Abstract
Adenylyl cyclases (AC) catalyze formation of cAMP, a critical component of G protein-coupled receptor signaling. So far, nine distinct membrane-bound AC isoforms (AC1-9) and one soluble AC (sAC) have been identified and, except for AC8, all of them are expressed in the kidney. While the role of ACs in renal cAMP formation is well established, we are just beginning to understand the function of individual AC isoforms, particularly with regard to hormonal regulation of transporter and channel phosphorylation, membrane abundance, and trafficking. This review focuses on the role of different AC isoforms in regulating renal water and electrolyte transport in health as well as potential pathological implications of disordered AC isoform function. In particular, we focus on modulation of transporter and channel abundance, activity, and phosphorylation, with an emphasis on studies employing genetically modified animals. As will be described, it is now evident that specific AC isoforms can exert unique effects in the kidney that may have important implications in our understanding of normal physiology as well as disease pathogenesis.
Keywords: homeostasis, parathyroid hormone, renal disease, signaling, vasopressin
nine different membrane-bound adenylyl cyclase (AC) isoforms in mammals have been described; all of them are key enzymes in catalyzing the conversion of ATP to cAMP. The membrane-bound ACs (which is what this review refers to, unless stated otherwise) have two membrane clusters that each contain six transmembrane domains and three large cytoplasmic domains (N, C1a/b and C2a/b). C1a and C2a form the catalytic core complex; they are highly conserved and homologous to one another. The N-terminal domain varies between AC isoforms and plays a regulatory role (46).
A given cell type can express several AC isoforms, binds numerous agonists that modify cAMP production, and has a wide range of cAMP-dependent effects. This begs the question as to how cAMP is able to mediate such a variety of effects yet do this in a specific and highly regulated manner. This review focuses on one aspect of such specificity, namely, the role of unique AC isoforms in mediating particular biological actions in the kidney. However, it is important to briefly discuss the other key factors that play a role in allowing cAMP to exert so many functions, yet in a highly directed manner.
The classic paradigm was that G proteins interacted randomly with ACs; i.e., each AC molecule's activity reflected the relative influence of potentially several G protein-coupled receptors (GPCR). However, studies using fluorescent energy transfer-based intracellular probes indicate that AC-derived cAMP (and its downstream effectors) is confined to specific regions within the cell (3, 22). Furthermore, recent studies suggest that cAMP synthesis by specific AC isoforms can occur in vesicular compartments due to sustained activity of specific internalized receptors (3). Such association between GPCRs and ACs is likely due, at least in part, to A-kinase-anchoring proteins (AKAPs) that act as scaffolds to attached ACs to the plasma membrane or to the cytoskeleton (21, 22). These AKAPs, of which there are >50 members, maintain cAMP levels within discrete ranges immediately surrounding ACs (comparable to a small cloud) through direct binding to specific phosphodiesterases (PDEs) and dephosphorylases (such as calcineurin). Of equal importance, AKAPs also maintain localized activation of the two major cAMP effectors through direct binding to protein kinase A (PKA; as the name implies) and indirectly complexing with exchange protein directly activated by cAMP (Epac; a third cAMP target, the cAMP-gated ion channels, has been much less studied) (21, 22). PKA has long been viewed as the primary effector of cAMP effects on a variety of renal transporters and channels; however, recent studies reveal that Epac, which activates the small G proteins Rap1 and Rap2 (73), also mediates several cAMP actions in the kidney, including Ca2+ mobilization, urea transport, and other effects (45, 87, 90).
In addition to varying subcellular localizations, the nine membrane-associated ACs may be differentially regulated by Gα and GβY G protein subunits, divalent cations, small molecules, and posttranslational modification. For a detailed description of individual AC regulation, the reader is referred to several excellent reviews (6, 34, 88); however, we will briefly summarize unique aspects of AC isoform regulation. At the outset, it is important to note that specific AC isoform inhibitors or activators, although in active development, have not yet been identified (76); most of our information about the characteristics of individual AC isoforms derives from heterologous expression systems, genetically modified mouse models, or inferences drawn from patterns of pharmacological manipulation of regulatory factors. With the exception of AC2, mice have been developed with global knockout of each of the nine membrane-bound AC isoforms (reviewed in Refs. 57 and 70). Unique phenotypes have been associated with each conventional AC isoform knockout with the exception of AC4, where no abnormal phenotype has been noted. A recent detailed and critical analysis of the literature pointed out that major gaps exist in our understanding of AC isoform properties (34); however, some generalities can be made. AC isoforms can be grouped according to response to changes in Ca2+: those that are directly stimulated by Ca2+ (AC1, AC8 and possibly AC3), directly inhibited by Ca2+ (AC5 and AC6) or those with no direct response to Ca2+ (AC2, AC4, AC7 and AC9). The AC isoforms also appear to have differential responsiveness to protein kinase C (PKC), wherein AC6 is inhibited while AC2, AC3, AC5, and AC7 are activated. Regulation of individual AC isoforms by G proteins is quite controversial and requires substantial elucidation (70).
Soluble AC
There is a single mammalian soluble AC (sAC) isoform (reviewed in Ref. 83). The sAC is diffusely located throughout the cytoplasm and also exists in discrete locations within various organelles. Its activity is increased by divalent cations (predominantly Ca2+) and HCO3−; unlike membrane-bound ACs, it is not stimulated by forskolin. The sAC can move between subcellular compartments in response to varying acid-base status, resulting in regional increases in cAMP that can affect cellular respiration and survival (83).
Expression of AC Isoforms Within the Nephron
Determination of the renal region- or cell-specific pattern of AC isoform expression originally was based upon inferences drawn from the use of agents that altered intracellular Ca2+ signaling. If agonist-stimulated cAMP accumulation was unaffected by modifying Ca2+ signaling, then the Ca2+-insensitive isoforms (AC2, AC4, AC7, AC9) were assumed to be involved. If increasing intracellular Ca2+ inhibited agonist-evoked cAMP, then AC5 or AC6 was implicated, while if this augmented cAMP levels, then AC1, AC3, or AC8 was invoked. However, such conclusions were fraught with potentially complicating factors and did not clearly identify individual AC isoforms. Subsequent studies assessed AC isoform mRNA presence; however, while the absence of a given AC isoform mRNA is generally reasonable evidence that the isoform is missing, its presence does not guarantee that the protein is expressed. Protein analysis, whether by Western blotting or immunostaining, has provided useful insights although some caution must be taken in interpretation of these studies given the difficulties in developing highly specific antibodies; all the membrane-bound ACs are structurally similar, so obtaining unique antigenicity has been challenging. In addition, only rodent kidney AC isoform expression has been explored in any detail (for a summary of the ensuing discussion, see Table 1). Given these caveats, the following describes our current understanding of AC isoform expression along the nephron.
Table 1.
Expression of AC isoforms within the nephron in rodents
| AC1 | AC2 | AC3 | AC4 | AC5 | AC6 | AC7 | AC8 | AC9 | sAC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Proximal tubule | − | + | + | ± | − | + | + | − | + | − |
| Thin limb | − | − | + | ± | − | + | − | − | + | − |
| Thick ascending limb | − | − | − | − | − | + | − | − | + | + |
| Macula densa | − | − | + | ± | − | + | − | − | + | − |
| Distal tubule | − | + | + | − | − | + | − | − | + | + |
| Cortical CD | − | ± | + | + | + | + | − | − | + | + |
| Outer medullary CD | − | − | + | + | + | + | − | − | + | + |
| Inner medullary CD | − | ± | + | + | − | + | − | − | + | − |
| Principal cell | − | − | + | + | − | + | − | − | + | + |
| Intercalated cell | − | − | + | + | + | + | − | − | + | + |
The table represents a distillation and interpretation of the data; specific information upon which this summary is based can be found in the text.
AC, adenylyl cyclase; CD, collecting duct.
Proximal tubule.
In studies examining rat proximal tubule, Bek et al. (7) determined that this nephron segment expressed AC2, AC3, AC6, AC7, and AC9 mRNA. They also noted immunostaining for AC2, AC3, AC4, and AC9 in the proximal tubule, while the antibodies for the other AC isoforms were deemed unreliable. Interestingly, AC isoforms were detected only on the luminal membrane, while Western blot analysis detected only AC2 and AC9 in brush-border membranes. These finding suggested that AC isoforms may be differentially located within the proximal tubule, raising the possibility that associated signaling molecules and receptors are similarly localized; this issue requires further examination. In addition, this group detected AC7 only in the proximal tubule and not elsewhere in the nephron. Chabardes et al. (12), examining only AC4, AC5, and AC6 mRNA expression, detected only AC6 in rat proximal tubule.
Loop of Henle.
The thin limb of Henle's loop in rats expresses AC3, AC4, AC6, and AC9 mRNA and protein (although, as stated above, AC6 staining was felt to be uninterpretable) (7). AC6, but not AC4 or AC5, mRNA was also reported in rat thin limb (12), while AC3 immunostaining was detected in rat thin limbs (38). In the thick ascending limb (TAL), only AC6 mRNA and AC9 mRNA and protein were detected (7). Another group also reported AC6, but not AC4 or AC5, mRNA in rat TAL (12). No AC3 immunostaining was observed in mouse TAL, although it was detected in the macula densa (60). In contrast, both AC3 and AC4 mRNA and protein have been reported in rat macula densa (7). Soluble AC immunostaining has been reported in TAL in two studies (33, 54).
Distal tubule.
The distal tubule in the rat contains AC2, AC3 (albeit inconsistently), AC4, AC6, and AC9 mRNA with a similar pattern of expression detected by immunostaining (7). AC3 immunostaining colocalized with the Na+-Cl− cotransporter (NCC) in the mouse distal convoluted tubule, and both were detected only apically (60). AC6, but not AC4 or AC5, mRNA was observed in rat and mouse distal tubule (12). Finally, sAC immunostaining was reported in the distal tubule (54).
Connecting tubule/collecting duct.
The collecting duct (CD) has been the most extensively evaluated region of the nephron with regard to AC isoform expression. Microdissected rat cortical CD (CCD) expressed AC2, AC4, AC5, AC6, and AC9 mRNA and protein (again, AC5 and AC6 staining could not reliably be determined) (7). AC5 and AC6, but not AC4, mRNA was observed in rat CCD (12, 36). Mouse CCD contained AC3, AC4, AC5, and AC6 mRNA (79). Immunostaining for AC3 was present in the mouse connecting segment and CCD (60). Soluble AC protein appears to be abundantly present in the CCD (33, 54, 55). The outer medullary CD (OMCD) has a similar pattern of AC isoform expression as the CCD (7, 12, 36, 79). Further analysis of these two regions of the CD reveals a differential pattern of expression between principal and intercalated cells. In particular, Pastor-Soler et al. (54) reported colocalization between sAC and the vacuolar ATPase (V-ATPase), but not aquaporin-2 (AQP2), in rat kidney, suggesting intercalated cells selectively express sAC within the kidney. In contrast, two other studies using a different antibody reported that sAC immunostaining was present in both rat principal and intercalated cells (33, 55). Paunescu et al. (55) noted that sAC and the V-ATPase co-localized apically and subapically in type A intercalated cells, while they colocalized basolaterally in type B intercalated cells. Finally, in situ hybridization studies detected AC5 only within intercalated cells based on colocalization with V-ATPase, but not AQP2 (36). Thus, while the CCD and OMCD express several AC isoforms, AC5 is uniquely found in this region of the nephron due to selective intercalated cell expression.
Studies on inner medullary CD (IMCD) AC isoforms have yielded somewhat conflicting results. Studies have consistently shown that the highest mRNA and/or protein expression was found for AC6 in rat and mouse IMCD (7, 12, 36, 38, 79, 84). Expression levels of AC6 were inversely correlated with fluid intake, pointing to a predominant role of this isoform in urinary concentration (65). AC5 expression was inconsistently found to be present, and AC3 mRNA and protein were observed in mouse IMCD by some investigators (65, 79) but not others (60). AC8 was consistently absent, and some studies found AC9 mRNA and possibly protein (7, 38, 65). AC4 mRNA was not detected in rat IMCD by one group (12); however, others have observed AC4 mRNA and/or protein in rat and mouse IMCD (38, 65, 79, 84). AC7 and AC9 mRNA, but not protein, were detected in rat and mouse IMCD (36, 65, 79), although one study in rats did find AC9 immunostaining in the IMCD (7).
Consideration of the above complex patterns of AC isoform expression in the nephron suggests that, as previously mentioned, these proteins may mediate region-specific effects. Certain isoforms (AC6 and AC9) appear to be fairly ubiquitously expressed along the nephron; while they may mediate the effects of specific agonists that are unique to a given nephron segment, such conclusions cannot be drawn from simple expression analysis. One study deserves special consideration wherein a green fluorescent protein (GFP) reporter gene was inserted into the mouse AC6 gene (18). GFP was expressed along the entire nephron, with the highest expression in the distal tubule and CD. Double labeling with a primary cilia marker indicated that, in addition to basolateral staining, the primary cilium at the luminal cell membrane was stained. Similarly, sAC appears to be primarily found in the proximal tubule, distal nephron, and particularly the CD; its role in bicarbonate sensing may explain this nephron localization (55, 83). Finally, it remains to be determined whether differences in AC isoform expression within the nephron vary between species.
Juxtaglomerular apparatus.
While not part of the nephron, consideration of AC expression in juxtaglomerular cells (JG) is included, since, besides the nephron, it is the region within the kidney where the function of individual AC isoforms has been relatively intensely studied. Most data derive from studies using cultures of JG cells. Ortiz-Capisano et al. (53) determined that mouse JG cells contain AC5, but not AC6 protein. Renin secretion by JG cells has been referred to as the “Ca2+ paradox” since agonists that increase intracellular Ca2+ typically elicit endocrine factor secretion, but paradoxically inhibit JG renin release. Since renin secretion is stimulated by cAMP, the conclusion was that Ca2+-inhibited AC isoforms (AC5 and/or AC6) were involved. This group observed that chelation of intracellular Ca2+ stimulated renin release and that this was prevented by a “selective AC5” inhibitor (NKY80). While these results support the notion that a Ca2+-inhibited AC isoform is involved, the inhibitor is not highly AC5 specific. Another study noted that parathyroid hormone (PTH) increased JG cell non-Ca2+-sensitive cAMP accumulation without increasing renin secretion, suggesting compartmentalized cAMP actions (4). In contrast to the hypothesis that AC5 might be the predominant isoform for renin secretion, Aldehni et al. (2) observed that under basal conditions AC5 knockout mice have similar plasma renin concentrations (PRC) compared with wild-type mice; however, AC6 knockout mice display significantly increased PRC under basal conditions. Agonist-induced increases in PRC were reduced in both AC5 and AC6 knockout mice, although the impairment was more severe in AC6 knockout mice.
Other regions of the kidney.
Information on AC expression/function in other parts of the kidney is limited. To our knowledge, only two studies exist which determined the role of AC1 or AC3 in podocytes (89) and glomeruli/renal artery (59), respectively. AC1 knockout mice show an aggravated protein loss in response to albumin overload, while having no other renal phenotype. The role of AC3 in glomeruli remains elusive; however, it was speculated to initiate signals leading to paracrine regulation of renin secretion. The role of AC3 in the renal artery needs to be determined.
Physiological Effects of AC Isoforms
Role of AC isoforms in mediating forskolin- and hormone-stimulated cAMP formation.
Basal renal AC activity of rats and rabbits was shown to be higher in the medulla vs. cortex (24, 77). In rat and chicken renal cortical slices, forskolin (unselective AC activator)-stimulated cAMP formation displayed a dose-response relationship, reaching a maximum of ∼10-fold stimulation over baseline at 300 μmol/l (26). Comparable observations were found in canine basolateral renal cortical membranes (48) or rat whole kidney membranes (75). In addition to the renal cortex, medullary forskolin-stimulated cAMP formation shows a comparable dose-response relationship. So far only one isoform, AC6, has been examined in terms of forskolin-stimulated cAMP formation. Studies in AC6 knockout mice indicated that medullary preparations (65) as well as whole kidney lysates (18) have markedly reduced forskolin-stimulated cAMP formation, providing evidence for a significant role of this AC isoform in overall renal cAMP formation.
Hormone-stimulated renal cAMP formation depends on the site of action and type of receptor expressed. The same hormone can stimulate cAMP formation in one nephron segment while it can inhibit cAMP formation in another (e.g., prostaglandin E2), drastically increasing the versatility of a given hormone (8, 50). In the kidney, the following hormones have been found to increase cAMP formation: calcitonin (15, 17), dopamine (62, 74), glucagon (5, 12), glucagon-like peptide (GLP-1) (11, 19), isoproterenol (15, 16), PTH (13, 14), arginine-vasopressin (AVP) (13, 41), and vasoactive intestinal peptide (VIP) (30, 31). Information provided will specifically focus on data related to AC isoform-specific cAMP formation.
So far, only two hormones acting on the proximal tubule have been tested in regard to activation of specific AC isoforms: 1) GLP-1 and 2) PTH. In rats, GLP-1 was able to increase urinary cAMP excretion dose dependently (19), an effect that was absent in both wild-type and AC6 knockout mice, while eliciting a clear effect on urinary Na+ excretion (63). Whether the results denote an effect of GLP-1 independently of the AC system or merely demonstrates that excretion of cAMP and cellular activation of AC are dissociable phenomena is unclear.
A stimulatory effect of PTH on urinary cAMP excretion has been reported in humans (42), mice (47), rats (27), birds (58), and dogs (78), whereas hamsters were found to be resistant to the effect of PTH (44). In wild-type mice, PTH increased urinary cAMP excretion (64); however, this effect was completely absent in AC6 knockout mice, suggesting that this isoform is required for PTH-stimulated cAMP formation in the kidney.
Studies in mice suggest that AC6 is a key isoform mediating AVP-induced cAMP formation. Mice with global AC6 knockout had absent AVP-stimulated cAMP formation in the IMCD (65). In a principal cell-specific AC6 knockout model, AVP-stimulated cAMP formation in acutely isolated IMCD was impaired compared with wild-type mice (68). Finally, Chien et al. (18) demonstrated that in whole kidney lysates AVP-induced AC activity was reduced by ∼60% in global AC6 knockout mice. The latter results possibly indicate that the remaining 40% of AVP-induced cAMP formation may be mediated by AC isoforms other than AC6, possibly localized to the renal cortex. In contrast to findings pointing toward a central role for AC6 in AVP-induced cAMP formation in the IMCD, Hoffert et al. (38) found that calmodulin inhibition markedly reduced AVP-stimulated cAMP in rat IMCD, suggesting a role for AC3. Data by Rieg et al. (65) in mouse inner medulla indicate that mRNA expression levels of AC3 are ∼100-fold lower compared with AC6 expression, which is in contrast to studies in rats (84) and possibly indicate species-specific expression patterns. In a recently developed principal cell-specific AC3 knockout mouse, basal and AVP-induced cAMP formation were comparable to wild-type mice (43), arguing against a significant role of AC3 in murine renal cAMP formation in this cell type.
Role of AC isoforms in modulating renal tubule electrolyte transport.
THICK ASCENDING LIMB.
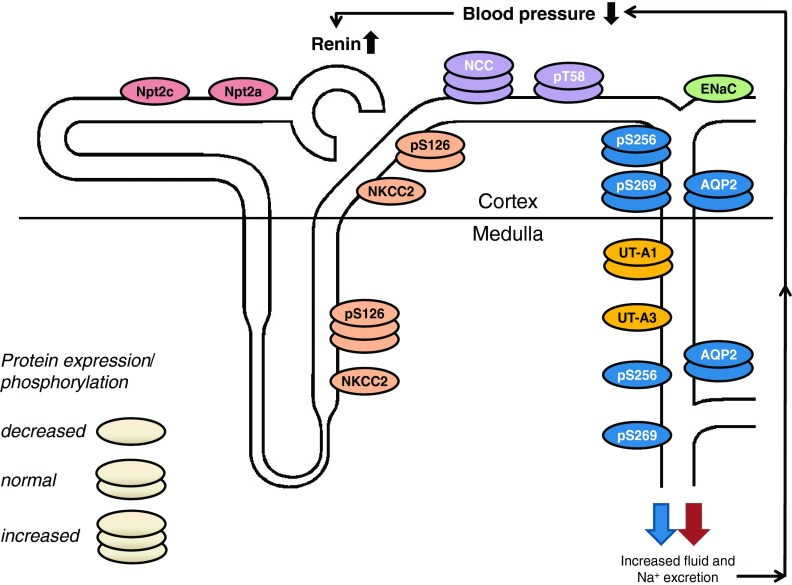
AVP stimulates Na+ transport in the TAL. Exposure to AVP increases Na+ transport in micropunctured rat and isolated mouse TAL (although there is some controversy on whether both cortical and medullary TAL [mTAL and cTAL, respectively] respond to AVP in the mouse) (20, 72). This stimulatory effect of AVP on TAL Na+ transport is mediated by modulation of the Na+-K+-2Cl− cotransporter (NKCC2). In isolated rat TAL, AVP increased steady-state surface NKCC2 via PKA activation but not via Epac (10). Short-term activation of NKCC2 by AVP involves phosphorylation: one study observed phosphorylation at threonine residues 96 and 101 (T96 and T101) (29), while another noted only T96 phosphorylation (32). Two additional serine phosphorylation sites of rat NKCC2, S126 and S874, have recently been identified (32); however, the role of these serine, as well as the threonine phosphorylation sites, in modulating NKCC2 activity and trafficking is incompletely understood. Rieg et al. (66) recently determined the role of AC6 in modulating NKCC2 protein expression, phosphorylation and localization. Unfortunately, despite extensive effort, the authors were unable to detect S874 NKCC2 protein in murine kidney. Under basal conditions, total renal NKCC2 expression was 50% lower in AC6 knockout compared with wild-type mice and this was associated with a 1.8-fold greater expression of pS126 NKCC2 (Fig. 1). The increased S126 phosphorylation might protect the AC6 knockout mouse from developing a more severe salt-losing phenotype (see below). Immunofluorescence labeling and confocal analysis confirmed a reduced total NKCC2 staining in kidneys of AC6 knockout mice compared with wild-type in both mTAL and cTAL. In the mTAL, the abundance of pS126 NKCC2 was low in wild-type mice and not detectable in AC6 knockout mice. In the cTAL, no apparent differences were observed in labeling intensity of pS126 NKCC2 between genotypes, which had overall greater labeling intensity than in the mTAL. The labeling intensity for total NKCC2 in mTAL and cTAL after AVP treatment was not different between genotypes. However, labeling of pS126 NKCC2 significantly increased in the mTAL of wild-type mice in response to AVP, a finding completely absent in AC6 knockout mice. In contrast to the mTAL, AVP did not affect labeling intensity of pS126 NKCC2 in cTAL of either genotype.
Fig. 1.
Integrated renal and blood pressure phenotype of AC6 knockout mice. AC6 may stimulate total renal Na+-K+-2Cl− cotransporter (NKCC2) protein abundance in medullary and cortical thick ascending limb (although the latter is controversial). Increased AC6-independent phosphorylation of NKCC2 at serine 126 (S126) in the medulla might help to stabilize NKCC2 activity in the absence of AC6. Upregulation of Na+-Cl− cotransporter (NCC) in AC6 knockout mice may compensate for reduced NKCC2 activity. In the aldosterone-sensitive distal nephron, the expression, but not single channel activity, of the epithelial sodium channel (ENaC) is reduced in AC6 knockout mice under basal conditions, while AVP-stimulated ENaC single-channel activity is absent. Total cortical and medullary aquaporin-2 (AQP2) were not different between genotypes; however, in the medullary portion of the collecting duct, phosphorylation of AQP2 at S256 and S269 (the latter only found in the apical plasma membrane) was severely reduced. In contrast to the medulla, cortical AQP2 phosphorylation was comparable to wild-type mice, indicating that possibly a different AC isoform regulates vasopressin-mediated water transport in the cortex. Impaired renal NaCl and fluid absorption decreases blood pressure and increases plasma renin concentration. AC6 knockout mice have comparable urinary urea excretion; however, inner medullary UT-A3 protein expression is reduced. The expression of proximal tubular Na+-phosphate transporters Npt2a and Npt2c are also severely reduced (unpublished observations), and AC6 knockout mice have phosphaturia (64). Possibly as a consequence of intact feedback mechanisms in AC6 knockout mice, renal resistance to vasopressin and parathyroid hormone causes both hormones to be significantly elevated. In summary, AC6 knockout mice have nephrogenic diabetes insipidus and a mild Bartter syndrome. See the text for a detailed description.
DISTAL TUBULE.
The activity of the distal tubule Na+-Cl− cotransporter (NCC) is subject to complex regulation, including AVP stimulation of Na+ reabsorption (23) through phosphorylation at multiple sites (T53, T58, S71, and S124; rodent nomenclature) (51, 56, 69). Under basal conditions, AC6 knockout mice had a 1.9-fold higher total NCC expression compared with wild-type mice, while pT58 NCC was not different between genotypes (66). The greater NCC expression is possibly a consequence of reduced NKCC2 expression/activity, resulting in greater delivery of NaCl from the TAL into the distal tubule. Qualitative immunohistochemistry and quantitative laser scanning confocal microscopy indicated that AVP treatment did not change the labeling intensity for total NCC in the DCT of either group. In contrast, labeling and protein expression of pT58 NCC significantly increased in response to AVP in wild-type, but not in AC6 knockout mice. The signaling pathway(s) involved in AVP-induced NCC phosphorylation are unknown, but in addition to AC6, might involve the “with-no-K[Lys] kinases”/STE20/SPS1-related proline/alanine-rich kinase/oxidative stress responsive kinase-1 pathway, which, when activated, results in increased NCC at the cell membrane and increased NCC activity (56, 71). Further analysis of AC6 knockout mice indicated 1) mild hypokalemia (66), 2) mild alkalosis (66), 3) mild urinary Na+/K+ loss (65, 66), 4) lower blood pressure (2), and 5) elevated plasma PRC (2). Taken together, the changes found in the TAL and DCT of AC6 knockout mice possibly result in a mild salt-losing phenotype and cause clinical features consistent with Bartter syndrome (shown in Fig. 1).
COLLECTING DUCT.
The CD epithelial Na+ channel (ENaC) is regulated by multiple factors, including by AVP-induced cAMP. Infusion of AVP in Brattleboro rats markedly increased ENaC β- and γ-subunit mRNA levels in the renal cortex (52). Similar results were obtained in Sprague-Dawley rats after either partial water restriction or AVP infusion. In isolated, perfused CCD of Brattleboro rats, AVP increased amiloride-sensitive Na+ transport (52). Bugaj et al. (9) demonstrated that AVP increases ENaC activity (open probability; Po) in acutely isolated split-open CCD; furthermore, this effect was dependent on AC and PKA. Recently, mice with principal cell-specific AC6 knockout were studied (67). Under basal conditions, these mice had decreased renal cortical mRNA content of α-, β-, and γ-ENaC as well as reduced total cell protein of α- and γ-ENaC compared with wild-type mice. Whereas baseline ENaC activity in acutely isolated split-open CCD was not different between genotypes, AVP-stimulated ENaC Po, and the increase in channel number was absent in principal cell-specific AC6 knockout compared with wild-type mice. In contrast, principal cell-specific AC3 knockout mice show a comparable Po and AVP-stimulated Po compared with wild-type mice (43). Blood pressure in principal cell-specific AC3 knockout mice was not different from wild-type mice under normal-, low-, or high-Na+ intake. Taken together, these results demonstrate that AC6 is required for normal expression and function of ENaC in principal cells.
Role of AC isoforms in modulating renal tubule water transport.
As discussed above, studies in rats and mice have provided evidence for AC3 and AC6 in the regulation of AVP-stimulated cAMP formation in the CD via activation of the vasopressin V2 receptor (V2R). With regard to water transport, AVP-stimulated cAMP induces sequential phosphorylation of aquaporin-2 (AQP2) at S256 (40) and S269 (39), resulting in apical plasma membrane accumulation of AQP2, and enhanced water reabsorption provided a favorable osmolar gradient (25, 49). Studies focusing on the role of specific AC isoforms in urinary concentration are limited. So far, only clear evidence exists for AC3 and AC6, and this is based on studies in genetically modified animals; other AC isoform knockout animals have not been challenged with water loading or water deprivation, and AVP responses have not been studied. However, with free access to food and water, global AC3 knockout mice, although having a lower glomerular filtration rate, have normal urine osmolality (60). In a principal cell-specific AC3 knockout mouse, total and S269 AQP2 expression were comparable to wild-type mice (43). Global AC5 knockout mice appear to have normal urine osmolality under baseline conditions (65) (Table 2). In contrast, global AC6 knockout mice show reduced urine osmolality under basal conditions associated with greater fluid intake and greater brain AVP mRNA expression compared with wild-type mice (18, 65, 68) (Table 2). In AC6 knockout mice no evidence for compensation by other AC isoforms was detected (65). Principal cell-specific AC6 knockout mice show similar findings: lower urine osmolality compared with control mice (68). Western blotting and immunohistochemistry indicated that AC6 knockout mice have lower amounts of pS256 AQP2 compared with wild-type mice; while pS269 AQP2 was not detectable (65). AVP-induced phosphorylation of AQP2 at S269 is completely absent in AC6 knockout mice; moreover, increases in pS256 AQP2 and total AQP2 trafficking into the apical membrane are attenuated in the IMCD of AC6 knockout compared with wild-type mice. Taken together with reduced TAL NKCC2 activity, it is possible that the markedly impaired urinary concentrating ability in global AC6 knockout mice is due to a combination of effects in the CD and the TAL (Fig. 1). Finally, it should be noted that there may be differences between the medulla and cortex with regard to AVP-stimulated AC6-mediated regulation of NKCC2 and AQP2: the function of these molecules is impaired in the medulla, but perhaps not in the cortex, in the setting of AC6 deficiency. It may be that different AC isoforms modulate AVP action in the cortex and the medulla; this intriguing possibility requires further exploration.
Table 2.
Summary of water homeostasis and renal arginine-vasopressin (AVP)-stimulated cAMP formation in genetically modified mice
| Water Intake, ml/day | Urine Osmolality, mmol/kgH2O | AVP-Stimulated cAMP Formation (Acutely Isolated Renal Tissue) | |
|---|---|---|---|
| WT | ND | 1,501 ± 277 | ND |
| Conventional AC3−/− (60) | ND | 2,361 ± 412 | |
| WT | ND | 1,988 ± 81 | ND |
| Conventional AC5−/− (65) | ND | 2,089 ± 53 | |
| WT | 6.9 ± 0.3 | 1,460 ± 43 | Comparable to WT |
| Conditional AC3-AQP2Cre (43) | 6.7 ± 0.5 | 1,392 ± 23 | |
| WT | 5.0 ± 0.3 | 2,027 ± 160 | Impaired vs. WT |
| Conventional AC6−/− (65) | 13.5 ± 0.9* | 875 ± 94* | |
| WT | 2.19 ± 0.1 | 3,721 ± 168 | Impaired vs. WT |
| Conventional AC6−/− (18) | 3.32 ± 0.4* | 1,612 ± 62* | |
| WT | 3.6 ± 0.8 | 1,938 ± 189 | Impaired vs. WT |
| Conditional AC6-AQP2Cre (68) | 4.61 ± 1.1* | 1,428 ± 91 | |
| WT | 4.62 ± 0.2 | 2,195 ± 147 | ND |
| Conditional AC6-Pax8Cre (unpublished observations) | 11.0 ± 0.2* | 952 ± 98* |
Values are means ± SE.
WT, wild-type; ND, not determined; AQP2, aquaporin-2.
P < 0.05 vs. WT.
Pathophysiology of AC Isoforms in Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most commonly inherited renal diseases, ultimately resulting in end-stage renal disease. The reader interested in the complex pathophysiological aspects of this disease is referred to excellent recent reviews (35, 81, 85, 91). There is now accumulating evidence indicating that the AVP-cAMP signaling pathway directly regulates cyst growth in ADPKD. In rat and mouse models of human ADPKD (named PCK and pcy, respectively), renal cAMP production and AQP2 protein expression are significantly increased compared with their wild-type counterparts, and treatment with the V2R antagonist OPC-31260 significantly inhibited renal cAMP formation and either stopped disease progression, inhibited disease development, or even caused regression of the disease (28). In addition, crossing PCK with Brattleboro rats (the latter lacks endogenous AVP) resulted in reduced renal cAMP formation and greatly inhibited cystogenesis, while administration of AVP to the F1 generation restored the cystic phenotype (86). Recent studies in humans found that the V2R antagonist OPC-41061 (tolvaptan) slowed cyst growth and improved renal function (37, 80, 82). Since AC6 had been implicated in mediating AVP-stimulated cAMP formation, and since AC6 has been reported to be expressed in primary cilia (18) (defects in cilia-mediated signaling activity is a key factor that leads to cyst formation), the role of AC6 in ADPKD was evaluated (61). Mice with principal cell-specific knockout of polycystin-1 were compared with mice with principal cell-specific knockout of polycystin-1 and AC6; deficiency of AC6 markedly ameliorated cyst formation and renal injury in this PKD model, even though double knockout mice showed slightly enlarged kidneys compared with wild-type mice. A reduced activation of the B-Raf/ERK/MEK pathway, important for cell proliferation in ADPKD, might explain the reduced kidney size and improved kidney function. Notably, urinary cAMP excretion and renal cAMP content were unchanged in double mutant mice compared with CD-specific polycystin-1 knockout mice, suggesting that the specific AC isoform may be more important than the total cellular cAMP content. In the final analysis, it is noteworthy that, in the major ADPKD trial, tolvaptan administration was complicated by high adverse events related to increased aquaresis (37, 80, 82); even more problematic is the recent FDA ruling limiting tolvaptan use to <30 days due to the risk of liver injury (1). Thus the need for better therapies for ADPKD, including possible treatments aimed at AC6 or specific pathways that AC6 activates, is evident.
Perspectives and Significance
Multiple AC isoforms are expressed in renal epithelial cells, often in the same cell. While knockout mice for each of the AC isoforms have been generated, for the majority of them their exact role in modulating kidney function is not known. There is accumulating evidence for an important role of AC6 in control of renal electrolyte and water transport involving transport systems in the proximal tubule, thick ascending limb, distal tubule, and CD. The complexity of the hormone-AC interaction may be further increased by the fact that one specific receptor could couple to different AC isoforms in different nephron segments. Important issues to be clarified in future studies include the identification and regulation of transport pathways by AC under physiological and pathological conditions, e.g., acute kidney injury, chronic kidney injury, polycystic kidney disease, and others. The role of apical vs. basolateral signaling deserves further consideration, as does the differences in AC action between the medulla and cortex. The use of conventional and conditional gene knockout mice will help to decipher the biological role of specific AC isoforms and may lead to a better understanding of their role in renal physiology and pathophysiology.
GRANTS
The authors were supported by grants provided by the National Institutes of Health (R01DK097007 to D. E. Kohan), O'Brien Center for Acute Kidney Injury Research (P30DK079337 to T. Rieg), the American Heart Association (10SDG2610034 to T. Rieg), a Carl W. Gottschalk Research Grant of the American Society of Nephrology (T. Rieg), and the Department of Veterans Affairs (T. Rieg and D. E. Kohan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.R. and D.E.K. conception and design of research; T.R. and D.E.K. performed experiments; T.R. and D.E.K. analyzed data; T.R. and D.E.K. interpreted results of experiments; T.R. and D.E.K. prepared figures; T.R. and D.E.K. drafted manuscript; T.R. and D.E.K. edited and revised manuscript; T.R. and D.E.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to the investigators of adenylyl cyclases in kidney function whose relevant publications were inadvertently not directly discussed or cited. We thank Dr. Jessica Dominguez Rieg for a critical reading of the manuscript.
REFERENCES
- 1. Anonymous. http://www.fda.gov/Drugs/DrugSafety/ucm350062.htm. [Google Scholar]
- 2.Aldehni F, Tang T, Madsen K, Plattner M, Schreiber A, Friis UG, Hammond HK, Han PL, Schweda F. Stimulation of renin secretion by catecholamines is dependent on adenylyl cyclases 5 and 6. Hypertension 57: 460–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoni FA. New paradigms in cAMP signalling. Mol Cell Endocrinol 353: 3–9, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Atchison DK, Harding P, Cecilia Ortiz-Capisano M, Peterson EL, Beierwaltes WH. Parathyroid hormone stimulates juxtaglomerular cell cAMP accumulation without stimulating renin release. Am J Physiol Renal Physiol 303: F1157–F1165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailly C, Imbert-Teboul M, Chabardes D, Hus-Citharel A, Montegut M, Clique A, Morel F. The distal nephron of rat kidney: a target site for glucagon. Proc Natl Acad Sci USA 77: 3422–3424, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beazely MA, Watts VJ. Regulatory properties of adenylate cyclases type 5 and 6: a progress report. Eur J Pharmacol 535: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA. Differential expression of adenylyl cyclases in the rat nephron. Kidney Int 60: 890–899, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol 63: 579–605, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297: F1411–F1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na+-K+-2Cl− cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Chabardes D, Firsov D, Aarab L, Clabecq A, Bellanger AC, Siaume-Perez S, Elalouf JM. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J Biol Chem 271: 19264–19271, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Chabardes D, Gagnan-Brunette M, Imbert-Teboul M, Gontcharevskaia O, Montegut M, Clique A, Morel F. Adenylate cyclase responsiveness to hormones in various portions of the human nephron. J Clin Invest 65: 439–448, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabardes D, Imbert M, Clique A, Montegut M, Morel F. PTH sensitive adenyl cyclase activity in different segments of the rabbit nephron. Pflügers Arch 354: 229–239, 1975 [DOI] [PubMed] [Google Scholar]
- 15.Chabardes D, Imbert-Teboul M, Gagnan-Brunette M, Morel F. Different hormonal target sites along the mouse and rabbit nephrons. Curr Probl Clin Biochem 8: 447–454, 1977 [PubMed] [Google Scholar]
- 16.Chabardes D, Imbert-Teboul M, Montegut M, Clique A, Morel F. Catecholamine sensitive adenylate cyclase activity in different segments of the rabbit nephron. Pflügers Arch 361: 9–15, 1975 [DOI] [PubMed] [Google Scholar]
- 17.Chabardes D, Imbert-Teboul M, Montegut M, Clique A, Morel F. Distribution of calcitonin-sensitive adenylate cyclase activity along the rabbit kidney tubule. Proc Natl Acad Sci USA 73: 3608–3612, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien CL, Wu YS, Lai HL, Chen YH, Jiang ST, Shih CM, Lin SS, Chang C, Chern Y. Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6). FEBS Lett 584: 2883–2890, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011 [DOI] [PubMed] [Google Scholar]
- 20.de Rouffignac C, Corman B, Roinel N. Stimulation by antidiuretic hormone of electrolyte tubular reabsorption in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 244: F156–F164, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Dessauer CW. Adenylyl cyclase-A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76: 935–941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards HV, Christian F, Baillie GS. cAMP: novel concepts in compartmentalised signalling. Semin Cell Dev Biol 23: 181–190, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Elalouf JM, Roinel N, de Rouffignac C. Effects of antidiuretic hormone on electrolyte reabsorption and secretion in distal tubules of rat kidney. Pflügers Arch 401: 167–173, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Erdorf M, Seifert R. Pharmacological characterization of adenylyl cyclase isoforms in rabbit kidney membranes. Naunyn Schmiedebergs Arch Pharmacol 383: 357–372, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Fenton RA, Pedersen CN, Moeller HB. New insights into regulated aquaporin-2 function. Curr Opin Nephrol Hypertens 22: 551–558, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Forte LR. Activation of renal adenylate cyclase by forskolin: assessment of enzymatic activity in animal models of the secondary hyperparathyroid state. Arch Biochem Biophys 225: 898–905, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Forte LR, Nickols GA, Anast CS. Renal adenylate cyclase and the interrelationship between parathyroid hormone and vitamin D in the regulation of urinary phosphate and adenosine cyclic 3′,5′-monophosphate excretion. J Clin Invest 57: 559–568, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Griffiths NM, Brick-Ghannam C, Siaume-Perez S, Chabardes D. Effect of prostaglandin E2 on agonist-stimulated cAMP accumulation in the distal convoluted tubule isolated from the rabbit kidney. Pflügers Arch 422: 577–584, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Griffiths NM, Chabardes D, Imbert-Teboul M, Siaume-Perez S, Morel F, Simmons NL. Distribution of vasoactive intestinal peptide-sensitive adenylate cyclase activity along the rabbit nephron. Pflügers Arch 412: 363–368, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA 107: 15653–15658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NM. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem 284: 5774–5783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol 3: a004143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PC. 2008 Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol 20: 1188–1198, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Helies-Toussaint C, Aarab L, Gasc JM, Verbavatz JM, Chabardes D. Cellular localization of type 5 and type 6 ACs in collecting duct and regulation of cAMP synthesis. Am J Physiol Renal Physiol 279: F185–F194, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS. Tolvaptan in autosomal dominant polycystic kidney disease: three years' experience. Clin J Am Soc Nephrol 6: 2499–2507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffert JD, Chou CL, Fenton RA, Knepper MA. Calmodulin is required for vasopressin-stimulated increase in cyclic AMP production in inner medullary collecting duct. J Biol Chem 280: 13624–13630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imbert M, Chabardes D, Montegut M, Clique A, Morel F. Vasopressin dependent adenylate cyclase in single segments of rabbit kidney tubule. Pflügers Arch 357: 173–186, 1975 [DOI] [PubMed] [Google Scholar]
- 42.Kaminsky NI, Broadus AE, Hardman JG, Jones DJ, Jr, Ball JH, Sutherland EW, Liddle GW. Effects of parathyroid hormone on plasma and urinary adenosine 3′,5′-monophosphate in man. J Clin Invest 49: 2387–2395, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kittikulsuth W, Stuart D, Van Hoek AN, Stockand JD, Bugaj V, Mironova E, Blount MA, Kohan DE. Lack of an effect of collecting duct-specific deletion of adenylyl cyclase 3 on renal sodium and water excretion or arterial pressure. Am J Physiol Renal Physiol (First published January 15, 2014). 10.1152/ajprenal.00505.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knox FG, Preiss J, Kim JK, Dousa TP. Mechanism of resistance to the phosphaturic effect of the parathyroid hormone in the hamster. J Clin Invest 59: 675–683, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem 277: 18598–18604, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Linder JU. Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell Mol Life Sci 63: 1736–1751, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda A, Okazaki M, Baron DM, Dean T, Khatri A, Mahon M, Segawa H, bou-Samra AB, Juppner H, Bloch KD, Potts JT, Jr, Gardella TJ. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA 110: 5864–5869, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin KJ, Stokes TJ, Jr, McConkey CL., Jr Influence of forskolin on the parathyroid hormone dependent adenylate cyclase system of canine kidney: evidence for noncatalytic effects of forskolin. Endocrinology 115: 1678–1682, 1984 [DOI] [PubMed] [Google Scholar]
- 49.Moeller HB, Fenton RA. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflügers Arch 464: 133–144, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Morath R, Klein T, Seyberth HW, Nusing RM. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol 10: 1851–1860, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Bohlick A, Bleich M, Shan Q, Bachmann S. Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298: F502–F509, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension 38: 1143–1149, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform v mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Pastor-Soler N, Beaulieu V, Litvin TN, Da SN, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem 278: 49523–49529, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paunescu TG, Da SN, Russo LM, McKee M, Lu HA, Breton S, Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–F138, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 8: 321–335, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Pines M, Polin D, Hurwitz S. Urinary cyclic AMP excretion in birds: dependence on parathyroid hormone activity. Gen Comp Endocrinol 49: 90–96, 1983 [DOI] [PubMed] [Google Scholar]
- 59.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rees S, Kittikulsuth W, Roos K, Strait KA, Van HA, Kohan DE. Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J Am Soc Nephrol 25: 232–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricci A, Collier WL, Rossodivita I, Amenta F. Dopamine receptors mediating inhibition of the cyclic adenosine monophosphate generating system in the rat renal cortex. J Auton Pharmacol 11: 121–127, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rieg T, Sharik M, Tang T, Aroonsakool A, Murray F. PTH-stimulated urinary phosphate and cAMP excretion are absent in mice lacking adenylyl cyclase 6. FASEB J 27: A1210.–14., 2013 [Google Scholar]
- 65.Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, Hammond HK, Vallon V. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol 21: 2059–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol 182: 96–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roos KP, Bugaj V, Mironova E, Stockand JD, Ramkumar N, Rees S, Kohan DE. Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J Am Soc Nephrol 24: 218–227, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE. Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302: F78–F84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenbaek LL, Assentoft M, Pedersen NB, MacAulay N, Fenton RA. Characterization of a novel phosphorylation site in the sodium-chloride cotransporter, NCC. J Physiol 590: 6121–6139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17: 5–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demaretz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K. SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24: 407–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasaki S, Imai M. Effects of vasopressin on water and NaCl transport across the in vitro perfused medullary thick ascending limb of Henle's loop of mouse, rat, and rabbit kidneys. Pflügers Arch 383: 215–221, 1980 [DOI] [PubMed] [Google Scholar]
- 73.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 65: 670–709, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Schoeppe W. Effects of dopamine on kidney function. Proc R Soc Med 70, Suppl 2: 36–42, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78: 3363–3367, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seifert R, Lushington GH, Mou TC, Gille A, Sprang SR. Inhibitors of membranous adenylyl cyclases. Trends Pharmacol Sci 33: 64–78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen T, Suzuki Y, Poyard M, Miyamoto N, Defer N, Hanoune J. Expression of adenylyl cyclase mRNAs in the adult, in developing, and in the Brattleboro rat kidney. Am J Physiol Cell Physiol 273: C323–C330, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Slatopolsky E, Mercado A, Morrison A, Yates J, Klahr S. Inhibitory effects of hypermagnesemia on the renal action of parathyroid hormone. J Clin Invest 58: 1273–1279, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strait KA, Stricklett PK, Chapman M, Kohan DE. Characterization of vasopressin-responsive collecting duct adenylyl cyclases in the mouse. Am J Physiol Renal Physiol 298: F859–F867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 Study. Am J Kidney Dis 57: 692–699, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int 79: 1277–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111: 39–53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM. Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Xiao Z, He L, Takemoto M, Jalanko H, Chan GC, Storm DR, Betsholtz C, Tryggvason K, Patrakka J. Glomerular podocytes express type 1 adenylate cyclase: inactivation results in susceptibility to proteinuria. Nephron Exp Nephrol 118: e39–e48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yip KP. Epac-mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Yoder BK, Mulroy S, Eustace H, Boucher C, Sandford R. Molecular pathogenesis of autosomal dominant polycystic kidney disease. Expert Rev Mol Med 8: 1–22, 2006 [DOI] [PubMed] [Google Scholar]