Abstract
Pancreatic acinar cells (PAC) obtain thiamin from the circulation via a carrier-mediated process that involves thiamin transporters 1 and 2 (THTR-1 and THTR-2; products of SLC19A2 and SLC19A3, respectively). Chronic alcohol exposure of PAC inhibits thiamin uptake, and, on the basis of in vitro studies, this inhibition appears to be transcriptionally mediated. The aim of this study was to confirm the involvement of a transcriptional mechanism in mediating the chronic alcohol effect in in vivo settings and to delineate the molecular mechanisms involved. Using transgenic mice carrying full-length SLC19A2 and SLC19A3 promoters, we found that chronic alcohol feeding led to a significant reduction in the activity of SLC19A2 and SLC19A3 promoters (as well as in thiamin uptake and expression of THTR-1 and -2). Similar findings were seen in 266-6 cells chronically exposed to alcohol in vitro. In the latter studies, the alcohol inhibitory effect was found to be mediated via the minimal SLC19A2 and SLC19A3 promoters and involved the cis-regulatory elements stimulating protein 1 (SP1)/gut-enriched Kruppel-like factor and SP1-GG-box and SP1/GC, respectively. Chronic alcohol exposure of PAC also led to a significant reduction in the expression of the SP1 transcription factor, which upon correction (via expression) led to the prevention of alcohol inhibitory effects on not only the activity of SLC19A2 and SLC19A3 promoters but also on the expression of THTR-1 and -2 mRNA and thiamin uptake. These results demonstrate that the inhibitory effect of chronic alcohol exposure on physiological/molecular parameters of thiamin uptake by PAC is mediated via specific cis-regulatory elements in SLC19A2 and SLC19A3 minimal promoters.
Keywords: SLC19A2, SLC19A3, alcohol feeding, thiamin transport, cis-regulatory elements
thiamin (vitamin b1) is essential for normal metabolic reactions and the health of all cell types, including pancreatic acinar cells (PAC). The vitamin acts as a cofactor for multiple enzymes (transketolase, pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and branched-chain ketoacid dehydrogenase); these enzymes participate in a variety of metabolic reactions related to oxidative energy (sugar) metabolism, ATP production (1, 26), and reduction of cellular oxidative stress (3, 14). Thus it is not surprising that cellular thiamin deficiency leads to acute energy failure and to a propensity for oxidative stress (2, 3, 5, 14, 26); such a deficiency also leads to functional and structural abnormalities in mitochondria (2).
The pancreas is a complex organ with important exocrine and endocrine functions. A variety of disease conditions and factors affect the health of this vital organ, leading to significant morbidity and mortality. The pancreas maintains a high level of thiamin (15), and deficiency of this vitamin negatively affects its exocrine and endocrine functions (16, 17, 21). As with all other mammalian cells, PAC cannot synthesize thiamin and must obtain the vitamin from circulation via transport across cell membranes. We have recently characterized the cellular and molecular features of the thiamin-uptake process of PAC and shown the involvement of specific carrier mediation. Using SLC19A2 and SLC19A3 knockout mouse models, we have also shown that both thiamin transporters 1 and 2 (THTR-1 and THTR-2) (products of the SLC19A2 and SLC19A3 genes, respectively) are involved in the vitamin-uptake process (22). We showed that chronic alcohol exposure leads to a significant inhibition in thiamin uptake (25) and, on the basis of preliminary in vitro investigations with rat-derived pancreatic acinar AR42J cells, this effect appeared to be mediated at the level of transcription of the SLC19A2 and SLC19A3 genes (25). Our aims in this study were to confirm the involvement of transcriptional mechanisms in mediating the alcohol inhibitory effects on the SLC19A2 and SLC19A3 promoters in an in vivo setting and to delineate the molecular mechanisms involved in this inhibition. For this, we used transgenic mice carrying the human SLC19A2 and SLC19A3 promoters to show that chronic alcohol feeding leads to inhibition in activity of the SLC19A2 and SLC19A3 promoters in PAC. Using the mouse-derived pancreatic acinar 266-6 cells, we also show that the chronic alcohol inhibitory effect is mediated via the minimal regions of the SLC19A2 and SLC19A3 promoters (the regions that confer significant basal promoter activity) and that it involves specific cis-regulatory elements [stimulating protein 1/gut-enriched Kruppel-like factor (SP1/GKLF) and SP1-GG-box in the case of SLC19A2, and SP1/GC in the case of SLC19A3]. Chronic alcohol exposure of PAC leads to a significant reduction in the level of expression of the SP1 transcription factor, and correction of this effect via expression of SP1 prevented the inhibitory effect of chronic alcohol exposure on the activity of the SLC19A2 and SLC19A3 promoters as well as on levels of expression of THTR-1 and -2 and thiamin uptake.
MATERIALS AND METHODS
Materials.
[3H]-Thiamin (specific activity 20 Ci/mmol; radiochemical purity >99%) was obtained from American Radiolabeled Chemicals (St. Louis, MO). Nitrocellulose filters (0.45-μm pore size) were from Millipore (Fisher Scientific, Rockford, IL). Unlabeled thiamin and other chemicals including molecular biology reagents were from commercial vendors and of analytical grade. Oligonucleotide primers used in this study were synthesized by Sigma Genosys (Sigma, Woodland, TX).
Chronic alcohol exposure of mice and thiamin uptake by freshly isolated PAC.
Transgenic mice carrying the full-length human SLC19A2 (−2,250 to −36) and SLC19A3 (−1,957 to +59) promoters fused to the firefly luciferase reporter gene [previously generated and characterized by this laboratory (11, 18)] were used (approval for their use was obtained from the Institutional Animal Care Use Committee of Long Beach VA Medical Center). Mice were fed the Lieber-DeCarli ethanol liquid diet (Dyets, Bethlehem, PA) [ethanol provided 25% of total ingested calories and was introduced gradually; calories were increased by 5% every day until we achieved 25% (8)] for 4 wk as described by us recently (25). Control littermates (sex-matched that had similar basal firefly luciferase mRNA expression) were pair fed with the same liquid diet but without ethanol (maltose-dextrin replaced ethanol isocalorically). After 4 wk, mice were euthanized; the pancreas was removed, and primary PAC were isolated from mice by a collagenase type-V (Sigma, St. Louis, MO) digestion method as described previously (24). Freshly isolated PAC were used for uptake analysis, with a portion stored at −80°C for protein, mRNA, and firefly luciferase analysis. Cells were suspended in Krebs-Ringer (K-R) buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5.0 glucose, 5.0 glutamine, 10 HEPES, and 10 MES; pH 7.4), and either [3H]-labeled or -unlabeled thiamin was added to initiate uptake. The uptake reaction was terminated by adding 1 ml ice-cold K-R buffer, and the cell suspension was placed on nitrocellulose filters under negative pressure by use of a vacuum manifold (Hoefer Scientific Instruments, Holliston, MA), washed with 5 ml of ice-cold K-R buffer, and dissolved in scintillation fluid. The radioactivity was measured using a scintillation counter (LS6500; Beckman Coulter, Brea, CA), and digested samples were used for determining protein concentration (Dc protein assay kit; Bio-Rad, Hercules, CA).
Culturing of 266-6 cells and chronic exposure to alcohol.
The mouse-derived pancreatic acinar 266-6 cells were obtained from American Type Tissue Collection (ATCC, Rockville, MD). Cells were used between passages 2 and 20 and were cultured in DMEM growth medium containing 10% FBS and an antibiotic cocktail. Cells were chronically (96 h) exposed to alcohol [50 mM; a concentration that mimics the level of alcohol found in blood of chronic alcoholics (4)] in DMEM growth medium containing 10% FBS as described before (13, 25). After 96 h, thiamin uptake was measured at 37°C in cells suspended in K-R buffer as described before (10). Labeled (and unlabeled) thiamin was added to the incubation medium at the onset of incubation, and uptake was examined during the initial linear period of uptake (5 min). The reaction was terminated by the addition of 2 ml of ice-cold K-R buffer, which was immediately removed by aspiration. After the cells were rinsed two times with ice-cold K-R buffer, they were digested with 1 ml of 1 N NaOH and then neutralized with 10 N HCl. Counting for radioactivity and protein determination was done as described above.
Western blot analysis.
Whole cell lysates prepared from mice primary PAC and 266-6 cells chronically fed/exposed to alcohol and their respective controls were used for Western blot analysis as described previously (10). The cells were suspended in 200 μl of RIPA buffer (Sigma) according to manufacturer's protocol and supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). Pancreatic acinar cell proteins (60 μg) were resolved onto premade 4–12% Bis-Tris minigel (Invitrogen, Carlsbad, CA). Proteins were electroblotted onto immobilon polyvinylidene difluoride membrane (Fisher Scientific) after electrophoresis and then blocked with Odyssey blocking solution (LI-COR Bioscience, Lincoln, NE) followed by overnight incubation with primary antibody of either mice THTR-1-, THTR-2-specific (1:200 dilution) polyclonal goat antibodies, or SP1 rabbit polyclonal antibody along with β-actin (1:3,000 dilution) monoclonal antibody. The THTR-1, THTR-2, SP1, and β-actin immunoreactive bands were detected by using donkey anti-goat IRDye-800 for THTR-1 and THTR-2, goat anti-rabbit IRDye 800 for SP1, and goat anti-mouse IRDye-680 for β-actin (LI-COR Bioscience) secondary antibodies (1:30,000 dilution). Signals were detected and quantitated with the Odyssey infrared imaging system (LI-COR Bioscience) accompanied with LI-COR software for quantification and normalized to β-actin as an internal control.
Real-time PCR analysis.
Total RNA (5 μg) isolated from primary acinar cells and 266-6 cells were treated with DNase I (Invitrogen) and subjected to cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad). The coding region of mice THTR-1, THTR-2, SP1, and acidic ribosomal phosphoprotein (ARPO) were PCR amplified using gene-specific primers (Table 1) for quantitative PCR study. Quantitative PCR conditions were the same as described previously (10). The data were normalized to ARPO and then quantified by a relative relationship method (9).
Table 1.
Primers used for amplifying coding region of the respective genes by quantitative PCR
| Gene Name | Forward and Reverse Primers (5′-3′) | Accession No. |
|---|---|---|
| Quantitative PCR Primers | ||
| mTHTR-1 | GTTCCTCACGCCCTACCTTC; GCATGAACCACGTCACAATC | NM_054087.3 |
| mTHTR-2 | TCATGCAAACAGCTGAGTTCT; ACTCCGACAGTAGCTGCTCA | NM_030556.2 |
| mARPO | GCTGAACATCTCCCCCTTCTC; ATATCCTCATCTGATTCCTCC | NM_007475.5 |
| mSP1 | TATGTTGTGGCTGCTACC; TGTGGGATTACTTGATACTGAA | NM_013672.2 |
m, mouse; THTR, thiamin transporter; ARPO, acidic ribosomal phosphoprotein; SP1, stimulating protein 1.
Transfection and reporter gene assay.
The SLC19A2 and SLC19A3 full-length, minimal, and mutated minimal promoter-luciferase reporter constructs utilized in this study were generated previously (11, 18). 266-6 Cells at <80% confluency were cotransfected in 12-well plates with 2 μg of each test construct and 100 ng of Renilla luciferase-thymidine kinase (pRL-TK) control plasmid (Promega, Madison, WI). For cotransfection with pPAC-Sp1/pPAC empty vector (pPAC-0), 1 μg of respective plasmids, 1 μg SLC19A2 and SLC19A3 minimal promoter, and 100 ng of the Renilla transfection control plasmid were used. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. On the second day posttransfection, the cells transfected with SLC19A2 and SLC19A3 constructs were exposed to alcohol (50 mM) for 96 h as described above. At the end of 96 h of alcohol exposure, luciferase activity was determined by using the Dual Luciferase Assay system (Promega) per the manufacturer's instructions.
Statistical analysis.
Uptake data with mouse primary pancreatic acinar and 266-6 cells presented in this study are means ± SE of at least three separate experiments from different mice and are expressed as percentages relative to simultaneously performed controls. Carrier-mediated thiamin uptake was determined by subtracting uptake by simple diffusion from total uptake. Protein, RNA, and luciferase activity determinations were performed from at least three sets of samples prepared at different occasions. The Student's t-test was used for statistical analysis, and P < 0.05 was considered statistically significant.
RESULTS
Effect of chronic alcohol feeding of SLC19A2 and SLC19A3 transgenic mice on promoter activity.
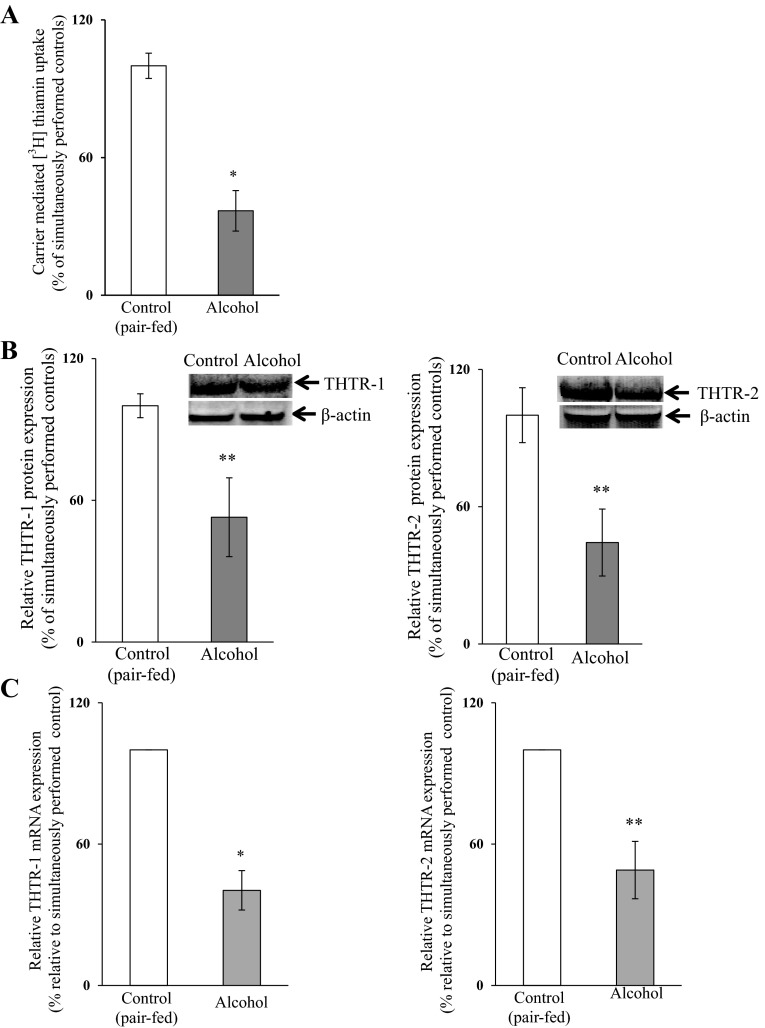
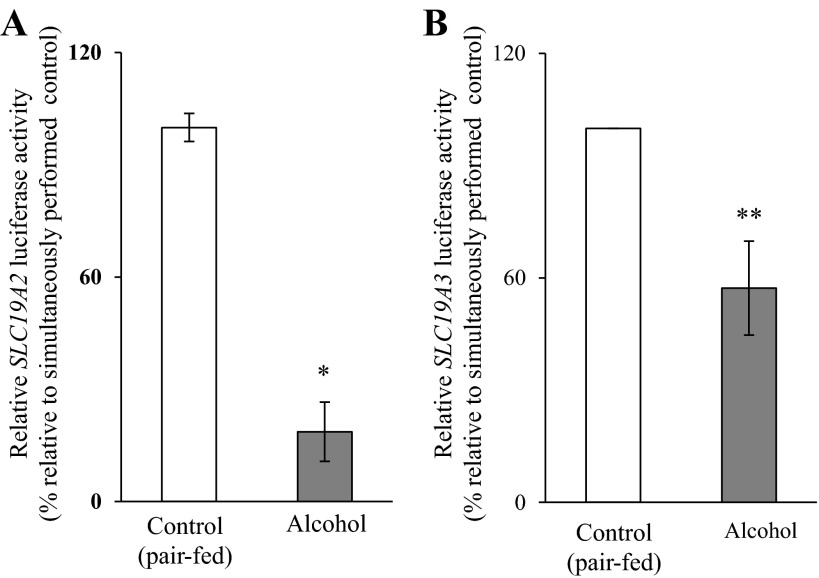
As stated earlier, one of our aims in this study was to confirm the involvement of transcriptional mechanisms in mediating the chronic alcohol inhibitory effects on the activity of the SLC19A2 and SLC19A3 promoters in an in vivo setting. We did this using transgenic mice carrying SLC19A2 and SLC19A3 promoters fused to luciferase reporter gene [that we have generated and characterized previously (11, 18)] that were fed the Lieber-DeCarli ethanol liquid diet (ethanol provided 25% of total ingested calories) for 4 wk; control transgenic mice were pair fed the same liquid diet but without alcohol (maltose-dextrin substituted for ethanol isocalorically). First, we confirmed that chronic alcohol feeding of mice inhibits thiamin uptake by freshly isolated PAC. The results indeed showed a significant (P < 0.01) inhibition in the initial rate of thiamin uptake by PAC in alcohol-fed mice compared with those from the pair-fed controls (Fig. 1A). This inhibition was associated with a significant decrease in the level of expression of mouse THTR-1 and -2 proteins (P < 0.05 for both) and mRNA (P < 0.01 for THTR-1 and < 0.05 for THTR-2) in PAC cells of the alcohol-fed animals compared with the pair-fed controls (Fig. 1, B and C, respectively). The effect of chronic alcohol feeding on the activity of the SLC19A2 and SLC19A3 promoters expressed in PAC cells of transgenic mice was then examined with the results showing a significantly (P < 0.01 and 0.05, respectively) lower activity of both promoters in PAC of the alcohol-fed transgenic mice compared with the activity in pair-fed control transgenic animals (Fig. 2). These results show that the effect of chronic alcohol exposure on thiamin uptake is transcriptionally mediated in the in vivo setting, as we observed previously in vitro (25).
Fig. 1.
Effect of chronic alcohol feeding of mice on carrier-mediated [3H]-thiamin uptake (A) and the expression of thiamin transporters 1 and 2 (THTR-1 and THTR-2) proteins (B) and mRNA (C) of pancreatic acinar cells (PAC). Primary PAC were isolated from mice fed alcohol (25% of total calories) for 4 wk and from their pair-fed transgenic mice controls. Carrier-mediated thiamin uptake was determined. Level of protein expression was determined by Western blotting. Levels of mRNA were determined by quantitative PCR. Data are means ± SE of at least 3 independent experiments from multiple sets of mice. *P < 0.01; **P < 0.05.
Fig. 2.
Effect of chronic alcohol feeding on activity of the human SLC19A2 and SLC19A3 promoters in transgenic mice carrying these promoters fused to luciferase. Luciferase activity of SLC19A2 (A) and SLC19A3 (B) promoters was determined and is presented as percentages relative to pair-fed transgenic mice controls. Data are means ± SE of at least 3 independent experiments from multiple sets of mice. *P < 0.01, **P < 0.05.
Molecular mechanisms involved in the transcriptional effect of chronic alcohol exposure on the activity of the SLC19A2 and SLC19A3 promoters in PAC.
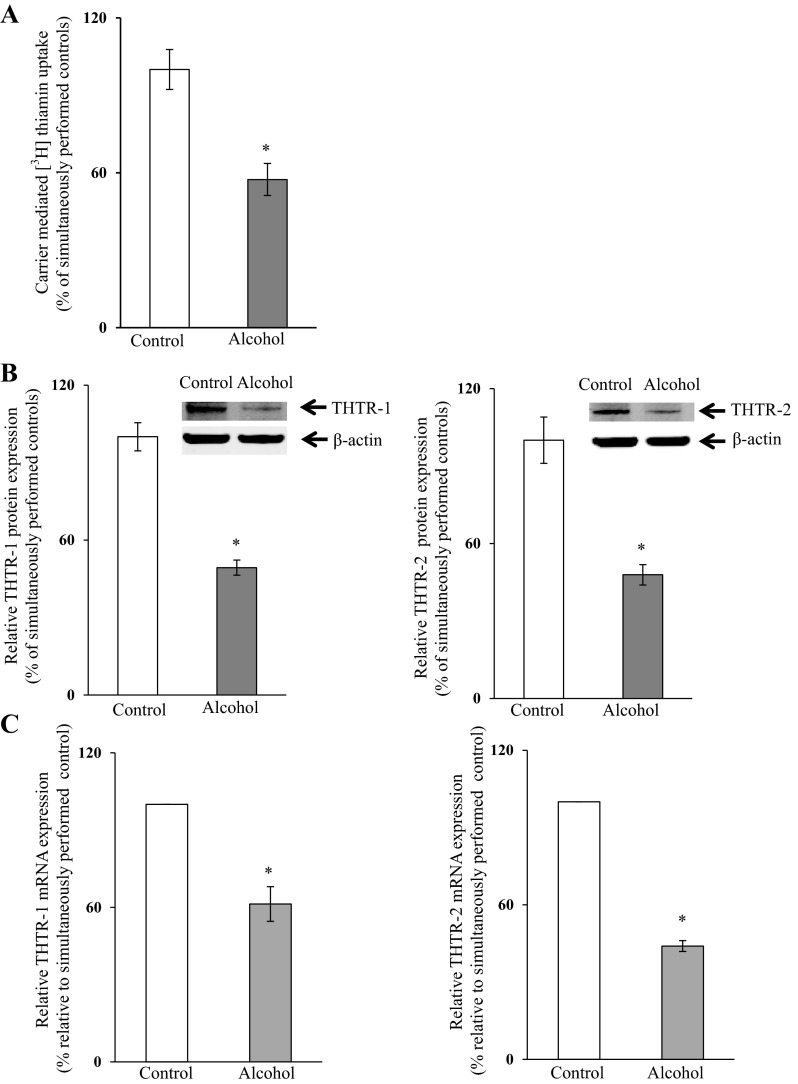
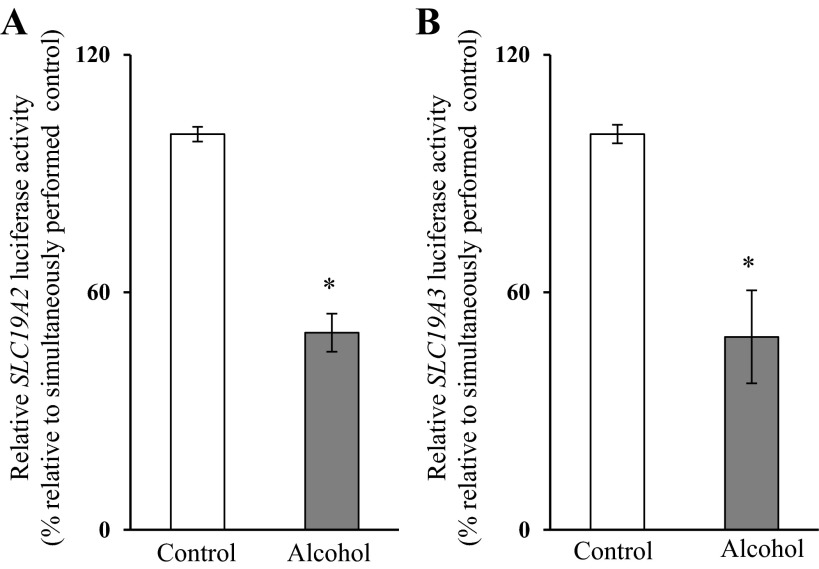
To understand the molecular mechanism(s) through which chronic alcohol exposure affects the transcriptional activity of the SLC19A2 and SLC19A3 promoters in PAC, we used the mouse-derived pancreatic acinar 266-6 cells as a model. We first established the suitability of these cells in such investigations by showing that chronic alcohol exposure [50 mM, 96 h (13, 25)] leads to a significant inhibition (P < 0.01) in carrier-mediated thiamin uptake (15 nM) (Fig. 3A) and in the level of expression of THTR-1 and -2 proteins (P < 0.01 for both) and mRNA (P < 0.01 for both) (Fig. 3, B and C, respectively). Chronic alcohol exposure also led to a significantly lower activity of the full-length SLC19A2 and SLC19A3 promoters transfected in these cells (P < 0.01 for both) (Fig. 4).
Fig. 3.
Effect of chronic alcohol exposure of pancreatic acinar 266-6 cells on carrier-mediated [3H]-thiamin uptake (A) and level of expression of THTR-1 and THTR-2 proteins (B) and mRNA (C). Cells were exposed chronically to alcohol (50 mM, 96 h), and carrier-mediated thiamin uptake was determined. Level of protein expression was determined by Western blotting. Levels of mRNA were determined by quantitative PCR. Data represent the means ± SE of at least 3 separate uptake determinations. *P < 0.01.
Fig. 4.
Effect of chronic exposure of pancreatic acinar 266-6 cells to alcohol on the activity of SLC19A2 and SLC19A3 promoters. 266-6 cells were transfected with full-length SLC19A2 (A) and SLC19A3 (B) promoters in pGL3-Basic and were exposed to 50 mM alcohol for 96 h followed by determination of luciferase activity. Data were normalized relative to Renilla luciferase activity and presented as means ± SE of at least 3 independent experiments. *P < 0.01.
Involvement of the minimal SLC19A2 and SLC19A3 promoters and specific cis-regulatory elements in mediating the chronic alcohol effect.
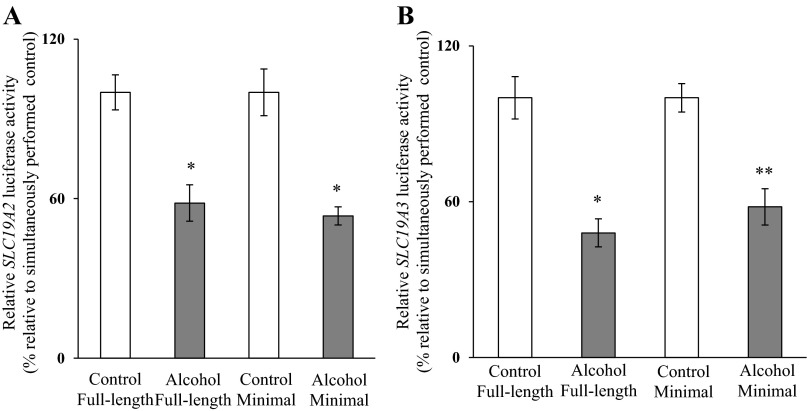
Using 266-6 cells, we examined whether the minimal regions of the SLC19A2 and SLC19A3 promoters were involved in mediating the chronic alcohol effect on promoter activity. The results (Fig. 5) showed that chronic alcohol exposure caused a similar degree of inhibition in the activity of the minimal SLC19A2 and SLC19A3 promoters as it did with the full-length promoters. These findings suggest that the minimal regions of these promoters are involved in mediating the chronic alcohol effect.
Fig. 5.
Effect of chronic alcohol exposure on activity of the full-length compared with minimal SLC19A2 and SLC19A3 promoters. Luciferase assays were performed on 266-6 cells transiently transfected with full-length or minimal SLC19A2 (A) and SLC19A3 (B) promoters in pGL3-Basic and exposed chronically to alcohol. Data are reported as relative firefly luciferase activity normalized to Renilla luciferase activity and represent means ± SE of at least 3 independent experiments. *P < 0.01, **P < 0.05.
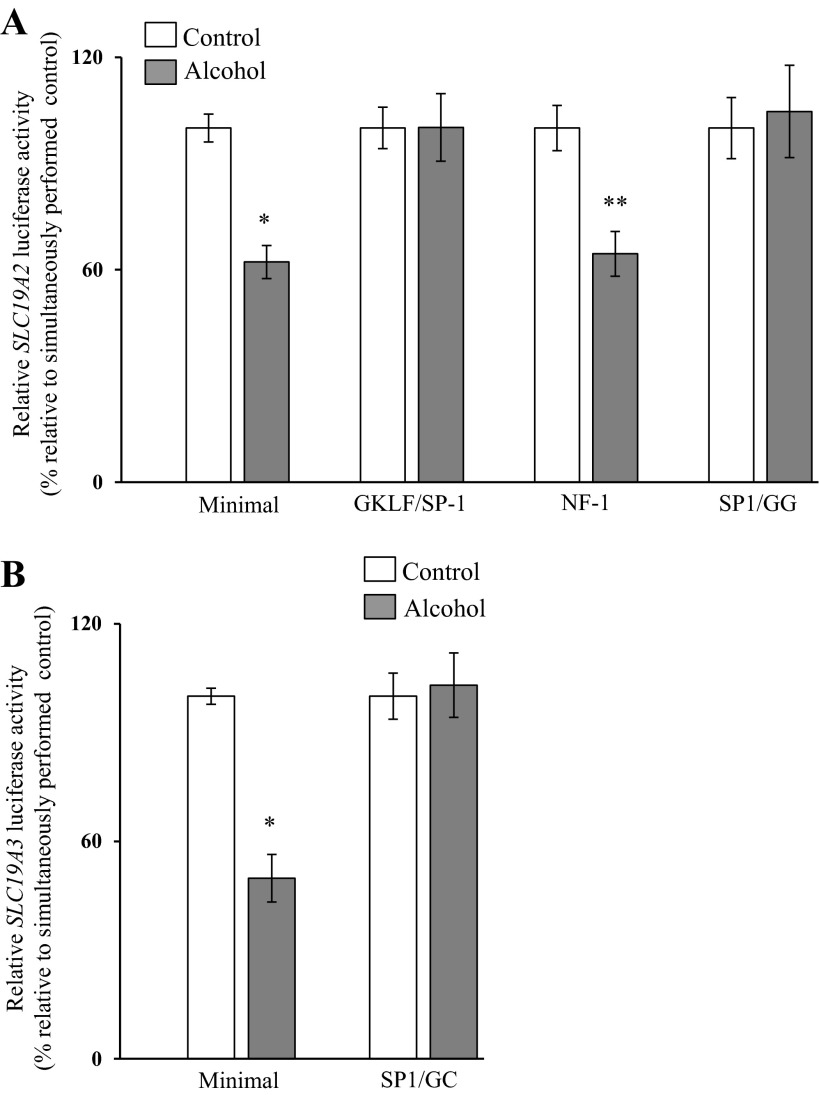
Previous studies from our laboratory have shown that the cis-regulatory elements GKLF/SP1, nuclear factor 1 (NF1), and SP1/GG-box in the SLC19A2 minimal promoter (18) and the SP1/GC-box in the SLC19A3 minimal promoter are important for the function of these promoters (11). Thus we also investigated whether the chronic alcohol exposure effect is mediated through these sites. For this, we examined the effect of mutating these sites (individually) on the ability of chronic alcohol exposure to inhibit the activity of the SLC19A2 and SLC19A3 minimal promoters. The results showed that mutating the GKLF/SP1 and SP1/GG-box sites (but not the NF1 site) in the SLC19A2 minimal promoter leads to a complete disappearance of the alcohol inhibitory effect on activity of this promoter (Fig. 6A). Similarly, mutating the SP1/GC-box in the SLC19A2 minimal promoter led to a complete disappearance of the inhibitory effect of alcohol on the activity of the SLC19A3 promoter (Fig. 6B).
Fig. 6.
Effect of mutating specific cis-regulatory elements in the SLC19A2 and SLC19A3 minimal promoters on the inhibitory effect of chronic alcohol exposure on promoter activity. Wild-type and mutated minimal SLC19A2 (A) and SLC19A3 (B) promoters in pGL3 were transiently transfected into 266-6 cells, and luciferase activity was determined. Data are reported as relative firefly luciferase activity normalized to Renilla luciferase activity and represent means ± SE of at least 3 independent experiments. *P < 0.01 **P < 0.05. GKLF, gut-enriched Kruppel-like factor; NF1, nuclear factor 1; SP1, stimulating protein 1.
Effect of chronic alcohol exposure on expression of the transcription element SP1.
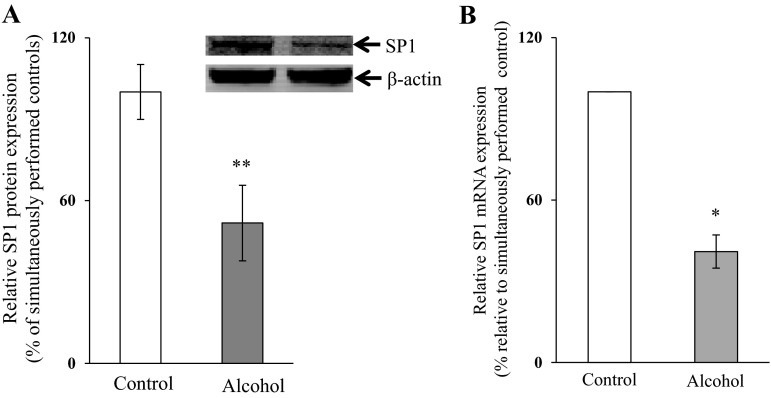
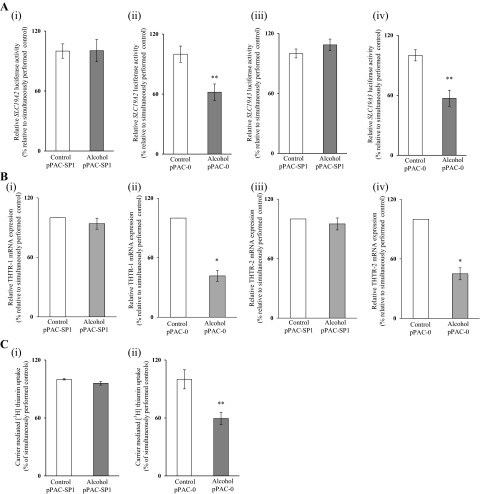
Because the cis-regulatory elements in the SLC19A2 and SLC19A3 minimal promoters that appear to mediate the chronic alcohol effect are potential SP1 binding sites (11, 18), we examined the effect of chronic exposure of 266-6 cells to alcohol [50 mM, for 96 h (13, 25)] on the level of SP1 expression. The results showed that chronic alcohol exposure of 266-6 cells leads to a significant decrease in the level of expression of SP1 at both the protein (P < 0.05) (Fig. 7A) and mRNA levels (P < 0.01) (Fig. 7B). The same was found in vivo in mice fed alcohol chronically (for 4 wk) in that the level of expression of SP1 mRNA in the pancreas was significantly lower (P < 0.01; 55 ± 10%) than that of pair-fed controls. To confirm the specific contribution of SP1 toward the effect of chronic alcohol exposure on the activity of the SLC19A2 and SLC19A3 promoters, we transfected 266-6 cells with pPAC-SP1 vector (which led to a 50% increase in SP1 protein expression; data not shown) then chronically exposed the cells to alcohol (50 mM, for 96 h). We examined the effect of chronic alcohol exposure of SP1-expressing pancreatic acinar 266-6 cells on the activity of the SLC19A2 and SLC19A3 promoters. The results showed no significant reduction in the activity of either of the SLC19A2 or SLC19A3 promoters in cells expressing SP1 compared with pPAC-0 controls (Fig. 8A). We also examined the level of expression of THTR-1 and -2 mRNA and the initial rate of thiamin (15 nM) uptake. The results showed that, in cells transfected with pPAC-SP1 and chronically exposed to alcohol, the level of expression of THTR-1 and -2 mRNA was not altered (Fig. 8B), and no inhibition in carrier-mediated thiamin uptake was seen compared with the inhibition seen in pPAC-0 control cells (Fig. 8C).
Fig. 7.
Effect of chronic exposure of pancreatic acinar 266-6 cells to alcohol on the level of expression of SP1 protein (A) and mRNA (B). Western blotting was performed on whole cell proteins (60 μg) isolated from alcohol-exposed and control 266-6 cells. Blot was incubated with rabbit polyclonal SP1 antibody. Q-PCR was performed using mice SP1 gene-specific primers and cDNA prepared from alcohol-exposed as well as control 266-6 cells mRNA. Data are means ± SE of at least 3 independent experiments and were normalized relative to ARPO and calculated by the relative relationship method. *P < 0.01, **P < 0.05.
Fig. 8.
Effect of chronic alcohol exposure of pancreatic acinar 266-6 cells transfected with pPAC-SP1 on activity of SLC19A2 and SLC19A3 promoters (A), level of expression of THTR-1 and THTR-2 mRNA (B), and carrier-mediated [3H] thiamin uptake (C). Cells cotransfected with pPAC-SP1 or pPAC-0 along with either of the minimal SLC19A2 (A, i and ii) and SLC19A3 (A, iii and iv) promoters in pGL3-Basic were exposed to alcohol (50 mM, 96 h) followed by determination of luciferase activity as described earlier. Levels of mRNA were determined by quantitative PCR using mouse THTR-1 (B, i and ii) and THTR-2 (B, iii and iv) gene-specific primers. Carrier-mediated thiamin uptake (C, i and ii) was determined. Data are normalized and presented as means ± SE of at least 3 independent experiments. *P < 0.01, **P < 0.05.
DISCUSSION
Thiamin assumes important roles in PAC and is transported into these cells via a specific carrier-mediated process that involves two plasma membrane transporters, THTR-1 and THTR-2 (22). Chronic alcohol use is associated with systemic thiamin deficiency that is believed to be, at least in part, mediated via inhibition in intestinal absorption and renal reabsorption of the vitamin (23, 24). Chronic alcohol use has also been shown to interfere with normal intracellular metabolism of thiamin that is tissue specific in nature (19).
In studies using rats and rat-derived PAC, AR42J, we have previously shown that chronic alcohol feeding/exposure leads to a significant inhibition in carrier-mediated thiamin uptake and in the expression of THTR-1 and -2. We also obtained preliminary evidence from in vitro studies that this effect may, at least in part, be mediated via transcriptional mechanism(s) affecting the SLC19A2 and SLC19A3 genes (25). Our aim in this study was to confirm that the effect of chronic alcohol exposure of the PAC thiamin uptake process is transcriptionally mediated in an in vivo setting of alcohol exposure and to delineate the molecular mechanism(s) involved. We used transgenic mice carrying the human SLC19A2 and SLC19A3 promoters (as an in vivo model of alcohol exposure) and the mouse-derived pancreatic acinar 266-6 cells in these investigations. It was important to first show that chronic alcohol feeding/exposure also inhibits the physiological and molecular parameters of thiamin uptake by mouse primary PAC, which indeed was found to be the case. Chronic alcohol feeding of the transgenic mice and chronic exposure of 266-6 cells to alcohol led to a significant inhibition in carrier-mediated thiamin uptake and in the level of expression of THTR-1 and -2. We then examined the effect of chronic alcohol feeding of transgenic mice on the activity of the SLC19A2 and SLC19A3 promoters in PAC and observed a significant inhibition in promoter activity. These findings provide physiological confirmation/relevance to our previous in vitro findings with regards to the involvement of transcriptional mechanism(s) in mediating the effect of chronic alcohol exposure on thiamin uptake by PAC (25).
To understand the molecular nature of the transcriptional mechanism(s) involved, we first investigated whether the effect of chronic alcohol exposure is mediated via the minimal SLC19A2 and SLC19A3 promoters. Results showed that the effect of chronic alcohol exposure could be fully accounted for by an action on the minimal SLC19A2 and SLC19A3 promoters. This conclusion is based on the finding of a similar degree of inhibition by chronic alcohol exposure on promoter activity regardless of whether the full-length or the minimal promoters were used. With the knowledge that these minimal SLC19A2 and SLC19A3 promoters contain specific cis elements that are important for their function, namely GKLF/SP1, NF1, and SP1/GG-box binding sites for the former minimal promoter (18) and SP1(SP-3)/GC-box binding site for the latter minimal promoter (11), we investigated the possible involvement of these sites in mediating the chronic alcohol inhibitory effects on promoter activity. This was done by examining the effect of mutations in these sites on the inhibitory effects of chronic alcohol exposure on activity of the SLC19A2 and SLC19A3 minimal promoters. The results showed that the inhibitory effect of chronic alcohol exposure on the activity of the SLC19A2 promoter disappeared when the SP1/GKLF and SP1-GG-box sites were mutated, suggesting involvement of these sites in mediating the inhibitory effect of alcohol. Similarly mutating the SP1(SP-3)/GC-box binding site of the SLC19A3 minimal promoter led to disappearance of the inhibitory effect of chronic alcohol exposure on activity of the SLC19A3 promoter, again suggesting involvement of this site in mediating the chronic alcohol inhibitory effect on activity of this promoter.
We have previously shown that the nuclear factor SP1 interacts with the GKLF/SP1 and SP1/GG-box cis-regulatory elements in the SLC19A2 minimal promoter (18) and with the SP1(SP-3)/GC-box cis-regulatory element of the SLC19A3 minimal promoter (11). Thus we also examined the effect of chronic alcohol exposure of pancreatic acinar 266-6 cells on the level of expression of SP1 and observed a significant reduction in the expression of this nuclear factor in cells chronically exposed to alcohol compared with control. This is not the first time that chronic alcohol feeding has been shown to affect the level of SP1 (20). Correcting the observed reduction in the level of expression of SP1 in alcohol-exposed 266-6 cells via expression of the transcription factor led to a significant reversal in the effect of chronic alcohol exposure on the activity of the SLC19A2 and SLC19A3 minimal promoters. In addition, expression of SP1 also led to a reversal in the inhibition of chronic alcohol exposure on the level of THTR-1 and -2 mRNA and functionality of the respective transporters.
The ubiquitous transcription factor SP1 affects the expression of a variety of genes. However, because chronic alcohol exposure affects (both up and down) the expression of a variety of genes, including those that encode activators and repressors (7), it would be difficult to draw a generalized conclusion about the role of SP1 in relation to the effect of alcohol on expression of other genes. Our focus on the role of SP1 in the context of the two thiamin transport genes (i.e., SLC19A2 and SLC19A3) is because we do know from previous investigations that this nuclear factor is involved in the regulation of these two genes (11, 18).
Our findings in the present study that chronic alcohol exposure leads to inhibition in the transport of thiamin into PAC may have relevance to the known deleterious effect of chronic alcohol use on the pancreas (6, 7, 12). This is because reducing the intracellular level of an essential micronutrient like thiamin [which is involved in cellular energy metabolism, reduction of oxidative stress, and normal function/integrity of the mitochondria (1–3, 14, 26)] may lead to acute energy failure, oxidative stress, and impairment in mitochondrial function (2, 3, 5, 14, 26). This may weaken the pancreas and lower its defense mechanisms against subsequent external/internal insults.
In summary, our findings demonstrate that transcriptional mechanisms affecting the SLC19A2 and SLC19A3 minimal promoters mediate the inhibitory effect of chronic alcohol feeding/exposure on thiamin uptake by PAC and that the effect may involve the nuclear transcription factor SP1.
GRANTS
This study was supported by grants from the National Institutes of Health (AA018071 and DK56061) and the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.S., V.S.S., and H.M.S. conception and design of research; P.S. and V.S.S. performed experiments; P.S. and V.S.S. analyzed data; P.S. and V.S.S. interpreted results of experiments; P.S. and V.S.S. prepared figures; P.S. and V.S.S. drafted manuscript; P.S., V.S.S., and H.M.S. edited and revised manuscript; P.S., V.S.S., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Berdanier CD. Advanced Nutrition Micronutrients. Boca Raton, FL: CRC, 1998, pp. 183–219 [Google Scholar]
- 2.Bettendorff L, Goessens G, Sluse F, Wins P, Bureau M, Laschet J, Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J Neurochem 64: 2013–2021, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 58: 946–958, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Devenyi P, Kapur BM, Roy JH. High-density lipoprotein response to alcohol consumption and abstinence as an indicator of liver function in alcoholic patients. Can Med Assoc J 130: 1445–1447, 1984 [PMC free article] [PubMed] [Google Scholar]
- 5.Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P, Grisar T, Bettendorff L. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One 5: e13616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gukovsky I, Pandol SJ, Gukovskaya AS. Organellar dysfunction in the pathogenesis of pancreatitis. Antioxid Redox Signal 15: 2699–2710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubisch CH, Gukovsky I, Lugea A, Pandol SJ, Kuick R, Misek DE, Hanash SM, Logsdon CD. Long-term ethanol consumption alters pancreatic gene expression in rats: a possible connection to pancreatic injury. Pancreas 33: 68–76, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM. Pancreatic β-cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am J Physiol Gastrointest Liver Physiol 297: G197–G206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabokina SM, Said HM. Characterization of the 5′-regulatory region of the human thiamin transporter SLC19A3: in vitro and in vivo studies. Am J Physiol Gastrointest Liver Physiol 287: G822–G829, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Pandol SJ, Lugea A, Mareninova OA, Smoot D, Gorelick FS, Gukovskaya AS, Gukovsky I. Investigating the pathobiology of alcoholic pancreatitis. Alcohol Clin Exp Res 35: 830–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pochareddy S, Edenberg HJ. Chronic alcohol exposure alters gene expression in HepG2 cells. Alcohol Clin Exp Res 36: 1021–1033, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Portari GV, Marchini JS, Vannucchi H, Jordao AA. Antioxidant effect of thiamine on acutely alcoholized rats and lack of efficacy using thiamine or glucose to reduce blood alcohol content. Basic Clin Pharmacol Toxicol 103: 482–486, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Prasannan KG, Sundaresan R, Venkatesan D. Thiamine deficiency and protein secretion by pancreatic slices in vitro. Experientia 33: 169–170, 1977 [DOI] [PubMed] [Google Scholar]
- 16.Rathanaswami P, Pourany A, Sundaresan R. Effects of thiamine deficiency on the secretion of insulin and the metabolism of glucose in isolated rat pancreatic islets. Biochem Int 25: 577–583, 1991 [PubMed] [Google Scholar]
- 17.Rathanaswami P, Sundaresan R. Effects of thiamine deficiency on the biosynthesis of insulin in rats. Biochem Int 24: 1057–1062, 1991 [PubMed] [Google Scholar]
- 18.Reidling JC, Said HM. In vitro and in vivo characterization of the minimal promoter region of the human thiamin transporter SLC19A2. Am J Physiol Cell Physiol 285: C633–C641, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Rindi G, Reggiani C, Patrini C, Laforenza U. Effect of ethanol administration on the in vivo kinetics of thiamine phosphorylation and dephosphorylation in different organs. I. Chronic effects. Alcohol Alcohol 26: 285–301, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Rulten SL, Ripley TL, Hunt CL, Stephens DN, Mayne LV. Sp1 and NFkappaB pathways are regulated in brain in response to acute and chronic ethanol. Genes Brain Behav 5: 257–273, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Singh M. Effect of thiamin deficiency on pancreatic acinar cell function. Am J Clin Nutr 36: 500–504, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Subramanian VS, Subramanya SB, Said HM. Relative contribution of THTR-1 and THTR-2 in thiamin uptake by pancreatic acinar cells: studies utilizing SLC19A2 and SLC19A3 knockout mouse models. Am J Physiol Gastrointest Liver Physiol 302: G572–G578, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol 299: F28–F34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: effect of chronic alcohol feeding/exposure. Am J Physiol Gastrointest Liver Physiol 301: G896–G904, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanphaichirt V. Modern Nutrition in Health and Disease. New York, NY: Lea and Febiger, 1994, pp. 359–375 [Google Scholar]