Abstract
Objective
Arsenic in drinking water has been linked with the risk of urinary bladder cancer, but the dose–response relationships for arsenic exposures below 100 µg/L remain equivocal. We conducted a population-based case–control study in southeastern Michigan, USA, where approximately 230,000 people were exposed to arsenic concentrations between 10 and 100 µg/L.
Methods
This study included 411 bladder cancer cases diagnosed between 2000 and 2004, and 566 controls recruited during the same period. Individual lifetime exposure profiles were reconstructed, and residential water source histories, water consumption practices, and water arsenic measurements or modeled estimates were determined at all residences. Arsenic exposure was estimated for 99% of participants’ person-years.
Results
Overall, an increase in bladder cancer risk was not found for time-weighted average lifetime arsenic exposure >10 µg/L when compared with a reference group exposed to <1 µg/L (odds ratio (OR) = 1.10; 95% confidence interval (CI): 0.65, 1.86). Among ever-smokers, risks from arsenic exposure >10 µg/L were similarly not elevated when compared to the reference group (OR = 0.94; 95% CI: 0.50, 1.78).
Conclusions
We did not find persuasive evidence of an association between low-level arsenic exposure and bladder cancer. Selecting the appropriate exposure metric needs to be thoughtfully considered when investigating risk from low-level arsenic exposure.
Keywords: Age factors, Arsenicals, Environmental exposure, Residential mobility, Urinary bladder
Introduction
Inorganic arsenic exposure is a risk factor for a range of adverse health outcomes including vascular diseases, skin ailments, cancers, and diabetes [1]. Drinking water is the predominant source of elevated inorganic arsenic exposure for most individuals due to naturally elevated arsenic concentrations in aquifers around the world [2]. Tens of millions of the world’s citizens are exposed, with much debate over quantifying risks at chronic, low-level exposures [1, 2].
Worldwide, bladder cancer is the ninth most frequent type of cancer, resulting in more than 350,000 new diagnoses each year [3]. Highest incidence occurs in industrialized regions of Western Europe and the United States where nearly all cases of bladder cancer are transitional cell carcinomas (TCC) [4]. Cigarette smoking and a host of occupational exposures are the major risk factors for TCC of the bladder; however, many cases remain unexplained [4].
The International Agency for Research on Cancer [5] considers there to be sufficient evidence for arsenic to increase risk of urinary bladder cancer in humans; yet risks related to exposure in the 10–100 µg/L range remain uncertain. Valid and reproducible animal models that may serve to explain how arsenic induces cancer do not exist [1, 6, 7]. Epidemiologic studies continue to be the primary source of data used to assess cancer risks for exposed populations [1].
Evidence in support of an association between arsenic and bladder cancer has been based on studies in high-arsenic regions (>150 µg/L) [8–11]. At lower levels of arsenic exposure (<100 µg/L arsenic in drinking water), epidemiologic studies have provided less evidence of increased risk for bladder cancer [12–20]. However, as others have noted, many of these studies have encountered two stumbling blocks for assessing risk from low-to-moderate arsenic concentrations: inadequate sample size and exposure misclassification which may bias results toward the null [15, 21, 22]. Carefully designed epidemiologic studies of low-level arsenic exposure <100 µg/L are critical for informed risk assessment and management [21]. Expected risk from low-level arsenic exposure remains uncertain.
In this report, we present results from a population-based bladder cancer case–control study which incorporate lifetime exposure assessment in a residentially stable population in Michigan. High rates of bladder cancer mortality have been reported for nearly 50 years in southeastern Michigan, yet these elevated rates are not well explained [23]. Concentrations of arsenic ≥50 µg/L were first reported in Michigan groundwater in 1981 [24]. Since then, arsenic has been identified in unconsolidated and bedrock aquifers throughout southeastern Michigan, with concentrations frequently exceeding the new Maximum Contaminant Level (MCL) of 10 µg/L set by the U.S. Environmental Protection Agency [25]. This region of Michigan has a population of 2.8 million people, with 1.6 million people relying on groundwater as their source of drinking water. Approximately 8% of the southeastern Michigan population (~ 230,000 people) are exposed to arsenic >10 µg/L in their drinking water, making this one of the largest populations with moderately elevated exposure in the United States [26].
Materials and methods
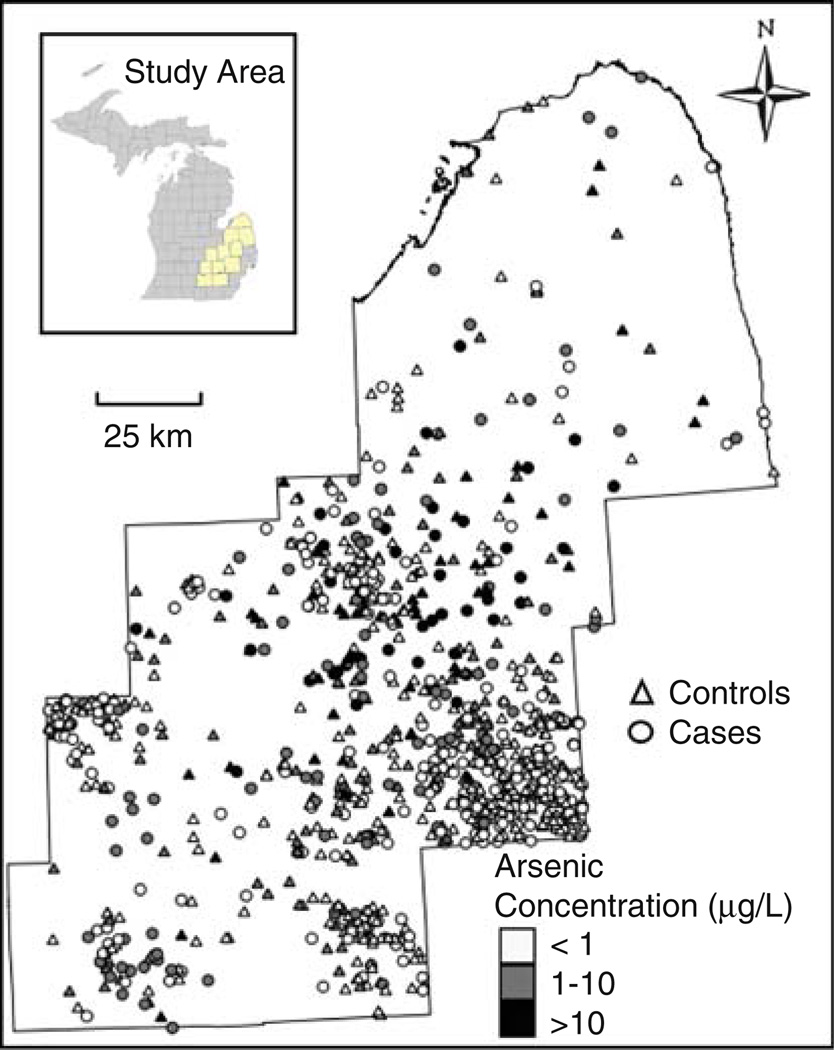
A population-based bladder cancer case–control study was conducted in eleven counties of southeastern Michigan (Fig. 1). These counties included Genesee, Huron, Ingham, Jackson, Lapeer, Livingston, Oakland, Sanilac, Shiawassee, Tuscola, and Washtenaw and were selected to investigate risk based on moderately elevated arsenic concentrations in the groundwater (<100 µg/L). Participants were offered a modest financial incentive; research was approved by the University of Michigan IRB-Health Committee and participants provided written informed consent. A total of 411 cases and 566 controls were determined to be eligible and completed all aspects of the study.
Fig. 1.
Arsenic concentrations in drinking water at current residence of cases and controls in eleven county study area of Southeastern Michigan, Enrolled 2002–2006
Cases diagnosed with urinary bladder cancer between 2000 and 2004 were recruited from the Michigan Cancer Surveillance Program (the state cancer registry), within the Division of Vital Records, Michigan Department of Community Health. Eligibility criteria included residence in the eleven county study area for at least the previous 5 years, age at diagnosis between 21 and 80 years, and no history of cancer per histology report in the cancer registry database (except for basal cell and squamous cell carcinomas of the skin which were not recorded by the registry). Cases were reported to the state cancer registry approximately 3–23 months following diagnosis, depending on each hospital’s reporting system. There were 1,634 potentially eligible cases. Approximately 22% died prior to contact; the registry was not permitted by hospital or physician to contact another 5% of cases. The remaining cases were mailed a letter by the registry asking for permission to release their name and contact information to the research team. Of these 1,178 cases, 50% agreed to have their name released. Among the 584 cases subsequently contacted by the research team, 411 cases (70%) completed all phases of participation, including telephone interview, in-person interview, and providing environmental and biological samples. Thus, of the 1,634 potentially eligible cases, 25% completed all phases of participation, resulting in 411 participating cases.
Controls were frequency matched to recruited cases by age (±5 years), gender, and race and were recruited by random digit dialing of age-weighted lists by the Michigan Public Health Institute (MPHI). Age-weighted lists purchased from Genesys Sampling Systems were weighted to be representative of the age distribution of cases in the study area and were generated from telephone directories, automobile and motorcycle registries, real estate listings, and driver’s license data. Random calls were made to 11,463 potential controls, and eligibility was determined based on answers to screening questions (at least 5 years of residence in the study area, no history of cancer except non-melanoma skin cancer, and appropriate case-matched frequencies of age, race, and gender). A number was dialed fifteen times during different times of day and days of week before it was retired. Of those numbers dialed, 3,341 were non-working/non-residential or were never answered, 3,333 resulted in hang-ups prior to screening, and 4,748 resulted in successful screening. Of those screened, 2,616 were found to be ineligible. Among the 2,132 eligible controls, 69% refused to participate, 4% failed to complete all requirements of participation, and 27% completed all phases of participation, resulting in 566 participating controls.
Participants answered a 30–45 min computer-aided telephone interview which included questions on water and other fluid consumption, dietary habits, smoking, and medical history. This was conducted by trained MPHI interviewers to ensure standardization of the administration of the survey questionnaire and response entry. With regard to fluid consumption, participants reported consumption of water, beverages made with water, and different types of fluids not made with water at home, at work, and at places other than home or work. Current and previous consumption patterns, including detailed characterization of major changes in fluid consumption were reported. These questions were used to estimate water consumption at home (water + beverages made with water measured in L/day) and total fluid intake over the adult lifetime.
In addition to the telephone interview, participants were mailed residential and occupational history forms; research team members met with participants at their home to review and collect the forms. Participants provided written residential and occupational histories of each home lived-in and each job, for at least 1 year. Individuals were asked to report name of employer, list job title and to describe the nature of the work activity for occupations held for at least 1 year. Duration of residence/occupation, drinking water source, change in water source, and street address were requested for each location. If exact street address was unknown, participants were asked to identify the closest cross streets. Written forms were double-entered into a database and checked for discrepancies. If discrepancies were found, the original document was checked and relied on. Each residence in the study area was geocoded and assigned a geographic coordinate in ArcGIS (Version 9.0; ESRI, Redlands, CA, USA); residences outside the study area were not geocoded. Geocoding settings were the following: spelling sensitivity of 70, minimum candidate score of 10, and minimum match score of 60.
Lifetime arsenic exposure assessment
The approach developed for individual lifetime exposure assessment has been previously described [27]. Briefly, locations of residences were used to generate lifetime exposure estimates; previous sensitivity analyses indicated limited exposure from food or drinking water at other locations (e.g., place of occupation) in this region [28]. The research team met with participants where they lived, and water samples were collected from sources used for drinking (including coffee) and cooking, as well as untreated well water if participants used a private well. Water samples were analyzed for arsenic using an inductively coupled plasma mass spectrometry unit (ICP-MS, Model 7500c, Agilent Technologies, Santa Clara, CA). Each batch of samples included a standard reference material (NIST SRM 1640, Trace Elements in Natural Water), and the set acceptance criterion was ± 10% of the certified arsenic concentration in the SRM. Non-detects (n = 40) were assigned a level of 0.02 µg/L, equal to half of the average instrument detection limit. Additional details about the analytic protocol can be found elsewhere [29].
A geostatistical model was developed for predicting arsenic concentrations at past residences on private well water [30]. A soft indicator kriging approach was adopted, incorporating the spatial pattern in the State’s arsenic database (n = 6,050 private untreated wells in the eleven county study area from 1993–2002) as well as secondary data such as geographic boundaries of different types of bedrock and unconsolidated geologic formations. The geostatistical model predicts arsenic concentration for 500 × 500 m2 pixels. This model was validated using a separate test dataset of 371 well water measurements, and showed a Pearson’s correlation r = 0.61; predicting above or below a 10 µg/L threshold resulted in sensitivity = 0.62, specificity = 0.80, and 75% agreement [31].
To estimate arsenic concentrations at past residences on public water supplies, 1,675 arsenic measurements in public well water supplies in the study area were extracted from a Michigan state arsenic database (1983–2004). A water supply history between 1920 and 2003 was also obtained by calling each municipality in the study area that serves water to at least 1,000 people (135 water suppliers). Changes in water source from groundwater to surface water and shut-down of high-arsenic wells were most common. The state arsenic database was combined with the water supply history to generate an arithmetic mean of arsenic concentration for each public groundwater supply over applicable time periods. Public surface water supplies were assigned an arsenic concentration of 0.30 µg/L, which is the average measured level in surface water in this study population (range 0.20–0.64 µg/L).
Altogether, within the study area (a) arsenic was measured in drinking water at each participant’s current residence, (b) estimates of arsenic were generated from a validated geostatistical model at past residences on private well water, and (c) average arsenic concentrations were derived for public water supplies from the State database and public water supply histories. Estimates of arsenic concentration in drinking water were linked to individual lifetime residential histories using automated functionality developed in space–time analytic software (STIS™, TerraSeer, Inc., Ann Arbor, MI). The procedure successively loops through each person’s residential history in the study area, retrieves their water source history, and assigns an arsenic level for each space–time interval.
For public supplies outside the study area, most participants drank water from Detroit, measured as 0.30 µg/L (consistent with surface water measurements within the study area). Outside of Detroit, estimates were generated from arsenic data compiled for public water supplies in the United States [32]. If no data existed for a particular city, which was common for cities relying on surface water, then a level of 0.30 µg/L was assigned. For private wells outside the study area, arsenic was estimated using city averages from a US Geological Survey (USGS) database of well water arsenic levels [33]. All locations outside of the United States were assigned a level of 0.30 µg/L, because public data were not readily available. The majority of time spent outside the United States was in a military capacity, relying on water at a military base or on a navy ship in the 1940s, 1950s, or 1960s.
Arsenic concentration estimates inside and outside of the study area were combined for each participant to provide estimates of arsenic concentration in the drinking water over the life-course. In generating these estimates, we assume that arsenic concentrations were temporally stable within a particular well, an assumption supported by studies in our region, and in other areas, across seasons, years, and decades [29, 34, 35]. Arsenic exposure estimates were constructed independent of case–control status.
Two arsenic exposure metrics were investigated for each participant: (a) arsenic concentration estimates (µg/L) from the lifetime exposure assessment; (b) arsenic intake (µg/day) over the adult life-course. The second metric was calculated by multiplying arsenic concentration by the sum of intake of tap water, coffee, and all other beverages made with water at home as an adult. These metrics were used to construct time-weighted average (TWA) exposure measures for arsenic concentration (µg/L) over the life-course and arsenic intake (µg/day) over the adult life-course.
In addition to long-term TWA exposure measures, researchers often examine lag effects and/or calculate exposure over decade-wide time windows. However, given limited knowledge about the temporal relationship between arsenic exposure and bladder cancer, we were concerned that these approaches could result in misspecification of the appropriate etiologic period, thereby introducing non-differential misclassification and bias the results toward the null as cautioned by Rothman [36]. Therefore, given that a temporal hypothesis could not be justified, we opted for an exploratory approach using temporally resolved exposure estimates. The two exposure metrics were calculated using moving 5 year averages over each year of a participant’s life; arsenic estimates were assigned to the middle year of each 5-year average and called yearly exposure estimates.
Epidemiologic analyses
Odds ratios (OR) and 95% confidence intervals (CI) were calculated for TWA exposure estimates using unconditional logistic regression in SAS (Cary, NC). Arsenic was treated both as a continuous variable (modeled linearly per 5 µg/L increment) and categorized a priori into <1 µg/L, 1–10 µg/L, and >10 µg/L. This categorization permitted examination of risk in the low-to-moderate exposure range above the current MCL (10 µg/L), where little information currently exists. Unadjusted (crude) analyses were conducted, as were analyses adjusted for factors shown to be associated with bladder cancer in univariate models or otherwise deemed important: age, race (white, black, other), sex, smoking (never smoker, former <20 pack-years, former ≥20 pack-years, current <20 pack-years, current ≥20 pack-years), education (highest level attained), history of urinary bladder cancer in an immediate relative (parent, sibling, or child), and at least 5 years of employment in a high-risk occupation (dye workers and users, aromatic amine manufacturing, leather workers, painting, driving trucks or other motor vehicles, aluminum workers, machinists, and automobile assemblers) [4, 37]. Employment classifications were made using job titles, place of occupation, and using the U.S. Census Bureau Index of Occupation [38].
To investigate possible interactions, stratified analyses were conducted. To compare results with previous studies [13, 14, 20], and because smoking is the most important known risk factor for bladder cancer, analyses were stratified by ever/never smoking. In addition, we were concerned about error associated with self-reported fluid consumption, and recent evidence suggests that classifications using the median are less likely to result in misclassification of water consumption-related exposures [39]. Therefore, for the arsenic concentration metric (µg/L), home water consumption was split at approximately the median (1 L/day) for stratified analyses. In addition, Bates et al. [13] hypothesized that dividing water consumption by total fluid consumption accounts for possible dilution of arsenic in the urine; this was investigated by calculating the percentage of total fluid intake that includes water from home and conducting stratified analyses using the median value (26%). These strata are characterized as above average home water consumption and above average percentage of fluids containing water from home, respectively, in the “Results” and “Discussion” sections.
The above logistic regression analyses were also conducted using yearly exposure estimates, in an exploratory attempt to generate a temporal hypothesis about the relationship between arsenic exposure and bladder cancer. Yearly exposure estimates were analyzed using three different temporal measures: calendar year, participant’s age, and years prior to diagnosis or interview. These are analogous to age-period-cohort models common in epidemiologic surveillance. Because of multiple testing, only signals that persisted for at least 5 years are reported.
Consistency of results was evaluated by conducting analyses for only those cases with transitional cell carcinoma (TCC) of the bladder (n = 400), and only those with papillary TCC (n = 306). Analyses were also repeated after removing the sixteen controls and ten cases who reported being diagnosed with non-melanoma skin cancer. Cumulative exposure estimates also were examined (as opposed to TWA). In addition, analyses were conducted using an arsenic concentration estimate of 0.30 µg/L (estimate of background exposure within the study area) for all residences outside of the study area (~ 33% of person-years), and for past residences with private wells in study area which were not geocoded to exact address or nearest cross street, representing ~ 5% of person-years.
Results
Demographics and exposure assessment
As a result of frequency matching, distributions of gender, race, and age were similar between bladder cancer cases and controls (Table 1). Cases and controls both averaged 65 years of age. Compared with controls, cases smoked more cigarettes over their lifetime, completed fewer years of schooling, and were more likely to have worked for at least 5 years in an occupation at high risk for bladder cancer. Cases were more likely to have a family member diagnosed with bladder cancer, compared with controls, albeit this only reached borderline significance.
Table 1.
Demographic characteristics of bladder cancer cases and controls, Southeastern Michigan, Enrolled 2002–2006
| Cases (n = 411) (No. (%)) |
Controls (n = 566) (No. (%)) |
p valuea | |
|---|---|---|---|
| Genderb | |||
| Male | 315 (76.6) | 418 (73.9) | 0.32 |
| Female | 96 (23.4) | 148 (26.1) | |
| Raceb | |||
| White | 380 (92.5) | 518 (91.5) | 0.81 |
| Black | 11 (2.7) | 15 (2.7) | |
| Other | 20 (4.8) | 33 (5.8) | |
| Ageb | |||
| < 45 | 10 (2.4) | 26 (4.6) | 0.29 |
| 45–54 | 58 (14.1) | 62 (11.0) | |
| 55–64 | 113 (27.5) | 158 (27.9) | |
| 65–74 | 135 (32.9) | 190 (33.5) | |
| 75 or > | 95 (23.1) | 130 (23.0) | |
| Cigarette smoking in pack-years | |||
| Nevers | 104 (25.3) | 263 (46.5) | <0.0001 |
| Former <20 | 80 (19.5) | 128 (22.6) | |
| Former ≥20 | 142 (34.5) | 120 (21.2) | |
| Current <20 | 6 (1.5) | 13 (2.3) | |
| Current ≥20 | 79 (19.2) | 42 (7.4) | |
| Education | |||
| High school or less | 154 (37.5) | 155 (27.4) | <0.0001 |
| Some college | 123 (29.9) | 145 (25.6) | |
| College graduate or more | 134 (32.6) | 266 (47.0) | |
| Worked ≥5 Years in high-risk occupationc | |||
| No | 329 (80.1) | 501 (88.5) | 0.0003 |
| Yes | 82 (20.0) | 65 (11.5) | |
| History of bladder cancer in immediated family members | |||
| No | 390 (94.9) | 550 (97.2) | 0.07 |
| Yes | 21 (5.1) | 16 (2.8) | |
| Usuale home water consumption >1 L/dayf | |||
| No | 169 (42.5) | 214 (39.9) | 0.27 |
| Yes | 229 (57.5) | 322 (60.1) | |
| Usuale percentage of fluid intake that includes water at home above mediang,h | |||
| No | 163 (53.4) | 161 (48.1) | 0.21 |
| Yes | 142 (46.6) | 174 (51.9) | |
| Usuali home water source | |||
| Public supply | 213 (51.8) | 304 (53.7) | 0.56 |
| Private well | 198 (48.2) | 262 (46.3) | |
| Exposed to arsenic >10 µg/L at ≥1 residence over life-course | |||
| No | 296 (72.0) | 430 (76.0) | 0.16 |
| Yes | 115 (28.0) | 136 (24.0) | |
Derived using a 2-sided chi-square test
Frequency matched between cases and controls
High-risk occupations include dye workers and users, aromatic amine manufacturing, leather workers, painting, driving trucks or other motor vehicles, aluminum workers, machinists, and automobile assemblers
Siblings, parents, or children
Refers to average amount over adult life
Missing data from 17 cases and 40 controls for calculating average home water consumption
Median value = 26%
Missing data from 106 cases and 231 controls for calculating percentage of fluid intake that includes water from home
Refers to predominant water source over adult life
In general, distributions of arsenic exposures and residential histories were similar for cases and controls. Cases reported an average of 9.1 residences per person, and 9.0 residences were reported for each control (Table 2). This resulted in a total of 8,823 residences for the study population accounting for 64,040 person-years, reflecting an average of 65 years of residential history per person. Cases and controls did not report a residence for 1% of their person-years and reported similar distributions of person-years on private well and public water supply (Table 2). Participants spent approximately 66% of their person-years within the study area, and another 13% of their person-years drinking Detroit city water, just outside the study area. Within the study area, cases and controls spent both approximately 28% of their person-years drinking private well water and another 38% of person-years on a public water supply. Bottled water was rarely reported and represented <1% of person-years for cases and controls. Highest arsenic concentrations were found in private well water, with a 90% range of approximately 0.02–25 µg/L for cases and controls (Table 2). Past residences on public water supply were associated with higher arsenic concentrations than current residences because of recent changes (post-1983) to reduce arsenic in water supplies.
Table 2.
Arsenic distribution by water supply and residential history strata for lifetime exposure reconstruction of cases and controls, South-eastern Michigan, Enrolled 2002–2006
| Cases | Controls | |||
|---|---|---|---|---|
| Person-years (%) |
Arsenic distribution (µg/L) (5%, 50%, 95%) |
Person-years (%) |
Arsenic distribution (µg/L) (5%, 50%, 95%) |
|
| Measurements at current residence | ||||
| Private well | 11.6 | 0.1, 5.3, 37.4 | 11.8 | 0.02, 1.8, 27.7 |
| Public supply | 15.7 | 0.1, 0.3, 3.6 | 15.0 | 0.02, 0.3, 3.5 |
| Bottled water | 0.6 | 0.02, 0.2, 1.0 | 0.6 | 0.02, 0.20, 0.20 |
| Past residencesa in study area | ||||
| Private wellb | 16.4 | 0.02, 4.0, 24.9 | 16.0 | 0.02, 4.3, 21.7 |
| Public supplyc | 23.0 | 0.3, 0.3, 11.1 | 21.8 | 0.3, 0.9, 8.4 |
| Past residencesa outside of study area | ||||
| Detroit public supply | 12.8 | 0.3, 0.3, 0.3 | 13.8 | 0.3, 0.3, 0.3 |
| Other public supplyd | 12.0 | 0.3, 0.3, 4.6 | 14.7 | 0.3, 0.3, 4.1 |
| Private wellse | 5.7 | 0.3, 0.3, 5.0 | 4.8 | 0.3, 0.3, 8.0 |
| International | 1.2 | 0.3, 0.3, 0.3 | 1.0 | 0.3, 0.3, 0.3 |
| Residence not provided | 1.0 | 0.5 | ||
| Residences | ||||
| Total | 3,741 | 5,082 | ||
| Average per person | 9.1 | 9.0 | ||
Bottled water not reported at past residences
Arsenic estimates from geostatistical model
Arsenic estimates from Michigan Department of Environmental Quality database, and water supplier history
Arsenic estimates from Natural Resources Defense Council database of arsenic in public water supplies
Arsenic estimates from United States Geological Survey database of arsenic in groundwater
Overall, geocoding accuracy was similar for cases and controls in the study area. Nearly 54% of controls’ person-years were geocoded to exact address or closest cross streets, and 11% geocoded to town center. Among cases, 53% were geocoded to exact address or closest cross streets, and 14% geocoded to town center. The remaining person-years outside the study area were not geocoded.
Epidemiologic findings
TWA life-course arsenic concentration and arsenic intake exposure metrics were not associated with the risk of bladder cancer in unadjusted or multivariate adjusted analyses (Table 3). The TWA life-course arsenic concentration metric resulted in higher ORs than the arsenic intake metric. Multivariate-adjusted analyses stratified by ever smoking also produced no significant findings (Table 4); these results held for both exposure metrics. However, among never-smokers, risk was significantly elevated for higher levels of TWA life-course arsenic concentration using continuous measures (OR = 1.29; 95% CI: 1.03, 1.63, per 5 µg/L increase). Results were non-significantly elevated among never-smokers using categorical measures of arsenic concentration, or the arsenic intake metric.
Table 3.
Unadjusted and multivariate-adjusted odds ratios and 95% confidence intervals for arsenic in drinking water and bladder cancer, Southeastern Michigan, Enrolled 2002–2006
| Unadjusted analyses | Multivariate-adjusted analysesa | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases (no.) | Controls (no.) | OR | 95% CI | Casesb (no.) | Controlsb (no.) | OR | 95% CI | |
| Arsenic concentration in water (TWA) | ||||||||
| Continuous (per 5 µg/L increase) | 411 | 566 | 1.11 | 0.98, 1.25 | 407 | 564 | 1.05 | 0.92, 1.20 |
| Categorical | ||||||||
| <1 µg/L | 188 | 264 | 1.00 | 187 | 264 | 1.00 | ||
| 1–10 µg/L | 185 | 263 | 0.99 | 0.76, 1.29 | 182 | 180 | 0.84 | 0.63, 1.12 |
| >10 µg/L | 38 | 39 | 1.37 | 0.84, 2.22 | 38 | 37 | 1.10 | 0.65, 1.86 |
| Arsenic intake from water (TWA)c | ||||||||
| Continuous (per 5 µg/day increase) | 398 | 536 | 1.06 | 0.97, 1.17 | 394 | 534 | 1.01 | 0.92, 1.12 |
| Categorical | ||||||||
| <1 µg/day | 190 | 254 | 1.00 | 189 | 252 | 1.00 | ||
| 1–10 µg/day | 165 | 234 | 0.99 | 0.75, 1.29 | 162 | 234 | 0.83 | 0.62, 1.11 |
| >10 µg/day | 43 | 48 | 1.25 | 0.80, 1.96 | 43 | 48 | 1.01 | 0.62, 1.64 |
OR odds ratio; CI confidence interval; TWA time-weighted average life-course exposure estimate
Adjusted for cigarette smoking history, education, history of employment in high-risk occupation, family history of bladder cancer, age, race, and sex
Differences in numbers of cases and controls in adjusted analyses attributable to missing pack-year data from six participants
Differences in numbers of cases and controls for arsenic intake metric attributable to missing water consumption data
Table 4.
Multivariate-adjusted odds ratios and 95% confidence intervals for arsenic in drinking water and bladder cancer, among ever- and never-smokers, Southeastern Michigan, Enrolled 2002–2006
| Ever smoker | Never smoker | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases (no.) | Controls (no.) | OR | 95% CI | Cases (no.) | Controls (no.) | OR | 95% CI | |
| Arsenic concentration in water (TWA) | ||||||||
| Continuous (per 5 µg/L increase) | 311 | 306 | 0.95 | 0.81, 1.11 | 100 | 260 | 1.29 | 1.03, 1.63 |
| Categorical | ||||||||
| <1 µg/L | 136 | 139 | 1.00 | 52 | 125 | 1.00 | ||
| 1–10 µg/L | 148 | 144 | 0.96 | 0.68, 1.36 | 37 | 119 | 0.72 | 0.43, 1.20 |
| >10 µg/L | 27 | 23 | 0.94 | 0.50, 1.78 | 11 | 16 | 1.62 | 0.68, 3.87 |
| Arsenic intake from water (TWA)a | ||||||||
| Continuous (per 5 µg/day increase) | 303 | 295 | 0.97 | 0.86, 1.09 | 95 | 241 | 1.14 | 0.94, 1.37 |
| Categorical | ||||||||
| <1 µg/day | 139 | 128 | 1.00 | 51 | 126 | 1.00 | ||
| 1–10 µg/day | 133 | 135 | 0.88 | 0.62, 1.25 | 32 | 99 | 0.80 | 0.47, 1.35 |
| >10 µg/day | 31 | 32 | 0.74 | 0.41, 1.31 | 12 | 16 | 2.01 | 0.87, 4.68 |
Adjusted for education, history of employment in high-risk occupation, family history of bladder cancer, age, race, and sex
OR odds ratio; CI confidence interval; TWA time-weighted average
Differences in numbers of cases and controls for arsenic intake metric attributable to missing water consumption data
Effect of TWA life-course arsenic concentration was also investigated in strata of home water consumption to examine interaction between water consumption and arsenic concentration in drinking water (Table 5). In multivariate-adjusted analyses, among those with home water consumption >1 L/day, arsenic approached a significant association with bladder cancer in continuous analyses (OR = 1.17; 95% CI: 0.94, 1.44, per 5 µg/L increase). Additionally stratifying into the subpopulation consuming a greater than average percentage of fluids containing water from home (>26%) resulted in an elevated, but non-significant association between arsenic and bladder cancer (OR = 2.62; 95% CI: 0.83, 8.25) for participants with home drinking water arsenic concentrations above 10 µg/L when compared to those with levels <1 µg/L (Table 5). Continuous analyses were also elevated, but not significantly (OR = 1.21; 95% CI: 0.91, 1.61, per 5 µg/L increase).
Table 5.
Multivariate-adjusted odds ratios and 95% confidence intervals for arsenic in drinking water and bladder cancer, stratified by home water consumption and percentage of fluids that include water from home, Southeastern Michigan, Enrolled 2002–2006
| Home water consumption above 1 L/day | Home water consumption above 1 L/day and above median percentage of fluids that contain water from homea |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases (no.) | Controls (no.) | OR | 95% CI | Cases (no.) | Controls (no.) | OR | 95% CI | |
| Arsenic concentration in water (TWA) | ||||||||
| Continuous (per 5 µg/L increase) | 202 | 262 | 1.17 | 0.94, 1.44 | 101 | 120 | 1.21 | 0.91, 1.61 |
| Categorical | ||||||||
| <1 µg/L | 79 | 123 | 1.00 | 36 | 58 | 1.00 | ||
| 1–10 µg/L | 104 | 124 | 1.05 | 0.69, 1.59 | 52 | 55 | 1.29 | 0.68, 2.45 |
| >10 µg/L | 19 | 15 | 1.62 | 0.72, 3.64 | 13 | 7 | 2.62 | 0.83, 8.25 |
Adjusted for cigarette smoking history, education, history of employment in high-risk occupation, family history of bladder cancer, age, race, and sex
OR odds ratio; CI confidence interval; TWA time-weighted average
Percentage of fluids that contain water from home: Median = 26%
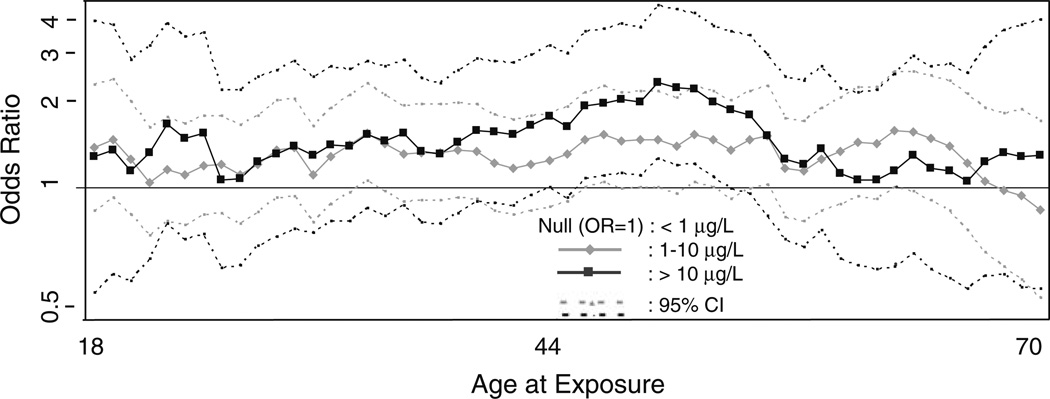
In addition to the TWA life-course exposure estimates, yearly estimates of exposure were analyzed for a relationship with bladder cancer. No significant results were observed in unstratified multivariate analyses using age, calendar year, or years prior to diagnosis/interview as temporal measures (results not shown). In analyses stratified by water consumption >1 L/day, multivariate analyses indicated that exposures occurring when participants were between 45 and 52 years old were associated with peaks in estimated risk using continuous or categorical measures of arsenic exposure (categorical measures shown in Fig. 2). Over this 8 year age-at-exposure period, 30–32 cases and 20–25 controls were in the above average water consumption and high-arsenic (>10 µg/L) strata contributing to these findings. Age at diagnosis did not influence these findings; cases contributing to the significant results included those diagnosed from age 50 (~ 5 years following peak exposure) through age 79 (~ 30 years following peak exposure). Although not significant for all years, risk was elevated for exposures between ages 30 and 55. Further stratification by above average percentage of fluids that contain water from home revealed a similar time period of elevated risk. Analyses using other temporal measures did not produce a significant relationship, nor did analyses limited to ever-smokers.
Fig. 2.
Multivariate-adjusted odds ratios and 95% confidence intervals for arsenic in drinking water and bladder cancer, among those who consume >1 L/day of water from home, by age of exposure; Southeastern Michigan, Enrolled 2002–2006. Adjusted for cigarette smoking history, education, history of employment in high-risk occupation, family history of bladder cancer, age, race, and sex. Exposure >10 µg/L between ages 45 and 52 significantly associated with bladder cancer
We also investigated whether the results were specific to arsenic, or if private well use also produced an elevated OR in stratified analyses. In multivariate-adjusted analyses, private well use was not associated with bladder cancer in full analyses (OR = 1.01; 95% CI: 0.76, 1.32); or among strata >1 L/day home water consumers (OR = 1.16; 95% CI: 0.78, 1.73), or among strata of above average home water consumers who also consumed a greater than average proportion of fluids from their home water source (OR = 1.13; 95% CI: 0.63, 2.04).
Analyses were conducted using subsets of the cases defined by TCC or papillary TCC. The effect estimates were consistent with those reported from analyses of the entire dataset, as were results among those not diagnosed with non-melanoma skin cancer. Similar results were also observed when using arsenic concentration estimates of 0.30 µg/L for all residences outside of study area (32.7% of person-years for cases, 34.8% for controls) and for past residences on private wells that were not geocoded to exact address or nearest cross street in study area (5.9% of person-years for cases, 4.8% for controls). Cumulative exposure estimates over the life-course also produced similar results.
Discussion
This case–control study included a detailed lifetime exposure assessment and had good statistical power to investigate the relationship between low-level arsenic exposure and bladder cancer. Our null findings from analyses of the full dataset are generally consistent with results from other epidemiologic studies of low-level arsenic and bladder cancer [12–20]. We also identified suggestive, albeit underpowered, evidence of a relationship between arsenic exposure and bladder cancer in analyses stratified by above average water consumption at home and above average percentage of total fluids that came from water at home. These variables were treated categorically, and analyses were stratified to address possible misclassification in self-reported fluid consumption, which could potentially bias results toward the null [40]. These analyses included small numbers of cases and controls in the high-exposure group (Table 5) highlighting the need to expand such studies. It is important to note that treating these fluid consumption variables in a continuous fashion and incorporating them directly into an exposure metric did not produce elevated associations. Other investigators of arsenic-bladder cancer relationships have not used categorical measures of fluid consumption variables, so we are unable to compare our results. Nonetheless, it is plausible that exposure to arsenic in home drinking water might produce stronger effects among individuals who consume more water from home. Furthermore, those who consume greater percentages of water from home out of total fluids would have less potential for dilution of arsenic in the bladder, as has previously been hypothesized [13, 14].
In addition to cumulative and TWA exposure measures, we opted to investigate temporally specific exposure estimates. When exploring varying induction-latency intervals and varying risk levels in relation to age-at-exposure intervals, we considered that cumulative measures alone would not have adequate sensitivity to demonstrate associations at low levels of exposure [36]. Lacking a suitable a priori temporal hypothesis, we investigated yearly averages of arsenic exposure using age, calendar year, and years prior to diagnosis/interview as temporal measures. A relationship between arsenic and bladder cancer was only observed using age as the temporal measure, with risk elevated for exposures occurring during ages 30–55 (significantly so for ages 45–52). These results appeared using strata defined by above average home water consumption or above average percentage of fluids containing water from home, and at a wide range of ages at diagnosis (ages 50–79), suggesting that age at diagnosis did not influence these findings. Previous studies have not examined whether participants’ ages of exposure affected the relationship between arsenic and bladder cancer. In light of our exploratory approach, which may be prone to spurious results due to the large number of statistical analyses carried out, we interpret these findings cautiously. Thus, the window of exposure that was identified should be examined using hypothesis-driven analyses in future studies.
One of the earlier epidemiologic studies of arsenic and bladder cancer identified increased risk among smokers [13]; however, more recent work from members of the same research team failed to find any interaction with smoking, using more detailed estimates of lifetime arsenic exposure [14]. While a couple studies have reported higher risk among smokers [16, 20], others have not [15, 19]. Our null findings among ever-smokers are not inconsistent with the weight of evidence available. Among never smokers, we found arsenic to be significantly associated with bladder cancer in continuous analyses, but not significant when treated categorically; these results were driven by only eleven highly exposed never-smoking cases. The significant finding among never smokers is inconsistent with the existing literature and requires confirmation before inferring that risk from arsenic exposure is higher among never-smokers.
One stumbling block for assessing cancer risk in such epidemiologic studies is characterization of individual-level long-term exposure to arsenic in drinking water [21, 22]. In addition to our study, three others involving relatively low-level arsenic exposure have incorporated long-term (30+ year) individual-level exposure reconstruction tracing residential histories [12, 14, 20]. Our study builds on these efforts by collecting lifetime residential history and assessing exposure inside and outside our study area using a combination of arsenic measurements and statistical predictions. In particular, two strengths of our study include the limited lifetime mobility of Michigan residents and our individual-level exposure reconstruction methodology. Residential histories were collected for 99% of case and control person-years, with an average 80% of person-years being spent in southeastern Michigan. Arsenic concentrations in drinking water were directly measured at then-current residences and modeled at past residences using historical databases compiled from MDEQ and municipal water suppliers, along with a geostatistical model that was validated using a separate test dataset. With respect to past private wells where residential information was inadequate for geocoding (~ 5% of person-years), analyses for consistency were conducted and logistic regression analyses revealed similar results whether arsenic concentrations were estimated from the geostatistical model or from background values (0.30 µg/L). Nonetheless, exposure misclassification is still possible; future work will involve deriving estimates of exposure uncertainty and propagating that uncertainty into epidemiologic analyses. Those efforts not withstanding, exposure misclassification is likely to be non-differential, and bias results toward the null.
In addition to exposure misclassification, selection bias could have occurred. Given that some potentially eligible individuals chose not to participate, we evaluated whether systematic selection bias was present among cases. In multiple logistic regression analyses, participating cases were compared with potentially eligible cases, and there was no evidence that the populations had different geographic distributions using counties or metropolitan statistical areas to represent urbanicity (6% vs. 7% rural, p = 0.39). No differences were observed with respect to race or gender between participating and non-participating cases. However, participating cases were younger (p <0.0001; average age in their 60 s at time of diagnosis, as opposed to their 70 s) and more likely to be diagnosed with less invasive, papillary TCC of the bladder (p = 0.0004; 75% vs. 66%) than their non-participating counterparts. These results suggest that our findings are applicable to younger cases with papillary TCC of the bladder. Given that 22% of cases died prior to contact, we also compared participating and deceased cases. Results from this comparison were similar to those described above between participating and potentially eligible cases, although deceased cases were more likely to come from rural areas (p = 0.02, 6% vs. 10%). We therefore cannot discount the possibility that arsenic exposure, being more prevalent in rural areas, may have contributed to earlier death and lack of participation by some individuals, thereby biasing our results toward the null. A study of arsenic-related chromosomal alterations in bladder cancer found evidence of more aggressive bladder tumors in arsenic-exposed patients compared with unexposed patients [41], and a hospital-based study reported poorer survival among bladder cancer patients living in a high-arsenic region of Taiwan [42], supporting the possibility that exclusion of early deceased cases may have biased results toward the null.
We evaluated the possibility of selection bias in controls using similar methods. Information pertaining to non-participating eligible controls was not available; therefore, controls were compared with the background population in relation to water source and county at current residence. Controls were not compared by age, race, or gender with the background population, because these variables were matched to the case population. Participating controls were better educated than the background age 45 and over population of the study area (p < 0.0001; 47% vs 32%). Participating controls were geographically representative of the background population whether compared by county or urbanicity (p = 0.95; 5% rural in both participants and non-participants). Nearly 95% of the population in the study area lived in a metropolitan statistical area at time of diagnosis/recruitment; nonetheless, groundwater was commonly used for drinking water throughout the region, even in suburban areas. Estimates of county-specific population on private well water were derived from US Census 2000 and Michigan Department of Environmental Quality population on public water supply database. Controls were more likely to use private well water at their current residence than the general population (p < 0.0001; 43% vs. 29%). Higher rates of private well use at current residence were reported by participants in every county other than two of the least populated, Shiawassee and Tuscola. Perhaps private well owners were more interested in participating in the study, because they were offered results of the water analyses as an incentive. Since controls had a higher percentage of private well users than the background population, and arsenic concentrations are highest in private well water, this again is likely to bias the results toward the null.
Recall bias also may be a concern in case–control studies. In our study, self-reports of water consumption history and residential mobility may be a source of recall bias. By knowing that the study was about arsenic in drinking water, cases might have recalled greater water consumption and had greater compliance with the residential history form. However, analysis demonstrates that this was not the case. Cases reported less water consumption at home over their adult lives than controls (p = 0.27), and both cases and controls reported mobility histories for 99% of their person-years, suggesting little evidence of bias from differential recall. This type of recall bias does not seem to be a problem in our study.
Another strategy for investigating the influence of bias that we employed was to compare our results when analyzing established risk factors for bladder cancer with results in other publications. Current smokers of >20 pack-years show a three- to five-fold increase in risk [43] consistent with our results (OR = 4.79, Table 1). In recent decades, bladder cancer risks from high-risk occupations have typically been less than two-fold [4], similar to what we see here (OR = 1.92, Table 1). Approximately 6% of cases and 4% of controls report family history of urinary bladder cancer [44], similar again to the distribution reported here (5% of cases, 3% of controls (Table 1)). The male-to-female ratio of bladder cancer in our study is 3.3, close to the expected ratio [4]. Overall, these comparisons provide little evidence of bias.
Selecting the appropriate exposure metric and time period for investigating an exposure–disease relationship is one of the major challenges in environmental epidemiologic research. This is especially true in studies of low-level exposures where improper evaluation of the exposure time–disease relationship may mask any true findings [45]. In our study, we considered time-aggregated measures (cumulative and TWA), time-specific estimates (as defined by age, calendar year, and years prior to diagnosis/interview), and different arsenic exposure metrics at times stratified by fluid consumption measures and smoking history. Incorporating this breadth of exposure measures allows for a rigorous analysis of the dataset, yet also introduces the possibility of spurious findings. Recognizing the frequency of false positive results in cancer epidemiology, we have interpreted our results cautiously and seek other verification [46].
Our findings indicate that, overall, low-level TWA arsenic concentration in drinking water and arsenic intake were not associated with bladder cancer. However, in underpowered stratified analyses, effects were suggested for people consuming >1 L/day of water containing arsenic >10 µg/L and who drink an above average percentage of fluids containing water from home. Exploratory time-specific analyses produced results consistent with those from the time-aggregated stratified analyses and suggested that arsenic exposure levels >10 µg/L among individuals ages 30 and 55 with above average home water consumption were associated with subsequent development of bladder cancer. Follow-up studies are needed to investigate effects of low-level arsenic exposure in analyses stratified by fluid consumption and to establish whether there are one or more age-specific intervals of exposure susceptibility.
Acknowledgments
We extend deep appreciation to study participants for taking part in this research. We also thank Joe Lovato, Sheridan Haack, Stacey Fedewa, Angela Hungerink, Roni Kobrosly, Zorimar Rivera-Núñez, Aaron Linder, Nicholas Mank, Caitlyn Meservey, Erin Zazzera, and Taylor Builee for valuable assistance with different aspects of this project. This research was funded by the National Cancer Institute, Grant R01 CA96002-10. The perspectives are those of the authors and do not necessarily represent the official position of the funding agency.
Contributor Information
Jaymie R. Meliker, Email: jrmeliker@gmail.com, Graduate Program in Public Health, Department of Preventive Medicine, Stony Brook University Medical Center, HSC L3, Rm 071, Stony Brook, NY 11794-8338, USA.
Melissa J. Slotnick, Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA
Gillian A. AvRuskin, BioMedware, Inc., Ann Arbor, MI, USA
David Schottenfeld, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Geoffrey M. Jacquez, Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA BioMedware, Inc., Ann Arbor, MI, USA.
Mark L. Wilson, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, USA
Pierre Goovaerts, BioMedware, Inc., Ann Arbor, MI, USA.
Alfred Franzblau, Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA.
Jerome O. Nriagu, Department of Environmental Health Sciences, School of Public Health, University of Michigan, Ann Arbor, MI, USA
References
- 1.National Research Council. Arsenic in Drinking Water: 2001 Update. Washington: National Academy of Sciences Press; 2001. [Google Scholar]
- 2.Smedley PL, Kinniburgh DG. A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem. 2002;17:517–568. [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT, Devesa SS, Moore LE, Rothman N. Bladder Cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer epidemiology and prevention. 3rd edn. New York: Oxford University Press; 2006. pp. 1101–1127. [Google Scholar]
- 5.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans: some drinking-water disinfectants and contaminants, including arsenic. vol 84. Lyon: International Agency for Research on Cancer; 2002. [PMC free article] [PubMed] [Google Scholar]
- 6.Sams R, II, Wolf DC, Ramasamy S, Ohanian E, Chen J, Lowit A. Workshop overview: arsenic research and risk assessment. Toxicol Appl Pharmacol. 2007;222:245–251. doi: 10.1016/j.taap.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Schoen A, Beck B, Sharma R, Dube E. Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol. 2004;198:253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Chen YC, Su HJJ, Guo YLL, et al. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003;14:303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Sasieni P, Evans S. Ingested arsenic, keratoses, and bladder cancer. Am J Epidemiol. 1992;136:417–421. doi: 10.1093/oxfordjournals.aje.a116514. [DOI] [PubMed] [Google Scholar]
- 10.Hopenhayn-Rich C, Biggs ML, Fuchs A, et al. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7:117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Tsuda T, Babazono A, Yamamoto E, et al. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141:198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- 12.Baastrup R, Sorensen M, Balstrom T, et al. Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Perspect. 2008;116:231–237. doi: 10.1289/ehp.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates MN, Smith AH, Cantor KP. Case-control study of bladder cancer and arsenic in drinking water. Am J Epidemiol. 1995;141:523–530. doi: 10.1093/oxfordjournals.aje.a117467. [DOI] [PubMed] [Google Scholar]
- 14.Bates MN, Rey OA, Biggs ML, et al. Case-control study of bladder cancer and exposure to arsenic in drinking water in Argentina. Am J Epidemiol. 2004;159:381–389. doi: 10.1093/aje/kwh054. [DOI] [PubMed] [Google Scholar]
- 15.Karagas MR, Tosteson TD, Morris JS, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control. 2004;15:465–472. doi: 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- 16.Kurttio P, Pukkala E, Kahelin H, Auvinen A, Pekkanen J. Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect. 1999;107:705–710. doi: 10.1289/ehp.99107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamm SH, Engel A, Kruse MB, et al. Arsenic in drinking water and bladder cancer mortality in the United States: An analysis based on 133 U.S. counties and 30 years of observation. J Occup Environ Med. 2005;46:298–306. doi: 10.1097/01.jom.0000116801.67556.8f. [DOI] [PubMed] [Google Scholar]
- 18.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Recnh J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107:359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud DS, Wright ME, Cantor KP, Taylor PR, Virtamo J, Albanes D. Arsenic concentrations in prediagnostic toenails and the risk of bladder cancer in a cohort study of male smokers. Am J Epidemiol. 2004;160:853–859. doi: 10.1093/aje/kwh295. [DOI] [PubMed] [Google Scholar]
- 20.Steinmaus C, Yuan Y, Bates MN, Smith AH. Case-control study of bladder cancer and drinking water arsenic in the western United States. Am J Epidemiol. 2003;158:1193–1201. doi: 10.1093/aje/kwg281. [DOI] [PubMed] [Google Scholar]
- 21.Cantor KP. Invited commentary: Arsenic and cancer of the urinary tract. Am J Epidemiol. 2001;153:419–421. doi: 10.1093/aje/153.5.419. [DOI] [PubMed] [Google Scholar]
- 22.Cantor KP, Lubin JH. Arsenic, internal cancers, and issues in inference from studies of low- level exposures in human populations. Toxicol Appl Pharmacol. 2007;222:252–257. doi: 10.1016/j.taap.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devesa SS, Grauman DG, Blot WJ, Pennello G, Hoover RN, Fraumeni JF., Jr . NIH Publication No. 99–4564. Washington: National Cancer Institute; 1999. Atlas of cancer mortality in the United States 1950–94. [Google Scholar]
- 24.Michigan Department of Public Health. Arsenic in drinking water–a study of exposure and clinical survey. Lansing, MI: Division of Environmental Epidemiology, Michigan Department of Public Health; 1982. [Google Scholar]
- 25.Kolker A, Haack SK, Cannon WF. Arsenic in southeastern Michigan. In: Welch AH, Stollenwerk KG, et al., editors. Arsenic in ground water. Norwell: Kluwer; 2003. pp. 281–294. [Google Scholar]
- 26.Frost FJ, Muller T, Petersen HV, Thomson B, Tollestrup K. Identifying US populations for the study of health effects related to drinking water arsenic. J Expo Sci Environ Epidemiol. 2003;13:231–239. doi: 10.1038/sj.jea.7500275. [DOI] [PubMed] [Google Scholar]
- 27.Meliker JR, Slotnick MJ, AvRuskin GA, et al. Individual lifetime exposure to inorganic arsenic using a Space-Time Information System. Int Arch Occup Environ Health. 2007;80:184–197. doi: 10.1007/s00420-006-0119-2. [DOI] [PubMed] [Google Scholar]
- 28.Meliker JR, Franzblau A, Slotnick MJ, Nriagu JO. Major contributors to inorganic arsenic intake in Southeastern Michigan. Int J Hyg Environ Health. 2006;209:399–411. doi: 10.1016/j.ijheh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Slotnick MJ, Meliker JR, Nriagu JO. Effects of time and point-of-use devices on arsenic levels in Southeastern Michigan drinking water, USA. Sci Total Environ. 2006;369:42–50. doi: 10.1016/j.scitotenv.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Goovaerts P, AvRuskin G, Meliker J, Slotnick M, Jacquez G, Nriagu J. Geostatistical modeling of the spatial variability of arsenic in groundwater of southeast Michigan. Water Resources Res. 2005;41:W07013. [Google Scholar]
- 31.Meliker JR, AvRuskin GA, Slotnick MJ, et al. Validity of spatial models of arsenic concentrations in private well water. Environ Res. 2008;106:42–50. doi: 10.1016/j.envres.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natural Resources Defense Council. Arsenic and old laws: a scientific and public health analysis of arsenic occurrence in drinking water, its health effects, and EPA’s outdated arsenic tap water standard. [Accessed 20 Sept 2005];2000 http://www.nrdc.org/water/drinking/arsenic/aolinx.asp. [Google Scholar]
- 33.USGS. Arsenic in Groundwater of the United States. [Accessed 20 Sept 2005];Data updated March 30, 2000. 2000 http://water.usgs.gov/nawqa/trace/arsenic/.
- 34.Cheng Z, Van Geen A, Seddique AA, Ahmed KM. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environ Sci Technol. 2005;39:4759–4766. doi: 10.1021/es048065f. [DOI] [PubMed] [Google Scholar]
- 35.Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res. 2005;99:164–168. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Rothman N. Induction and latent periods. Am J Epidemiol. 1981;114:253–259. doi: 10.1093/oxfordjournals.aje.a113189. [DOI] [PubMed] [Google Scholar]
- 37.Kobrosly RW, Meliker JR, Nriagu JO. Automobile industry occupations and bladder cancer: a population-based case-control study in southeastern Michigan, USA. Occup Environ Med. 2009;66:650–656. doi: 10.1136/oem.2008.041616. [DOI] [PubMed] [Google Scholar]
- 38.Bureau of the Census. Alphabetical index of industries and occupations. Washington: Bureau of the Census; 2000. [Google Scholar]
- 39.Wright JM, Murphy PA, Nieuwenhuijsen MJ, Savitz DA. The impact of water consumption, point-of-use filtration and exposure categorization on exposure misclassification of ingested drinking water contaminants. Sci Total Environ. 2006;366:65–73. doi: 10.1016/j.scitotenv.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Shimokura GH, Savitz DA, Symanski E. Assessment of water use for estimating exposure to tap water contaminants. Environ Health Perspect. 1998;106:55–59. doi: 10.1289/ehp.9810655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore LE, Smith AH, Eng C, et al. P53 alterations in bladder tumors from arsenic and tobacco exposed patients. Carcinogenesis. 2003;24:1785–1791. doi: 10.1093/carcin/bgg136. [DOI] [PubMed] [Google Scholar]
- 42.Chen CH, Chiou HY, Hsueh YM, Chen CJ, Yu HJ, Pu YS. Clinicopathologic characteristics and survival outcome of arsenic related bladder cancer in Taiwan. J Urol. 2009;181:547–553. doi: 10.1016/j.juro.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Puente D, Hartge P, Greiser E, et al. A pooled analysis of bladder cancer case-control studies evaluating smoking in men and women. Cancer Causes Control. 2006;17:71–79. doi: 10.1007/s10552-005-0389-0. [DOI] [PubMed] [Google Scholar]
- 44.Kantor AF, Hartge PF, Hoover RN, Fraumeni JF., Jr Familial and environmental interactions in bladder cancer risk. Int J Cancer. 1985;35:703–706. doi: 10.1002/ijc.2910350602. [DOI] [PubMed] [Google Scholar]
- 45.Richardson DB, Wing S. Methods for investigating age differences in the effects of prolonged exposures. Am J Ind Med. 1998;33:123–130. doi: 10.1002/(sici)1097-0274(199802)33:2<123::aid-ajim4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Boffetta P, McLaughlin JK, La Vecchia C, Tarone RE, Lipworth L, Blot WJ. False-positive results in cancer epidemiology: a plea for epistemological modesty. J Natl Cancer Inst. 2008;100:988–995. doi: 10.1093/jnci/djn191. [DOI] [PMC free article] [PubMed] [Google Scholar]