Abstract
Intracellular calcium (Ca2+) plays pivotal roles in distinct cellular functions through global and local signaling in various subcellular compartments, and subcellular Ca2+ signal is the key factor for independent regulation of different cellular functions. In vascular smooth muscle cells, subsarcolemmal Ca2+ is an important regulator of excitation-contraction coupling, and nucleoplasmic Ca2+ is crucial for excitation-transcription coupling. However, information on Ca2+ signals in these subcellular compartments is limited. To study the regulation of the subcellular Ca2+ signals, genetically encoded Ca2+ indicators (cameleon), D3cpv, targeting the plasma membrane (PM), cytoplasm, and nucleoplasm were transfected into rat pulmonary arterial smooth muscle cells (PASMCs) and Ca2+ signals were monitored using laser scanning confocal microscopy. In situ calibration showed that the Kd for Ca2+ of D3cpv was comparable in the cytoplasm and nucleoplasm, but it was slightly higher in the PM. Stimulation of digitonin-permeabilized cells with 1,4,5-trisphosphate (IP3) elicited a transient elevation of Ca2+ concentration with similar amplitude and kinetics in the nucleoplasm and cytoplasm. Activation of G protein-coupled receptors by endothelin-1 and angiotensin II preferentially elevated the subsarcolemmal Ca2+ signal with higher amplitude in the PM region than the nucleoplasm and cytoplasm. In contrast, the receptor tyrosine kinase activator, platelet-derived growth factor, elicited Ca2+ signals with similar amplitudes in all three regions, except that the rise-time and decay-time were slightly slower in the PM region. These data clearly revealed compartmentalization of Ca2+ signals in the subsarcolemmal regions and provide the basis for further investigations of differential regulation of subcellular Ca2+ signals in PASMCs.
Keywords: cameleon, FRET, Ca2+ signaling, endothelin, angiotensin
Ca2+ is a ubiquitous second messenger that plays crucial roles in almost every cellular process from egg fertilization, cell proliferation, muscle contraction, transcriptional regulation to apoptosis (7). Specific Ca2+ signals are encoded in amplitude and frequency depending on the activities and dynamic properties of the Ca2+ influx/release channels and transporters. They are decoded locally by specific effectors in various subcellular compartments and organelles (5, 7, 11, 12). The local concentration of Ca2+ ([Ca2+]) in various organelles may differ during cell activation, and subcellular Ca2+ signal is the key factor for independent regulation of different cellular functions. Hence, elucidating local Ca2+ signals in various subcellular compartments is important for the understanding of the physiological regulation and pathophysiological dysregulation of specific cellular processes.
In vascular smooth muscle cells (VSMCs), subsarcolemmal Ca2+ signals play pivotal roles in the regulation of vascular functions. Ca2+ influx through plasmalemmal Ca2+-permeating channels generates local Ca2+ microdomains that develop rapidly under plasma membrane (PM) and trigger a wide range of physiological responses, including Ca2+-induced Ca2+ release for excitation-contraction coupling, and activation of Ca2+-dependent proteins/enzymes to modulate channel activities and transcriptional factors (6, 40, 44). Local Ca2+ release events from the peripherally coupled sarcoplasmic reticulum (SR) can also generate Ca2+ signals in the subsarcolemmal space to activate Ca2+-activated K+ channel to generate spontaneous transient outward currents (21, 37) and/or Ca2+-activated Cl− channels to elicit spontaneous transient inward currents to cause vasorelaxation and vasoconstriction, respectively (16, 55, 56). Besides subsarcolemmal Ca2+ signals, nucleoplasmic Ca2+ is known to play crucial roles in gene transcription and cell proliferation (2, 41). It is regulated by the propagation of cytoplasmic Ca2+ into the nucleoplasm and by local Ca2+ release via 1,4,5-trisphosphate (IP3) receptors (IP3Rs) and ryanodine receptors of perinuclear Ca2+ stores, nuclear envelope, and nucleoplasmic reticulum (13, 20, 30, 31, 33, 57). However, information regarding subsarcolemmal and nucleoplasmic Ca2+ signals in VSMCs is limited, and direct comparison of Ca2+ signals between the subcellular compartments during agonist stimulation is unavailable.
Although small molecule fluorescent dyes like rhod-2 and Fluo-5N have been used successfully to measure Ca2+ signals in mitochondria (23, 53) and sarcoplasmic reticulum (24, 47), respectively, under certain conditions, the study of Ca2+ signaling in other specific intracellular compartments has been limited by technical difficulties. For example, many subcellular organelles in live cells are not readily discernible by fluorescence microscopy, hence the commonly used fluorescent Ca2+ indicators, including Fluo-3, Fluo-4, Fura-2, and Indo-1, are unable to distinguish Ca2+ signals of specific organelles from the neighboring cytoplasmic Ca2+ signals. These limitations can be overcome by using genetically encoded Ca2+ indicators with targeting sequences to express selectively into specific organelles. As our first effort to study local Ca2+ signals in subcellular compartments, we used the second-generation fluorescence resonance energy transfer (FRET)-based Ca2+-sensor (cameleon) D3cpv (38, 39) to generate specific probes targeting the plasma membrane and the nucleus. We verified their subcellular locations and performed in situ calibration of the Ca2+ biosensors in rat pulmonary arterial smooth muscle cells (PASMCs). Simultaneous detection of subsarcolemmal, nucleoplasmic, and cytoplasmic Ca2+ signals was performed by coexpression of the Ca2+ biosensors. We successfully recorded Ca2+ signals in these subcellular compartments activated by IP3, endothelin-1 (ET-1), angiotensin II (ANG II), and platelet-derived growth factor (PDGF) and reveal for the first time the differentially regulated subcellular Ca2+ signals activated by the vasoactive agonists in PASMCs.1
MATERIALS AND METHODS
Generation of targeted and nontargeted constructs.
The nontargeted cameleon (D3cpv) was generated by using 4mtD3cpv (kindly provided by Dr. R. Tsien) (38). The mitochondria targeting sequence (4mt) of 4mtD3cpv was excised by restriction digestion (HindIII). The digested construct was purified and religated to generate D3cpv. Nuclear targeted (3NLS-D3cpv) and membrane targeted (Lyn-D3cpv) cameleons were generated by cloning synthetic oligonucleotides coding for three tandem repeats of the nuclear localization signal from simian virus large T-antigen (3NLS, DPKKKRKVDPKKKRKVDPKKKRKV) (25), and the myristoylation-palmitoylation sequence from lyn kinase (Lyn, MGCIKSKRKDNLNDDGVDMKT) (60), respectively, to the NH2 terminus of D3cpv (Fig. 1A). The oligonucleotide inserts were designed in such a way that the translation initiation codon (ATG) of targeted sequences was surrounded by Kozak consensus sequence, GCCACCATGG, for optimal expression (28). Synthetic oligonucleotides encompassing the Kozak consensus sequence, coding sequence of targets (3NLS and Lyn,) and sticky ends for HindIII site were synthesized and annealed. The annealed inserts were directly ligated into the HindIII-restricted D3cpv and transformed into Escherichia coli. Clones were selected after screening by PCR and restriction digestion, and the inserted sequences were confirmed by automated sequencing.
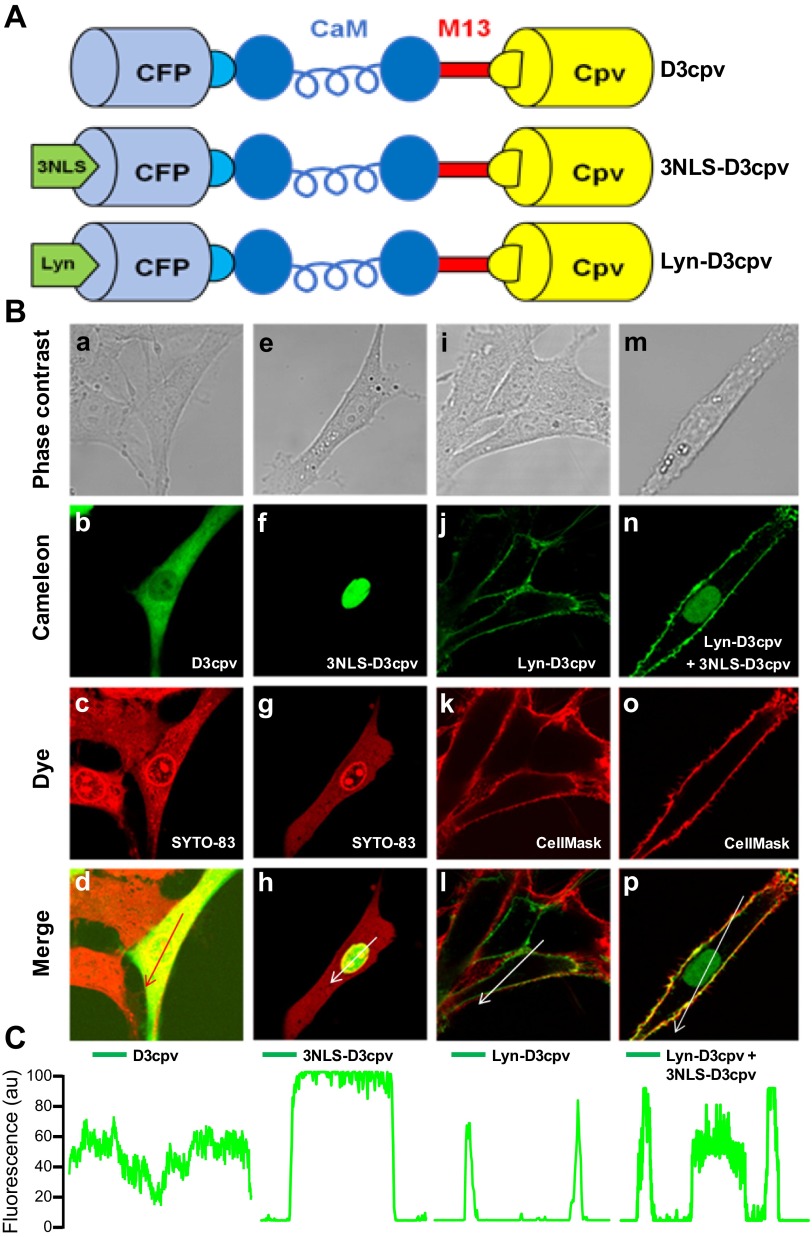
Fig. 1.
Subcellular distribution of D3cpv, 3NLS-D3cpv, and Lyn-D3cpv in transiently transfected rat pulmonary arterial smooth muscle cells (PASMCs). A: schematic structure of the D3cpv, 3NLS-D3cpv, and Lyn-D3cpv cameleon probes. D3cpv containing improved calmodulin (CaM) and M13 sequences (38) (top) was modified by inserting 3NLS (middle) and Lyn (bottom) sequences at the NH2 terminus. CFP, cyan fluorescent protein. B: confocal images of rat PASMCs transfected with D3cpv (a–d), 3NLS-D3cpv (e–h), Lyn-D3cpv (i–l) and cotransfected with 3NLS-D3cpv and Lyn-D3cpv (m–p). Phase contrast images (a, e, i, and m), cameleon cpV fluorescence (b, f, j, and n), SYTO 83 Orange staining (c and g), and CellMask Orange plasma membrane staining (k and o) are shown. Overlay images of b and c (d), f and g (h), j and k (l), and n and o (p) are indicated. C: linear fluorescence profiles of cpV fluorescence distribution in the cells across the arrow. au, Arbitrary units.
Isolation and culture of rat PASMCs.
All experiments were performed under protocols approved by the Johns Hopkins University Animal Care and Use Committee. Rat PASMCs were enzymatically isolated and cultured as described earlier (59). In brief, male Wistar rats (150–250 g) were anesthetized with pentobarbital sodium (130 mg/kg ip). Lungs were removed quickly and immersed in ice-cold HEPES-buffered salt solution (HBSS) containing (in mM) 130 NaCl, 5 KCl, 1.2 MgCl2, 1.5 CaCl2, 10 HEPES, and 10 glucose (pH 7.2, adjusted with NaOH). Second- and third-generation intrapulmonary arteries were isolated from the lungs. The surrounding connective tissues were carefully removed. The arteries were cut opened to expose the endothelial surface, and the endothelium was removed by gentle rubbing with a cotton swab. The tissues were allowed to recover for 30 min in ice-cold HBSS, followed by 20 min in reduced-Ca2+ (20 μM) HBSS at room temperature. The tissues were digested at 37°C for 20 min in 20 μmol-Ca2+ HBSS containing type I collagenase (1,750 U/ml), papain (9.6 U/ml), bovine serum albumin (2 mg/ml), and DTT (1 mM). After digestion was stopped by washing the tissue with nominal Ca2+-free HBSS, PASMCs were mechanically dispersed by gentle trituration with a wide bore pipette tip in Ca2+-free HBSS at room temperature. Cells were then placed on 100-mm petri dishes, allowed to settle for 20 min at room temperature, and cultured overnight in Ham's F-12 medium (with l-glutamine) supplemented with 0.5% FCS, 100 U/ml streptomycin, and 0.1 mg/ml penicillin at 37°C in a humidified atmosphere containing 5% CO2. The Ham's medium was replaced with smooth muscle cell growth basal medium (SmBM, Lonza) supplemented with growth factors, 5% FCS, 100 U/ml streptomycin, and 0.1 mg/ml penicillin. The cells were cultured for 2–3 days before passage. Cells at passages 2-3 were used for the experiments.
Transfection.
Rat PASMCs were transiently transfected with 0.5–1 μg of cameleon plasmids using an Amaxa Nucleofector kit for primary smooth muscle cells (Lonza). The transfected cells were then seeded onto 25-mm glass coverslips in petri dishes containing prewarmed SmBM supplemented with growth factors, FCS, and antibiotics and incubated in a humidified atmosphere containing 5% CO2. Fluorescence experiments were performed 48 h after transfection. For the experiments on agonist-induced Ca2+ response, PASMCs were starved overnight by replacing the complete medium with SmBM without growth factors and FCS.
Confocal microscopy.
Rat PASMCs were transfected with D3cpv, 3NLS-D3cpv, and/or Lyn-D3cpv and cultured for 24–48 h before imaging. They were washed thrice with Hanks' balanced salt solution (Invitrogen/Life Technologies) buffered with 20 mM HEPES and containing 2 g/l d-glucose (HHBSS, pH 7.4). Confocal images were acquired under a Zeiss LSM-510 inverted confocal microscope (Zeiss) with a Zeiss Plan-Neofluor ×40 oil immersion objective (numerical aperture 1.3). To confirm the targeted expression, cameleons were excited by the 458 nm line of a HeNe laser, and the emitted fluorescence signal was captured at both 475–515 nm [cyan fluorescent protein (CFP)] and 530 nm (cpV). The cells were then loaded with the cell-permeant fluorescent nucleic acid stain SYTO 83 Orange or the plasma membrane marker CellMask Orange (Invitrogen) at room temperature. Cells were then washed twice with HHBSS, and images were taken. Both of the dyes were excited with an argon laser line (543 nm), and emission was recorded at 560–615 nm. To eliminate the possibility of signal contamination by cameleon fluorescence, the laser intensity was decreased to the level at which cameleon fluorescence was undetectable. The whole cell staining was done by using excess amounts of SYTO 83 Orange, and the image was taken before the dyes diffused out of the cell or moved completely into the nucleus.
For Ca2+ imaging experiments, cells were rinsed thrice and then maintained in HHBSS for at least 10 min at room temperature. Cells were exposed to different agonists, and images were recorded for different time courses. For IP3-induced Ca2+ signals, cells were permeabilized and maintained in an internal medium before agonist treatment. Cell permeabilization was done as described earlier (14) with some modification. Briefly, cells were exposed to 15 μM digitonin in standard solution containing (in mM) 100 K+ aspartate, 15 KCl, 5 KH2PO4, 0.75 MgCl2, 10 HEPES, and dextran (MW: 40,000) 8%, pH 7.2 with KOH supplemented with 100 μM EGTA, for 30–60 s and washed with the same solution without digitonin. The cells were then maintained in internal solution (standard solution supplemented with MgATP 5 mM, EGTA 1 mM, CaCl2 0.55 mM, phosphocreatine 10 mM, and creatine phosphokinase 5 U/ml, [Ca2+]Free was 300 nM) for 10 min. Internal solution was washed with standard solution (without Ca2+ and ATP but with 100 μM EGTA), and 10 μM IP3 was applied. Images were captured at a rate of 0.3–1 s/frame. Images were analyzed by using ImageJ software (National Institutes of Health, Bethesda, MD) with Ratio Plus plugin.
In situ calibration of D3cpv, 3NLS-D3cpv, and/or Lyn-D3cpv.
For in situ calibration experiments, PASMCs transfected with the cameleons were permeabilized with digitonin (12.5–25 μM, 30–45 s) or Staphylococcus aureus (S. aureus) α-toxin (25 μM, 45 min at 37°C) in an intracellular-like medium containing (in mM): 130 K-gluconate, 10 NaCl, 1 KH2PO4, 1 MgSO4, 20 HEPES (pH 7.0), and EGTA (100 μM). The permeabilizing agent was removed, and the medium was replaced stepwise to achieve [Ca2+]Free ranging from 0 μM to 39 μM by using the calcium calibration kit no. 1 (Invitrogen). Higher [Ca2+] levels were obtained by adding CaCl2 into the medium. The apparent Kd for Ca2+ binding was estimated by fitting the FRET ratio as a function of [Ca2+]Free with the Hill equation. In some cells, [Ca2+] within nucleoplasm and the PM region was calibrated according to Grynkiewicz et al. (17). The minimal and maximal fluorescence ratios (Rmin and Rmax) were determined by adding 3 mM EGTA and 10 mM CaCl2, respectively, in the presence of 5 μM Ca2+ ionophore (4-bromo-Ca2+ ionophore, A23187, Sigma) at the end of the experiment, and the proportionality factor was determined by the ratio of emission intensities of the Ca2+-free to the Ca2+-bound cameleon (34).
Data analysis.
Results are presented as means ± SE with n equal to the number of cells. Statistical comparisons were conducted with one-way ANOVA or paired t-test, according to the experimental design. Statistical significance was defined as P < 0.05.
RESULTS
Verification of targeted Ca2+ indicators.
The nontargeted D3cpv, nucleus-targeted 3NLS-D3cpv, and plasma membrane-targeted Lyn-D3cpv were transfected into rat PASMCs. Confocal imaging showed that D3cpv was expressed in a diffuse pattern indistinguishable from that of cytoplasmic dye, and with a lower expression in the nucleus region (Fig. 1B, a–d, and 1C). In contrast, 3NLS-D3cpv was exclusively localized in the nucleus (Fig. 1B, f and 1C), and Lyn-D3cpv was expressed solely in the plasma membrane of PASMCs (Fig. 1B, j and 1C). Specific targeting of the organelles by the cameleons was verified by staining the same cells with the fluorescent nucleic acid stain SYTO 83 and the plasma membrane marker CellMask. The SYTO 83 and CellMask fluorescence were colocalized with 3NLS-D3cpv (Fig. 1B, e–h) and Lyn-D3pcv (Fig. 1B, i–l) respectively. When the cells were cotransfected with 3NLS-D3cpv and Lyn-D3cpv, the nuclear and PM targeting cameleons were clearly distinguishable and highly localized (Fig. 1B, m–p, and 1C), indicating that they can be employed for simultaneous measurement of nucleoplasmic and subsarcolemmal Ca2+ signals in the same cell.
In situ calibration of D3cpv, 3NLS-D3cpv, and Lyn-D3cpv in rat PASMCs.
The Kd for Ca2+ of genetically encoded Ca2+ indicators can be different in vivo and in vitro (19) and may vary in different subcellular compartments (14, 32). Hence, we performed in situ calibration of the targeted and nontargeted cameleons. Rat PASMCs expressing 3NLS-D3cpv were permeabilized with digitonin (25 μM for 45 s) and exposed to different [Ca2+]Free. Figure 2A shows original images of two nucleus of PASMCs obtained before and during the calibration procedure at various [Ca2+]Free. Increase in [Ca2+]Free caused a rapid decrease in CFP fluorescence and increase in cpV fluorescence (Fig. 2B), resulting in an overall increase in FRET fluorescence ratio (Fig. 2C), which was saturated at [Ca2+]Free of >10 μM. The same permeabilization procedure, however, resulted in rapid loss of D3cpv and Lyn-D3cpv in the transfected cells. The leak of cytoplasmic D3cpv was prevented by using a lower concentration of digitonin at a reduced exposure time (12.5 μM for 30 s), and the loss of Lyn-D3cpv from the plasma membrane was avoided by using S. aureus α-toxin for membrane perforation (8). The apparent Kd for Ca2+ binding was estimated by fitting the FRET ratio as a function of [Ca2+]Free using the Hill equation (Fig. 2D). The apparent Kd of 3NLS-D3cpv for Ca2+ (digitonin-permeabilized cells: 0.22 ± 0.02 μM, n = 34) was similar to that of D3cpv (digitonin-permeabilized cells: 0.22 ± 0.01 μM, n = 11) (Fig. 2E). However, the Kd value of Lyn-D3cpv (α-toxin-permeabilized cells: 0.64 ± 0.03 μM, n = 12, P < 0.001) was significantly higher than those of D3cpv and 3NLS-D3cpv (Fig. 2E). The Kd of 3NLS-D3cpv obtained by α-toxin and digitonin permeabilization were basically the same (α-toxin-permeabilized cells: 0.27 ± 0.05 μM, n = 5). These results indicate that Ca2+ binding affinity of D3cpv is similar in nucleoplasmic and cytoplasmic compartments but is lower in subsarcolemmal regions of rat PASMCs.
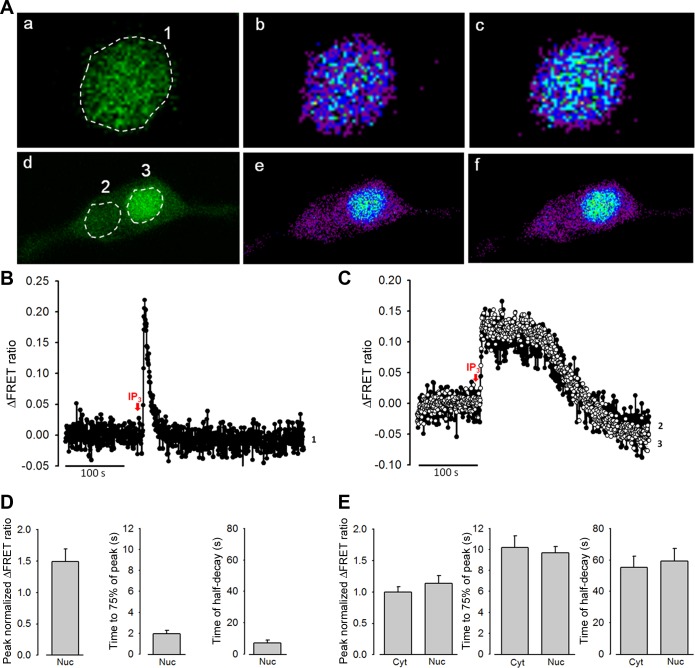
Fig. 2.
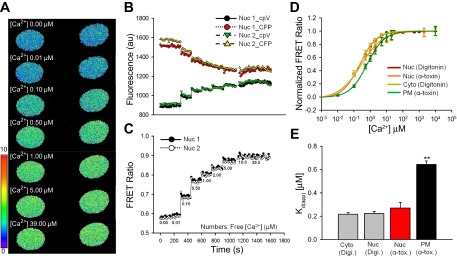
In situ calibration of the cameleon D3cpv in the cytoplasm (Cyto), nucleus (Nuc), and plasma membrane (PM) of rat PASMCs. A: original two-dimensional (2D) confocal images of fluorescence resonance energy transfer (FRET) fluorescence of rat PASMCs transfected with 3NLS-D3cpv at various [Ca2+]Free values during the calibration protocol. B: representative traces generated from the nuclei of digitonin-permeabilized rat PASMCs in A showing individual fluorescence intensities of CFP and cpV of 3NLS-D3cpv in the presence of different [Ca2+]Free (as in C), obtained by excitation at 458 nm. C: ratio traces derived from the individual fluorescence intensities of CFP and cpV in B. D: concentration-response curves of the FRET ratio as a function of [Ca2+]Free in the cytoplasm, nucleoplasm, and PM of rat PASMCs. E: apparent dissociation constants for Ca2+ binding (Kd(app)) in the cytoplasm, nucleoplasm, and PM. Digi, digitonin; α-tox, α-toxin. One-way ANOVA was used to calculate the P values.**P < 0.001.
IP3-induced nucleoplasmic and cytoplasmic Ca2+ signals in permeabilized rat PASMCs.
IP3Rs are expressed on the nuclear envelope and nucleoplasmic reticulum and have been implicated in nucleoplasmic Ca2+ signaling. We first characterized the nucleoplasmic Ca2+ signals by examining IP3-induced Ca2+ release in digitonin-permeabilized PASMCs transfected with 3NLS-D3cpv. Application of IP3 (10 μM) elicited a transient elevation of nucleoplasmic [Ca2+] (Fig. 3A, a–c, and 3B), which lasted for a brief period of time (time to 75% of peak: 2.0 ± 0.3 s and time of half-decay: 7.1 ± 1.7 s, n = 9, Fig. 3D). The transient nucleoplasmic Ca2+ signals in fully permeabilized PASMCs indicate rapid Ca2+ released and removal from the nucleoplasm. To examine whether direct activation of IP3Rs differentially regulates nuclear and cytoplasmic Ca2+ signals, PASMCs were cotransfected with D3cpv and 3NLS-D3cpv and partially permeabilized with digitonin to retain cytoplasmic D3cpv. Under this condition, application of IP3 elicited cytoplasmic and nucleoplasmic Ca2+ signals with significantly longer durations (Fig. 3A, d–f, and 3C). The peak change in fluorescence ratio (cytoplasmic = 0.098 ± 0.008, nucleoplasmic = 0.112 ± 0.013, n = 5), rise-time (time to 75% of peak, cytoplasmic = 10.2 ± 1.1 s, nucleoplasmic = 9.7 ± 0.6 s, n = 5), and time of half-decay (cytoplasmic = 55.4 ± 7.0 s, nucleoplasmic = 59.3 ± 8.0 s, n = 5) (Fig. 3E) of the nucleoplasmic and cytoplasmic Ca2+ transients were similar, suggesting efficient Ca2+ diffusion between the nucleus and cytoplasm.
Fig. 3.
Effects of inositol 1,4,5-trisphosphate (IP3) on the nucleoplasmic and cytoplasmic Ca2+ increase in digitonin-permeabilized rat PASMCs. A: 2D confocal images of PASMCs cotransfected with D3cpv and 3NLS-D3cpv and permeabilized with digitonin in excess (a–c) and under controlled conditions (d–f). IP3 (10 μM)-induced FRET ratio changes were analyzed in different regions of interest (ROIs 1-3) in the nucleus, and the cytoplasm. Cells showing only cpV fluorescence (a and d), and Ca2+ mobilization before (b and e) and after (c and f) IP3 treatment. B: ΔFRET fluorescence ratio trace showing Ca2+ mobilization in the nucleoplasm of rat PASMCs (ROI 1) after IP3 treatment. C: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the cytoplasm (●) and nucleoplasm (○) of rat PASMCs (ROI 2 and 3, respectively) after IP3 treatment. D: statistical analysis of the peak normalized ΔFRET fluorescence ratio, time to 75% of the peak, and time of half-decay of IP3-induced Ca2+ transients in the nucleoplasm of rat PASMCs under excess permeabilization conditions.E: statistical analysis of the peak normalized ΔFRET fluorescence ratio, time to 75% of the peak, and time of half-decay of IP3-induced Ca2+ transients in the nucleoplasm and cytoplasm of rat PASMCs under moderate permeabilization conditions.
ET-1 and ANG II preferentially triggered subsarcolemmal Ca2+ increase in PASMCs.
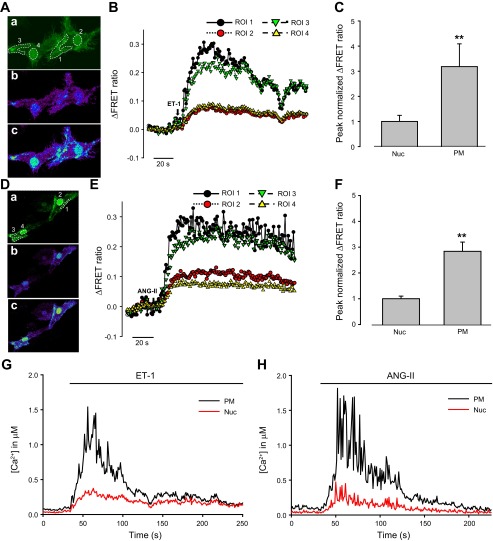
We next compared Ca2+ signals in the PM region, cytoplasm, and nucleoplasm activated by the physiological agonists ET-1 and ANG II. Rat PASMCs were cotransfected either with 3NLS-D3cpv and Lyn-D3cpv or with D3cpv and 3NLS-D3cpv. ET-1 (30 nM) and ANG II (100 nM) both elicited nucleoplasmic and PM Ca2+ signals in the 3NLS-D3cpv and Lyn-D3cpv cotransfected cells (Fig. 4, A–H). The Ca2+ signal was significantly stronger in the PM region (Fig. 4, A–H), with the peak change in FRET fluorescence ratio approximately threefold higher in the PM compared with the nucleoplasm (ET-1: PM = 0.237 ± 0.021, nucleoplasmic = 0.076 ± 0.011, n = 8, P < 0.001; ANG II: PM = 0.255 ± 0.020; nucleoplasmic = 0.093 ± 0.009, n = 12, P < 0.001) (Fig. 4, C and F, shown as peak normalized intensity values). Significant difference between [Ca2+] within nucleoplasm and the PM region was verified by converting the FRET signals to absolute [Ca2+], according to Grynkiewicz et al. (17)(Fig. 4, G and H). In contrast, both agonists caused similar changes in fluorescence ratio in the nucleoplasm and the cytoplasm of D3cpv and 3NLS-D3cpv cotransfected PASMCs (Fig. 5, A–F). The kinetics of Ca2+ signals were not different between nucleoplasm and PM, or between nucleoplasm and cytoplasm (data not shown). These data indicate that ET-1 and ANG II preferentially activate PM Ca2+ signals compared with nucleoplasmic and cytoplasmic Ca2+ and for the first time demonstrate the differential regulation of subcellular Ca2+ signals in PASMCs.
Fig. 4.
Effects of endothelin-1 (ET-1) and angiotensin II (ANG II) on the nucleoplasmic and PM Ca2+ increase in rat PASMCs. A: 2D confocal images of PASMCs cotransfected with Lyn-D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the PM and nucleoplasm before (b) and after (c) ET-1 (30 nM) treatment. B: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the PM (ROI 1 and 3) and nucleoplasm (ROI 2 and 4) of rat PASMCs (from A) after ET-1 treatment. C: statistical analysis of the peak normalized ΔFRET fluorescence ratio of ET-1-induced Ca2+ transients in the nucleoplasm and PM of rat PASMCs. One-way ANOVA was used to calculate P values. **P < 0.001; n = 8. Error bars denote SE. D: 2D confocal images of the cells cotransfected with Lyn-D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the PM and nucleoplasm before (b) and after (c) ANG II (100 nM) treatment. E: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the PM (ROI 1 and 3) and nucleoplasm (ROI 2 and 4) of rat PASMCs (from D) after ANG II treatment. F: statistical analysis of the peak normalized ΔFRET fluorescence ratio of ANG II-induced Ca2+ transients in the nucleoplasm and PM of rat PASMCs. One-way ANOVA was used to calculate P values. **P < 0.001; n = 12. Error bars denote SE. G and H: time course showing changes in nucleoplasmic and PM [Ca2+] in a single cell upon treatment with ET-1 and ANG II.
Fig. 5.
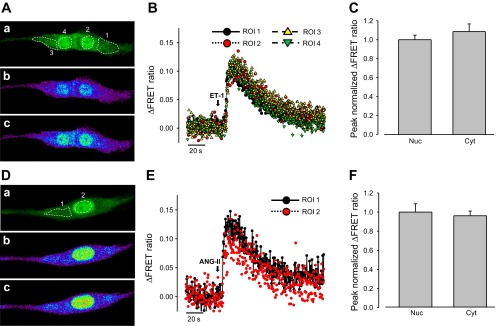
Effects of ET-1 and ANG II on the nucleoplasmic and cytoplasmic Ca2+ increase in rat PASMCs. A: 2D confocal images of a PASMC cotransfected with D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the cytoplasm and nucleoplasm before (b) and after (c) ET-1 (30 nM) treatment. B: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the cytoplasm (ROI 1 and 3) and nucleoplasm (ROI 2 and 4) of rat PASMCs (from A) after ET-1 treatment. C: statistical analysis of the peak normalized ΔFRET fluorescence ratio of ET-1 induced Ca2+ transients in the nucleoplasm and cytoplasm of rat PASMCs. One-way ANOVA was used to calculate P values. P > 0.05; n = 10. Error bars denote SE. D: 2D confocal images of a PASMC cotransfected with D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the cytoplasm and nucleoplasm before (b) and after (c) ANG II (100 nM) treatment. E: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the cytoplasm (ROI 1) and nucleoplasm (ROI 2) of rat PASMCs (from D) after ANG II treatment. F: statistical analysis of the peak normalized ΔFRET fluorescence ratio of ANG II-induced Ca2+ transients in the nucleoplasm and cytoplasm of rat PASMCs. One-way ANOVA was used to calculate P values. P > 0.05; n = 12. Error bars denote SE.
PDGF-induced comparable Ca2+ increase in the PM, nucleoplasm, and cytoplasm.
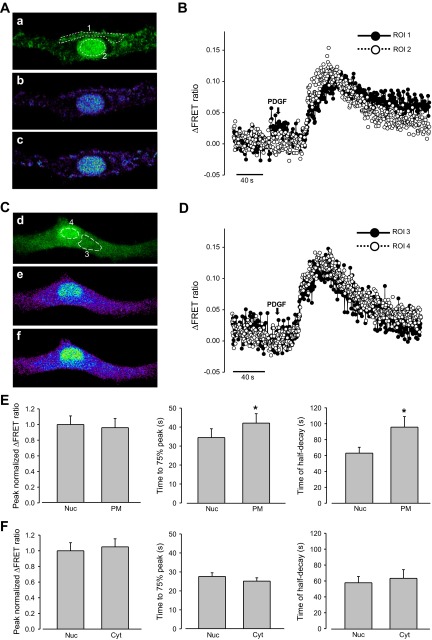
We further examined the subcellular Ca2+ signals induced by the receptor tyrosine kinase agonist PDGF. PDGF elicited Ca2+ transients with a slower kinetics compared with ET-1 and ANG II-treated cells. Time to 75% of peak was 33.8 ± 3.2 s for PDGF compared with 13.1 ± 1.9 s for ET-1 or 11.1 ± 1.1 s for ANG II; the time of half-decay was 81.2 ± 12.4 s for PDGF compared with 25.7 ± 4.1 s for ET-1 or 23.6 ± 4.90 s for ANG II. There was no clear difference in the amplitude of the Ca2+ signals in PM (peak ΔFRET ratio: 0.079 ± 0.01, n = 8) versus nucleoplasm (0.083 ± 0.009, n = 8) and the cytoplasm (0.090 ± 0.009, n = 6) versus nucleoplasm (0.086 ± 0.009, n = 8) (Fig. 6, A–F). The kinetics of PDGF-induced Ca2+ signals in the PM region, however, was significantly slower in terms of the rise-time and the half-decay time compared with those of nucleoplasm and cytoplasm (Fig. 6E). Our data suggest that PDGF regulates subcellular Ca2+ signals in a manner essentially different from that of ET-1 and ANG II.
Fig. 6.
Effects of platelet-derived growth factor (PDGF) on PM, cytoplasmic, and nucleoplasmic Ca2+ increase in rat PASMCs. A: 2D confocal images of a PASMC cotransfected with Lyn-D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the PM and nucleoplasm before (b) and after (c) PDGF (20 ng/ml) treatment. B: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the PM (ROI 1) and nucleoplasm (ROI 2) of rat PASMCs (from A) after PDGF treatment. C: 2D confocal images of a PASMC cotransfected with D3cpv and 3NLS-D3cpv, showing cpV fluorescence (a), and Ca2+ mobilization in the cytoplasm and nucleoplasm before (b) and after (c) PDGF treatment. D: ΔFRET fluorescence ratio traces showing Ca2+ mobilization in the cytoplasm (ROI 3) and nucleoplasm (ROI 4) of rat PASMCs (from C) after PDGF treatment. E: statistical analysis of the peak normalized ΔFRET fluorescence ratio, time to 75% of peak, and time of half-decay of PDGF-induced Ca2+ transients in the nucleoplasm and PM of rat PASMCs. F: statistical analysis of the normalized ΔFRET fluorescence ratio peak intensity, time to 75% of peak, and time of half-decay of PDGF-induced Ca2+ transients in the nucleoplasm and cytoplasm of rat PASMCs. Paired t-test was used to calculate P values. *P < 0.05 compared with nucleoplasmic. Error bars denote SE.
DISCUSSION
Intracellular Ca2+ is a second messenger for the regulation of numerous cellular functions. Despite being a key player of cellular processes, the regulation of local and compartmentalized Ca2+ signal remains particularly difficult to study. It is because Ca2+ probes with adequate dynamic range, suitable Ca2+ sensitivity, and specific subcellular localization are required to detect and quantify the Ca2+ signals (2). In the present study, we used the FRET-based cameleon D3cpv to generate specific Ca2+ probes to measure cytoplasmic, nucleoplasmic, and subsarcolemmal Ca2+ signals in PASMCs. D3cpv was chosen among the other genetically encoded Ca2+ probes because it has a wide dynamic range of emission fluorescence and a Kd (0.6 μM) for Ca2+ that is suitable for monitoring the moderate Ca2+ elevations in cytoplasm and nucleoplasm (35, 38, 39). Since the in vivo and in vitro Kd of genetically encoded Ca2+ indicators can be different (19), we performed in situ calibration of the targeted and nontargeted cameleons in our PASMCs. The apparent Kd for Ca2+ of D3cpv and 3NLS-D3cpv determined in situ are similar, but they are slightly lower than the Kd of 0.6 μM determined in vitro using recombinant D3cpv (38). This is in agreement with earlier findings on several types of cameleons showing a decrease in the Kd value when calibrated in vivo compared with in vitro (19). In contrast, the PM-targeted Lyn-D3cpv has a slightly higher in situ Kd compared with those of D3cpv and 3NLS-D3cpv. This could be related to the local ionic environment of the membrane bound cameleon. The higher apparent Kd of Lyn-D3cpv is not due to the use of α-toxin for membrane permeabilization because Kd values of the nucleoplasmic-targeted 3NLS-D3cpv are similar when calibrated using digitonin or α-toxin. Our finding is congruent with previous observations that the same cameleon may show different Kd values in different subcellular compartments (14, 32). One potential limitation of D3cpv is the slower kinetics compared with the Ca2+ fluorescent dyes (19), so it may miss the detection of very rapid transient events. However, D3cpv has been used successfully to detect single Ca2+ spikes in neuronal cells (54), suggesting that it should be adequate for reporting the usually slower Ca2+ response in PASMCs.
Using the targeted Ca2+ probes, we compared the Ca2+ signals induced by exogenous IP3 in the cytoplasmic and nucleoplasmic compartments. IP3Rs are expressed in SR of PASMCs, and photorelease of caged-IP3 can generate Ca2+ sparks and regenerative global Ca2+ release through cross-activation of ryanodine receptors (61). In PASMCs permeabilized with a mild treatment of digitonin, exogenous application of IP3 induced cytoplasmic and nucleoplasmic Ca2+ signals, which had no clear difference in magnitude and kinetics, suggesting efficient Ca2+ diffusion between the cytosol and the nucleus under our experimental conditions. This is consistent with the notion that the nuclear pore complexes are freely permeable to small ions and molecules (1, 49), even though cytoplasmic and nucleoplasmic Ca2+ can be regulated independently by specific signaling pathways (4, 30). When PASMCs were treated under excessive permeabilization conditions, exogenous IP3 elicited a rapid nucleoplasmic Ca2+ response with a much shorter duration. This could be related to the shorter diffusion time of IP3 and faster dispersal of released Ca2+ in the fully permeabilized cells. It is interesting, however, to note that the rapid transient nucleoplasmic Ca2+ response is similar to Ca2+ release from IP3Rs in the nuclear envelope and/or nucleoplasmic reticulum reported in isolated nucleus of other cell types (9, 15, 63). Since the expression of functional IP3Rs in the nuclear envelope or nucleoplasmic reticulum of PASMCs has not been examined, their relative contribution to nuclear Ca2+ signaling requires further investigation.
An important observation of the present study is the differential regulation of Ca2+ signals in subcellular regions by the agonists of G protein-coupled receptors. ET-1 and ANG II elicited Ca2+ signals were significantly stronger in the subsarcolemmal region than in the cytoplasm or nucleoplasm of PASMCs. Since the PM-targeted Lyn-D3cpv is inserted into the plasma membrane, it detects Ca2+ signals within a few nanometers underneath the plasma membrane. This is the site where numerous Ca2+ signaling processes occur, including Ca2+ entry via voltage-dependent and voltage-independent Ca2+ channels (e.g., store-operated and receptor-operated Ca2+ channels), and cross-signaling between Ca2+ release channels and membrane channels (e.g., voltage-gated Ca2+ channels, Ca2+-activated K+ and Cl−-channels)(45, 46, 52, 59, 61). ET-1 and ANG II mobilize intracellular Ca2+ through G protein stimulation of PLC-β to generate IP3 and diacylglycerol (DAG), leading to the activation of multiple Ca2+-permeating cation channels (29, 43, 62) and Ca2+ release channels (22, 48, 61) in VSMCs. Since [Ca2+] in the microdomain of an opened Ca2+-permeating channel can reach hundreds of micromolar (36, 45, 50), the remarkably high [Ca2+] detected by Lyn-D3cpv in the PM region likely reflects Ca2+ signals generated near the membrane Ca2+ channels and perhaps from Ca2+ release channels of peripherally coupled SR during agonist stimulation (10, 59). The lesser Ca2+ signals in the cytoplasm and nucleus might relate to Ca2+ release from the centrally located SR and lysosomal Ca2+ stores, which are known to be activated by ET-1 in PASMCs (22, 61).
In contrast, PDGF-induced Ca2+ signal in the plasma membrane region was similar in amplitude compared with those of cytoplasm and nucleoplasm, except with slower onset and decay. The cytoplasmic and nucleoplasmic Ca2+ transients induced by PDGF were also significantly slower than those activated by ET-1 and ANG II. This is consistent with previous reports that ANG II elicits larger and faster increases in IP3 than PDGF in vascular smooth muscle cells (26) and that ET-1 and PDGF show different profiles of intracellular Ca2+ responses (27). These contrasting Ca2+ responses are probably related to the differences in signaling mechanisms of the tyrosine kinase receptor agonist PDGF and the G protein-coupled receptor agonists (26, 27, 42, 51). PDGF activates PDGF receptors (PDGFR-α and PDGFR-β), causing tyrosine phosphorylation of PLC-γ to generate DAG and IP3, as well as activation of other signaling pathways including Ras-MAPK and PI3K (3). Depending on the subcellular location and spatial association of the signaling molecules and their effectors, PDGF may preferentially activate Ca2+ release from the centrally located Ca2+ stores, instead of the membrane Ca2+-permeating channels. It is consistent with reports that mitogens and growth factors stimulate Ca2+ release from Ca2+ stores in the perinuclear regions and/or nucleoplasmic reticulum to regulate gene transcription and cell proliferation in other cell types (2, 9, 18, 20, 41, 57). The faster PDGF-induced Ca2+ signals in the cytoplasm and nucleus compared with those in the PM region observed in this study suggest that PDGF-induced Ca2+ increase is initiated in the central Ca2+ sources of PASMCs. However, there was no clear disparity between the PDGF-induced cytoplasmic and nucleoplasmic Ca2+ signals in PASMCs under our experimental conditions. It is likely that the Ca2+ signal of IP3Rs in the nuclear envelope and/or nucleoplasmic reticulum was masked by cytoplasmic Ca2+ release, which can easily diffuse through nuclear pore complexes. Since fast transient local Ca2+ signals may escape detection due to the limitations in the imaging speed (2 frames/s) of our experiments, future investigations using techniques with improved spatial and temporal resolution are needed for further elucidation of the local regulation of nucleoplasmic Ca2+ signals by the growth factors.
Taken together, we have successfully applied the genetically encoded cameleon D3cpv to probe specific Ca2+ signals in the subsarcolemmal, cytosolic, and nucleoplasmic compartments in PASMCs. Our results provide the first experimental evidence that subsarcolemmal Ca2+ signals is preferentially stimulated by the G protein-coupled receptor agonists ET-1 and ANG II in PASMCs. The use of organelle-specific Ca2+ biosensors and the observation of compartmentalized Ca2+ signals in this study lay the foundation for further investigation of the differential regulation of subcellular Ca2+ signals in PASMCs, as well as other cell types.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL071835 and R01 HL075134 (to J. S. K. Sham).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.P.S. and J.S.K.S. conception and design of research; K.P.S. and O.P. performed experiments; K.P.S. analyzed data; K.P.S. and J.S.K.S. interpreted results of experiments; K.P.S. prepared figures; K.P.S. drafted manuscript; K.P.S. and J.S.K.S. edited and revised manuscript; J.S.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. R. Tsien for providing the cameleon 4mtD3cpv for the study.
Footnotes
This article is the topic of an Editorial Focus by Ningyong Xu, Michael Francis, Donna L. Cioffi, and Troy Stevens (58).
REFERENCES
- 1.Allbritton NL, Oancea E, Kuhn MA, Meyer T. Source of nuclear calcium signals. Proc Natl Acad Sci USA 91: 12458–12462, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso MT, Garcia-Sancho J. Nuclear Ca(2+) signalling. Cell Calcium 49: 280–289, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badminton MN, Campbell AK, Rembold CM. Differential regulation of nuclear and cytosolic Ca2+ in HeLa cells. J Biol Chem 271: 31210–31214, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. The AM and FM of calcium signalling. Nature 386: 759–760, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium 40: 405–412, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol 182: 167–175, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Cardenas C, Liberona JL, Molgo J, Colasante C, Mignery GA, Jaimovich E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci 118: 3131–3140, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Clark JH, Kinnear NP, Kalujnaia S, Cramb G, Fleischer S, Jeyakumar LH, Wuytack F, Evans AM. Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle. J Biol Chem 285: 13542–13549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem 278: 39224–39234, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 80: 439–444, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Gordienko DV, Zholos AV, Bolton TB. Membrane ion channels as physiological targets for local Ca2+ signalling. J Microsc 196: 305–316, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 18.Hardingham GE, Bading H. Nuclear calcium: a key regulator of gene expression. Biometals 11: 345–358, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Hendel T, Mank M, Schnell B, Griesbeck O, Borst A, Reiff DF. Fluorescence changes of genetic calcium indicators and OGB-1 correlated with neural activity and calcium in vivo and in vitro. J Neurosci 28: 7399–7411, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E, Lavandero S. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res 112: 236–245, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Jiang YL, Lin AH, Xia Y, Lee S, Paudel O, Sun H, Yang XR, Ran P, Sham JS. Nicotinic acid adenine dinucleotide phosphate (NAADP) activates global and heterogeneous local Ca2+ signals from NAADP- and ryanodine receptor-gated Ca2+ stores in pulmonary arterial myocytes. J Biol Chem 288: 10381–10394, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jou MJ, Peng TI, Sheu SS. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J Physiol 497: 299–308, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabbara AA, Allen DG. The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol 534: 87–97, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell 39: 499–509, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Kawahara Y, Kariya K, Araki S, Fukuzaki H, Takai Y. Platelet-derived growth factor (PDGF)-induced phospholipase C-mediated hydrolysis of phosphoinositides in vascular smooth muscle cells–different sensitivity of PDGF- and angiotensin II-induced phospholipase C reactions to protein kinase C-activating phorbol esters. Biochem Biophys Res Commun 156: 846–854, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Kojima N, Hori M, Murata T, Morizane Y, Ozaki H. Different profiles of Ca2+ responses to endothelin-1 and PDGF in liver myofibroblasts during the process of cell differentiation. Br J Pharmacol 151: 816–827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196: 947–950, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Lee HA, Baek EB, Park KS, Jung HJ, Kim JI, Kim SJ, Earm YE. Mechanosensitive nonselective cation channel facilitation by endothelin-1 is regulated by protein kinase C in arterial myocytes. Cardiovasc Res 76: 224–235, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM, Ehrlich BE, Nathanson MH. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci USA 100: 2975–2980, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipp P, Thomas D, Berridge MJ, Bootman MD. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J 16: 7166–7173, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljubojevic S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskamper J. In situ calibration of nucleoplasmic versus cytoplasmic Ca2+ concentration in adult cardiomyocytes. Biophys J 100: 2356–2366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium 39: 65–73, 2006 [DOI] [PubMed] [Google Scholar]
- 34.McCombs JE, Palmer AE. Measuring calcium dynamics in living cells with genetically encodable calcium indicators. Methods 46: 152–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neher E. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. In: Calcium Electrogenesis and Neuronal Functioning, edited by Heinemann U, Klee M, Neher E, Singer W. Berlin: Springer-Verlag, 1986, p. 80–96 [Google Scholar]
- 37.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol 13: 521–530, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc 1: 1057–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol 586: 3043–3054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem 282: 17061–17068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roe MW, Hepler JR, Harden TK, Herman B. Platelet-derived growth factor and angiotensin II cause increases in cytosolic free calcium by different mechanisms in vascular smooth muscle cells. J Cell Physiol 139: 100–108, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol 587: 5361–5375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santana LF, Navedo MF. Molecular and biophysical mechanisms of Ca2+ sparklets in smooth muscle. J Mol Cell Cardiol 47: 436–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sham JSK. Ca2+ release-induced inactivation of Ca2+ current in rat ventricular myocytes: evidence for local Ca2+ signalling. J Physiol 500: 285–295, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sham JSK, Cleemann L, Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci USA 92: 121–125, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res 93: 40–45, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Shimoda LA, Sylvester JT, Sham JSK. Mobilization of intracellular Ca2+ by endothelin-1 in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L157–L164, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Stehno-Bittel L, Perez-Terzic C, Clapham DE. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science 270: 1835–1838, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Stern MD. Buffering of calcium in the vicinity of a channel pore. Cell Calcium 13: 183–192, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Sultzman L, Ellis C, Lin LL, Pawson T, Knopf J. Platelet-derived growth factor increases the in vivo activity of phospholipase C-gamma 1 and phospholipase C-gamma 2. Mol Cell Biol 11: 2018–2025, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol 590: 3507–3521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun 236: 738–742, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Wallace DJ, Meyer zum Alten Borgloh S, Astori S, Yang Y, Bausen M, Kugler S, Palmer AE, Tsien RY, Sprengel R, Kerr JN, Denk W, Hasan MT. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nat Methods 5: 797–804, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34: 211–229, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Williams BA, Sims SM. Calcium sparks activate calcium-dependent Cl− current in rat corpus cavernosum smooth muscle cells. Am J Physiol Cell Physiol 293: C1239–C1251, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu N, Francis M, Cioffi DL, Stevens T. Studies on the resolution of subcellular free calcium concentrations: a technological advance. Focus on “Detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells.” Am J Physiol Cell Physiol (February 19, 2013). 10.1152/ajpcell.00046.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JSK. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L338–L348, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Zhang XF, Iwamuro Y, Okamoto Y, Kawanabe Y, Masaki T, Miwa S. Endothelin-1-induced contraction of rat thoracic aorta depends on calcium entry through three types of calcium channel. J Cardiovasc Pharmacol 36: S105–S106, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol 584: 601–611, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]