger R, Lipowsky G, Simbruner G. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet 352: 1271–1277, 1998 [DOI] [PubMed] [Google Scholar]
- 204. Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 40: 754–761, 2012 [DOI] [PubMed] [Google Scholar]
- 205. Lamar CD, Hurley RA, Taber KH. Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci 23: 237–241 [DOI] [PubMed] [Google Scholar]
- 206. LaRosa SP, Opal SM. Tissue factor pathway inhibitor and antithrombin trial results. Crit Care Clin 21: 433–448, 2005 [DOI] [PubMed] [Google Scholar]
- 207. Latour-Perez J, Alcala-Lopez A, Garcia-Garcia MA, Sanchez-Hernandez JF, Abad-Terrado C, Viedma-Contreras JA, Masia M, Gonzalez-Tejera M, Arizo-Leon D, Porcar MJ, Bonilla-Rovira F, Gutierrez F. Diagnostic accuracy of sTREM-1 to identify infection in critically ill patients with systemic inflammatory response syndrome. Clin Biochem 43: 720–724, 2010 [DOI] [PubMed] [Google Scholar]
- 208. Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16: 452–466, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med 23: 1294–1303, 1995 [DOI] [PubMed] [Google Scholar]
- 210. LeMay LG, Vander AJ, Kluger MJ. Role of interleukin 6 in fever in rats. Am J Physiol Regul Integr Comp Physiol 258: R798–R803, 1990 [DOI] [PubMed] [Google Scholar]
- 211. Levi M, de Jonge E, van der PT. Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis 16: 43–47, 2003 [DOI] [PubMed] [Google Scholar]
- 212. Levi M, Schouten M, van't Veer C, van der Poll T. Factor V Leiden mutation in severe infection and sepsis. Semin Thromb Hemost 37: 955–960, 2011 [DOI] [PubMed] [Google Scholar]
- 213. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145: 24–33, 2009 [DOI] [PubMed] [Google Scholar]
- 214. Levin J, Cluff LE. Endotoxaemia and adrenal hemorrhage. A mechanism for the Waterhouse-Friderichsen syndrome. J Exp Med 121: 247–260, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 36: 222–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, Deutschman CS. Evidence of myocardial hibernation in the septic heart. Crit Care Med 33: 2752–2756, 2005 [DOI] [PubMed] [Google Scholar]
- 217. Lichtenstern C, Brenner T, Bardenheuer HJ, Weigand MA. Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infectious Dis 25: 328–336, 2012 [DOI] [PubMed] [Google Scholar]
- 218. Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, Jo EK, Park JK, Song CH. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS One 6: e28531, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Lozon TI, Eastman AJ, Matute-Bello G, Chen P, Hallstrand TS, Altemeier WA. PKR-dependent CHOP induction limits hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 300: L422–L429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Lu M, Varley AW, Ohta S, Hardwick J, Munford RS. Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following gram-negative bacterial infection. Cell Host Microbe 4: 293–302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lucas S. The autopsy pathology of sepsis-related death. Curr Diagn Pathol 13: 375–388, 2007 [Google Scholar]
- 222. Lustig MK, Bac VH, Pavlovic D, Maier S, Grundling M, Grisk O, Wendt M, Heidecke CD, Lehmann C. Colon ascendens stent peritonitis–a model of sepsis adopted to the rat: physiological, microcirculatory and laboratory changes. Shock 28: 59–64, 2007 [DOI] [PubMed] [Google Scholar]
- 223. Ma T, Han L, Gao Y, Li L, Shang X, Hu W, Xue C. The endoplasmic reticulum stress-mediated apoptosis signal pathway is involved in sepsis-induced abnormal lymphocyte apoptosis. Eur Surg Res 41: 219–225, 2008 [DOI] [PubMed] [Google Scholar]
- 224. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med 127: 257–266, 1997 [DOI] [PubMed] [Google Scholar]
- 225. Mancini N, Carletti S, Ghidoli N, Cichero P, Burioni R, Clementi M. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin Microbiol Rev 23: 235–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mancini N, Carletti S, Ghidoli N, Cichero P, Ossi CM, Ieri R, Poli E, Burioni R, Clementi M. Molecular diagnosis of polymicrobial sepsis. J Clin Microbiol 47: 1274–1275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Manjuck J, Saha DC, Astiz M, Eales LJ, Rackow EC. Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. J Lab Clin Med 135: 153–160, 2000 [DOI] [PubMed] [Google Scholar]
- 228. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med 31: 141–145, 2003 [DOI] [PubMed] [Google Scholar]
- 229. Marik PE, Zaloga GP. The effect of aging on circulating levels of proinflammatory cytokines during septic shock. Norasept II Study Investigators. J Am Geriatr Soc 49: 5–9, 2001 [DOI] [PubMed] [Google Scholar]
- 230. Marlar RA, Kleiss AJ, Griffin JH. Human protein C: inactivation of factors V and VIII in plasma by the activated molecule. Ann NY Acad Sci 370: 303–310, 1981 [DOI] [PubMed] [Google Scholar]
- 231. Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med 37: 2290–2298, 2009 [DOI] [PubMed] [Google Scholar]
- 232. Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, Beale R. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care 13: R103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 234. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34: 15–21, 2006 [DOI] [PubMed] [Google Scholar]
- 235. Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. McCue JD. Antibiotic use in the elderly: issues and nonissues. Clin Infect Dis 28: 750–752, 1999 [DOI] [PubMed] [Google Scholar]
- 237. McCulloh R, Opal SM. Human recombinant lactoferrin for sepsis: too good to be true? Crit Care Med 41: 908–909, 2013 [DOI] [PubMed] [Google Scholar]
- 238. McDunn JE, Husain KD, Polpitiya AD, Burykin A, Ruan J, Li Q, Schierding W, Lin N, Dixon D, Zhang W, Coopersmith CM, Dunne WM, Colonna M, Ghosh BK, Cobb JP. Plasticity of the systemic inflammatory response to acute infection during critical illness: development of the riboleukogram. PLoS One 3: e1564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 180: 640–648, 2009 [DOI] [PubMed] [Google Scholar]
- 240. Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, Carstina D, Oltean M. Multiplex cytokine profiling in patients with sepsis. Acta Pathol Microbiol Immunol Scand 119: 155–163, 2011 [DOI] [PubMed] [Google Scholar]
- 241. Mi Q, Li NY, Ziraldo C, Ghuma A, Mikheev M, Squires R, Okonkwo DO, Verdolini-Abbott K, Constantine G, An G, Vodovotz Y. Translational systems biology of inflammation: potential applications to personalized medicine. Per Med 7: 549–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 37: 1670–1677, 2009 [DOI] [PubMed] [Google Scholar]
- 243. Miller RA. The aging immune system: primer and prospectus. Science 273: 70–74, 1996 [DOI] [PubMed] [Google Scholar]
- 244. Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107: 1071–1082, 2010 [DOI] [PubMed] [Google Scholar]
- 245. Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract 15: 353–369, 2009 [DOI] [PubMed] [Google Scholar]
- 246. Mohr A, Polz J, Martin EM, Griessl S, Kammler A, Potschke C, Lechner A, Broker BM, Mostbock S, Mannel DN. Sepsis leads to a reduced antigen-specific primary antibody response. Eur J Immunol 42: 341–352, 2012 [DOI] [PubMed] [Google Scholar]
- 247. Monneret G, Finck ME, Venet F, Debard AL, Bohe J, Bienvenu J, Lepape A. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett 95: 193–198, 2004 [DOI] [PubMed] [Google Scholar]
- 248. Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, Thizy H, Bienvenu J, Gueyffier F, Vanhems P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 32: 1175–1183, 2006 [DOI] [PubMed] [Google Scholar]
- 249. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial Agents Chemother 49: 3640–3645, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood 104: 1740–1744, 2004 [DOI] [PubMed] [Google Scholar]
- 251. Mosnier LO, Lisman T, van den Berg HM, Nieuwenhuis HK, Meijers JC, Bouma BN. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost 86: 1035–1039, 2001 [PubMed] [Google Scholar]
- 252. Mosnier LO, Meijers JC, Bouma BN. Regulation of fibrinolysis in plasma by TAFI and protein C is dependent on the concentration of thrombomodulin. Thromb Haemost 85: 5–11, 2001 [PubMed] [Google Scholar]
- 253. Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem 282: 33022–33033, 2007 [DOI] [PubMed] [Google Scholar]
- 254. Mrus JM, Braun L, Yi MS, Linde-Zwirble WT, Johnston JA. Impact of HIV/AIDS on care and outcomes of severe sepsis. Crit Care 9: R623–630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 134: 769–781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med 29: 1404–1407, 2001 [DOI] [PubMed] [Google Scholar]
- 257. Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest 88: 1747–1754, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest 136: 102–109, 2009 [DOI] [PubMed] [Google Scholar]
- 259. Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke KD, Spies C. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15: R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, bd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369: 836–843 [DOI] [PubMed] [Google Scholar]
- 261. Nakamura D, Tsuru A, Ikegami K, Imagawa Y, Fujimoto N, Kohno K. Mammalian ER stress sensor IRE1beta specifically down-regulates the synthesis of secretory pathway proteins. FEBS Lett 585: 133–138, 2011 [DOI] [PubMed] [Google Scholar]
- 262. Nakashima M, Uematsu T, Umemura K, Maruyama I, Tsuruta K. A novel recombinant soluble human thrombomodulin, ART-123, activates the protein C pathway in healthy male volunteers. J Clin Pharmacol 38: 540–544, 1998 [DOI] [PubMed] [Google Scholar]
- 263. Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. Humane endpoints in shock research. Shock 21: 17–25, 2004 [DOI] [PubMed] [Google Scholar]
- 264. Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock 10: 110–117, 1998 [DOI] [PubMed] [Google Scholar]
- 265. Ng PC, Li K, Chui KM, Leung TF, Wong RP, Chu WC, Wong E, Fok TF. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res 61: 93–98, 2007 [DOI] [PubMed] [Google Scholar]
- 266. Nguyen HB, Loomba M, Yang JJ, Jacobsen G, Shah K, Otero RM, Suarez A, Parekh H, Jaehne A, Rivers EP. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J Inflamm 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Nicolle LE. Infection control in long-term care facilities. Clin Infectious Dis 31: 752–756, 2000 [DOI] [PubMed] [Google Scholar]
- 268. Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PGM, Have KT, Eijsman LN, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation 96: 3534–3541, 1997 [DOI] [PubMed] [Google Scholar]
- 269. Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, Karulf MR, Rom WN, Weiden MD, Gold JA. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PloS One 4: e6600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med 177: 301–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology 217: 616–621, 2012 [DOI] [PubMed] [Google Scholar]
- 272. Nuutila J, Hohenthal U, Laitinen I, Kotilainen P, Rajamaki A, Nikoskelainen J, Lilius EM. Simultaneous quantitative analysis of FcgammaRI (CD64) expression on neutrophils and monocytes: a new, improved way to detect infections. J Immunol Methods 328: 189–200, 2007 [DOI] [PubMed] [Google Scholar]
- 273. O'Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med 35: 345–350, 2007 [DOI] [PubMed] [Google Scholar]
- 274. O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Crit Care Med and the Infectious Diseases Society of America. Crit Care Med 36: 1330–1349, 2008 [DOI] [PubMed] [Google Scholar]
- 275. Oberhoffer M, Vogelsang H, Russwurm S, Hartung T, Reinhart K. Outcome prediction by traditional and new markers of inflammation in patients with sepsis. Clin Chem Lab Med 37: 363–368, 1999 [DOI] [PubMed] [Google Scholar]
- 276. Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16: 83–96, 2001 [DOI] [PubMed] [Google Scholar]
- 277. Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348: 550–552, 1990 [DOI] [PubMed] [Google Scholar]
- 278. Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Yao J, Paton AW, Paton JC, Kitamura M. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am J Physiol Renal Physiol 295: F1495–F1503, 2008 [DOI] [PubMed] [Google Scholar]
- 279. Opal SM. Clinical trial design and outcomes in patients with severe sepsis. Shock 20: 295–302, 2003 [DOI] [PubMed] [Google Scholar]
- 280. Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 25: 1115–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 281. Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, Vincent JL. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309: 1154–1162 [DOI] [PubMed] [Google Scholar]
- 282. Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, Chierego ML, Silva TO, Fonseca A, Vincent JL, De Backer D. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 36: 949–955 [DOI] [PubMed] [Google Scholar]
- 283. Osuchowski M, Welch K, Yang H, Siddiqui J, Remick D. Sepsis: always in Mars. Shock 25: 5, 2006 [Google Scholar]
- 284. Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med 37: 1567–1573, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Osuchowski MF, Craciun F, Weixelbaumer KM, Duffy ER, Remick DG. Sepsis chronically in MARS: systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J Immunol 189: 4648–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Osuchowski MF, Craciun FL, Schuller E, Sima C, Gyurko R, Remick DG. Untreated type 1 diabetes increases sepsis-induced mortality without inducing a prelethal cytokine response. Shock 34: 369–376, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 177: 1967–1974, 2006 [DOI] [PubMed] [Google Scholar]
- 288. Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J Immunol 179: 623–630, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Oude Nijhuis CS, Vellenga E, Daenen SM, van der Graaf WT, Gietema JA, Groen HJ, Kamps WA, de Bont ES. Lipopolysaccharide-binding protein: a possible diagnostic marker for Gram-negative bacteremia in neutropenic cancer patients. Intensive Care Med 29: 2157–2161, 2003 [DOI] [PubMed] [Google Scholar]
- 290. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Pacher R, Redl H, Frass M, Petzl DH, Schuster E, Woloszczuk W. Relationship between neopterin and granulocyte elastase plasma levels and the severity of multiple organ failure. Crit Care Med 17: 221–226, 1989 [DOI] [PubMed] [Google Scholar]
- 292. Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M, Teoh L, Van Meter L, Daum L, Lemeshow S, Hicklin G, Doig C. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med 32: 2173–2182, 2004 [DOI] [PubMed] [Google Scholar]
- 293. Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg 88: 22–30, 2001 [DOI] [PubMed] [Google Scholar]
- 294. Parmar A, Langenberg C, Wan L, May CN, Bellomo R, Bagshaw SM. Epidemiology of septic acute kidney injury. Curr Drug Targets 10: 1169–1178, 2009 [DOI] [PubMed] [Google Scholar]
- 295. Pavare J, Grope I, Kalnins I, Gardovska D. High-mobility group box-1 protein, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in children with community acquired infections and bacteraemia: a prospective study. BMC Infect Dis 10: 28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Penack O, Buchheidt D, Christopeit M, von Lilienfeld-Toal M, Massenkeil G, Hentrich M, Salwender H, Wolf HH, Ostermann H. Management of sepsis in neutropenic patients: guidelines from the infectious diseases working party of the German Society of Hematology and Oncology. Ann Oncol 22: 1019–1029, 2011 [DOI] [PubMed] [Google Scholar]
- 297. Peng SC, Wong DS, Tung KC, Chen YY, Chao CC, Peng CH, Chuang YJ, Tang CY. Computational modeling with forward and reverse engineering links signaling network and genomic regulatory responses: NF-kappaB signaling-induced gene expression responses in inflammation. BMC Bioinformatics 11: 308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Perez EA, Dueck AC, McCullough AE, Reinholz MM, Tenner KS, Davidson NE, Gralow J, Harris LN, Kutteh LA, Hillman DW, Jenkins RB, Chen B. Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst 104: 159–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med 5: 115–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Perl TM, Dvorak L, Hwang T, Wenzel RP. Long-term survival and function after suspected gram-negative sepsis. JAMA 274: 338–345, 1995 [PubMed] [Google Scholar]
- 301. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 14: R15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest 103: 565–575, 1993 [DOI] [PubMed] [Google Scholar]
- 303. Pitts LR, Simpson SQ. From mice to men: systematic reviews of animal data could make sepsis trials safer and more productive. Crit Care Med 38: 2420–2422, 2010 [DOI] [PubMed] [Google Scholar]
- 304. Ploder M, Pelinka L, Schmuckenschlager C, Wessner B, Ankersmit HJ, Fuerst W, Redl H, Roth E, Spittler A. Lipopolysaccharide-induced tumor necrosis factor alpha production and not monocyte human leukocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock 25: 129–134, 2006 [DOI] [PubMed] [Google Scholar]
- 305. Pluschke G, Achtman M. Antibodies to O-antigen of lipopolysaccharide are protective against neonatal infection with Escherichia coli K1. Infection Immunity 49: 365–370, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock 30 Suppl 1: 53–59, 2008 [DOI] [PubMed] [Google Scholar]
- 307. Pope JV, Jones AE, Gaieski DF, Arnold RC, Trzeciak S, Shapiro NI. Multicenter study of central venous oxygen saturation (ScvO2) as a predictor of mortality in patients with sepsis. Ann Emerg Med 55: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Povoa P, Teixeira-Pinto AM, Carneiro AH. C-reactive protein, an early marker of community-acquired sepsis resolution: a multi-center prospective observational study. Crit Care 15: R169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chiolero R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 35: 1738–1748, 2009 [DOI] [PubMed] [Google Scholar]
- 310. Pruitt JH, Welborn MB, Edwards PD, Harward TR, Seeger JW, Martin TD, Smith C, Kenney JA, Wesdorp RI, Meijer S, Cuesta MA, Abouhanze A, Copeland EM, 3rd, Giri J, Sims JE, Moldawer LL, Oldenburg HS. Increased soluble interleukin-1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood 87: 3282–3288, 1996 [PubMed] [Google Scholar]
- 311. Raeven P, Feichtinger GA, Weixelbaumer KM, Atzenhofer S, Redl H, Van Griensven M, Bahrami S, Osuchowski MF. Homogeneity versus diversity: inhibition of plasma PAI-1 in murine sepsis proved lethal in homogeneous cohorts but not in all-inclusive populations. Critical Care 11 Suppl 3: 16, 2012 [Google Scholar]
- 312. Rajagopalan S, Yoshikawa TT. Antimicrobial therapy in the elderly. Med Clin North Am 85: 133–147, vii, 2001 [DOI] [PubMed] [Google Scholar]
- 313. Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 6: 252–262, 1986 [DOI] [PubMed] [Google Scholar]
- 314. Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366: 2055–2064, 2012 [DOI] [PubMed] [Google Scholar]
- 315. Redl H, Schlag G, Togel E, Assicot M, Bohuon C. Procalcitonin release patterns in a baboon model of trauma and sepsis: relationship to cytokines and neopterin. Crit Care Med 28: 3659–3663, 2000 [DOI] [PubMed] [Google Scholar]
- 316. Reinhart K, Meisner M, Brunkhorst FM. Markers for sepsis diagnosis: what is useful? Crit Care Clinics 22: 503–519, ix-x, 2006 [DOI] [PubMed] [Google Scholar]
- 317. Remick DG. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr Pharmaceutical Design 9: 75–82, 2003 [DOI] [PubMed] [Google Scholar]
- 318. Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infection Immunity 73: 2751–2757, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Remick DG, Bolgos GE, Siddiqui J. Inflammatory status in sepsis alters efficacy of interleukin-18 binding protein therapy. Crit Care Med 31: 2096–2101, 2003 [DOI] [PubMed] [Google Scholar]
- 320. Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17: 463–467, 2002 [DOI] [PubMed] [Google Scholar]
- 321. Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13: 110–116, 2000 [DOI] [PubMed] [Google Scholar]
- 322. Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock 24 Suppl 1: 7–11, 2005 [DOI] [PubMed] [Google Scholar]
- 323. Remick DG, Xioa H. Hypothermia and sepsis. Front Biosci 11: 1006–1013, 2006 [DOI] [PubMed] [Google Scholar]
- 324. Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Bard Ermentrout G. A reduced mathematical model of the acute inflammatory response. I. Derivation of model and analysis of anti-inflammation. J Theor Biol 242: 220–236, 2006 [DOI] [PubMed] [Google Scholar]
- 325. Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, Ii M, Matsuda H, Mouri K, Cohen J. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 38: 1685–1694 [DOI] [PubMed] [Google Scholar]
- 326. Riche FC, Cholley BP, Panis YH, Laisne MJ, Briard CG, Graulet AM, Gueris JL, Valleur PD. Inflammatory cytokine response in patients with septic shock secondary to generalized peritonitis. Crit Care Med 28: 433–437, 2000 [DOI] [PubMed] [Google Scholar]
- 327. Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8: 776–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukocyte Biol 81: 137–143, 2007 [DOI] [PubMed] [Google Scholar]
- 329. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 330. Rivers EP. Point: adherence to early goal-directed therapy: does it really matter? Yes. After a decade, the scientific proof speaks for itself. Chest 138: 476–480 [DOI] [PubMed] [Google Scholar]
- 331. Roger PM, Hyvernat H, Breittmayer JP, Dunais B, Dellamonica J, Bernardin G, Bernard A. Enhanced T-cell apoptosis in human septic shock is associated with alteration of the costimulatory pathway. Eur J Clin Microbiol Infectious Dis 28: 575–584, 2009 [DOI] [PubMed] [Google Scholar]
- 332. Root RK, Lodato RF, Patrick W, Cade JF, Fotheringham N, Milwee S, Vincent JL, Torres A, Rello J, Nelson S. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med 31: 367–373, 2003 [DOI] [PubMed] [Google Scholar]
- 333. Rosenberg AL, Seneff MG, Atiyeh L, Wagner R, Bojanowski L, Zimmerman JE. The importance of bacterial sepsis in intensive care unit patients with acquired immunodeficiency syndrome: implications for future care in the age of increasing antiretroviral resistance. Crit Care Med 29: 548, 2001 [DOI] [PubMed] [Google Scholar]
- 334. Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, Ankersmit HJ. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochem Biophys Res Commun 308: 840–846, 2003 [DOI] [PubMed] [Google Scholar]
- 336. Ruokonen E, Ilkka L, Niskanen M, Takala J. Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand 46: 398–404, 2002 [DOI] [PubMed] [Google Scholar]
- 337. Rutkowski DT, Kaufman RJ. All roads lead to ATF4. Dev Cell 4: 442–444, 2003 [DOI] [PubMed] [Google Scholar]
- 338. Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, Hirayama A, Matsuda T, Asakura H, Nakashima M, Aoki N. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thrombosis Haemostasis 5: 31–41, 2007 [DOI] [PubMed] [Google Scholar]
- 339. Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev 124: 1047–1058, 2003 [DOI] [PubMed] [Google Scholar]
- 340. Sapey E, Stockley RA. Exacerbations COPD 2: aetiology. Thorax 61: 250–258, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Sarafidis K, Soubasi-Griva V, Piretzi K, Thomaidou A, Agakidou E, Taparkou A, Diamanti E, Drossou-Agakidou V. Diagnostic utility of elevated serum soluble triggering receptor expressed on myeloid cells (sTREM)-1 in infected neonates. Intensive Care Med 36: 864–868, 2010 [DOI] [PubMed] [Google Scholar]
- 342. Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg 133: 1200–1205, 1998 [DOI] [PubMed] [Google Scholar]
- 343. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med 9: 107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Int Med 171: 1322–1331, 2011 [DOI] [PubMed] [Google Scholar]
- 345. Schwulst SJ, Grayson MH, DiPasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic monoclonal antibody against CD40 receptor decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol 177: 557–565, 2006 [DOI] [PubMed] [Google Scholar]
- 346. Scott MJ, Hoth JJ, Stagner MK, Gardner SA, Peyton JC, Cheadle WG. CD40-CD154 interactions between macrophages and natural killer cells during sepsis are critical for macrophage activation and are not interferon gamma dependent. Clin Exp Immunol 137: 469–477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Seam N, Suffredini AF. Mechanisms of sepsis and insights from clinical trials. Drug Discov Today Dis Mech 4: 83–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Kohl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med 28: 2793–2798, 2000 [DOI] [PubMed] [Google Scholar]
- 349. Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 45: 524–528, 2005 [DOI] [PubMed] [Google Scholar]
- 351. Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson KJ, Milzman D, Gaieski DF, Goyal M, Cairns CB, Ngo L, Rivers EP. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 37: 96–104, 2009 [DOI] [PubMed] [Google Scholar]
- 352. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunol 8: 239–245, 2007 [DOI] [PubMed] [Google Scholar]
- 353. Sherwin C, Broadbent R, Young S, Worth J, McCaffrey F, Medlicott NJ, Reith D. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol 25: 629–636, 2008 [DOI] [PubMed] [Google Scholar]
- 353a. Shoda J, Kano M, Oda K, Kamiya J, Nimura Y, Suzuki H, Sugiyama Y, Miyazaki H, Todoroki T, Stengelin S, Kramer W, Matsuzaki Y, Tanaka N. The expression levels of plasma membrane transporters in the cholestatic liver of patients undergoing biliary drainage and their association with the impairment of biliary secretory function. Am J Gastroenterol 96: 3368–3378, 2001 [DOI] [PubMed] [Google Scholar]
- 354. Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatric Crit Care Med 9: 407–413, 2008 [DOI] [PubMed] [Google Scholar]
- 355. Simpson AJ, Smith MD, Weverling GJ, Suputtamongkol Y, Angus BJ, Chaowagul W, White NJ, van Deventer SJ, Prins JM. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J Infectious Dis 181: 621–625, 2000 [DOI] [PubMed] [Google Scholar]
- 356. Slotman GJ. Prospectively validated prediction of organ failure and hypotension in patients with septic shock: the Systemic Mediator Associated Response Test (SMART). Shock 14: 101–106, 2000 [DOI] [PubMed] [Google Scholar]
- 357. Solis MM, Cook C, Cook J, Glaser C, Light D, Morser J, Yu SC, Fink L, Eidt JF. Intravenous recombinant soluble human thrombomodulin prevents venous thrombosis in a rat model. J Vasc Surg 14: 599–604, 1991 [DOI] [PubMed] [Google Scholar]
- 358. Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone therapy for patients with septic shock. N Engl J Med 358: 111–124, 2008 [DOI] [PubMed] [Google Scholar]
- 359. Stanilova SA, Karakolev ZT, Dimov GS, Dobreva ZG, Miteva LD, Slavov ES, Stefanov CS, Stanilov NS. High interleukin 12 and low interleukin 10 production after in vitro stimulation detected in sepsis survivors. Intensive Care Med 31: 401–407, 2005 [DOI] [PubMed] [Google Scholar]
- 360. Stearns-Kurosawa DJ, Collins V, Freeman S, Tesh VL, Kurosawa S. Distinct physiologic and inflammatory responses elicited in baboons after challenge with Shiga toxin type 1 or 2 from enterohemorrhagic Escherichia coli. Infectious Immunity 78: 2497–2504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA 93: 10212–10216, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol 6: 19–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Stocker M, Fontana M, El Helou S, Wegscheider K, Berger TM. Use of procalcitonin-guided decision-making to shorten antibiotic therapy in suspected neonatal early-onset sepsis: prospective randomized intervention trial. Neonatology 97: 165–174, 2010 [DOI] [PubMed] [Google Scholar]
- 364. Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly 138: 512–519, 2008 [DOI] [PubMed] [Google Scholar]
- 365. Stoller JK, Aboussouan LS. A Review of alpha1-Antitrypsin Deficiency. Am J Respir Crit Care Med 185: 246–259, 2011 [DOI] [PubMed] [Google Scholar]
- 366. Strassburg CP. Shock liver. Best Pract Res Clin Gastroenterol 17: 369–381, 2003 [DOI] [PubMed] [Google Scholar]
- 367. Strohmaier W, Redl H, Schlag G, Inthorn D. d-Erythro-neopterin plasma levels in intensive care patients with and without septic complications. Crit Care Med 15: 757–760, 1987 [DOI] [PubMed] [Google Scholar]
- 368. Su EJ, Geyer M, Wahl M, Mann K, Ginsburg D, Brohmann H, Petersen KU, Lawrence DA. The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J Thrombosis Haemostasis 9: 1174–1182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355: 1018–1028, 2006 [DOI] [PubMed] [Google Scholar]
- 370. Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, Thomas R. Procalcitonin: a valuable indicator of infection in a medical ICU? Intensive Care Med 26: 1232–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 371. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Tabata Y, Takano K, Ito T, Iinuma M, Yoshimoto T, Miura H, Kitao Y, Ogawa S, Hori O. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am J Physiol Cell Physiol 293: C411–C418, 2007 [DOI] [PubMed] [Google Scholar]
- 373. Takemitsu T, Wada H, Hatada T, Ohmori Y, Ishikura K, Takeda T, Sugiyama T, Yamada N, Maruyama K, Katayama N, Isaji S, Shimpo H, Kusunoki M, Nobori T. Prospective evaluation of three different diagnostic criteria for disseminated intravascular coagulation. Thrombosis Haemostasis 105: 40–44, 2011 [DOI] [PubMed] [Google Scholar]
- 374. Tamayo E, Fernandez A, Almansa R, Carrasco E, Heredia M, Lajo C, Goncalves L, Gomez-Herreras JI, de Lejarazu RO, Bermejo-Martin JF. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Network 22: 82–87, 2011 [DOI] [PubMed] [Google Scholar]
- 375. Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet 7: 210–217, 2007 [DOI] [PubMed] [Google Scholar]
- 376. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation: On behalf of the Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Thrombosis Haemostasis 86: 1327–1330, 2001 [PubMed] [Google Scholar]
- 377. Tegtmeyer FK, Horn C, Richter A, van Wees J. Elastase alpha 1 proteinase inhibitor complex, granulocyte count, ratio of immature to total granulocyte count, and C-reactive protein in neonatal septicaemia. Eur J Pediatr 151: 353–356, 1992 [DOI] [PubMed] [Google Scholar]
- 378. Thirumala R, Ramaswamy M, Chawla S. Diagnosis and management of infectious complications in critically ill patients with cancer. Crit Care Clin 26: 59–91 [DOI] [PubMed] [Google Scholar]
- 379. Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 99: 275–282, 2006 [DOI] [PubMed] [Google Scholar]
- 380. Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38: 72–83 [DOI] [PubMed] [Google Scholar]
- 381. Tieri P, Termanini A, Bellavista E, Salvioli S, Capri M, Franceschi C. Charting the NF-kappaB Pathway Interactome Map. PLoS One 7: e32678, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol 171: 909–914, 2003 [DOI] [PubMed] [Google Scholar]
- 383. Toh CH, Hoots WK, Isthsodic OT. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thrombosis Haemostasis 5: 604–606, 2007 [DOI] [PubMed] [Google Scholar]
- 384. Toltl LJ, Austin RC, Liaw PC. Activated protein C modulates inflammation, apoptosis and tissue factor procoagulant activity by regulating endoplasmic reticulum calcium depletion in blood monocytes. J Thrombosis Haemostasis 9: 582–592, 2011 [DOI] [PubMed] [Google Scholar]
- 385. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330: 662–664, 1987 [DOI] [PubMed] [Google Scholar]
- 387. Trzeciak S, Dellinger RP, Chansky ME, Arnold RC, Schorr C, Milcarek B, Hollenberg SM, Parrillo JE. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med 33: 970–977, 2007 [DOI] [PubMed] [Google Scholar]
- 388. Tsalik EL, Jaggers LB, Glickman SW, Langley RJ, van Velkinburgh JC, Park LP, Fowler VG, Cairns CB, Kingsmore SF, Woods CW. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emergency Med 43: 97–106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Tschaikowsky K, Hedwig-Geissing M, Braun GG, Radespiel-Troeger M. Predictive value of procalcitonin, interleukin-6, and C-reactive protein for survival in postoperative patients with severe sepsis. J Crit Care 26: 54–64, 2011 [DOI] [PubMed] [Google Scholar]
- 390. Tschaikowsky K, Hedwig-Geissing M, Schiele A, Bremer F, Schywalsky M, Schuttler J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med 30: 1015–1023, 2002 [DOI] [PubMed] [Google Scholar]
- 391. Tschaikowsky K, Hedwig-Geissing M, Schmidt J, Braun GG. Lipopolysaccharide-binding protein for monitoring of postoperative sepsis: complemental to C-reactive protein or redundant? PloS One 6: e23615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock 21: 121–125, 2004 [DOI] [PubMed] [Google Scholar]
- 393. Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock 19: 310–313, 2003 [DOI] [PubMed] [Google Scholar]
- 394. Ulrichts H, Silence K, Schoolmeester A, de Jaegere P, Rossenu S, Roodt J, Priem S, Lauwereys M, Casteels P, Van Bockstaele F, Verschueren K, Stanssens P, Baumeister J, Holz JB. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood 118: 757–765, 2011 [DOI] [PubMed] [Google Scholar]
- 395. Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol 184: 3768–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Urano F, Bertolotti A, Ron D. IRE1 and efferent signaling from the endoplasmic reticulum. J Cell Sci 113: 3697–3702, 2000 [DOI] [PubMed] [Google Scholar]
- 397. Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med 34: 1996–2003, 2006 [DOI] [PubMed] [Google Scholar]
- 398. Valenti WM, Trudell RG, Bentley DW. Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N Engl J Med 298: 1108–1111, 1978 [DOI] [PubMed] [Google Scholar]
- 399. Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med 31: 1901–1907, 2003 [DOI] [PubMed] [Google Scholar]
- 400. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med 354: 449–461, 2006 [DOI] [PubMed] [Google Scholar]
- 401. Van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351: 950–953, 1998 [DOI] [PubMed] [Google Scholar]
- 402. Van Iersel T, Stroissnig H, Giesen P, Wemer J, Wilhelm-Ogunbiyi K. Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb Haemost 105: 302–312, 2011 [DOI] [PubMed] [Google Scholar]
- 403. Van Loon JE, de Jaegere PP, Ulrichts H, van Vliet HH, de Maat MP, de Groot PG, Simoons ML, Leebeek FW. The in vitro effect of the new antithrombotic drug candidate ALX-0081 on blood samples of patients undergoing percutaneous coronary intervention. Thromb Haemost 106: 165–171, 2011 [DOI] [PubMed] [Google Scholar]
- 404. Vaneycken I, D'Huyvetter M, Hernot S, De Vos J, Xavier C, Devoogdt N, Caveliers V, Lahoutte T. Immuno-imaging using nanobodies. Curr Opin Biotechnol 22: 877–881, 2011 [DOI] [PubMed] [Google Scholar]
- 405. Vaneycken I, Devoogdt N, Van Gassen N, Vincke C, Xavier C, Wernery U, Muyldermans S, Lahoutte T, Caveliers V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J 25: 2433–2446, 2011 [DOI] [PubMed] [Google Scholar]
- 406. Vanska M, Koivula I, Hamalainen S, Pulkki K, Nousiainen T, Jantunen E, Juutilainen A. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica 96: 1385–1389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettila V. Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med 35: 1277–1283, 2007 [DOI] [PubMed] [Google Scholar]
- 408. Verboon-Maciolek MA, Thijsen SF, Hemels MA, Menses M, van Loon AM, Krediet TG, Gerards LJ, Fleer A, Voorbij HA, Rijkers GT. Inflammatory mediators for the diagnosis and treatment of sepsis in early infancy. Pediatr Res 59: 457–461, 2006 [DOI] [PubMed] [Google Scholar]
- 409. Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery 138: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 410. Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clinics 27: 241–251, 2011 [DOI] [PubMed] [Google Scholar]
- 411. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–353, 2006 [DOI] [PubMed] [Google Scholar]
- 412. Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis 17: 445–451, 2006 [DOI] [PubMed] [Google Scholar]
- 413. Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol 289: R1048–R1053, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med 169: 333–338, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Wada H, Gabazza EC, Asakura H, Koike K, Okamoto K, Maruyama I, Shiku H, Nobori T. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC): diagnostic criteria of the International Society of Thrombosis and Hemostasis and of the Japanese Ministry of Health and Welfare for overt DIC. Am J Hematol 74: 17–22, 2003 [DOI] [PubMed] [Google Scholar]
- 416. Wada H, Mori Y, Shimura M, Hiyoyama K, Ioka M, Nakasaki T, Nishikawa M, Nakano M, Kumeda K, Kaneko T, Nakamura S, Shiku H. Poor outcome in disseminated intravascular coagulation or thrombotic thrombocytopenic purpura patients with severe vascular endothelial cell injuries. Am J Hematol 58: 189–194, 1998 [DOI] [PubMed] [Google Scholar]
- 417. Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J 5: 338–343, 1991 [DOI] [PubMed] [Google Scholar]
- 418. Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 419. Warren HS. Strategies for the treatment of sepsis. N Engl J Med 336: 952–953, 1997 [DOI] [PubMed] [Google Scholar]
- 420. Warren HS, Suffredini AF, Eichacker PQ, Munford RS. Risks and benefits of activated protein C treatment for severe sepsis. N Engl J Med 347: 1027–1030, 2002 [DOI] [PubMed] [Google Scholar]
- 421. Watkins RR, Lemonovich TL. Serum procalcitonin in the diagnosis and management of intra-abdominal infections. Exp Rev Anti-infective Ther 10: 197–205, 2012 [DOI] [PubMed] [Google Scholar]
- 422. Weixelbaumer KM, Raeven P, Redl H, van Griensven M, Bahrami S, Osuchowski MF. Repetitive low-volume blood sampling method as a feasible monitoring tool in a mouse model of sepsis. Shock 34: 420–426 [DOI] [PubMed] [Google Scholar]
- 423. Weksler ME, Goodhardt M, Szabo P. The effect of age on B cell development and humoral immunity. Springer Semin Immunopathol 24: 35–52, 2002 [DOI] [PubMed] [Google Scholar]
- 424. Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukocyte Biol 78: 325–337, 2005 [DOI] [PubMed] [Google Scholar]
- 425. Westerheide SD, Bosman JD, Mbadugha BNA, Kawahara TLA, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem 279: 56053–56060, 2004 [DOI] [PubMed] [Google Scholar]
- 426. Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med 340: 207–214, 1999 [DOI] [PubMed] [Google Scholar]
- 427. Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock 30: 267–273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. White M, Mahon V, Grealy R, Doherty DG, Stordeur P, Kelleher DP, McManus R, Ryan T. Post-operative infection and sepsis in humans is associated with deficient gene expression of gammac cytokines and their apoptosis mediators. Crit Care 15: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock–a review of laboratory models and a proposal. J Surg Res 29: 189–201, 1980 [DOI] [PubMed] [Google Scholar]
- 430. Wiedermann CJ, Hoffmann JN, Juers M, Ostermann H, Kienast J, Briegel J, Strauss R, Keinecke HO, Warren BL, Opal SM. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med 34: 285–292, 2006 [DOI] [PubMed] [Google Scholar]
- 431. Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300: 933–944, 2008 [DOI] [PubMed] [Google Scholar]
- 432. Wiley JC, Meabon JS, Frankowski H, Smith EA, Schecterson LC, Bothwell M, Ladiges WC. Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of app proteolysis and prevents apoptosis in neuronal cells. PLoS One 5: e9135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Wilke MH, Grube RF, Bodmann KF. The use of a standardized PCT-algorithm reduces costs in intensive care in septic patients: a DRG-based simulation model. Eur J Med Res 16: 543–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care 8: R291–298, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Williams SC. After Xigris, researchers look to new targets to combat sepsis. Nature Med 18: 1001. [DOI] [PubMed] [Google Scholar]
- 436. Wilson JX, Young GB. Progress in clinical neurosciences: sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci 30: 98–105, 2003 [DOI] [PubMed] [Google Scholar]
- 437. Wong HR. Clinical review: sepsis and septic shock–the potential of gene arrays. Crit Care 16: 204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 11: 1473–1480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Wu HP, Shih CC, Lin CY, Hua CC, Chuang DY. Serial increase of IL-12 response and human leukocyte antigen-DR expression in severe sepsis survivors. Crit Care 15: R224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13: 374–384, 2006 [DOI] [PubMed] [Google Scholar]
- 441. Wuillemin WA, Fijnvandraat K, Derkx BH, Peters M, Vreede W, ten Cate H, Hack CE. Activation of the intrinsic pathway of coagulation in children with meningococcal septic shock. Thromb Haemost 74: 1436–1441, 1995 [PubMed] [Google Scholar]
- 442. Wunderink RG, Laterre PFo, Francois B, Perrotin D, Artigas A, Vidal LO, Lobo SM, Juan JS, Hwang SC, Dugernier T, LaRosa S, Wittebole X, Dhainaut JF, Doig C, Mendelson MH, Zwingelstein C, Su G, Opal S, Group obotCT Recombinant tissue factor pathway inhibitor in severe community-acquired pneumonia. Am J Respir Crit Care Med 183: 1561–1568, 2011 [DOI] [PubMed] [Google Scholar]
- 443. Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol 29: 79–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Xiao H, Remick DG. Correction of perioperative hypothermia decreases experimental sepsis mortality by modulating the inflammatory response. Crit Care Med 33: 161–167, 2005 [DOI] [PubMed] [Google Scholar]
- 445. Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infection Immunity 74: 5227–5235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med 15: 1318–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Yamakawa K, Fujimi S, Mohri T, Matsuda H, Nakamori Y, Hirose T, Tasaki O, Ogura H, Kuwagata Y, Hamasaki T, Shimazu T. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care 15: R123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Yasui HIRO, Gabazza EC, Tamaki SHIG, Kobayashi TETS, Hataji OSAM, Yuda HISA, Shimizu SHIN, Suzuki KOJI, Adachi YUKI, Taguchi OSAM. Intratracheal administration of activated protein C inhibits bleomycin-induced lung fibrosis in the mouse. Am J Respir Crit Care Med 163: 1660–1668, 2001 [DOI] [PubMed] [Google Scholar]
- 449. Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol 9: 145–152, 1992 [DOI] [PubMed] [Google Scholar]
- 450. Zakariah AN, Cozzi SM, Van Nuffelen M, Clausi CM, Pradier O, Vincent JL. Combination of biphasic transmittance waveform with blood procalcitonin levels for diagnosis of sepsis in acutely ill patients. Crit Care Med 36: 1507–1512, 2008 [DOI] [PubMed] [Google Scholar]
- 451. Zanetti G, Heumann D, Gerain J, Kohler J, Abbet P, Barras C, Lucas R, Glauser MP, Baumgartner JD. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-alpha and to lipopolysaccharide. J Immunol 148: 1890–1897, 1992 [PubMed] [Google Scholar]
- 452. Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 25: 106–110, 1997 [DOI] [PubMed] [Google Scholar]
- 453. Zhang J, She D, Feng D, Jia Y, Xie L. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infectious Dis 11: 53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care 15: R70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Zhang Y, Zhou Y, Lou J, Li J, Bo L, Zhu K, Wan X, Deng X, Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care 14: R220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Zuev SM, Kingsmore SF, Gessler DD. Sepsis progression and outcome: a dynamical model. Theor Biol Med Model 3: 8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]