Abstract
Picrotoxin, an antagonist for γ-aminobutyric acid receptor subtype A (GABAA), was used to investigate the role of GABAA receptors in nociceptive and nonnociceptive reflex bladder activities and pudendal inhibition of these activities in cats under α-chloralose anesthesia. Acetic acid (AA; 0.25%) was used to irritate the bladder and induce nociceptive bladder overactivity, while saline was used to distend the bladder and induce nonnociceptive bladder activity. To modulate the bladder reflex, pudendal nerve stimulation (PNS) was applied at multiple threshold (T) intensities for inducing anal sphincter twitching. AA irritation significantly (P < 0.01) reduced bladder capacity to 34.3 ± 7.1% of the saline control capacity, while PNS at 2T and 4T significantly (P < 0.01) increased AA bladder capacity to 84.0 ± 7.8 and 93.2 ± 15.0%, respectively, of the saline control. Picrotoxin (0.4 mg it) did not change AA bladder capacity but completely removed PNS inhibition of AA-induced bladder overactivity. Picrotoxin (iv) only increased AA bladder capacity at a high dose (0.3 mg/kg) but significantly (P < 0.05) reduced 2T PNS inhibition at low doses (0.01–0.1 mg/kg). During saline cystometry, PNS significantly (P < 0.01) increased bladder capacity to 147.0 ± 7.6% at 2T and 172.7 ± 8.9% at 4T of control capacity, and picrotoxin (0.4 mg it or 0.03–0.3 mg/kg iv) also significantly (P < 0.05) increased bladder capacity. However, picrotoxin treatment did not alter PNS inhibition during saline infusion. These results indicate that spinal GABAA receptors have different roles in controlling nociceptive and nonnociceptive reflex bladder activities and in PNS inhibition of these activities.
Keywords: urinary bladder, neuromodulation, GABAA receptor, cat
overactive bladder (OAB), which is characterized by urgency with or without urge incontinence usually with frequency and nocturia (1), affects ∼30 million people in United States (6). The etiology and pathophysiology of OAB are still unknown (22). However, it is known that reflex bladder activity is controlled by two distinct neural pathways (9): a spinobulbospinal micturition reflex pathway activated by nonnociceptive Aδ afferents and a spinal micturition reflex pathway activated by nociceptive C-fiber afferents (9, 10). Understanding these two micturition reflexes is important for developing new OAB treatments.
Currently, antimuscarinic drugs are the first-line pharmacotherapy for OAB, which have a moderate efficacy and significant side effects such as dry mouth, constipation, and blurred vision (2, 5). Therefore, FDA-approved sacral or tibial neuromodulation becomes an attractive option for OAB patients after the failure of pharmacotherapy (29, 34). Recent clinical trials also showed that pudendal neuromodulation can be used to treat the OAB patients who are refractory to sacral neuromodulation (27, 28). However, the mechanisms underlying neuromodulation of OAB are still uncertain.
Gamma-aminobutyric acid (GABA), which is a major inhibitory neurotransmitter at both spinal and supraspinal synapses (13, 18), has been implicated in the control of micturition in animals. In cats application of GABA or GABAA receptor agonists by the intrathecal route (11), iontophoretically to bladder parasympathetic preganglionic neurons in the sacral spinal cord (7), or by local injection into the pontine micturition center (19) inhibits preganglionic neuron firing and/or reflex bladder activity. Conversely, intrapontine administration of a GABAA receptor antagonist (bicuculline) facilitates bladder reflexes (19). In rats intrathecal injection of a GABAA agonist also inhibits the micturition reflex (12, 15, 23), and electrical stimulation of local interneurons elicits GABAergic inhibitory postsynaptic currents in parasympathetic preganglionic neurons (4), indicating an inhibitory role of spinal GABAA receptors.
Because pudendal nerve stimulation (PNS) also elicits inhibitory postsynaptic potentials in bladder parasympathetic preganglionic neurons in the cat (8), we hypothesize that pudendal neuromodulation might inhibit bladder overactivity by stimulating spinal GABAergic inhibitory mechanisms. In this study using anesthetized cats, the bladder was distended with either saline to activate the nonnociceptive Aδ-afferent fiber-mediated supraspinal micturition reflex or irritated with dilute (0.25%) acetic acid (AA) to activate the nociceptive C-fiber afferent-mediated spinal micturition reflex. PNS was applied to inhibit these reflexes and mimic pudendal neuromodulation. Picrotoxin (a GABAA receptor antagonist) was administered intravenously (iv) or intrathecally (it) to examine the role of GABAA receptors in PNS inhibition. Understanding neurotransmitter mechanisms involved in PNS inhibition could identify new molecular targets for pharmacological treatment of OAB or develop new neuromodulation therapies combined with pharmacological treatments.
MATERIALS AND METHODS
The Animal Care and Use Committee of University of Pittsburgh approved the protocol and animal use in our study.
Surgical procedure.
Thirty-three cats (15 males and 18 females, 2.2–4.2 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–3% in oxygen) during surgery and then with α-chloralose anesthesia (initial iv dose of 65 mg/kg followed by 2 mg·kg−1·h−1) during data collection. Pancuronium (initial iv dose of 0.05 mg/kg followed by 0.05 mg·kg−1·h−1) was also given during data collection to prevent striated muscle contractions. Right and left cephalic veins were catheterized for intravenous administration of drugs and fluid. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue.
Through an abdominal incision, the ureters were isolated and cut for external drainage. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen was connected to a pump to slowly (1–2 ml/min) infuse saline or 0.25% AA. The other lumen was attached to a pressure transducer to measure bladder pressure. The right pudendal nerve was dissected via a 3- to 4-cm incision in the sciatic notch lateral to the tail. A tripolar cuff electrode (NC223pt; MicroProbe, Gaithersburg, MD) was implanted around the pudendal nerve and connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA) via a constant voltage stimulus isolator (SIU5; Grass Medical Instruments). In 10 cats, a small incision was made to remove the L3 spinal process and expose the spinal cord. Then, the spinal dura was pierced and a fine catheter (PE10) was inserted caudally underneath the dura to position the catheter tip at the level of S1 sacral spinal cord for intrathecal administration of picrotoxin. The location of the intrathecal catheter was confirmed by a postmortem laminectomy between the L5-S3 spinal processes. After the surgery, the skin and muscle layers were closed by sutures.
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-ms pulse width) were used to stimulate the pudendal nerve. The stimulation threshold (T), which was defined as the minimal intensity for inducing anal sphincter twitching, was determined before administration of pancuronium. PNS of multiple intensity thresholds (2T/4T) was used to inhibit bladder activity.
Initially cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that is defined as the bladder volume threshold required to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Multiple CMGs were performed to ensure reproducibility of the saline control capacity. Then, the animals were divided into two groups.
In the first group (n = 13), repeated CMGs were performed with infusion of 0.25% AA to irritate the bladder, activate nociceptive bladder afferent C fibers, and induce bladder overactivity. When the bladder capacity stabilized, four CMGs were performed with AA infusion before administration of picrotoxin: 1) control CMG without PNS, 2) CMG during 2T PNS, 3) CMG during 4T PNS, and 4) control CMG without PNS to determine any poststimulation effect. The bladder was emptied at the end of each CMG, and a 3- to 5-min rest period was inserted between CMGs. After the predrug CMGs were performed, cumulative doses (0.01, 0.03, 0.1, and 0.3 mg/kg iv) of picrotoxin (Sigma-Aldrich, St. Louis, MO) were given in eight cats. Ten minutes after each dose of picrotoxin was administered, the four AA CMGs were again performed under the four conditions (control, 2T PNS, 4T PNS, and post-PNS control). In another five cats instead of intravenous administration, a single intrathecal dose (0.4 mg) of picrotoxin was given. Ten minutes after intrathecal injection, the four AA CMGs were performed again.
In the second group (n = 20), repeated saline CMGs were performed to activate nonnociceptive Aδ-afferent-initiated micturition reflexes. Before administration of picrotoxin, four saline CMGs were performed: 1) control CMG without PNS, 2) CMG during 2T PNS, 3) CMG during 4T PNS, and (4) control CMG without PNS to determine any poststimulation effect. Then, cumulative doses (0.01, 0.03, 0.1, and 0.3 mg/kg iv) of picrotoxin were given in 15 cats. Ten minutes after each dose of picrotoxin was administered, the saline CMGs were again performed under the four conditions (control, 2T PNS, 4T PNS, and post-PNS control). In another five cats, instead of intravenous administration, a single intrathecal dose (0.4 mg) of picrotoxin was given. Ten minutes after intrathecal injection, the four saline CMGs were performed again.
Data analysis.
For each CMG bladder capacity was normalized to the initial saline control capacity in the same animal, which allowed for comparisons between animals. The bladder capacities were averaged for each condition and are reported with SE. Student's t-test or ANOVA followed by Dunnett or Bonferroni posttests was used to determine statistical significance (P < 0.05).
RESULTS
Effect of picrotoxin on PNS inhibition of nociceptive bladder overactivity.
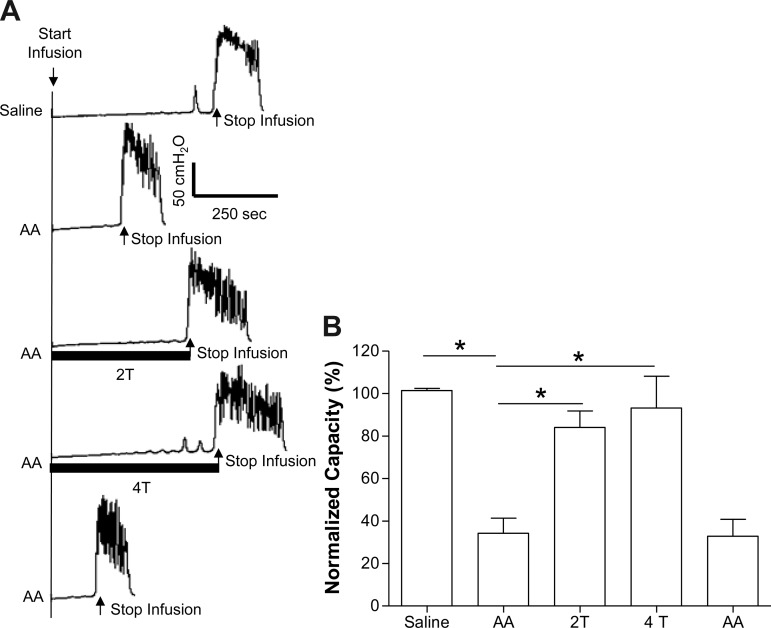
After saline control CMGs, AA-induced bladder irritation significantly (P < 0.01) reduced bladder capacity to 34.3 ± 7.1% (2.8 ± 1.2 ml) of saline control capacity (8.3 ± 1.3 ml; Fig. 1). PNS significantly (P < 0.01) inhibited bladder overactivity and increased capacity to 84.0 ± 7.8% at 2T and 93.2 ± 15.0% at 4T of saline control (T = 1.1 ± 0.2 V). Poststimulation AA control capacity was not different from prestimulation AA control demonstrating that there was no poststimulation effect (Fig. 1).
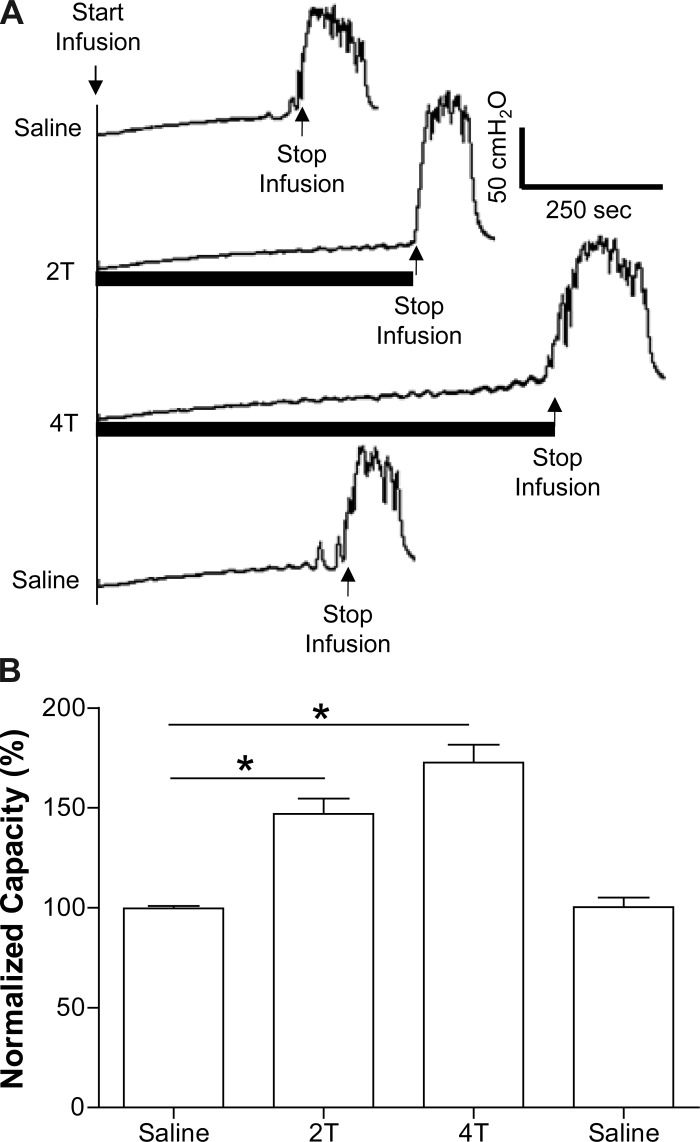
Fig. 1.
Acetic acid (AA) irritation decreases bladder capacity and pudendal nerve stimulation (PNS) increases bladder capacity. A: cystometrograms (CMGs) during saline or 0.25% AA infusion with/without PNS. T: stimulation intensity threshold to induce anal sphincter twitch. Black bars under pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T =1 V). Infusion rate = 1 ml/min. B: summarized results from 8 cats. The bladder capacity is normalized to the measurement during saline infusion. *Significantly different (one-way ANOVA). Infusion rate = 1–2 ml/min.
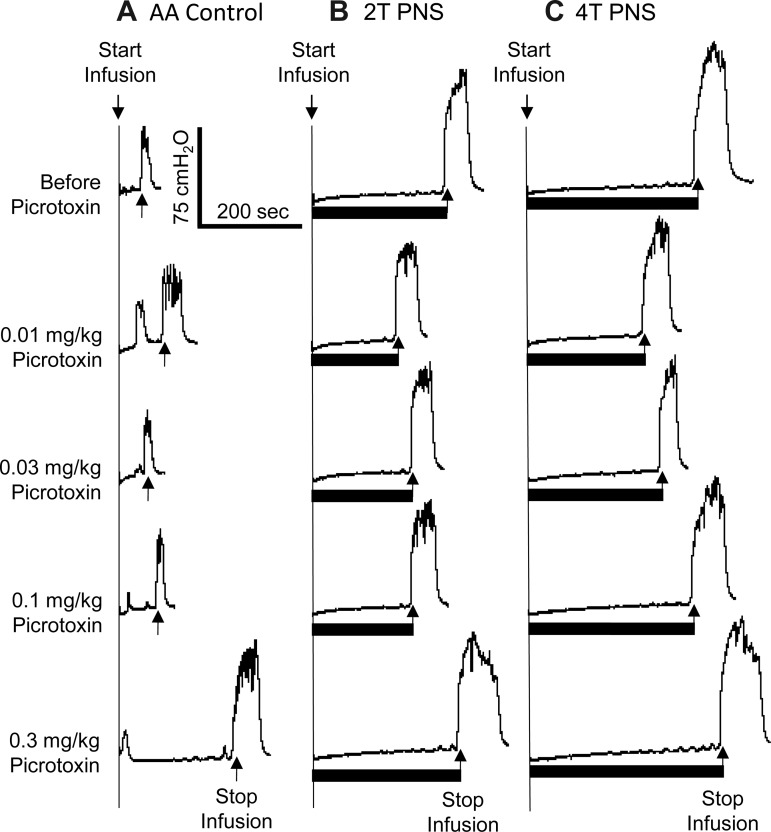
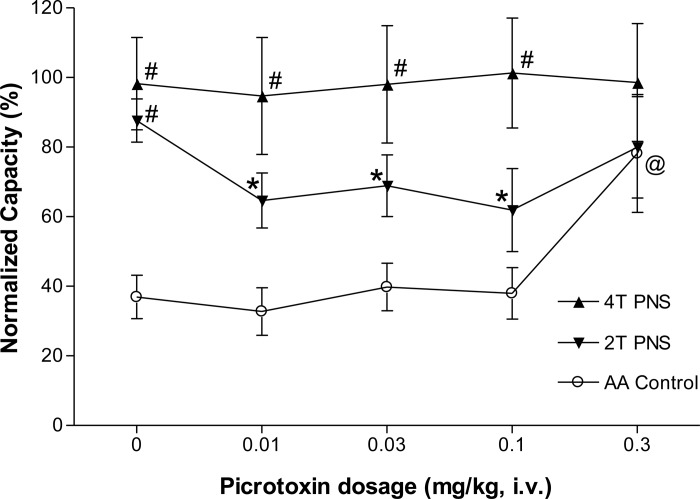
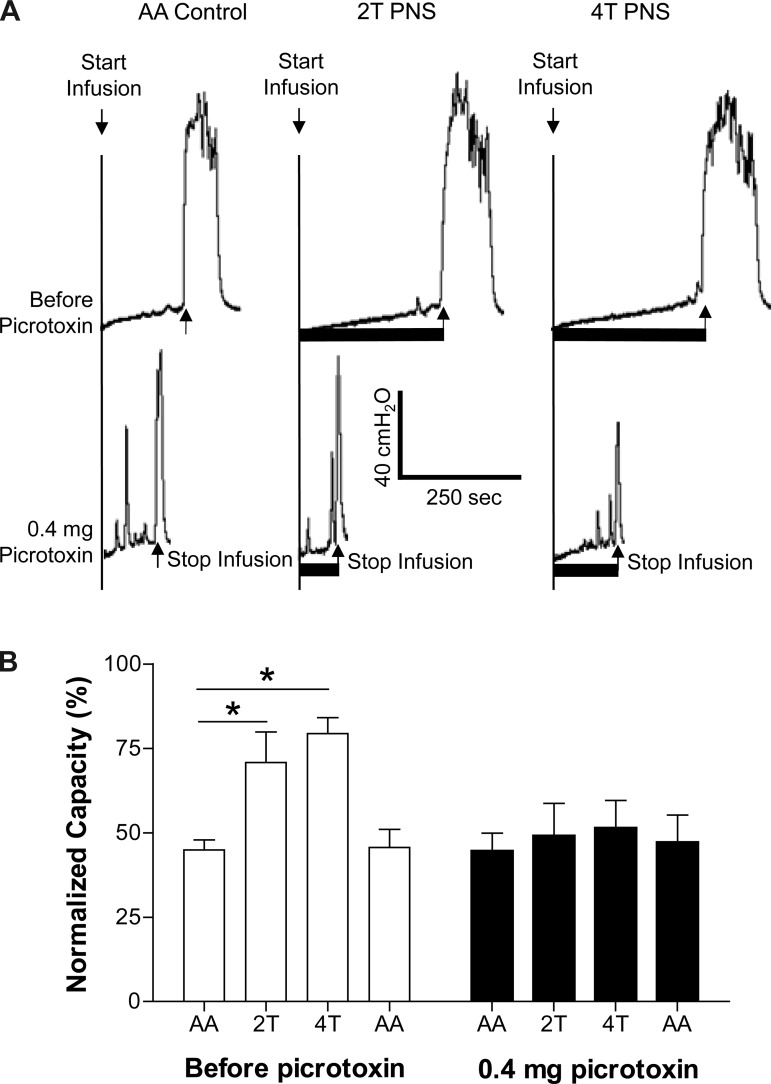
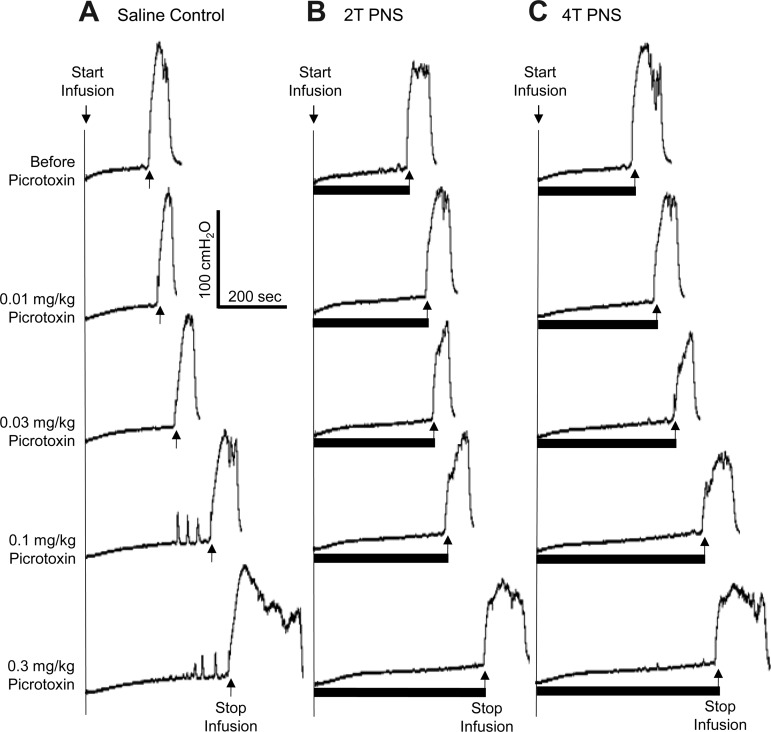
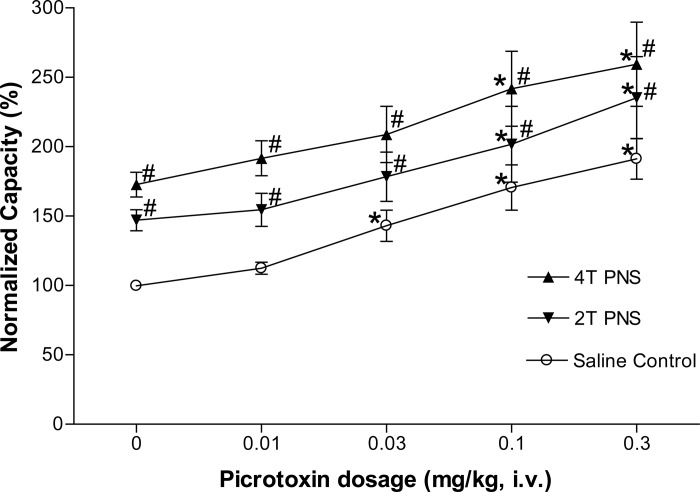
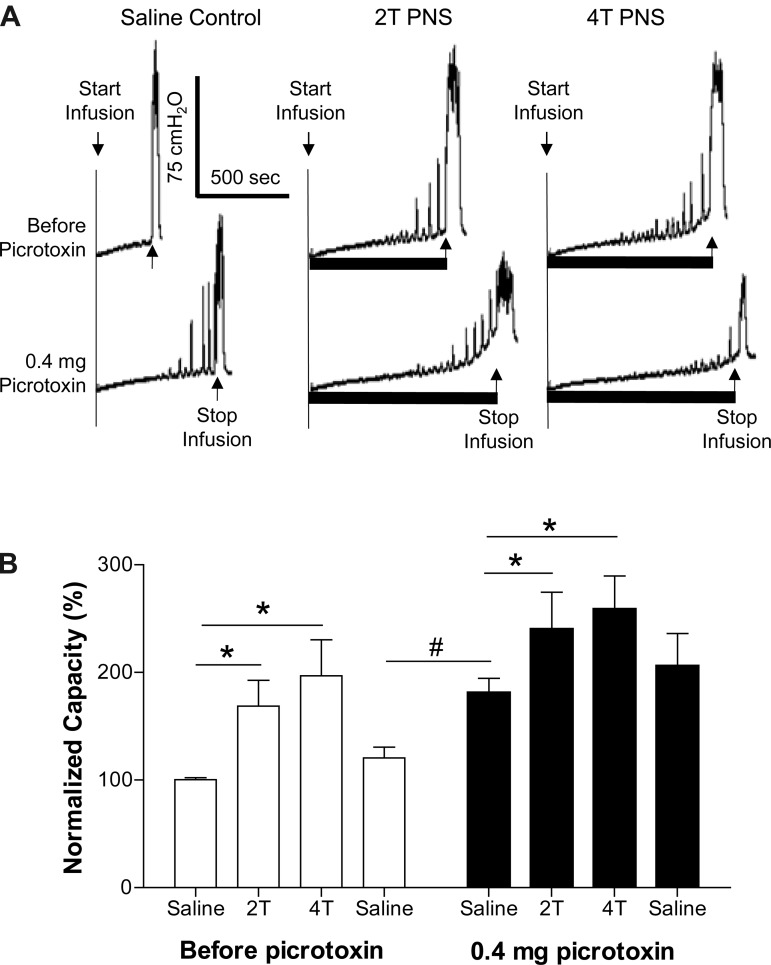
Picrotoxin at low doses (0.01–0.1 mg/kg iv) significantly (P < 0.05) reduced the inhibition induced by 2T PNS but did not significantly change the inhibition elicited by 4T PNS (Figs. 2 and 3). After the high dose of picrotoxin (0.3 mg/kg), which significantly (P < 0.05) increased AA control bladder capacity, neither 2T nor 4T PNS significantly increased bladder capacity. In another group of cats, intrathecal administration of picrotoxin (0.4 mg) did not change the AA control bladder capacity but completely eliminated the inhibition induced by both 2T and 4T PNS (Fig. 4).
Fig. 2.
Effect of picrotoxin (iv) on the inhibition induced by PNS during CMGs with bladder infusion of 0.25% AA. The CMGs at increasing cumulative doses of picrotoxin were performed in sequence from left to right and from top to bottom in A–C. A: AA CMGs without PNS. B: AA CMGs during 2T PNS. C: AA CMGs during 4T PNS. Black bars under the pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T = 1.2 V). Infusion rate = 1 ml/min.
Fig. 3.
Summarized results of picrotoxin (iv) effect on the inhibition induced by PNS during bladder infusion of 0.25% AA. #Significantly different from AA control data (two-way ANOVA). *Significantly different from the bladder capacity measured before picrotoxin treatment (0 mg/kg) during 2T PNS. @Significantly different from the bladder capacity measured before picrotoxin treatment (0 mg/kg) without PNS (one-way ANOVA). PNS: 5 Hz, 0.2 ms, T = 0.4–1.6 V; n = 8 cats.
Fig. 4.
Effect of picrotoxin (it) on the inhibition induced by PNS during CMGs with bladder infusion of 0.25% AA. A: CMGs performed before and after intrathecal picrotoxin. Black bars under the pressure trace indicate PNS duration. PNS: 5 Hz, 0.2 ms, T = 0.3 V. Infusion rate = 2 ml/min. B: summarized results of picrotoxin (it) effect on PNS inhibition of bladder overactivity. *Significantly different (one-way ANOVA). PNS: 5 Hz, 0.2 ms, T = 0.25–1.8 V; n = 5 cats.
Effect of picrotoxin on PNS inhibition of nonnociceptive bladder activity.
During saline distention, PNS significantly (P < 0.01) increased capacity to 147.0 ± 7.6% at 2T and 172.7 ± 8.9% at 4T of saline control (9.2 ± 1.0 ml; Fig. 5). Poststimulation control capacity was not different from prestimulation saline control demonstrating that there was no poststimulation effect (Fig. 5).
Fig. 5.
Inhibition of normal bladder reflex by PNS during saline infusion. A: CMGs during saline infusion with/without PNS. T: stimulation intensity threshold to induce anal sphincter twitch. Black bars under pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T = 0.35 V). Infusion rate = 1 ml/min. B: summarized results from 15 cats. The bladder capacity is normalized to the measurement during first CMG without PNS. *Significantly different (one-way ANOVA). PNS: 5 Hz, 0.2 ms, T = 0.25–2.2 V. Infusion rate = 1–2 ml/min.
Picrotoxin (iv) significantly (P < 0.05) increased the saline control bladder capacity at doses of 0.03–0.3 mg/kg (Figs. 6 and 7). Bladder inhibition induced by either 2T or 4T PNS was unchanged (50–80% increase in bladder capacity) after each dose of picrotoxin (Figs. 6 and 7). Picrotoxin (0.4 mg it) also significantly (P < 0.05) increased the saline control bladder capacity and the inhibition induced by either 2T or 4T PNS was maintained (Fig. 8).
Fig. 6.
Dose-dependent effect of picrotoxin (iv) on the bladder reflex and inhibition induced by PNS during saline infusion. The CMGs at increasing cumulative doses of picrotoxin were performed in sequence from left to right and from top to bottom in A–C. A: saline CMGs without stimulation. B: saline CMGs during 2T PNS. C: saline CMGs during 4T PNS. The black bars under the pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T = 1.4 V). Infusion rate = 2 ml/min.
Fig. 7.
Dose-dependent effect of picrotoxin (iv) on bladder reflex and inhibition induced by PNS during saline infusion CMG. #Significantly different from the saline control data (two-way ANOVA). *Significantly different from the bladder capacity measured before picrotoxin treatment (0 mg/kg) without PNS or with 2T/4T PNS (5 Hz, 0.2 ms, T = 0.25–2.2 V, one-way ANOVA); n = 15 cats.
Fig. 8.
Effect of picrotoxin (it) on inhibition induced by PNS during saline infusion CMG. A: CMGs were performed before and after picrotoxin (it) treatment. The black bars under the pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T = 1.2 V). B: summarized results. *Significantly different by one-way ANOVA. #Significantly different by student t-test. PNS: 5 Hz, 0.2 ms, T = 0.32–1.4 V; n = 5 cats.
DISCUSSION
This study in chloralose-anesthetized cats examined the role of spinal GABAA receptor inhibitory mechanisms in the regulation of the micturition reflex and in pudendal neuromodulation of the micturition reflex in normal bladders during saline CMGs and in irritated overactive bladders during AA CMGs. During saline CMGs administration (iv or it) of picrotoxin, a GABAA receptor antagonist, increased bladder capacity but did not alter the increase in bladder capacity elicited by 2T or 4T PNS (Figs. 7 and 8). On the other hand, in AA-irritated bladders picrotoxin in low intravenous doses (0.01–0.1 mg/kg) reduced 2T PNS inhibition or after an intrathecal dose (0.4 mg) suppressed both 2T and 4T PNS inhibition without altering the control bladder capacity (Figs. 3 and 4). These results indicate different roles of spinal GABAA receptors in the control of the micturition reflex and in PNS inhibition in normal and irritated overactive bladders.
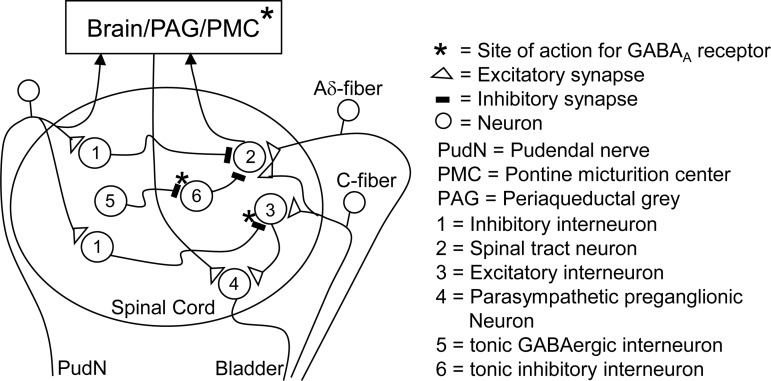
The effect of intrathecal (0.4 mg) or low intravenous doses (0.03–0.1 mg/kg) of picrotoxin to increase bladder capacity during saline CMGs was unexpected because blocking receptors for a major inhibitory transmitter would be expected to facilitate the micturition reflex. Indeed, injections of a GABAA receptor antagonist into the pontine micturition center (PMC) of cats (19, 25) or the periaqueductal gray (PAG) area in rats (30) reduces bladder capacity and/or increases the frequency of reflex activity. The opposite result in the present experiments after intrathecal or intravenous administration might have several explanations. First, the ascending limb of spinobulbospinal micturition reflex triggered by nonnociceptive Aδ-afferent nerves might be tonically suppressed by a mechanism (inhibitory interneuron 6 in Fig. 9) that is in turn tonically suppressed by activation of GABAA receptors located at the synapse between neurons 5 and 6 in Fig. 9. Blocking the latter with picrotoxin would remove the GABAergic control and enhance the inhibition of neuron 2 in the micturition reflex pathway by neuron 6 and thereby reduce excitatory input to the PMC and increase bladder capacity but not necessarily block the micturition reflex. It is known that the descending efferent limb of the spinobulbospinal micturition reflex pathway is inhibited at the spinal level by coactivation of glycinergic and GABAergic mechanisms (33); however, the effect of picrotoxin on bladder capacity in our study is not likely due to its action on the descending limb of the micturition reflex because this should increase the amplitude of bladder contractions rather than increase bladder capacity. The sensitivity of the spinobulbospinal pathway to the inhibitory effect of intravenous picrotoxin could also reflect the actions of the drug on other sites in the brain (Fig. 9) that modulate the PMC because injections of GABA into other pontine areas in cats can either excite or inhibit the bladder (26), indicating complex brain GABAergic mechanisms in micturition control.
Fig. 9.
The possible locations (*) in the sacral spinal cord and brain stem of GABAA receptors that participate in 1) the tonic inhibition of the spinobulbospinal micturition reflex pathway or 2) the micturition inhibitory mechanism evoked by PNS. *Possible sites of action of the GABAA receptor antagonist.
The lack of effect of intrathecal (0.4 mg) or low intravenous doses (0.01–0.1 mg/kg) of picrotoxin on bladder capacity after AA irritation of the bladder suggests that the bladder reflexes triggered by nociceptive and nonnociceptive stimuli are mediated by distinct reflex pathways (spinal and spinobulbospinal, see Fig. 9) that are controlled by different inhibitory mechanisms. These data are consistent with previous conclusions regarding the existence of two micturition reflex mechanisms based on the differences in the effects of naloxone (an opioid receptor antagonist) (32) or MTEP (a metabotropic glutamate receptor-5 antagonist) (16) on reflex bladder activity elicited by nociceptive and nonnociceptive stimuli.
However, it is noteworthy that the largest intravenous dose of picrotoxin (0.3 mg/kg) did increase AA bladder capacity. Because this effect did not occur with intrathecal administration of a large dose of the drug, it is reasonable to conclude that it was due to an action of picrotoxin at sites in the brain (Fig. 9), which influenced descending inputs to the sacral spinal cord.
The suppression by intrathecal or intravenous picrotoxin of 2T PNS inhibition of bladder overactivity induced by AA irritation (Figs. 3 and 4) and the resistance to picrotoxin of PNS inhibition of normal bladder reflexes during saline CMGs (Figs. 7 and 8) provide further evidence for the two distinct micturition reflex mechanisms. Furthermore, because PNS inhibition was eliminated by intrathecal picrotoxin during AA CMG (Fig. 4), the inhibition must occur at the level of the spinal cord where the pudendal afferent input activates GABAA inhibitory interneuron (interneuron 1 in Fig. 9) to inhibit the excitatory interneuron (interneuron 3 in Fig. 9) in the bladder C-fiber afferent-mediated spinal micturition reflex pathway. However, because intrathecal picrotoxin did not alter bladder capacity in the absence of PNS (Fig. 4), it is also reasonable to conclude that the putative inhibitory pathway is inactive in the absence of PNS and does not control gating of the micturition reflex under nociceptive conditions.
Although intrathecal administration of picrotoxin antagonized the inhibition of bladder overactivity induced by 2T and 4T PNS (Fig. 4), intravenous administration of picrotoxin in doses (0.01–0.1 mg/kg) that antagonized 2T PNS-induced inhibition did not antagonize the inhibition elicited by 4T PNS (Fig. 3). This difference between the sensitivity of 2T and 4T PNS to intravenous picrotoxin raises the possibility that the latter is mediated by a non-GABAergic inhibition. However, we believe that this is unlikely because intrathecal picrotoxin blocked the effect of both intensities of stimulation. Thus higher intravenous doses of picrotoxin might be necessary to block the inhibitory effect of more intense PNS. After the highest intravenous dose of picrotoxin (0.3 mg/kg), 4T PNS failed to significantly increase capacity (Fig. 3) suggesting that the inhibition was completely blocked. However, the interpretation of this result is complicated because this dose of picrotoxin alone significantly increased bladder capacity, which might simply occlude the response to PNS. However, when picrotoxin increased bladder capacity during saline CMGs, the 4T PNS still further increased bladder capacity, a finding that argues against the possibility of occlusion.
The role of central GABAA mechanisms in the control of nonnociceptive bladder activity has also been investigated in rats (3). GABAA agonists (it or icv) inhibited reflex bladder activity (12, 14, 15, 17), while a GABAA antagonist (it) excited the bladder (12, 17, 23). In addition, a GABAA agonist or antagonist injected into the PAG inhibited and excited, respectively, reflex bladder activity (30). In the neonatal rat isolated spinal cord-bladder preparation, a GABAA antagonist also enhanced reflex bladder activity (31). These results indicate that the central GABAA mechanisms mediate a tonic inhibition of nonnociceptive bladder activity in rats, which is different from the results in cats (Figs. 7 and 8).
A previous study in rats showed that spinal GABAA receptors are involved in the rectal distention-induced inhibition of nonnociceptive bladder activity (24). Rectal distention can activate the pudendal afferent nerve branch innervating the distal bowel and therefore might be expected to mimic the effects of PNS. Since our study in the cat failed to demonstrate a role of spinal GABAA receptors in PNS inhibition of nonnociceptive bladder activity, these data indicate either a difference in the activation of different pudendal afferent nerve branches or another difference between rats and cats.
However, there are also reports in rats showing that bicuculline (iv or icv) can produce variable responses including a transient inhibition of isovolumetric bladder contractions, a significant increase in bladder capacity, or no effect (14, 17, 21). These variable responses might be due to variations in dosages or routes of administration in different studies. In addition, in rats a high intravenous dose of bicuculline can induce an initial excitation and/or convulsions lasting for 3–10 min followed by a prolonged (15–30 min) inhibition of neural activity (14, 17). Hence, the different results between cats and rats might be due to studying the effects of the drug at different times after administration.
A comparison of the present findings with the results of previous studies of tibial nerve neuromodulation of bladder activity in the cat indicates that the mechanisms of neuromodulation of reflex bladder activity can vary markedly between different somatic afferent pathways. Opioid receptors play a major role in tibial neuromodulation of nociceptive bladder overactivity (32) but have no role in PNS inhibition of this activity (20). Instead, spinal GABAA receptors play a key role in this type of PNS inhibition (Fig. 4).
This study also revealed a differential role of spinal GABAA receptors in nociceptive and nonnociceptive bladder reflexes and PNS inhibition of these reflexes (Figs. 4 and 8). A similar differential role of opioid receptors has also been observed with tibial neuromodulation of these bladder reflexes (32). These results suggest a more general concept regarding the different role of neurotransmitter mechanisms in micturition control and in somatic neuromodulation. GABAA and opioid inhibitory mechanisms are tonically activated under normal (i.e., nonnociceptive) physiological conditions to either facilitate (Figs. 7 and 8) or suppress (32) the Aδ-fiber afferent-mediated spinobulbospinal bladder reflex. Therefore, somatic afferent neuromodulation of normal bladder activity does not use these mechanisms to inhibit the normal bladder reflex (Figs. 7 and 8) (32). However, in a pathological condition after bladder irritation, GABAA and opioid inhibitory neurotransmitter mechanisms do not tonically regulate the nociceptive C-fiber afferent-mediated spinal reflex (Fig. 4) (32). Therefore, these inhibitory neurotransmitter mechanisms can be recruited by PNS (Fig. 4) or tibial nerve stimulation (32) to induce an antinociceptive effect and correct the abnormal bladder overactivity.
This study in cats revealed an important role of spinal GABAA receptors in PNS inhibition of bladder overactivity. It also revealed a tonic facilitatory role of GABAA receptors in the control of normal bladder reflex activity. Understanding the neurotransmitter mechanisms involved in bladder reflexes and neuromodulation of these reflexes may identify molecular targets for new OAB therapies including the combination of drugs with neuromodulation to enhance the efficacy of treatments.
GRANTS
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-094905, DK-090006, DK-068566, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. conception and design of research; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; Z.X., J.R., Z.S., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urol 63, Suppl 1: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63: 2595–2611, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J Neurophysiol 72: 2903–2910, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54: 543–562, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77: 1081–1087, 2011 [DOI] [PubMed] [Google Scholar]
- 7.de Groat WC. The effect of glycine, GABA and strychnine on sacral parasympathetic preganglionic neurons. Brain Res 18: 542–544, 1970 [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol 200: 87–108, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in cat. Brain Res 298: 51–65, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Igawa Y, Mattiasson A, Andersson KE. Effects of GABA-receptor stimulation and blockade on micturition in normal rats and rats with bladder outflow obstruction. J Urol 150: 537–542, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Jursky F, Tamura S, Tamura A, Mandiyan S, Nelson H, Nelson N. Structure, function and brain localization of neurotransmitter transporters. J Exp Biol 196: 283–295, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Kanie S, Yokoyama O, Komatsu K, Kodama K, Yotsuyanagi S, Niikura S, Nagasaka Y, Miyamoto KI, Namiki M. GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol Regul Integr Comp Physiol 279: R1230–R1238, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kontani H, Kawabata Koshiura YR. In vivo effects of gamma-aminobutyric acid on the urinary bladder contraction accompanying micturition. Jpn J Pharmacol 45: 45–53, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi CA, Furio M, Santicioli P, Conte B, Meli A. Spinal and supraspinal components of GABAergic inhibition of the micturition reflex in rats. J Pharmacol Exp Ther 240: 998–1005, 1987 [PubMed] [Google Scholar]
- 18.Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci 17: 457–462, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Mallory BS, Roppolo JR, de Groat WC. Pharmacological modulation of the pontine micturition center. Brain Res 546: 310–320, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuta Y, Yusup A, Tanase K, Ishida H, Akino H, Yokoyama O. Melatonin increases bladder capacity via GABAergic system and decreases urine volume in rats. J Urol 184: 386–391, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Miller J, Hoffman E. The causes and consequences of overactive bladder. J Womens Health 15: 251–260, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am J Physiol Regul Integr Comp Physiol 295: R336–R342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazato M, Sugaya K, Nishijima S, Ashitomi K, Ohyama C, Ogawa Y. Rectal distention inhibits bladder activity via glycinergic and GABAergic mechanisms in rats. J Urol 171: 1353–1356, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Munata A, Iwata T, Iuchi H, Taniguchi N, Kita M, Wada N, Kato Y, Kakizaki H. Micturition-suppression region in the periaqueductal gray of the mesencephalon of the cat. Am J Physiol Regul Integr Comp Physiol 294: R1996–R2000, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Nishizawa O, Sugaya K, Shimoda N. Pontine and spinal modulation of the micturition reflex. Scand J Urol Nephrol 175, Suppl: 15–19, 1995 [PubMed] [Google Scholar]
- 27.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29: 1267–1271, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Stone E, Coote JH, Allard J, Lovick TA. GABAergic control of micturition within the periaqueductal grey matter of the male rat. J Physiol 589: 2065–2078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugaya K, de Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol Regul Integr Comp Physiol 266: R658–R667, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomson TD, Franz DN. Amino acid and monoamine inhibition of sacral parasympathetic preganglionic neurons. Prog Clin Biol Res 68: 181–189, 1981 [PubMed] [Google Scholar]
- 34.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama a Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007 [DOI] [PubMed] [Google Scholar]