Abstract
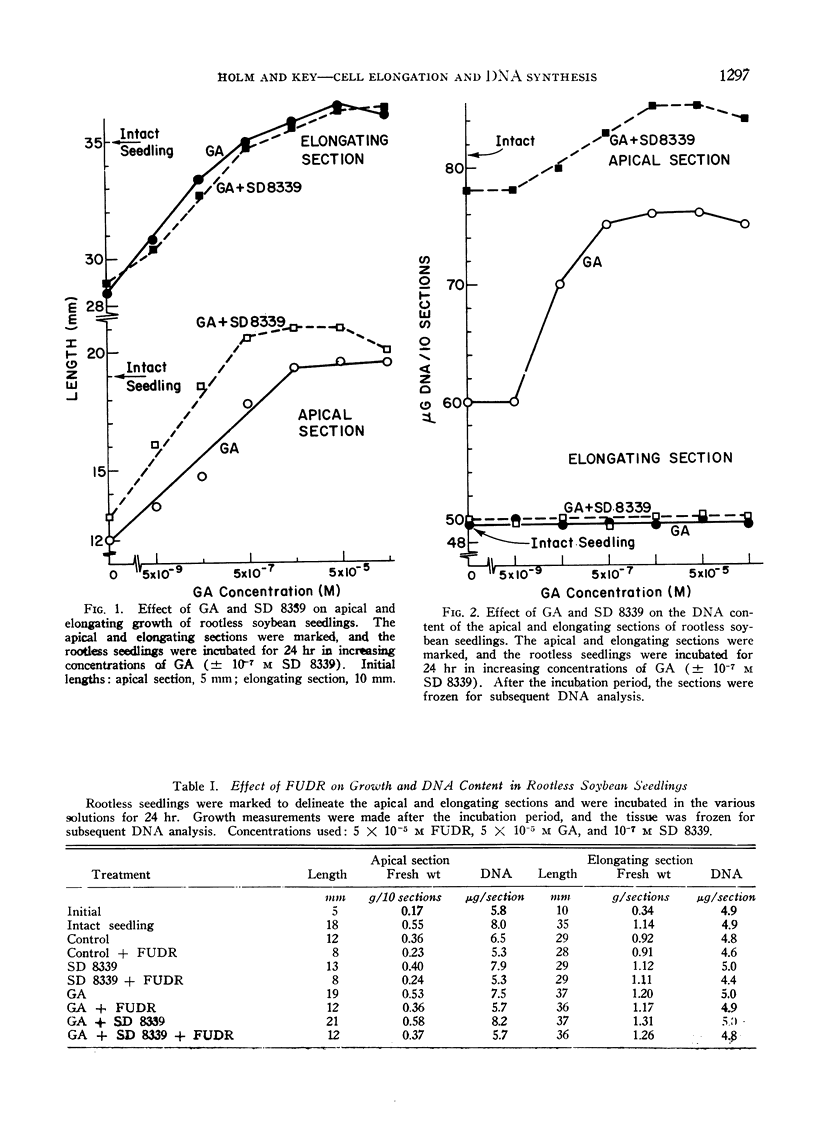
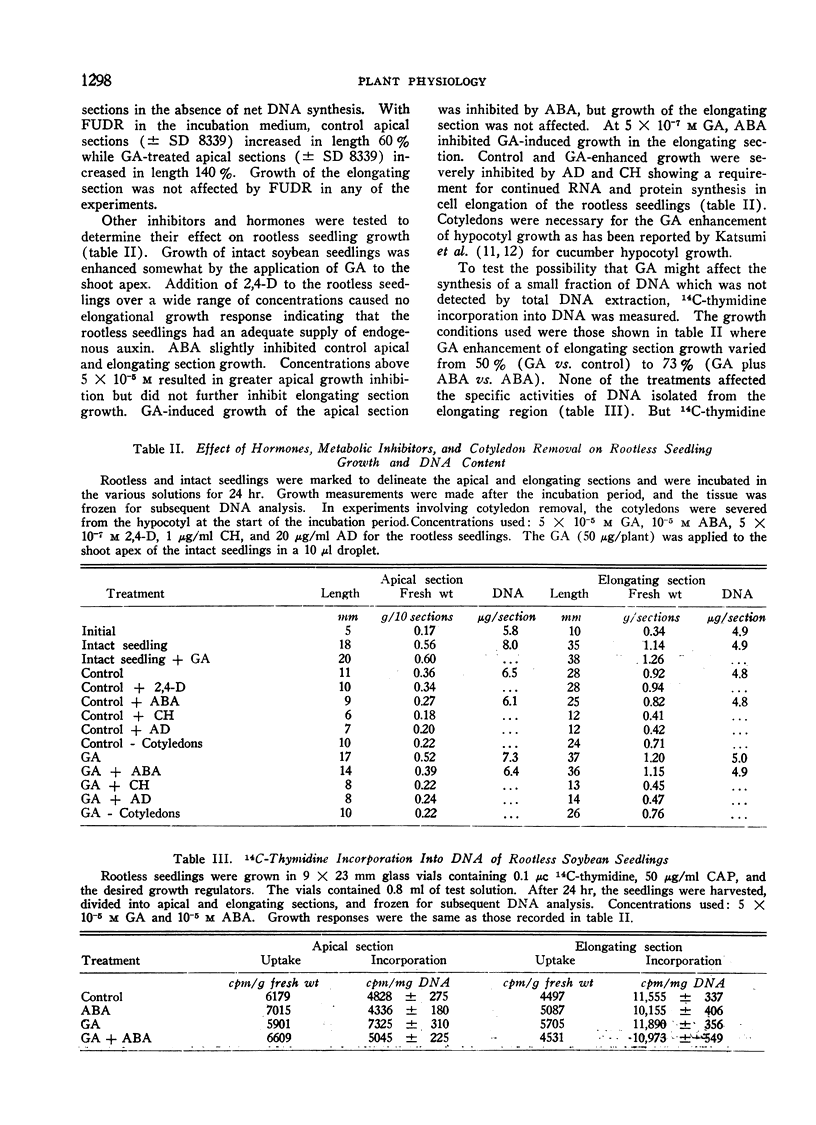
A method was developed where soybean seedlings were grown without roots to study the influence of hormones of root origin on shoot growth. Excision of the root resulted in inhibition of apical section growth and DNA synthesis and inhibited elongating section growth. A synthetic cytokinin restored DNA synthesis in the apical section, but did not influence growth in either the apical or elongating sections. Low concentrations of gibberellin with the cytokinin restored growth in the apical section. Gibberellin alone was sufficient to restore growth in the elongating section.
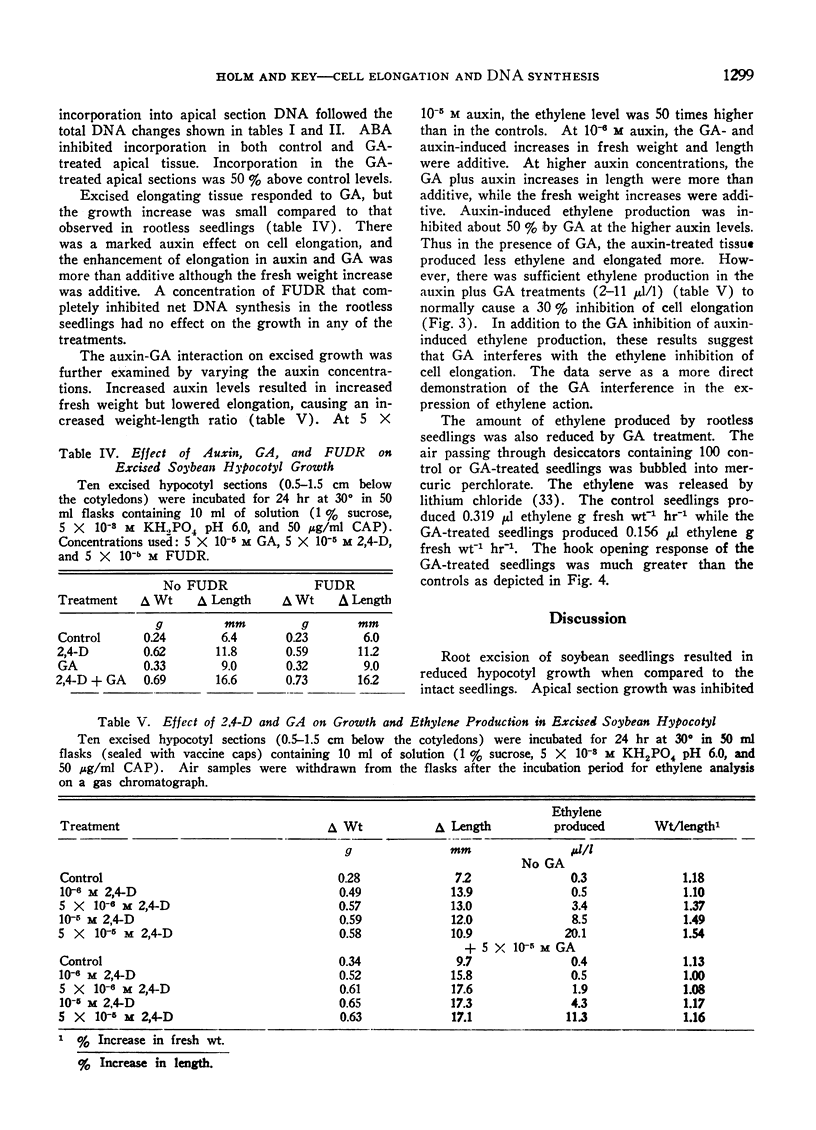
An inhibitor of DNA synthesis, 5-fluorodeoxyuridine, inhibited the increase in apical section DNA without inhibiting control or gibberellin-induced growth in the elongating section. Experiments with 14C-thymidine resulted in no DNA labeling differences in the elongating section under conditions where gibberellin-induced elongation varied from 50% to 73% above controls. It was concluded that gibberellin-induced elongation in soybean hypocotyl occurred in the absence of DNA synthesis. Gibberellin does stimulate DNA synthesis in the apical tissue apart from its effect on cell elongation.
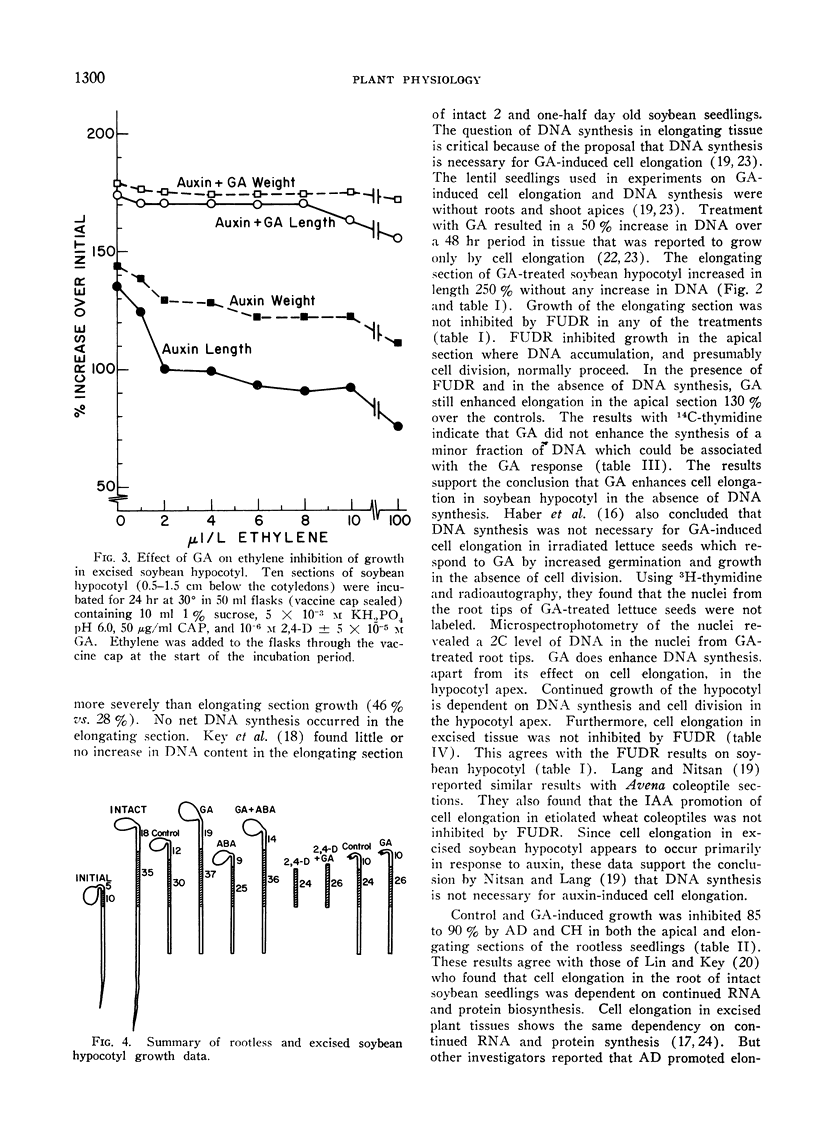
Excised soybean hypocotyl elongated maximally at 10−6m auxin. At higher auxin concentrations, fresh weight and ethylene production increased, but elongation was reduced. Addition of GA to the higher auxin concentrations resulted in a 50% inhibition in auxin-induced ethylene production and resumption in maximal elongation. Added ethylene inhibited elongation 30% at 2 μl/l. Addition of up to 100 μl/l ethylene did not inhibit elongation with GA present in the incubation medium. Thus GA may counteract ehtylene inhibition of cell elongation in addition to inhibiting ethylene production in auxin-treated tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRELIN E. S. Mitosis in adult cartilage. Science. 1957 Apr 5;125(3249):650–650. doi: 10.1126/science.125.3249.650. [DOI] [PubMed] [Google Scholar]
- Datko A. H., Maclachlan G. A. Indoleacetic Acid and the synthesis of glucanases and pectic enzymes. Plant Physiol. 1968 May;43(5):735–742. doi: 10.1104/pp.43.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN K. U., HEIDELBERGER C. Studies on fluorinated pyrimidines. XIII. Inhibition of thymidylate synthetase. J Biol Chem. 1961 Nov;236:3006–3013. [PubMed] [Google Scholar]
- Haber A. H., Foard D. E., Perdue S. W. Actions of gibberellic and abscisic acids on lettuce seed germination without actions on nuclear DNA synthesis. Plant Physiol. 1969 Mar;44(3):463–467. doi: 10.1104/pp.44.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. KINETINLIKE FACTORS IN THE ROOT EXUDATE OF SUNFLOWERS. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1302–1307. doi: 10.1073/pnas.53.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Ribonucleic Acid and Protein Synthesis as Essential Processes for Cell Elongation. Plant Physiol. 1964 May;39(3):365–370. doi: 10.1104/pp.39.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIBY P. D., OLSEN O. W. THE CESTODE ECHINOCOCCUS MULTILOCULARIS IN FOXES IN NORTH DAKOTA. Science. 1964 Sep 4;145(3636):1066–1066. doi: 10.1126/science.145.3636.1066. [DOI] [PubMed] [Google Scholar]
- Lang A., Nitsan J. Relations among cell growth, DNA synthesis, and gibberellin action. Ann N Y Acad Sci. 1967 Aug 9;144(1):180–190. doi: 10.1111/j.1749-6632.1967.tb34012.x. [DOI] [PubMed] [Google Scholar]
- Nitsan J., Lang A. DNA synthesis in the elongating nondividing cells of the lentil epicotyl and its promotion by gibberellin. Plant Physiol. 1966 Jun;41(6):965–970. doi: 10.1104/pp.41.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsan J., Lang A. Inhibition of cell division and cell elongation in higher plants by inhibitors of DNA synthesis. Dev Biol. 1965 Dec;12(3):358–376. doi: 10.1016/0012-1606(65)90003-5. [DOI] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. C., Leopold A. C. Opposing effects of gibberellin and ethylene. Plant Physiol. 1967 Jul;42(7):1021–1022. doi: 10.1104/pp.42.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota A. E., Leaver C. J., Key J. L. A detailed evaluation of the possible contribution of bacteria to radioactive precursor incorporation into nucleic acids of plant tissues. Plant Physiol. 1968 Jun;43(6):907–913. doi: 10.1104/pp.43.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Vaadia Y. Kinetin-like activity in root apices of sunflower plants. Life Sci. 1965 Jul;4(13):1323–1326. doi: 10.1016/0024-3205(65)90084-6. [DOI] [PubMed] [Google Scholar]
- Went F. W. SPECIFIC FACTORS OTHER THAN AUXIN AFFECTING GROWTH AND ROOT FORMATION. Plant Physiol. 1938 Jan;13(1):55–80. doi: 10.1104/pp.13.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]



