Abstract
Epidermal growth factor (EGF) family peptides are ligands for the EGF receptor (EGFR). Here, we elucidate functional differences among EGFR ligands and mechanisms underlying these distinctions. In 32D/EGFR myeloid and MCF10A breast cells, soluble amphiregulin (AR), transforming growth factor alpha (TGFα), neuregulin 2 beta, and epigen stimulate greater EGFR coupling to cell proliferation and DNA synthesis than do EGF, betacellulin, heparin-binding EGF-like growth factor, and epiregulin. EGF competitively antagonizes AR, indicating that its functional differences reflect dissimilar intrinsic activity at EGFR. EGF stimulates much greater phosphorylation of EGFR Tyr1045 than does AR. Moreover, the EGFR Y1045F mutation and z-cbl dominant-negative mutant of the c-cbl ubiquitin ligase potentiate the effect of EGF but not of AR. Both EGF and AR stimulate phosphorylation of EGFR Tyr992. However, the EGFR Y992F mutation and phospholipase C gamma inhibitor U73122 reduce the effect of AR much more than that of EGF. Expression of TGFα in 32D/EGFR cells causes greater EGFR coupling to cell proliferation than does expression of EGF. Moreover, expression of EGF in 32D/EGFR cells causes these cells to be largely refractory to stimulation with soluble EGF. Thus, EGFR ligands are functionally distinct in models of paracrine and autocrine signaling and EGFR coupling to biological responses may be specified by competition among functionally distinct EGFR ligands.
Keywords: Amphiregulin, EGF, TGFα, EGF receptor, ligand specificity, autocrine and paracrine growth regulation
Introduction
The epidermal growth factor (EGF) receptor (EGFR/ErbB1) is a member of the ErbB family of receptor tyrosine kinases, a family that also includes ErbB2/HER2/Neu, ErbB3/HER3, and ErbB4/HER4 (Citri and Yarden 2006). EGFR ligands are members of the EGF family of peptide growth factors and include amphiregulin (AR), epiregulin (EPR), transforming growth factor alpha (TGFα), heparin-binding EGF-like growth factor (HB-EGF), epigen (EPG), betacellulin (BTC), neuregulin 2 beta (NRG2β), and EGF (Wilson et al. 2009).
EGFR ligands can be functionally distinct (Wilson et al. 2009). For example, in MCF10A human mammary epithelial cells, AR stimulates greater motility and invasiveness than does EGF (Willmarth and Ethier 2006). EGFR expression in the interleukin 3 (IL3)-dependent 32D mouse myeloid cell line enables EGF to stimulate IL3-independent survival but enables NRG2β to stimulate IL3-independent proliferation (Gilmore et al. 2006). Likewise, EGFR ligands can stimulate distinct biological outcomes in mouse developmental model systems (Wilson et al. 2009).
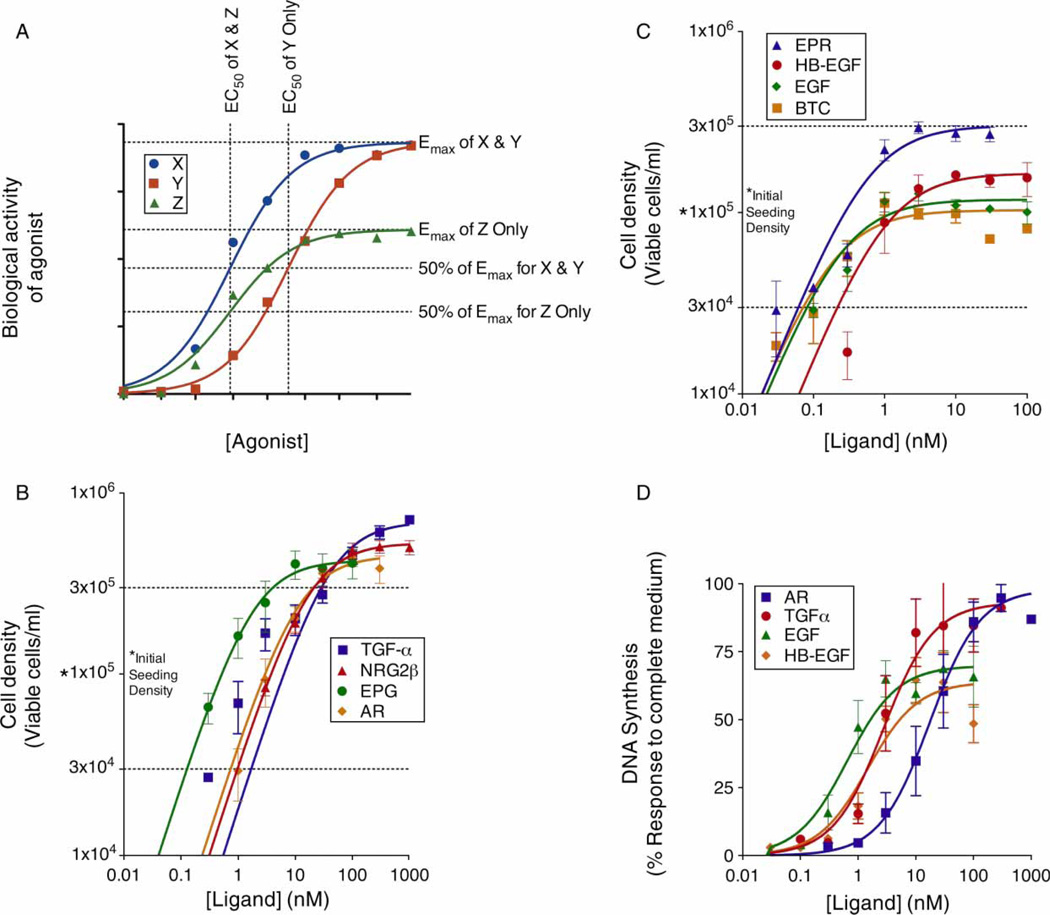
However, there are multiple hypotheses concerning the mechanism of these functional distinctions among EGFR ligands. One hypothesis is that functional distinctions reflect differences in ligand affinity and potency (Figure 1A, compare ligands X and Y). Functional distinctions may also be independent of differences in ligand affinity or potency and may instead reflect differences in ligand efficacy/intrinsic activity (Figure 1A, compare ligands X and Z).
Figure 1.
EGFR agonists differentially stimulate EGFR coupling in 32D/EGFR cells and MCF10A cells. Data in panels B, C, and D are compiled from at least three independent assays. Error bars indicate the standard error of the means. (A) The simulated agonist dose–response curves depicted here illustrate fundamental principals of pharmacology. Saturating concentrations of agonists X and Y yield the same degree of biological response (Emax). Thus, the concentration- and affinity-independent activities (efficacies or intrinsic activities) of these ligands are identical. Note that the efficacy/intrinsic activity of Z is lower than the efficacies/intrinsic activities of X and Y. The concentration of an agonist that is required to elicit a half-maximal biological response to that agonist (50% of Emax) is termed the EC50. Therefore, the EC50 of X is less than the EC50 of Y and X is a more potent agonist than Y. If X and Y share the same receptor, in many cases X possesses higher affinity for the receptor than does Y. Despite the fact that X possesses greater efficacy/intrinsic activity than does Z, these two agonists possess identical potencies. (B and C) 32D/EGFR cells were treated with increasing concentrations of the indicated EGFR agonists. IL3 independence was evaluated; ligand potency (EC50) and efficacy (Emax) are provided in Table I. (D) DNA synthesis in MCF10A cells was determined following treatment with increasing concentrations of the indicated EGFR agonists. DNA synthesis is expressed as a percentage of that stimulated by complete medium. Ligand potency (EC50) and efficacy (Emax) are provided in Table I.
One potential mechanism for differences in the efficacy of EGFR agonists is differential stimulation of EGFR coupling to ubiquitination via the ubiquitin ligase c-cbl and differential EGFR signaling duration, trafficking, recycling, and turnover. These functional distinctions may reflect differences in the capacity of EGFR agonists to stimulate phosphorylation of EGFR Tyr1045, which couples EGFR to c-cbl (Baulida et al. 1996; Duan et al. 2003; Roepstorff et al. 2009; Sorkin and Goh 2009; Foley et al. 2010; Eden et al. 2011). Here, we use detailed pharmacological analyses to demonstrate that EGFR ligands display distinctions in efficacy/intrinsic activity in both autocrine and paracrine model systems and to explore whether differential EGFR tyrosine phosphorylation and coupling to c-cbl may underlie these differences in efficacy/intrinsic activity.
Methods
Recombinant growth factors
We have previously described the preparation of recombinant NRG2β using S2 insect cells and E. coli (Hobbs et al. 2004; Wilson et al. 2007). EGF (PeproTec: Rocky Hill, NJ, USA), HB-EGF (Sigma: St. Louis, MO, USA), AR (R&D Systems: Minneapolis, MN, USA), TGFα (PeproTec), BTC (PeproTec), EPG (R&D Systems), and EPR (PeproTec) were handled according to vendor instructions.
Cell lines
The 32D/EGFR derivative of the 32D mouse myeloid cell line and MCF10A human mammary epithelial cell line were maintained according to published procedures (VanBrocklin et al. 2005; Gilmore et al. 2006).
IL3-independent assays
32D cells are dependent upon IL3 for survival and proliferation. However, in 32D cells engineered to express EGFR (32D/EGFR), stimulation with an EGFR agonist induces IL3-independent survival and proliferation (Gilmore et al. 2006). Briefly, 32D cell lines were seeded at a density of 105 cells/ml in medium devoid of IL3. Cells were treated with EGFR ligands for 5–6 days, after which the viable cell density of each sample was determined. In some cases, ligand potency (EC50) and efficacy (Emax) were calculated using Prism (GraphPad Software) and values are provided. When appropriate, a two-way ANOVA with a Bonferroni post-test or one-way ANOVA was used to evaluate statistical significance (Prism).
DNA synthesis assays
MCF10A human mammary epithelial cells exhibit endogenous EGFR expression and require EGF for proliferation in culture. We have established a procedure for assaying agonist-induced EGFR coupling to DNA synthesis in these cells (VanBrocklin et al. 2005; Wilson et al. 2009). Briefly, MCF10A cells were seeded in a 96-well plate and starved of serum and growth factors for 24 h. Cells were treated with EGFR agonists for 16 h. DNA synthesis was determined by assaying the incorporation of tritiated thymidine and is expressed as a percentage of that stimulated by the positive control. Ligand potency (EC50) and efficacy (Emax) were calculated using Prism (GraphPad Software, La Jolla, CA, USA).
Immunoblotting
32D/EGFR cells or derivatives were stimulated with an EGFR agonist or Phosphate-buffered saline (PBS) (diluent control) and lysed. In 32D/EGFR/EGF mature, 32D/EGFR/TGFα mature, and 32D/EGFR/TGFα precursor cell lines, EGFR was precipitated using Concanavalin A Sepharose (Con A) as previously described (Gilmore et al. 2006). EGFR expression and tyrosine phosphorylation were assayed as previously described (Gilmore et al. 2006, 2008). Briefly, the cell lysate or precipitate was resolved by SDS–PAGE and electroblotted onto polyvinylidene fluoride (PVDF). The blot was probed with anti-phosphotyrosine antibody 4G10 (Millipore, Billerica, MA, USA) an anti-phospho Tyr992 EGFR rabbit polyclonal antibody (Cell Signaling Technologies, Boston, MA, USA) or an anti-phospho Tyr1045 EGFR rabbit polyclonal antibody (Cell Signaling Technologies). In some cases, a parallel blot was probed with an anti-EGFR sheep polyclonal antibody (Capralogics, Gilbertville, MA, USA). When appropriate, EGFR tyrosine phosphorylation was quantified and the potency of EGF expressed in the 32D/EGFR/EGF mature cell line was determined using published methodologies (Gilmore et al. 2006).
EGFR mutants
The EGFR Y992F and Y1045F mutants were constructed by standard molecular biology techniques, using the recombinant retroviral vector pLXSN-EGFR (Riese et al. 1995) as a template. High-titer amphotropic recombinant retrovirus stocks were generated from these constructs (Penington et al. 2002) and used to infect 32D cells (Gilmore et al. 2006) in order to create stable cell lines for analysis.
Cbl mutants
Z-cbl was subcloned from pRVY-z-cbl (Walker-Daniels et al. 2002) via standard molecular biology techniques into the recombinant retroviral vector pBabe-puro (Morgenstern and Land 1990). High-titer amphotropic recombinant retrovirus stocks were generated from these constructs (Penington et al. 2002) and used to infect 32D/EGFR cells in order to create stable cell lines for analysis.
Growth factor expression
The region of the human EGF cDNA that encodes the soluble, mature form of EGF (amino acid residues 969–1021 of the precursor protein) was subcloned from pCMV6/XL4/EGF (Origene, Rockville, MD, USA; SC127840) using standard molecular biology techniques and the shuttle vector pENTR1A (Invitrogen, Grand Island, NY, USA) into the recombinant lentiviral vector pLenti6/V5-DEST (Invitrogen). The regions of the human TGFα cDNA that encodes the transmembrane, precursor form of TGFα (amino acid residues 1–160) or the soluble, mature form of TGFα (amino acid residues 38–87 of the precursor protein) were subcloned from pCMV5/XL5/TGFα (Origene SC125863) into pLenti6/V5-DEST in an analogous manner. Recombinant lentivirus stocks were packaged according to vendor recommendations and published studies (Mill et al. 2011) and were used to infect 32D/EGFR cells in order to create stable cell lines for analysis.
Results
There have been numerous reports that the various EGF family peptide hormones stimulate distinct effects via the EGFR. However, it has not been apparent that these distinctions are independent of differences in ligand affinity for EGFR (Wilson et al. 2009). Here, we demonstrate that saturating concentrations of TGFα, AR, NRG2β, and EPG stimulate greater IL3 independence (as measured by cell density) in 32D/EGFR myeloid cells than do saturating concentrations of EGF, BTC, and HB-EGF (Figure 1B,C and Table I). EPR stimulates an intermediate response (Figure 1C and Table I). Similarly, saturating concentrations of TGFα and AR stimulate greater DNA synthesis in MCF10A human mammary epithelial cells than do saturating concentrations of EGF and HB-EGF (Figure 1D and Table I). Thus, in models of paracrine growth regulation by ectopic and endogenous EGFR signaling, TGFα and AR exhibit greater intrinsic activity than do EGF and HB-EGF.
Table I.
EGF ligands differentially stimulate EGFR coupling to cell proliferation and DNA synthesis
| Cell proliferation 32D/EGFR cells |
DNA synthesis MCF10A cells |
|||||
|---|---|---|---|---|---|---|
| Ligand | EC50 (nM) | Emax (cell density × 104) | N | EC50 (nM) | Emax (% complete media) | N |
| AR | 10 ± 3 | 45 ± 3 | 4 | 18 ± 4 | 98 ± 6 | 3 |
| BTC | 0.18 ± 0.07 | 10.3 ± 0.8 | 4 | |||
| EGF | 0.24 ± 0.09 | 11.8 ± 0.7 | 5 | 0.7 ± 0.2 | 70 ± 4 | 6 |
| EPG | 1.7 ± 0.7 | 42 ± 4 | 3 | |||
| EPR | 0.6 ± 0.2 | 30 ± 2 | 4 | |||
| HB-EGF | 1.0 ± 0.4 | 16 ± 1 | 3 | 1.5 ± 0.5 | 64 ± 5 | 4 |
| NRG2β | 16 ± 3 | 53 ± 2 | 12 | |||
| TGFα | 37 ± 8 | 69 ± 4 | 4 | 2.7 ± 0.9 | 93 ± 6 | 4 |
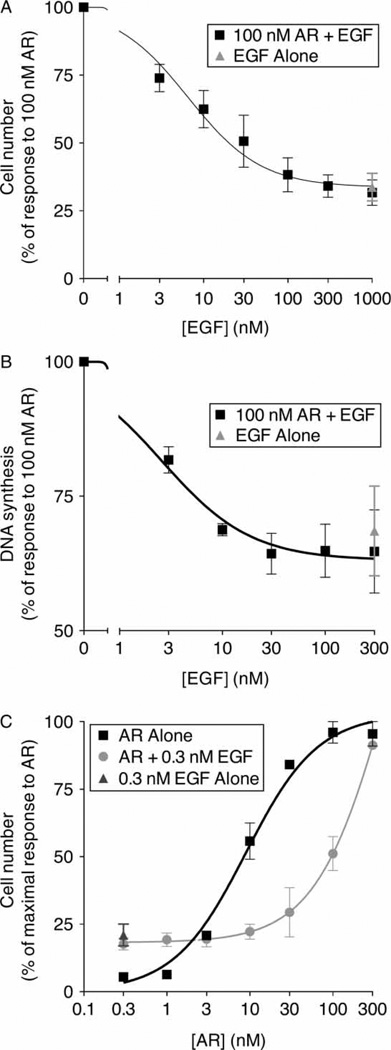
Since EGF and AR share a common binding site on EGFR (Shoyab et al. 1989; Johnson et al. 1993), we hypothesized that EGF would competitively antagonize stimulation by AR. Indeed, EGF is a potent partial antagonist of AR (100 nM) stimulation of proliferation and DNA synthesis, displaying an IC50 of 7 ± 2 and 3 ± 2 nM, respectively (Figure 2A,B). AR potently stimulates proliferation, displaying an EC50 of 8.3 ± 0.8 nM. However, 0.3 nM EGF reduces the potency (changes the EC50) of AR to 65 ± 18 nM, but does not affect the response to a saturating concentration of AR. This rightward shift in the AR dose–response curve in the presence of EGF indicates that EGF competitively antagonizes AR stimulation of EGFR coupling to proliferation (Figure 2C).
Figure 2.
EGF competitively antagonizes AR stimulation of EGFR coupling to cell proliferation and DNA synthesis. Each data point represents the mean value calculated from at least three independent experiments. Error bars represent the standard error of these means. (A) 32D/EGFR cells were treated with 100 nM AR and/or increasing concentrations of EGF. IL3 independence was determined and is expressed as a percentage of the response to 100 nM AR. (B) DNA synthesis in MCF10A cells was determined following treatment with 100 nM AR and/or increasing concentrations of EGF. DNA synthesis is expressed as a percentage of that stimulated by 100 nM AR. (C) 32D/EGFR cells were treated with increasing concentrations of AR in the presence or absence of 0.3 nM EGF. As a control, cells were treated with 0.3 nM EGF alone. IL3 independence was determined and is expressed as a percentage of the maximal response to AR.
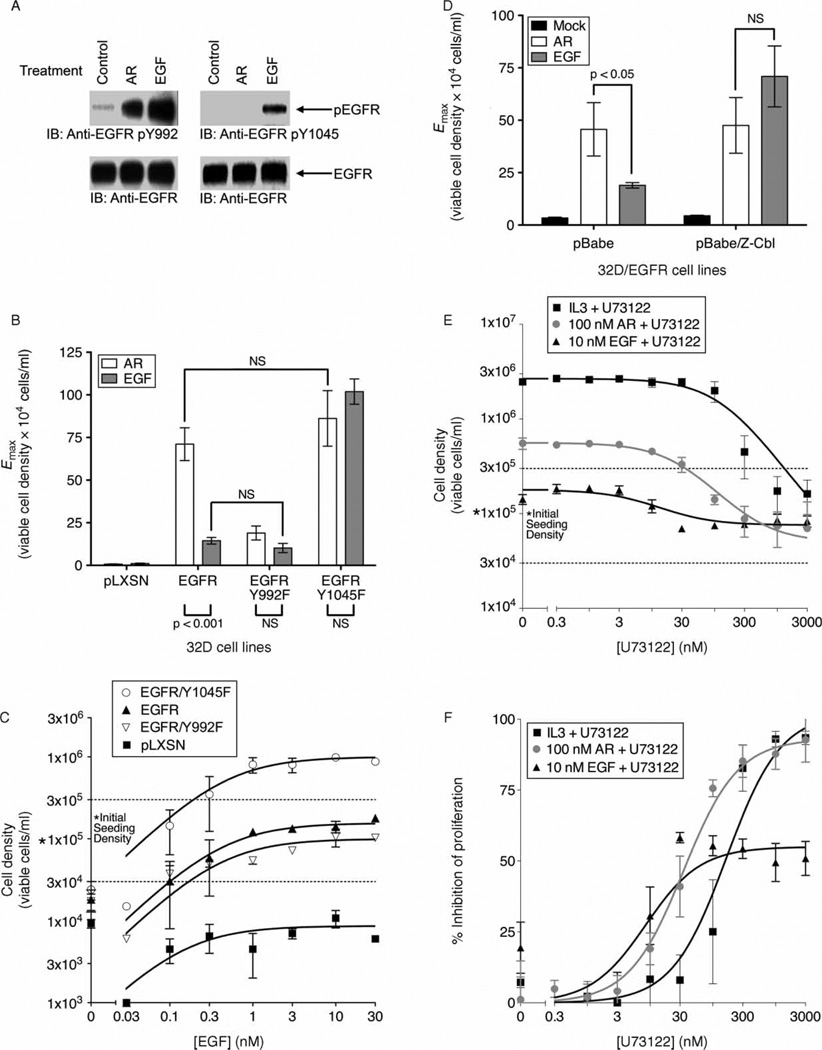
We hypothesized that functionally different EGFR ligands stimulate EGFR phosphorylation on distinct sets of tyrosine residues, resulting in ligand-specific effector binding to EGFR, effector activity, and coupling to biological responses. Indeed, EGF stimulates EGFR phosphorylation on Tyr1045 in 32D/EGFR cells, but AR does not (Figure 3A). At first blush, it would appear that this result is inconsistent with our observation that AR possesses greater intrinsic activity at EGFR than does EGF. However, phosphorylation of EGFR at Tyr1045 enables the binding of the ubiquitin ligase c-cbl to EGFR, resulting in EGFR ubiquitination and degradation (Levkowitz et al. 1999; Grovdal et al. 2004; Ravid et al. 2004). Moreover, EGF stimulates much greater EGFR ubiquitination and degradation than does AR (Stern et al. 2008; Baldys et al. 2009; Roepstorff et al. 2009; Willmarth et al. 2009).
Figure 3.
Phosphorylation of EGFR Tyr992 and Tyr1045 specifies EGFR coupling in response to stimulation with EGF or AR. (A) 32D/EGFR cells were stimulated with 10 nM EGF, 100 nM AR, or PBS (diluent control). Immunoblotting was used to assess phosphorylation atTyr992 of EGFR (upper left panel), phosphorylation atTyr1045 of EGFR (upper right panel), and EGFR expression (lower panels). The position of phospho-EGFR (pEGFR) or EGFR on the blots is indicated. Blots are representative of three independent experiments. (B and C) IL3 independence in 32D/LXSN (vector control), 32D/LXSN-EGFR, 32D/LXSN-EGFR/Y992F, and 32D/LXSN-EGFR/Y1045F cells was determined following the treatments described below. (B) Cells were treated with 100 nM AR or 10 nM EGF. Data are averages of at least three independent experiments. Error bars indicate the standard error of the means. A two-way ANOVA with a Bonferroni post-test was used to evaluate statistical significance (p value is indicated) or insignificance (NS). (C) Cells were treated with increasing concentrations of EGF. Data points are averaged from multiple independent experiments. Error bars indicate the standard error of the means. (D) IL3 independence in 32D/LXSN-EGFR/pBabe (vector control) and 32D/LXSN-EGFR/pBabe-z-cbl cells was determined following treatment with 100 nM AR or 10 nM EGF. Data are averages of at least three independent experiments. Error bars indicate the standard error of the means. A two-way ANOVA with a Bonferroni post-test was used to evaluate statistical significance (p value is indicated) or insignificance (NS). (E and F) 32D/EGFR cells were treated with IL3, 10 nM EGF, or 100 nM AR. In addition, cells were treated with increasing concentrations of the PLCγ inhibitor U73122 (Tocris) or DMSO (vehicle control). Data points are averaged from at least three independent experiments. Error bars indicate the standard error of the means. (E) Cell density is plotted as a function of U73122 concentration. (F) Data from the preceding panel are used to plot percent inhibition (relative to maximal stimulation) as a function of U73122 concentration.
We and others have observed that EGF stimulates greater phosphorylation of Tyr1045 than does AR (Figure 3A) (Gilmore et al. 2008; Willmarth et al. 2009). Consequently, we have postulated that the difference in EGFR phosphorylation at Tyr1045 enables EGF to stimulate EGFR coupling to a negative regulatory pathway to a greater extent than does AR and is thereby responsible for the disparity in the intrinsic activities of EGF and AR. Indeed, at a saturating concentration of EGF, mutation of EGFR Tyr1045 to phenylalanine (Y1045F) allows EGF to stimulate a greater degree of cell proliferation. However, the Y1045F mutation has minimal effect on the activity of AR (Figure 3B), indicating that the amount of EGFR Tyr1045 phosphorylation stimulated by AR is irrelevant to the activity of AR. In fact, the EGFR Y1045F mutant permits EGF and AR to exhibit roughly equivalent intrinsic activities (Figure 3B). Moreover, increasing concentrations of EGF do not permit wild-type EGFR to exhibit the same degree of coupling to proliferation that the Y1045F mutant displays (Figure 3C). Thus, mutation of EGFR Tyr1045 to phenylalanine abrogates the disparity in the intrinsic activities of AR and EGF by increasing the intrinsic activity of EGF.
Given the importance of EGFR Tyr1045 to specifying the difference in the intrinsic activities of AR and EGF, we postulated that c-cbl may also play a critical role in specifying the intrinsic activity of EGFR ligands. The z-cbl dominant-negative mutant of c-cbl permits EGF to exhibit much greater intrinsic activity in 32D/EGFR cells, but has a minimal effect on the intrinsic activity of AR (Figure 3D). Thus, z-cbl abrogates the disparity in the intrinsic activities of AR and EGF. In summary, a difference in EGFR ubiquitination and degradation via differential EGFR phosphorylation at Tyr1045 and differential EGFR binding to c-cbl appears to underlie the disparity in the intrinsic activities of AR and EGF.
Both AR and EGF stimulate EGFR phosphorylation at Tyr992 (Figure 3A), one of the major sites of EGFR phosphorylation following stimulation with AR (Gilmore et al. 2008). We note that EGF stimulates greater EGFR phosphorylation at Tyr992 than does AR. Thus, we used an EGFR mutant in which Tyr992 is changed to phenylalanine (Y992F) to evaluate whether phosphorylation of EGFR Tyr992 is critical for AR stimulation of EGFR coupling to cell proliferation. Upon stimulation with a saturating concentration of AR, the EGFR Y992F mutant displays a lesser degree of coupling to cell proliferation than does wild-type EGFR (Figure 3B). In contrast, the Y992F mutation has minimal effect on the activity of a saturating concentration of EGF (Figure 3B), and increasing concentrations of EGF do not cause a significant difference between the coupling of wild-type EGFR to cell proliferation and the coupling of the EGFR Y992F mutant to cell proliferation (Figure 3C). Thus, mutation of EGFR Tyr992 to phenylalanine abrogates the disparity in the intrinsic activities of AR and EGF by decreasing the intrinsic activity of AR, despite the fact that EGF stimulates greater phosphorylation at EGFR Tyr992 than does AR.
EGFR phosphorylation at Tyr992 enables phospholipase C gamma (PLCγ) binding to EGFR (Rotin et al. 1992) and increased PLCγ enzymatic activity (Suh et al. 2008). We postulated that the difference in the intrinsic activities of AR and EGF is due to differences in agonist-induced EGFR coupling to PLCγ. The PLCγ inhibitor U73122 abolishes stimulation of cell proliferation by IL3 (Figure 3E,F) and almost completely blocks stimulation of cell proliferation by AR (Imax = 93%; Figure 3E,F). In contrast, U73122 blocks stimulation of cell proliferation by EGF to a much lesser extent (Imax = 55%; Figure 3E,F), thereby causing AR to display approximately the same intrinsic activity as EGF (Figure 3E). Consequently, differential stimulation of EGFR coupling to PLCγ appears to underlie the disparity in the intrinsic activities of EGF and AR.
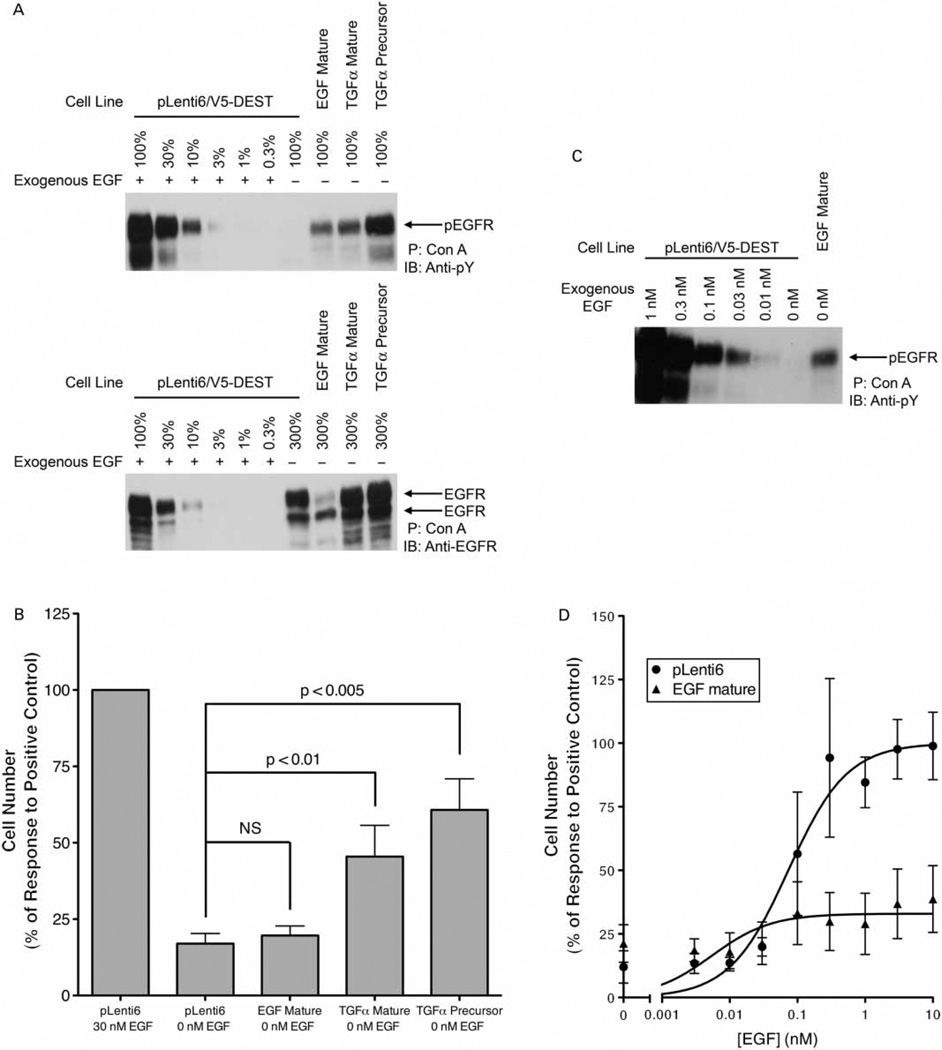
Differential ligand depletion has also been proposed as a mechanism that underlies functional distinctions among EGFR ligands (van de Poll et al. 2005). We have partially addressed this issue by noting that a 1-h exposure to ErbB receptor ligands (and subsequent rinsing of the cells) is sufficient to elicit differential stimulation of ErbB receptor coupling to IL3 independence (data not shown). We have also addressed this issue by exploring whether EGFR ligands are functionally distinct in an autocrine model of growth regulation by EGFR signaling (in which differential ligand depletion would not occur). We expressed the mature (soluble) form of EGF, the mature (soluble) form of TGFα, or the precursor (transmembrane) form of TGFα in 32D/EGFR cells. Cells that express the precursor form of TGFα exhibit a marked increase in EGFR tyrosine phosphorylation, whereas cells that express the mature form of EGF or the mature form of TGFα exhibit a more modest increase in EGFR tyrosine phosphorylation (Figure 4A, upper panel). Thus, EGFR ligands expressed in these cells bind EGFR and stimulate its phosphorylation. Expression of the mature form of EGF causes a marked decrease in EGFR expression, but expression of either form of TGFα does not (Figure 4A, lower panel).
Figure 4.
Autocrine expression of EGF and TGFα differentially stimulates EGFR coupling. (A) EGFR was precipitated from 32D/EGFR/EGF mature, 32D/EGFR/TGFα mature, and 32D/EGFR/TGFα precursor cell lines. Immunoblotting was used to assess tyrosine phosphorylation (upper panel) and EGFR expression (lower panel). The position of pEGFR or EGFR on the blots is indicated. Increasing amounts of an EGFR precipitate generated from the 32D/EGFR/pLenti6/V5-DEST vector control cell line following stimulation with 30 nM EGF were used to compare EGFR expression and phosphorylation in the experimental cell lines. The figure shown is representative of four independent trials. (B) IL3 independence of 32D/EGFR/EGF mature, 32D/EGFR/TGFα mature, and/or 32D/EGFR/TGFα precursor cell lines was determined. The 32D/EGFR/pLenti6/V5-DEST vector control cell line treated with 30 nM EGF served as the positive control. IL3 independence is expressed as a percentage of this control. The data are averages of at least seven independent experiments; error bars indicate the standard error of the means. A one-way ANOVA was used to evaluate whether differences in viable cell density are statistically significant (p value is indicated) or insignificant (NS). (C) The 32D/EGFR/EGF mature cell line was mock stimulated using diluent (PBS). As a control, the 32D/EGFR/pLenti6/V5-DEST vector control cell line was treated with increasing concentrations of EGF as indicated. EGFR tyrosine phosphorylation was assessed by immunoblotting. The figure shown is representative of five independent experiments. (D) IL3 independence in 32D/EGFR/EGF mature and 32D/EGFR/pLenti6/V5-DEST vector control cell lines was determined following treatment with increasing concentrations of EGF. The data are compiled from seven independent experiments. For each data point, the error bar indicates the standard error of the mean value. EGF potency (EC50) and efficacy (Emax) in each cell line are provided in Table II.
Expression of either form of TGFα causes constitutive EGFR coupling to cell proliferation, whereas expression of mature EGF does not (Figure 4B). The amount of EGFR phosphorylation stimulated by expression of mature EGF is approximately 10% of that stimulated by treatment with a saturating concentration of soluble EGF (Figure 4A, upper panel). This modest amount of EGFR tyrosine phosphorylation in the 32D/EGFR/EGF mature cell line may be the consequence of the dramatic decrease in steady-state EGFR expression in that cell line (Figure 4A, lower panel), suggesting that mature EGF expressed in the 32D/EGFR cell line is capable of stimulating EGFR phosphorylation and signaling. The amount of EGFR phosphorylation stimulated by expression of mature EGF is roughly equivalent to that stimulated by expression of mature TGFα (Figure 4A, upper panel). Finally, the amount of EGFR phosphorylation stimulated by expression of mature EGF is equivalent to that caused by stimulation with 31.6 ± 5.8 pM soluble EGF (Figure 4C), a concentration that stimulates a modest level of cell proliferation (Figure 4D). These data suggest that mature EGF expressed in the 32D/EGFR cell line exhibits less intrinsic activity than do the mature and precursor forms of TGFα expressed in this same cell line.
An alternative interpretation of these data is that EGF is not expressed at a level sufficient to stimulate EGFR coupling to cell proliferation in the 32D/EGFR cell line. We have addressed this possibility by comparing the effects of soluble EGF in the 32D/EGFR/EGF mature cell line to the effects of soluble EGF in the 32D/EGFR/pLenti6 vector control cell line. We predicted that if the level of EGF expression in the 32D/EGFR/EGF mature cell line is insufficient to stimulate EGFR coupling to cell proliferation, then the response of the 32D/EGFR/EGF mature cell line to soluble EGF should be roughly equivalent to the response of the 32D/EGFR/pLenti6 vector control cell line to soluble EGF.
Saturating concentrations of soluble EGF stimulate much greater cell proliferation in the 32D/EGFR/pLenti6 vector control cell line (EGF Emax of 88.5 ± 5.0 × 104 cells/ml) than in the 32D/EGFR/EGF mature cell line (EGF Emax of 29.2 ± 3.8 × 104 cells/ml, Figure 4D and Table II). In addition, the potency of soluble EGF in the 32D/EGFR/EGF mature cell line (EGF EC50 of 5.5 ± 5.0 pM) is greater than the potency of soluble EGF in the 32D/EGFR/pLenti6 vector control cell line (EGF EC50 of 73.4 ± 21.7 pM, Figure 4D and Table II). Together, these data suggest that EGF expression in the 32D/EGFR/EGF mature cell line is sufficient to stimulate EGFR phosphorylation and turnover; nonetheless, EGF exhibits less intrinsic activity than do the mature or precursor forms of TGFα.
Table II.
Expression of EGF markedly reduces stimulation of cell proliferation by subsequent treatment with soluble EGF.
| Cell line | EGF EC50 (pM) | EGF Emax (cell density × 104) | N |
|---|---|---|---|
| 32D/EGFR/pLenti6/V5-DEST | 73.4 ± 21.7 | 88.5 ± 5.0 | 7 |
| 32D/EGFR/EGF mature | 5.5 ± 5.0 | 29.2 ± 3.8 | 7 |
In fact, this reduced intrinsic activity in our model of growth regulation by autocrine EGFR signaling is so profound that it renders the 32D/EGFR/EGF cells largely refractile to stimulation of EGFR coupling to cell proliferation by soluble EGF. This reduced intrinsic activity is likely to reflect the fact that EGF stimulates greater EGFR turnover and reduced steady-state EGFR expression than does TGFα (Figure 4A, lower panel). Parenthetically, the reduction in steady-state EGFR expression observed in the 32D/EGFR/EGF mature cell line is also likely to account for the fact that soluble EGF is a more potent (but less efficacious) EGFR agonist in this cell line than in the vector control 32D/EGFR/pLenti6 cell line (Figure 4D and Table II).
Discussion
The EGFR Y992F mutation reduces the efficacy of AR, causing EGF and AR to display equivalent efficacy at the EGFR. These data suggest that phosphorylation at EGFR Tyr992 is critical for specifying the efficacy of EGFR ligands. Although both AR and EGF stimulate EGFR phosphorylation at Tyr992, we and others have observed that EGF stimulates greater EGFR phosphorylation at Tyr992 than does AR (Gilmore et al. 2008). We propose to resolve this apparent paradox by postulating that these differences in the amount of ligand-stimulated phosphorylation at EGFR Tyr992 are not relevant. Instead, we postulate that differences in the duration of ligand-stimulated EGFR phosphorylation of Tyr992 and coupling to PLCγ specify EGFR coupling to biological responses and underlie the disparity in the intrinsic activities of AR and EGF. Definitive testing of this hypothesis awaits a comparison of the duration of EGFR/PLCγ complex formation or a comparison of the duration of PLCγ signaling following stimulation with AR or EGF. Such experiments lie outside of the work at hand. Nonetheless, this hypothesis is supported by our observation that EGF stimulates much greater phosphorylation of EGFR Tyr1045 than does AR. Moreover, the EGFR Y1045F mutant and z-cbl, both of which presumably increase the duration of EGF-induced EGFR signaling, increase the intrinsic activity of EGF but not of AR. Finally, this hypothesis is consistent with the observation that an increase in the duration of EGFR coupling to Extracellular-signal-related kinase (Erk) is associated with greater cell migration (Joslin et al. 2007).
The mechanism underlying the difference in the ability of EGF and AR to stimulate EGFR phosphorylation at Tyr1045 remains unknown. We postulate that different EGFR ligands stabilize the EGFR extracellular regions in subtly distinct conformations. This could alter the juxtapositioning of the intracellular domains of the receptors in the observed asymmetric receptor dimer, in turn influencing which cytoplasmic tyrosine residues of one receptor monomer are most efficiently phosphorylated by the kinase domain of the other monomer (Wilson et al. 2009). Evidence for ligand-specific receptor conformations can be seen in a comparison of the structure of the EGFR extracellular region when bound to EGF or TGFα. The conformation of EGFR extracellular subdomain II (a site for receptor–receptor interactions within a receptor dimer) appears to be subtly different in the EGFR–EGF and EGFR–TGFα complexes and these differences may alter EGFR dimer geometry and sites of EGFR tyrosine phosphorylation (Wilson et al. 2009).
The data presented here indicate that EGFR ligands display differences in intrinsic activity in both paracrine and autocrine models of growth regulation by EGFR signaling. It will be interesting to see whether the mechanisms underlying the functional differences among EGFR ligands in autocrine models of growth regulation are identical to the mechanisms that underlie the functional differences in paracrine models. Comparisons of EGFR cell surface expression, recycling kinetics, and subcellular localization will help address this question, but lie outside of the descriptive comparison of autocrine growth regulation by EGFR ligands at hand here.
Nonetheless, the data presented here are consistent with the observation that EGF expression in breast tumor samples is associated with a more favorable prognosis, whereas TGFα expression is associated with more aggressive tumors (Revillion et al. 2008). In non-small-cell lung carcinoma (NSCLC) patients, TGFα and AR serum concentrations positively correlate with tumor aggressiveness, but the serum concentration of EGF does not. Indeed, the serum concentration of EGF is higher in healthy individuals (Lemos-Gonzalez et al. 2007). Finally, NSCLC and head and neck squamous cell carcinoma cell lines that overexpress AR are more sensitive to the EGFR tyrosine kinase inhibitor gefitinib and the EGFR-specific monoclonal antibody cetuximab than are cells with lower levels of AR expression. This suggests that AR contributes to the malignant phenotypes of these cells (Yonesaka et al. 2008). These data and ours suggest that TGFα and AR stimulate EGFR coupling to tumor cell aggressiveness and chemoresistance, whereas EGF does so to a much lesser extent and may antagonize stimulation of pathogenic EGFR signaling by TGFα and AR. Consequently, the assumption that all agonists for a given receptor tyrosine kinase are functionally equivalent should be applied with great care, particularly when predicting or considering the consequences of agonist-induced receptor signaling on tumor behavior.
Acknowledgement
We thank Allison Lange for minor contributions to the work described here. We acknowledge support from the National Cancer Institute of the National Institutes of Health (R21CA080770 and R01CA114209 to DJR; R01CA115830 to JS; P30CA023168 to the Purdue University Center for Cancer Research), the US Army Medical Research and Materiel Command Breast Cancer Research Program (DAMD17-00-1-0416 to DJR), the Indiana Elks Foundation (to DJR) and the American Cancer Society (IRG-58-006 to the Purdue University Center for Cancer Research). KJW was supported in part by a National Institutes of Health predoctoral training grant to the Purdue University interdisciplinary graduate program in Biochemistry and Molecular Biology and a FIRST postdoctoral fellowship (K12GM000680) through Emory University.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baldys A, Gooz M, Morinelli TA, Lee MH, Raymond JR, Jr, Luttrell LM, Raymond JR., Sr Essential role of c-Cbl in amphiregulin-induced recycling and signaling of the endogenous epidermal growth factor receptor. Biochemistry. 2009;48:1462–1473. doi: 10.1021/bi801771g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- Eden ER, Huang F, Sorkin A, Futter CE. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01305.x. in press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Nickerson NK, Nam S, Allen KT, Gilmore JL, Nephew KP, Riese DJ., 2nd EGFR signaling in breast cancer: Bad to the bone. Semin Cell Dev Biol. 2010;21:951–960. doi: 10.1016/j.semcdb.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JL, Gallo RM, Riese DJ. The epidermal growth factor receptor (EGFR)-S442F mutant displays increased affinity for neuregulin-2beta and agonist-independent coupling with downstream signalling events. Biochem J. 2006;396:79–88. doi: 10.1042/BJ20051687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JL, Scott JA, Bouizar Z, Robling A, Pitfield SE, Riese DJ, 2nd, Foley J. Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat. 2008;110:493–505. doi: 10.1007/s10549-007-9748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hobbs SS, Cameron EM, Hammer RP, Le AT, Gallo RM, Blommel EN, Coffing SL, Chang H, Riese DJ., 2nd Five carboxyl-terminal residues of neuregulin2 are critical for stimulation of signaling by the ErbB4 receptor tyrosine kinase. Oncogene. 2004;23:883–893. doi: 10.1038/sj.onc.1207250. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Kannan B, Shoyab M, Stromberg K. Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. J Biol Chem. 1993;268:2924–2931. [PubMed] [Google Scholar]
- Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J Cell Sci. 2007;120:3688–3699. doi: 10.1242/jcs.010488. [DOI] [PubMed] [Google Scholar]
- Lemos-Gonzalez Y, Rodriguez-Berrocal FJ, Cordero OJ, Gomez C, Paez de la Cadena M. Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br J Cancer. 2007;96:1569–1578. doi: 10.1038/sj.bjc.6603770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Mill CP, Gettinger KL, Riese DJ., 2nd Ligand stimulation of ErbB4 and a constitutively-active ErbB4 mutant result in different biological responses in human pancreatic tumor cell lines. Exp Cell Res. 2011;317:392–404. doi: 10.1016/j.yexcr.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington DJ, Bryant I, Riese DJ., 2nd Constitutively active ErbB4 and ErbB2 mutants exhibit distinct biological activities. Cell Growth Differ. 2002;13:247–256. [PubMed] [Google Scholar]
- Ravid T, Heidinger JM, Gee P, Khan EM, Goldkorn T. c-Cbl-mediated ubiquitinylation is required for epidermal growth factor receptor exit from the early endosomes. J Biol Chem. 2004;279:37153–37162. doi: 10.1074/jbc.M403210200. [DOI] [PubMed] [Google Scholar]
- Revillion F, Lhotellier V, Hornez L, Bonneterre J, Peyrat JP. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann Oncol. 2008;19:73–80. doi: 10.1093/annonc/mdm431. [DOI] [PubMed] [Google Scholar]
- Riese DJ, 2nd, van Raaij TM, Plowman GD, Andrews GC, Stern DF. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM, van Deurs B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Margolis B, Mohammadi M, Daly RJ, Daum G, Li N, Fischer EH, Burgess WH, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: Identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. Embo J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M, Plowman GD, McDonald VL, Bradley JG, Todaro GJ. Structure and function of human amphiregulin: A member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- VanBrocklin HF, Lim JK, Coffing SL, Hom DL, Negash K, Ono MY, Gilmore JL, Bryant I, Riese DJ., 2nd Anilinodialkoxyquinazolines: Screening epidermal growth factor receptor tyrosine kinase inhibitors for potential tumor imaging probes. J Med Chem. 2005;48:7445–7456. doi: 10.1021/jm050607w. [DOI] [PubMed] [Google Scholar]
- van de Poll ML, van Rotterdam W, Gadellaa MM, Jacobs-Oomen S, van Zoelen EJ. Ligand depletion negatively controls the mitogenic activity of epidermal growth factor. Exp Cell Res. 2005;304:630–641. doi: 10.1016/j.yexcr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J, Riese DJ, 2nd, Kinch MS. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res. 2002;1:79–87. [PubMed] [Google Scholar]
- Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37737. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- Willmarth NE, Baillo A, Dziubinski ML, Wilson K, Riese DJ, 2nd, Ethier SP. Altered EGFR localization and degradation in human breast cancer cells with an amphiregulin/EGFR autocrine loop. Cell Signal. 2009;21:212–219. doi: 10.1016/j.cellsig.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Mill CP, Cameron EM, Hobbs SS, Hammer RP, Riese DJ., 2nd Inter-conversion of neuregulin2 full and partial agonists for ErbB4. Biochem Biophys Res Commun. 2007;364:351–357. doi: 10.1016/j.bbrc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ., 2nd Functional selectivity of EGF family peptide growth factors: Implications for cancer. Pharmacol Ther. 2009;122:1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, Rogers AM, Johnson BE, Janne PA. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14:6963–6973. doi: 10.1158/1078-0432.CCR-08-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]