Abstract
The aims of this paper are to report hepatitis B virus reactivation in 12 patients with rheumatic disease undergoing immunosuppressive therapy and to evaluate whether pre-emptive antiviral therapy is necessary in patients receiving disease-modifying anti-rheumatic drugs. From January 2008 to March 2012, a total of 12 HBV-infected patients with rheumatic diseases were consecutively enrolled in the long-term follow-up. Liver function, HBV DNA, and serum aminotransferase level were tested during the follow-up. We also reviewed the published reports and summarized the clinical characteristics of HBV reactivation during immunosuppressive therapy in patients with rheumatic diseases. The medium duration of follow-up was 41 months (range 16–48). Patients were treated with prednisone, disease-modifying anti-rheumatic drugs (DMARDs) or tumor necrosis factor-alpha-blocking agents (TNFBA). HBV reactivation was only documented in two patients treated with prednisone without pre-emptive antiviral therapy. One hundred patients from literature review were identified as having HBV reactivation; 20.8 % of the patients receiving prednisone experienced HBV reactivation compared to only 4.46 and 9.52 % of patients treated with DMARDs or TNFBA, respectively. This long-term follow-up of serial cases suggests that pre-emptive antiviral therapy should be administered in patients receiving prednisone therapy for rheumatic disease. In contrast, DMARDs and TNFBA are relatively safe to HBV-infected patients with rheumatic diseases. Close monitoring of HBV DNA and ALT levels is necessary in the management of HBV reactivation.
Keywords: Disease-modifying anti-rheumatic drugs, Hepatitis B, Rheumatic disease, Steroid, Tumor necrosis factor-alpha-blocking agent
Introduction
Hepatitis B virus (HBV) infection is a global health problem, resulting in more than 350 million people worldwide [1]. Chronic infection with HBV can lead to cirrhosis, hepatic decompensation, and hepatocellular carcinoma.
HBV reactivation in patients undergoing chemotherapy or immunosuppressive therapy has been a well-recognized complication [2]. However, most of these reports have come from the fields of oncology and transplantation. The emergence of immunosuppressive therapy as a key therapeutic option for patients with rheumatoid disease has been associated with increasing reports of HBV reactivation.
EASL clinical practice guidelines updated its recommendations for management of chronic hepatitis in 2012, claiming that HBsAg-positive candidates for chemotherapy and immunosuppressive therapy should be tested for HBV DNA levels and should receive pre-emptive nucleotide or nucleoside analogue administration during therapy (regardless of HBV DNA levels) and lasting for 12 months after cessation of therapy [3]. However, pre-emptive therapy in patients with rheumatic diseases treated with disease-modifying anti-rheumatic drugs (DMARDs) or tumor necrosis factor-alpha-blocking (TNFBA) is still a matter of controversy.
We conducted this long-term follow-up of serial cases and literature review to access and summarize the current evidence of HBV reactivation in HBV-infected patients with rheumatic diseases who receive different immunosuppressive therapy, including steroids, DMARDs, and TNFBA. We also evaluated whether pre-emptive antiviral therapy is necessary in different drug administration.
Materials and methods
Patients
From January 2008 to March 2012, HBV-infected patients who were candidates for immunosuppressive therapy for newly diagnosed rheumatic diseases were consecutively enrolled in the long-term follow-up. Patients were excluded if they had the evidence of autoimmune hepatitis, prior exposure to immunosuppressive therapy or coinfection with hepatitis C or D before the administration. Finally, a total of 12 patients were consecutively enrolled in the long-term follow-up. Patients were treated with prednisone, DMARDs, or TNFBA. HBV markers, HBV DNA, and ALT levels were tested at baseline and every 2–3 months during the follow-up. This study protocol was approved by the ethics committee of our hospital, and informed consent was obtained from enrolled patients.
Review of the literature
We search the PubMed databases using the MeSH term “hepatitis B virus” combined with the terms “DMARDs”, “steroid”, “prednisone”, “methotrexate”, “leflunomide”, “hydroxychloroquine”, “salicylazosulfapyridine”, “cyclophosphamide”, “azathioprine”, “etanercept”, “infliximab”, “adalimumab”, “rituximab” and “rheumatoid disease”. Thirty-seven articles describing 991 patients having HBV reactivation were retrieved. These patients were identified as having chronic HBV infection or past HBV infection.
Definitions
Past HBV infection was defined as positive for anti-HBc (anti-HBc+) and negative for HBsAg (HBsAg−) [4]. Chronic HBV infection was defined as the persistent positivity of HBsAg in serum. HBV reactivation was defined as an elevation of both serum level of ALT and HBV DNA following immunosuppressive therapy.
Results
Baseline characteristics
From January 2008 to March 2012, a total of 12 HBV-infected patients with rheumatic diseases were consecutively enrolled in the long-term follow-up. Table 1 showed the demographic characteristic of the 12 patients. Medium age of the 12 patients was 42.5 years old (range 17–74). Seven of the 12 patients were male. Only one patient was negative for HBsAg at baseline. The level of HBV DNA was not obtained at baseline in two patients. Patients were treated with prednisone, DMARDs, and TNFBA. The medium duration of follow-up was 41 months (range 16–48). Treatment regimens were summarized in Table 1.
Table 1.
The baseline characteristics and treatment regimen of the 12 patients enrolled
| PatientNo. | Diagnosis | Sex/age | HBsAg | HBeAg | HBVDNA | Pre-emptive therapy | Therapy /follow-up months | Medications |
|---|---|---|---|---|---|---|---|---|
| 1 | DM | M/41 | + | − | <103 | No | Pred/48 | Pred 50 mg qd, now reduce to 5 mg qd |
| 2 | DM | F/74 | − | − | Not done | No | Pred + AZA/16, died of cerebral infarction | Pred 50–20 mg qd + AZA 50 mg qd * 9 m |
| 3 | SLE | F/63 | + | + | 2.36 × 105 | ETV | Pred/45 | Pred 40 mg qd, now reduce to 5 mg qd |
| 4 | SCLE | F/49 | + | + | 1 × 107 | ETV | Pred/30 | Pred 50 mg qd, now reduce to 5 mg qd |
| 5 | AS | M/50 | + | − | <103 | No | SASP + MTX/34 | SASP 0.5 tid * 2 m MTX 10 mg qw * 24 m |
| 6 | AS | M/29 | + | − | 1.6 × 103 | No | SASP + MTX/44 | SASP 1.0 bid + MTX 10 mg qw * 6 m now SASP 1.0 bid * 26 m |
| 7 | AS | M/20 | + | + | 2.35 × 107 | No | SASP/48 | SASP 1.0 bid |
| 8 | RA | F/67 | + | + | 4.46 × 104 | No | MTX/47 | MTX 7.5 mg qw |
| 9 | AS | M/17 | + | − | <103 | No | SASP + MTX/47 | SASP 1.0 bid + MTX 5 mg qw * 3 m now withdraw |
| 10 | AS | M/24 | + | + | 2.43 × 105 | No | ETN/46 | ETN 25 mg qw * 3 m, 25 mg q2w * 2 m, 12.5 mg qm * 21 m |
| 11 | RA | F/44 | + | − | <103 | No | HCQ/LEF + ETN/44 | HCQ 0.2 bid + ETN 25 mg biw * 5 m |
| LEF 10 mg qn + ETN 25 mg qw * 2 m | ||||||||
| ETN 25 mg qw * 20 m | ||||||||
| 12 | AS | M/20 | + | + | Not done | No | SASP + ETN/43 | SASP 1.0 bid + ETN 25 mg biw * 3 m |
| Now withdraw |
AS ankylosing spondylitis, AZA azathioprine, DM dermatomyositis, ETN entanercept, ETV entecavir, HBeAg hepatitis B e antigen, HBsAg hepatitis B surface antigen, HBV hepatitis B virus, HCQ hydroxychloroquine, LEF leflunomide, MTX methotrexate, Pred prednisone, RA rheumatoid arthritis, SASP salazosulfapyridine, SCLE subacute cutaneous lupus erythematosus, SLE systemic lupus erythematosus
Hepatitis reactivation in different treatment regimens
Prednisone was administered in four of the 12 patients (patients 1, 2, 3 and 4). Patients 3 and 4 were prescribed pre-emptive antiviral therapy. DMARDs were administered in five patients (patients 5, 6, 7, 8 and 9). The remaining three patients (patients 10, 11 and 12) were treated with the TNFBA.
HBV reactivation was only documented in two patients (patients 1 and 2). Patient 1 was treated with prednisone alone, and patient 2 was treated combination therapy of prednisone and azathioprine. Neither of them received the pre-emptive therapy (Fig. 1). In contrast, the other two patients who received pre-emptive antiviral therapy before prednisone administration did not develop the HBV reactivation (Fig. 2). HBV reactivation was not observed in patients treated with DMARDs (Fig. 3) or TNFBA (Fig. 4) during the follow-up.
Fig. 1.
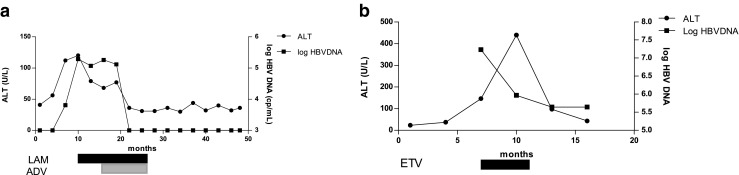
The clinical course of the two patients who developed HBV reactivation during prednisone therapy without pre-emptive therapy. a The clinical course of patient 1, a 41-year-old man diagnosed as DM and treated with predinisolone. b The clinical course of patient 2, a 74-year-old female diagnosed as DM and treated with predinisolone plus AZA. ADV adefovir, AZA azathioprine, DM dermatomysitotis, ETV entecavir, LAM lamivudine
Fig. 2.
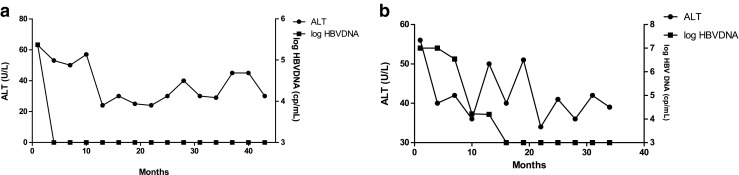
The clinical course of the two patients who received steroid therapy with pre-emptive therapy. Neither of them developed HBV reactivation. a Patient 3. b Patient 4
Fig. 3.
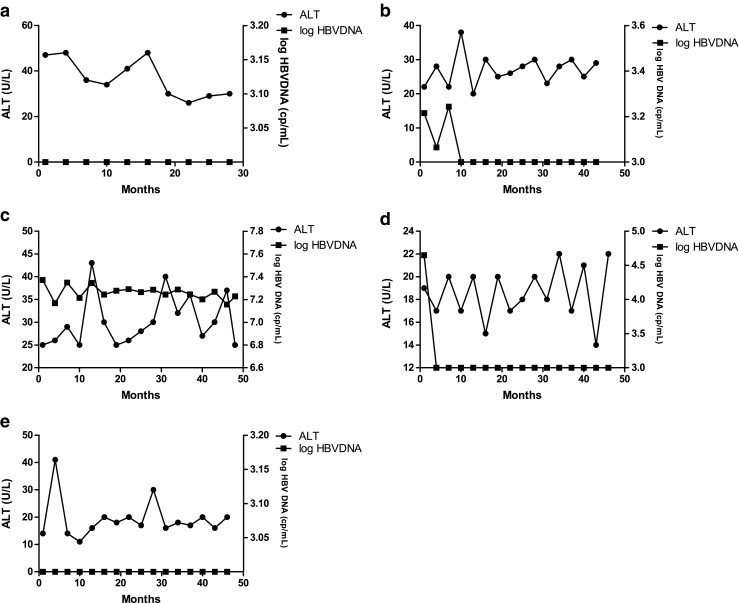
The clinical course of the five patients who received DMARDs without pre-emptive therapy. None of them developed HBV reactivation. a Patient 5. b Patient 6. c Patient 7. d Patient 8. e Patient 9
Fig. 4.
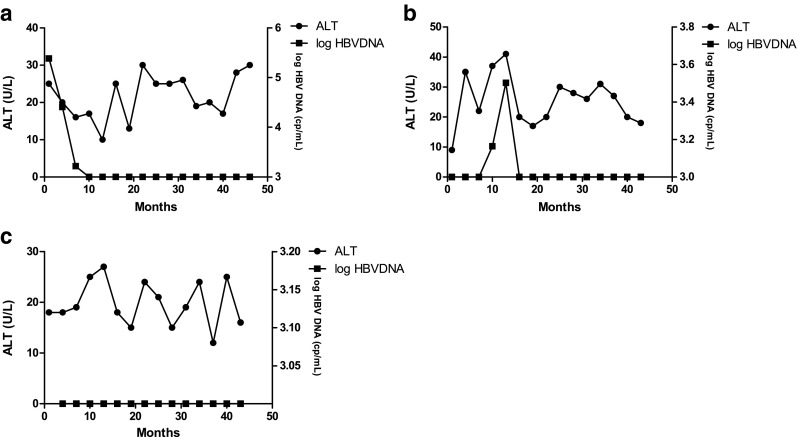
The clinical course of the three patients who underwent TNFBA without pre-emptive therapy. None of them developed HBV reactivation. a Patient 10. b Patient 11. c Patient 12
HBV DNA level increased in one patient after LEF administration. After the withdrawal of LEF, the level of HBV DNA turned to normal. The level of ALT remained normal during the 3-year follow-up in patients who did not develop HBV reactivation.
Hepatitis reactivation in two cases
A 41-year-old man who was diagnosed with dermatomyositis in January 2008. Tests for hepatitis B virus markers, HBsAg, anti-HBc and anti-HBe yielded positive findings. The level of serum HBV DNA was under detectable limit and liver function was normal. He was treated with methylpredinisolone therapy (40 mg/day). One month later, serum level of ALT increased to 112 U/L (more than two-folds of normal limit), and serum level of HBV DNA increased to 6.41 × 103 copies/ml meanwhile. Despite antiviral therapy with lamivudine, the level of ALT and HBV DNA was still high. Adefovir was added to lamivudine (10 mg/day) 4 months later. After 9 months of combination therapy, the level of ALT and HBV DNA returned to normal. LEF was added in October 2008. Serum levels of ALT and HBV DNA remained persistently normal in the following 3 years (Fig. 1a).
A 74-year-old woman who was diagnosed with dermatomyositis in March 2008. Regular laboratory test revealed normal serum ALT levels, and HBV serological marker was negative for HBsAg but positive for anti-HBc and anti-HBe. Serum levels of HBV DNA were undetectable before treatment with methylpredinisolone (40 mg/day) and AZA (50–100 mg/day). Six months later, no flare of ALT was observed but serological test revealed that she was positive for HBsAg. Twelve months later, hepatitis reactivation occurred. Serum levels of ALT increased to 146 U/L, and circulating HBV DNA was detectable at levels of 1.7 × 107 copies/ml. Entecavir at a daily dose of 0.5 mg was started. After 3 months of antiviral therapy, serum levels of ALT decreased to 43 U/L, and serum levels of HBV DNA decreased to 1.68 × 103 copies/ml (Fig. 1b). Unfortunately, the patient died of cerebral infarction during the follow-up.
Literature review
Thirty-seven articles describing 991 patients with rheumatic diseases exposed to different immunosuppressive therapy were retrieved [5–41]. One hundred forty-four patients received steroids. Two hundred twenty-four patients received DMARDs and 620 patients received TNFBA. There were also three patients treated with anti-CD20. The summary of the literature review was shown in Table 2.
Table 2.
The results of the literature review
| Reference | Type | Patient number | HBV status (number of patients) | Medications | Pre-emptive therapy | Number of patients developing reactivation |
|---|---|---|---|---|---|---|
| [4, 20, 33, 38] | Case report | 5 | CHB (4), Past infection (1) | Prednisone | N | 5 |
| [23] | Prospective study | 41 | CHB (41) | Prednisone | N | 21 |
| [5] | Retrospective study | 98 | CHB (21), Not applied (77) | Prednisone | N | 4 |
| [8, 27] | Case report | 2 | CHB (1), past infection (1) | DMARDs | N | 2 |
| [12] | Prospective study | 215 | CHB (27), past infection (188) | DMARDs | Y (4 patients received pre-emptive therapy) | 4 |
| [13] | Prospective study | 50 | CHB (5), past infection (45) | DMARDs (6 patients), TNFBA (44 patients) | N | 3 |
| [31] | Retrospective study | 8 | CHB (8) | TNFBA | N | 1 |
| [14] | Retrospective study | 49 | CHB (49) | TNFBA | Y (20 patients received pre-emptive therapy) | 3 |
| [10] | Prospective study | 52 | CHB (14), HBV-vaccinated (19), past infection (19) | TNFBA | N | 1 |
| [11] | Prospective analysis | 135 | Past infection (135) | TNFBA | N | 7 |
| [32] | Retrospective study | 7 | CHB (7) | TNFBA | N | 3 |
| [19] | Retrospective study | 92 | CHB (92) | TNFBA (91 patients), DMARDs (1 patient) | N | 27 |
| [24] | Retrospective study | 88 | CHB (18), past infection (60) | TNFBA | Y (10 patients received pre-emptive therapy) | 6 |
| [21] | Retrospective study | 60 | Past infection (60) | TNFBA | N | 2 |
| [34] | Prospective study | 21 | Past infection (21) | TNFBA | N | 0 |
| [36] | Prospective study | 67 | Past infection (67) | TNFBA | N | 0 |
| [6, 7, 16, 18, 22, 25, 26, 28–30, 35, 37, 39] | Case report | 17 | Past infection (3), CHB (14) | TNFBA | Y (6 patients received pre-emptive therapy) | 9 |
| [15, 17, 40] | Case report | 3 | CHB (3) | Anti-CD30 | Y (2 patients received pre-emptive therapy) | 1 |
CHB chronic hepatitis b, DMARDs disease-modifying anti-rheumatic drugs, HBV hepatitis B virus, TNFBA tumor necrosis factor-alpha-blocking agents
With regard to HBV infection status, 609 patients had past infection and 305 patients were considered to have chronic hepatitis B. Seventy-seven patients were not detected for the serum HBV markers at baseline.
Patients treated with DMARDS [9, 12–14, 28]
Two hundred twenty-four patients were treated with DMARDS. Among them, 192 patients had past HBV infection and the other 32 patients were considered to have chronic hepatitis B. A total of four patients with chronic hepatitis B infection received antiviral prophylaxis therapy. HBV reactivation was reported in ten cases [9, 12–14, 28] (4.46 %) with a median follow-up of 13.2 months. MTX was administered in eight of the ten patients with HBV reactivation [9, 12–14, 28]. HBV reactivation after the initiation of LEF [13], HCQ [13], SASP [13] therapy were also reported. Antiviral therapy was started after HBV reactivation. ETV seemed to be the preferred option. A satisfied response to the antiviral therapy was observed in most cases. Two patients [13] did not receive antiviral therapy despite the HBV reactivation. Serum levels of HBV DNA were markedly elevated in one case but remained persistently normal in the other case. Patient with bad outcome was only reported by Satoshi Ito [28], in which the patient died of fulminant hepatitis after a 28-month follow-up in spite of the treatment with interferon-b.
Patients treated with TNFBA [7, 8, 10–12, 14, 15, 17, 19, 20, 22, 23, 25–27, 29–33, 35–38, 40]
Six hundred twenty patients were treated with TNFBA. Among them, 416 patients had past infection with HBV and 204 patients had chronic HBV infection. Antiviral prophylaxis therapy was administered in 36 patients with chronic viral infection. HBV reactivation was reported in 59 (9.52 %) patients, in whom 13 patients were with past infection while the remaining 46 patients were chronic infection. Treatment with ETA or INF for rheumatic diseases was reported in most cases. Followed by the appearance of viral reactivation, the antiviral therapy was immediately started. Bad outcome was seen in only one case [23], in which the patient suffered liver failure and died after 26 months of follow-up.
Patients treated with steroids [5, 6, 21, 24, 34, 39]
Steroids were usually co-administered with DMARDs or TNFBA for treating rheumatic diseases. This may explain why only 6 articles reporting 144 patients were included. However, the effect of steroids on hepatitis reactivation cannot be denied. A total of 30 (one with past infection and the others with chronic infection) of the 144 patients experienced the HBV reactivation after a median time of 9.8 months. Serum HBV markers prior to steroid therapy were detailed in 67 patients. Among them, only one patient was defined as past infection and the other 66 patients were chronic infection. Four out of the seven articles were case reports [5, 21, 34, 39] concerning six patients with rheumatic diseases affected by hepatitis B virus. Bae [39] reported a fatal case of hepatitis B virus (HBV) reactivation during long-term, very-low-dose steroid treatment in an inactive HBV carrier. The patients was treated with prednisone combined with DMARDs (SSZ and HCQ), however the reactivation of HBV was mainly attributed to the steroid according to the author's opinion. There are two cases [21, 34] that reported steroid monotherapy for treating rheumatoid disease. None of the patients received prophylaxis treatment and developed HBV reactivation after 5 and 9 months of the administration, respectively. Another case [5] showed hepatic failure due to fibrosing cholestatic hepatitis in a patient with pre-surface mutant hepatitis B virus and mixed connective tissue disease treated with prednisolone and chloroquine. Similar to the patient treated with low dose of steroid [39], chloroquine was prescribed to supplement the steroid treatment. Both patients died of liver failure. HBV reactivation in patient with past HBV infection after steroid therapy [34] was also reported. A brief report of prospective study on a comparison of a standard-dose prednisone regimen and mycophenolate mofetil combined with a lower prednisone dose in Chinese adults with idiopathic nephrotic syndrome who were carriers of hepatitis B surface antigen [24] were included in this study. The result of study showed that rate of reactivation was higher in prednisone regimen than in combination therapy group, confirming that the impact of prednisone on the viral reactivation. Another retrospective study [6] reported HBV reactivation following GC therapy for a case series of pemphigus vulgaris and dermatomyositis. Four out of the 98 patients finally had a reactivation after a median time of 10.5 months.
Patients treated with anti-CD20 [16, 18, 41]
Rituximab is the representative of anti-CD20, which is also a kind of biological DMARD. Only one patient [18] underwent the HBV reactivation and was treated with LAM combined with tenofovir. A good response to the treatment was received.
Discussion
Clinical cases about HBV reactivation in patients with rheumatic disease after immunosuppressive therapy have been increasing, which suggests the possibility of HBV reactivation in the patients receiving immunosuppressive treatment for rheumatic diseases. Recent researches of HBV reactivation leading to serious complications have been described in patients with rheumatic disease undergoing treatment with biological agents, mainly including infliximab and rituximab [19, 42]. Considering the potential risk of biological agents, pre-emptive antiviral treatment is recommended in HBsAg-positive patients at the commencement of TNFBA treatment [8].
We presented the result of three patients who received biological agents (entanercept) in this study. HBV reactivation occurred in none of them. There are, however, observations from our literature review suggesting that 59 patients in 620 cases finally experienced the hepatitis reactivation. The result suggests the potential of TNFBA to induce viral reactivation. Once the anti-TNF agents were withdrawn and antiviral therapy was given, the level of liver enzyme and HBV DNA decreased to normal [43] and patients came through in most cases. Hepatitis reactivation usually occurred 30–60 days after immunosuppressive therapy. Antiviral prophylaxis treatment is recommended in HBsAg-positive patients. Since the publication and selection bias cannot be avoided, a bigger and longer follow-up study is required to clarify the influence on hepatitis reactivation after anti-TNF-alpha treatment.
SASP and MTX are the commonly single-use DMARDs in rheumatic patients. The possibility of DMARDs to induce HBV reactivation is still a controversial issue in recent studies. In our serial cases, five patients received SASP therapy, and none of them developed hepatitis reactivation regardless of serum levels of HBV DNA prior to SASP therapy. No research approves that SASP induce hepatitis reactivation. We believe SASP is safe on hepatitis B patients. Four patients were treated with MTX therapy and no HBV reactivation was observed during follow-up. In our literature review, most cases were treated with MTX as a preferred choice. A few researches reported HBV reactivation during or after MTX therapy [17, 28, 44]. One of them progressed to fatal hepatic failure in the end [44]. According to the result of the literature review, DMARDs seem to be the safest medicine considering only 4.46 % of the patients experienced HBV reactivation after the treatment.
The efficacy of prednisone on hepatitis reactivation is proved again. In this study, HBV reactivation occurred in patients who did not receive pre-emptive therapy. Results from some research suggested that the risk of hepatitis reactivation depends on the dosage of prednisone [24]. However, in our literature review, this assumption was not proved. Prophylaxis antiviral therapy is necessary in those hepatitis B-infected patients who will receive prednisone therapy. Optimal agent for pre-emptive therapy is still controversial. The benefits of LAM may be limited due to resistance and entecavir is recommended as first choice for pre-emptive therapy. HBV reactivation induced by prednisone has been investigated thoroughly in cancer patients receiving chemotherapy treatment and some consensus has been made. However, in the field of rheumatism, the subject seemed to be anything but a hotspot. Thirty of the 144 patients experienced HBV reactivation in the literature review, which strongly suggested the necessity to prescribe antiviral pre-emptive therapy.
HBV reactivation also occurs in HBV-infected patients who were negative for HBsAg but positive for anti-HBc. In this study, a 74-year-old woman who was negative for HBsAg before the commencement of prednisone therapy experienced HBV reactivation 11 months after prednisone administration. Reactivation of HBV infection has been reported in HBsAg-negative patients who received chemotherapy [45] for lymphoma. Observation of the literature review also confirmed the possibility of viral reactivation in patients with past HBV infection treated with immunosuppressive therapy for rheumatic diseases. Nineteen of the 609 patients (3.20 %) with past infection experienced reactivation after immunosuppressive therapy. Taking these lines of evidence together, serum levels of ALT and HBV DNA should be monitored not only in patients who were chronically infected, but also in patients who have past infection (negative for HBsAg and positive for anti-HBc).
The time of HBV reactivation is still not confirmed, based on our study. Not surprisingly, reactivation was observed during the treatment with immunosuppressive therapy. However, even after the drug withdrawal, the reactivation still occurred [8]. Therefore, the whole course of the therapy, instead of the first few months, ought to be monitored closely.
In conclusion, HBV reactivation also occurs in patients with rheumatic diseases treated with immunosuppressive therapy. Pre-emptive therapy is proved beneficial in cancer patients with hepatitis B. However, pre-emptive therapy in patients with rheumatic diseases treated with different immunosuppressive therapy is still a matter of controversy. In terms of our study, the benefits of pre-emptive therapy definitely exceed the harm of it in patients receiving steroids. Entecavir is recommended as the optimal agent against HBV reactivation. On the other hand, DMARDs is shown to be quite safe in rheumatic patients with HBV infection so that pre-emptive therapy may not be recommended in these patients. TNFBA is considered safe in HBsAg-negative patients, while pre-emptive is required in patients who are positive for HBsAg. Unnecessary antiviral therapy may induce virus mutation and further increases the economic burden. Despite the lower risk of HBV reactivation in patients with rheumatic diseases who are candidates for DMARDs and TNFNA therapy, such patients should be followed-up periodically and tested for ALT and HBV DNA.
Acknowledgments
We thank Dr. Weiguo Wan, Dr. Ling Lv , Dr. Yu Xue, Dr. Jiong Zhang for providing patients.
Financial support
The study was supported by Program for New Century Excellent Talents in University (NCET-08-0133) and Shanghai new hundred talents program: training plan of excellent talents in Shanghai city health system (XBR2011001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interests
The authors have declared that no competing interests exist.
Abbreviations
- ADV
Adefovir
- ALT
Alanine aminotransferase
- AS
Ankylosing spondylitis
- AZA
Azathioprine
- CHB
Chronic hepatitis B
- DM
Dermatomyositis
- DMARDs
Disease-modifying anti-rheumatic drugs
- ETN
Entanercept
- ETV
Entecavir
- HBeAg
Hepatitis B e antigen
- HBsAg
Hepatitis B surface antigen
- HBV
Hepatitis B virus
- HCQ
hydroxychloroquine
- LAM
Lamivudine
- LEF
Leflunomide
- MTX
Methotrexate
- Pred
Prednisone
- RA
Rheumatoid arthritis
- SASP
Salazosulfapyridine
- SCLE
Subacute cutaneous lupus erythematosus
- SLE
Systemic lupus erythematosus
- TNFBA
Tumor necrosis factor-alpha-blocking agents
Contributor Information
Wenhong Zhang, Phone: +86-21-52888123, FAX: +86-21-62489015, Email: zhangwenhong@fudan.edu.cn.
Hejian Zou, Email: hjzou@163.com.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337(24):1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120(4):1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 3.EASL clinical practice guidelines Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol. 2007;46(1):160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Zanati SA, Locarnini SA, Dowling JP, Angus PW, Dudley FJ, Roberts SK. Hepatic failure due to fibrosing cholestatic hepatitis in a patient with pre-surface mutant hepatitis B virus and mixed connective tissue disease treated with prednisolone and chloroquine. J Clin Virol. 2004;31(1):53–57. doi: 10.1016/j.jcv.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Yang CH, Wu TS, Chiu CT. Chronic hepatitis B reactivation: a word of caution regarding the use of systemic glucocorticosteroid therapy. Br J Dermatol. 2007;157(3):587–590. doi: 10.1111/j.1365-2133.2007.08058.x. [DOI] [PubMed] [Google Scholar]
- 7.Wendling D, Di Martino V, Prati C, Toussirot E, Herbein G. Spondyloarthropathy and chronic B hepatitis. Effect of anti-TNF therapy. Jt Bone Spine. 2009;76(3):308–311. doi: 10.1016/j.jbspin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Wendling D, Auge B, Bettinger D, Lohse A, Le Huede G, Bresson-Hadni S, Toussirot E, Miguet JP, Herbein G, Di Martino V. Reactivation of a latent precore mutant hepatitis B virus related chronic hepatitis during infliximab treatment for severe spondyloarthropathy. Ann Rheum Dis. 2005;64(5):788–789. doi: 10.1136/ard.2004.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe K, Takase K, Ohno S, Ideguchi H, Nozaki A, Ishigatsubo Y. Reactivation of hepatitis B virus in a hepatitis B surface antigen-negative patient with rheumatoid arthritis treated with methotrexate. Mod Rheumatol. 2012;22(3):470–473. doi: 10.3109/s10165-011-0521-9. [DOI] [PubMed] [Google Scholar]
- 10.Verhelst X, Orlent H, Colle I, Geerts A, De Vos M, Van Vlierberghe H. Subfulminant hepatitis B during treatment with adalimumab in a patient with rheumatoid arthritis and chronic hepatitis B. Eur J Gastroenterol Hepatol. 2010;22(4):494–499. doi: 10.1097/MEG.0b013e3283329d13. [DOI] [PubMed] [Google Scholar]
- 11.Vassilopoulos D, Apostolopoulou A, Hadziyannis E, Papatheodoridis GV, Manolakopoulos S, Koskinas J, Manesis EK, Archimandritis AI. Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis. 2010;69(7):1352–1355. doi: 10.1136/ard.2009.127233. [DOI] [PubMed] [Google Scholar]
- 12.Urata Y, Uesato R, Tanaka D, Kowatari K, Nitobe T, Nakamura Y, Motomura S. Prevalence of reactivation of hepatitis B virus replication in rheumatoid arthritis patients. Mod Rheumatol. 2011;21(1):16–23. doi: 10.3109/s10165-010-0337-z. [DOI] [PubMed] [Google Scholar]
- 13.Tan J, Zhou J, Zhao P, Wei J. Prospective study of HBV reactivation risk in rheumatoid arthritis patients who received conventional disease-modifying antirheumatic drugs. Clin Rheumatol. 2012;31(8):1169–1175. doi: 10.1007/s10067-012-1988-2. [DOI] [PubMed] [Google Scholar]
- 14.Tamori A, Koike T, Goto H, Wakitani S, Tada M, Morikawa H, Enomoto M, Inaba M, Nakatani T, Hino M, Kawada N. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol. 2011;46(4):556–564. doi: 10.1007/s00535-010-0367-5. [DOI] [PubMed] [Google Scholar]
- 15.Ryu HH, Lee EY, Shin K, Choi IA, Lee YJ, Yoo B, Park MC, Park YB, Bae SC, Yoo WH, Kim SI, Lee EB, Song YW. Hepatitis B virus reactivation in rheumatoid arthritis and ankylosing spondylitis patients treated with anti-TNFalpha agents: a retrospective analysis of 49 cases. Clin Rheumatol. 2012;31(6):931–936. doi: 10.1007/s10067-012-1960-1. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Escalera C, Fernandez-Nebro A. The use of rituximab to treat a patient with ankylosing spondylitis and hepatitis B. Rheumatology (Oxford) 2008;47(11):1732–1733. doi: 10.1093/rheumatology/ken362. [DOI] [PubMed] [Google Scholar]
- 17.Robinson H, Walker-Bone K. Anti-TNF-alpha therapy for rheumatoid arthritis among patients with chronic hepatitis B infection. Rheumatology (Oxford) 2009;48(4):448–450. doi: 10.1093/rheumatology/kep003. [DOI] [PubMed] [Google Scholar]
- 18.Pyrpasopoulou A, Douma S, Vassiliadis T, Chatzimichailidou S, Triantafyllou A, Aslanidis S. Reactivation of chronic hepatitis B virus infection following rituximab administration for rheumatoid arthritis. Rheumatol Int. 2011;31(3):403–404. doi: 10.1007/s00296-009-1202-2. [DOI] [PubMed] [Google Scholar]
- 19.Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis. 2003;62(7):686–687. doi: 10.1136/ard.62.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oshima Y, Tsukamoto H, Tojo A. Association of hepatitis B with antirheumatic drugs: a case–control study. Mod Rheumatol. 2012 doi: 10.1007/s10165-012-0709-7. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi K, Ishikawa M, Nakauchi M, Sakurai A, Doi K, Taniguchi Y. Antibody to hepatitis B e positive hepatitis induced by withdrawal of steroid therapy for polymyositis: response to interferon-alpha and cyclosporin A. Intern Med. 1998;37(6):519–522. doi: 10.2169/internalmedicine.37.519. [DOI] [PubMed] [Google Scholar]
- 22.Mori S. Past hepatitis B virus infection in rheumatoid arthritis patients receiving biological and/or nonbiological disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21(6):621–627. doi: 10.3109/s10165-011-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto T, Marusawa H, Dogaki M, Suginoshita Y, Inokuma T. Adalimumab-induced lethal hepatitis B virus reactivation in an HBsAg-negative patient with clinically resolved hepatitis B virus infection. Liver Int. 2010;30(8):1241–1242. doi: 10.1111/j.1478-3231.2010.02238.x. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Tian J, Wu J, He Q, Li H, Han F, Li Q, Chen Y, Ni Q, Chen J. A comparison of a standard-dose prednisone regimen and mycophenolate mofetil combined with a lower prednisone dose in Chinese adults with idiopathic nephrotic syndrome who were carriers of hepatitis B surface antigen: a prospective cohort study. Clin Ther. 2009;31(4):741–750. doi: 10.1016/j.clinthera.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Lan JL, Chen YM, Hsieh TY, Chen YH, Hsieh CW, Chen DY, Yang SS. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011;70(10):1719–1725. doi: 10.1136/ard.2010.148783. [DOI] [PubMed] [Google Scholar]
- 26.Kishida D, Okuda Y, Onishi M, Takebayashi M, Matoba K, Jouyama K, Yamada A, Sawada N, Mokuda S, Takasugi K. Successful tocilizumab treatment in a patient with adult-onset Still's disease complicated by chronic active hepatitis B and amyloid A amyloidosis. Mod Rheumatol. 2011;21(2):215–218. doi: 10.3109/s10165-010-0365-8. [DOI] [PubMed] [Google Scholar]
- 27.Kaur PP, Chan VC, Berney SN. Histological evaluation of liver in two rheumatoid arthritis patients with chronic hepatitis B and C treated with TNF-alpha blockade: case reports. Clin Rheumatol. 2008;27(8):1069–1071. doi: 10.1007/s10067-008-0896-y. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Nakazono K, Murasawa A, Mita Y, Hata K, Saito N, Kikuchi M, Yoshida K, Nakano M, Gejyo F. Development of fulminant hepatitis B (precore variant mutant type) after the discontinuation of low-dose methotrexate therapy in a rheumatoid arthritis patient. Arthritis Rheum. 2001;44(2):339–342. doi: 10.1002/1529-0131(200102)44:2<339::AID-ANR51>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Germanidis G, Hytiroglou P, Zakalka M, Settas L. Reactivation of occult hepatitis B virus infection, following treatment of refractory rheumatoid arthritis with abatacept. J Hepatol. 2012;56(6):1420–1421. doi: 10.1016/j.jhep.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Durmus O, Tekin L, Carli AB, Cakar E, Acar A, Ulcay A, Dincer U, Kiralp MZ. Hepatitis B virus reactivation in a juvenile rheumatoid arthritis patient under treatment and its successful management: a complicated case. Rheumatol Int. 2013;33(5):1345–1349. doi: 10.1007/s00296-011-2244-9. [DOI] [PubMed] [Google Scholar]
- 31.Doubrawa E, Ricca RA, Malucelli TO, Pizzol VI, Barros DH, Paiva ES. Use of infliximab in a patient with rheumatoid arthritis and chronic hepatitis B. Rev Bras Reumatol. 2012;52(4):653–655. doi: 10.1590/S0482-50042012000400015. [DOI] [PubMed] [Google Scholar]
- 32.Chung SJ, Kim JK, Park MC, Park YB, Lee SK. Reactivation of hepatitis B viral infection in inactive HBsAg carriers following anti-tumor necrosis factor-alpha therapy. J Rheumatol. 2009;36(11):2416–2420. doi: 10.3899/jrheum.081324. [DOI] [PubMed] [Google Scholar]
- 33.Cho YT, Chen CH, Chiu HY, Tsai TF. Use of anti-tumor necrosis factor-alpha therapy in hepatitis B virus carriers with psoriasis or psoriatic arthritis: a case series in Taiwan. J Dermatol. 2012;39(3):269–273. doi: 10.1111/j.1346-8138.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J, Li JB, Sun QL, Li X. Reactivation of hepatitis B virus after steroid treatment in rheumatic diseases. J Rheumatol. 2011;38(1):181–182. doi: 10.3899/jrheum.100692. [DOI] [PubMed] [Google Scholar]
- 35.Charpin C, Guis S, Colson P, Borentain P, Mattei JP, Alcaraz P, Balandraud N, Thomachot B, Roudier J, Gerolami R. Safety of TNF-blocking agents in rheumatic patients with serology suggesting past hepatitis B state: results from a cohort of 21 patients. Arthritis Res Ther. 2009;11(6):R179. doi: 10.1186/ar2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll MB, Bond MI. Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis B infection. Semin Arthritis Rheum. 2008;38(3):208–217. doi: 10.1016/j.semarthrit.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Caporali R, Bobbio-Pallavicini F, Atzeni F, Sakellariou G, Caprioli M, Montecucco C, Sarzi-Puttini P. Safety of tumor necrosis factor alpha blockers in hepatitis B virus occult carriers (hepatitis B surface antigen negative/anti-hepatitis B core antigen positive) with rheumatic diseases. Arthritis Care Res (Hoboken) 2010;62(6):749–754. doi: 10.1002/acr.20130. [DOI] [PubMed] [Google Scholar]
- 38.Cansu DU, Kalifoglu T, Korkmaz C. Short-term course of chronic hepatitis B and C under treatment with etanercept associated with different disease modifying antirheumatic drugs without antiviral prophylaxis. J Rheumatol. 2008;35(3):421–424. [PubMed] [Google Scholar]
- 39.Bae JH, Sohn JH, Lee HS, Park HS, Hyun YS, Kim TY, Eun CS, Jeon YC, Han DS. A fatal case of hepatitis B virus (HBV) reactivation during long-term, very-low-dose steroid treatment in an inactive HBV carrier. Clin Mol Hepatol. 2012;18(2):225–228. doi: 10.3350/cmh.2012.18.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anelli MG, Torres DD, Manno C, Scioscia C, Iannone F, Covelli M, Schena FP, Lapadula G. Improvement of renal function and disappearance of hepatitis B virus DNA in a patient with rheumatoid arthritis and renal amyloidosis following treatment with infliximab. Arthritis Rheum. 2005;52(8):2519–2520. doi: 10.1002/art.21216. [DOI] [PubMed] [Google Scholar]
- 41.Andres M, Courtney P. No hepatitis B reactivation in a patient with refractory antisynthetase syndrome successfully treated with rituximab. Jt Bone Spine. 2011;78(6):653–654. doi: 10.1016/j.jbspin.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Ghrenassia E, Mekinian A, Rouaghe S, Ganne N, Fain O. Reactivation of resolved hepatitis B during rituximab therapy for rheumatoid arthritis. Jt Bone Spine. 2012;79(1):100–101. doi: 10.1016/j.jbspin.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Nordgaard-Lassen I, Dahlerup JF, Belard E, Gerstoft J, Kjeldsen J, Kragballe K, Ravn P, Sorensen IJ, Theede K, Tjellesen L. Guidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatment. Dan Med J. 2012;59(7):C4480. [PubMed] [Google Scholar]
- 44.Gwak GY, Koh KC, Kim HY. Fatal hepatic failure associated with hepatitis B virus reactivation in a hepatitis B surface antigen-negative patient with rheumatoid arthritis receiving low dose methotrexate. Clin Exp Rheumatol. 2007;25(6):888–889. [PubMed] [Google Scholar]
- 45.Wu JM, Huang YH, Lee PC, Lin HC, Lee SD. Fatal reactivation of hepatitis B virus in a patient who was hepatitis B surface antigen negative and core antibody positive before receiving chemotherapy for non-Hodgkin lymphoma. J Clin Gastroenterol. 2009;43(5):496–498. doi: 10.1097/MCG.0b013e3181945942. [DOI] [PubMed] [Google Scholar]


