Abstract
Mammary gland (MG) de novo lipogenesis contributes significantly to milk fat in animals but little is known in humans. Objective: To test the hypothesis that the incorporation of 13C carbons from [U-13C]glucose into fatty acids (FA) and glycerol in triglycerides (TG) will be greater: 1) in milk than plasma TG, 2) during a high-carbohydrate (H-CHO) diet than high-fat (H-FAT) diet, and 3) during feeding than fasting. Seven healthy, lactating women were studied on two isocaloric, isonitrogenous diets. On one occasion, subjects received diets containing H-FAT or H-CHO diet for 1 wk. Incorporation of 13C from infused [U-13C]glucose into FA and glycerol was measured using GC-MS and gene expression in RNA isolated from milk fat globule using microarrays. Incorporation of 13C2 into milk FA increased with increased FA chain length from C2:0 to C12:0 but progressively declined in C14:0 and C16:0 and was not detected in FA>C16. During feeding, regardless of diets, enrichment of 13C2 in milk FA and 13C3 in milk glycerol were ∼3- and ∼7-fold higher compared with plasma FA and glycerol, respectively. Following an overnight fast during H-CHO and H-FAT diets, 25 and 6%, respectively, of medium-chain FA (MCFA, C6–C12) in milk were derived from glucose but increased to 75 and 25% with feeding. Expression of genes involved in FA or glycerol synthesis was unchanged regardless of diet or fast/fed conditions. The human MG is capable of de novo lipogenesis of primarily MCFA and glycerol, which is influenced by the macronutrient composition of the maternal diet.
Keywords: fatty acid, glycerol, GC-MS, gene expression, lipogenesis, stable isotopes
triglycerides (TG) make up 98% of the lipid content and, in humans, contributes 40–50% of the total energy content (28). Total milk lipid content ranges, depending on the species, from 0 to 50% of the total milk volume (20). The fatty acid (FA) composition of the TG is dependents on the dietary FA composition, the dietary carbohydrate/lipid ratio and the species. Milk TG contains FA that are derived from three sources: de novo mammary gland (MG) synthesis, dietary lipids, and endogenous fat stores (adipose or hepatic lipids) (7). Data from a study comparing the milk fats from seven different species (4) revealed that the milk fats of humans, dogs, and guinea pigs are largely (92, 93, and 97%, respectively) made up of long-chain FA (LCFA). The milk fats of horses contain large amounts (∼33%) of medium-chain FA (MCFA); however, the milk fats of cows, sheep, and goats are enriched (13–15%) in short-chain FA (SCFA) (4). The MG is one of three primary lipid-synthesizing organs in the body; the other two are liver and adipose tissue (48). In fact, many of the initial pathways of FA biosynthesis were defined using mammary tissue from lactating ruminants and rodents (1). The unique feature of FA synthesis in the MG is that saturated FA with 6–14 carbons constitutes the major product of de novo FA synthesis in these animals (10, 38, 39). This is because the mammary alveolar cells contain thioesterase II (OLAH), which terminates FA synthesis after the addition of 8–16 carbons (21). Thus, the vast majority of longer-chain FA in milk are derived from the diet or mobilization of endogenous TG (37). Generally, the rate of TG synthesis in the lactating MG depends on the stage of mammary development and is decreased by fasting and starvation in ruminants and rodents but not in species that fast during lactation, such as seals and hibernating bears (14, 34). A number of factors may play regulatory roles in the de novo FA synthetic processes such as insulin, prolactin, and nonesterified FA(32).
The FAs in milk TG are metabolized differently based on their carbon chain length. SCFA and MCFA differ from LCFA in that they are absorbed directly and rapidly oxidized (2), induce satiety (40), and form precursors of important biological molecules (18, 33). MCFA in premature infants fed formulae containing medium chain triglycerides (MCT) have been reported to increase absorption of calcium and magnesium (44) and improve fat and nitrogen absorption (45). Therefore, the possibility to alter favorably the composition of milk lipid through dietary manipulation of maternal diet is appealing.
Hachey et al. (9) used 2H2O to measure the endogenous FA synthesis of C10:0 to C18:0 saturated FA in both milk and plasma TG during consumption of high-fat (H-FAT) and high-carbohydrate (H-CHO) diets in humans. Perhaps, due to the relatively insensitive technique, they did not provide enrichments for either FA ≤C10 in milk or FA ≤C14 in plasma (9). Additionally, enrichments of C16 and C18 were similar or even higher in plasma compared with milk. As a result, they were unable to determine whether these FA in milk were synthesized within or were external to the MG or somewhere else (liver, adipose tissue) and then transported to the MG to be incorporated into milk (9).
In the present study, we used the incorporation of 13C from [U-13C]glucose to measure de novo FA synthesis into specific milk and plasma FA. In addition, we measured the incorporation of 13C into glycerol, the carbon backbone of TG, in both plasma and milk. We applied this approach to study milk FA composition and de novo TG synthesis from [U-13C]glucose in blood and milk compartments following an overnight fast and during continuous feeding of either a H-FAT or a H-CHO diet. We hypothesized that de novo lipogenesis as reflected by the incorporation of 13C carbons from [U-13C]glucose into FA and glycerol in TG would be greater 1) in milk than in plasma TG, 2) during a H-CHO diet than a H-FAT diet under isocaloric and isonitrogenous conditions, and 3) during feeding than during fasting.
MATERIALS AND METHODS
Procedure
Detailed information regarding subjects and procedures has already been published (28). Briefly, following approval by the IRB at Baylor College of Medicine and the General Clinical Research Center (GCRC) Advisory Committee, written consent was obtained from each of seven healthy, lean exclusively breast-feeding women, who were 28.5 ± 1.2 yr of age (mean ± SE), weighed 60.0 ± 1.0 kg, and had a BMI of 22.2 ± 0.7 kg/m2. At the time of these studies, their babies were 10.0 ± 2.0 wk of age and weighed 5.4 ± 0.5 kg.
Protocol
Each woman was studied on two occasions for 8 days each separated by 1–2 wk. On each study occasion, the subjects were admitted to the GCRC for 4 days and 3 nights. The subjects were randomly assigned to receive either a low-carbohydrate/high-fat diet (H-FAT: 30% carbohydrate, 55% fat, and 15% protein) on one occasion or a high-carbohydrate/low-fat diet (H-CHO: 60% carbohydrate, 25% fat, and 15% protein) on the other occasion. Total energy intake for each woman was set as a multiple of 1.3 times basal metabolic rate (BMR). For 4 days before admission, 3 meals and 3 snacks/day were weighed, prepacked, and sent to the subject's home by our research kitchen [an example of a 1-day menu of the diets has been presented previously as supplemental material (28)]. Following the 4-day diet period at home, the subjects were admitted at ∼4 PM to the GCRC for 4 days and 3 nights. During the first 3 days, the subjects received the same intake and diet composition as that consumed at home. During the stay in the GCRC, women breastfed their babies every 3 h and expressed 2.5 ml of milk from each breast at the beginning, middle, and after the feeding (a total of 15 ml each feeding). Milk samples for each collection time were pooled for milk analyses. The babies were weighed before and after each breastfeeding to estimate the milk volume consumed (16). After breastfeeding, the mothers again were asked to empty their breasts completely using a breast pump. This extra milk was weighed. The milk volume produced during each feeding was calculated from the sum of the delta in the baby's weight over each feeding plus the pumped milk.
On the evening of day 3, the subjects were provided dinner at 6 PM and a snack at 8 PM. Thereafter, except for water, they were fasted until 9 AM the following morning (day 4). In the evening of study day 3, two intravenous catheters were placed under ELAMAX cream analgesia (Astra Pharmaceuticals, Wayne, PA). One was placed in an antecubital vein for isotope infusion and a second in a vein in the contralateral arm for blood sampling. At 4 AM on study day 3, a primed constant infusion of [U-13C]glucose (61 ± 0.7 μmol/kg and 1.02 ± 0.01 μmol·kg−1·min−1) was initiated to measure glucose rate of appearance and glucose production from gluconeogenesis (28). From 9 AM until 4 PM, the women consumed small, frequent meals (q15 min). The food had the same composition and provided the same calories as that consumed on a daily basis over the previous week. At the initiation of the feedings, a second priming dose of [U-13C]glucose (161 ± 4 μmol/kg) was given, and the infusion rate was increased to 2.7 ± 0.1 μmol·kg−1·min−1 to maintain sufficient [U-13C]glucose enrichment to measure accurately the rate of gluconeogenesis (28). At 3 AM, a baseline blood sample (10 ml) was obtained. Subsequently, 5 ml of blood was drawn every 15 min from 8 AM to 9 AM and from 3 PM to 4 PM. Milk samples were obtained at 3 AM (baseline), 9 AM, 12 PM, and 4 PM.
Gene Expression Analysis
In milk samples from 9 AM and 4 PM of day 4 during each study occasion, milk fat globule was collected and processed for gene expression according to our previous reports (24, 26).
Materials
Acetone, chloroform, ethanol hexane methanol, and all solvents were HPLC grade. [U-13C]glucose (99 atom %); [13C3]- and [2H5]glycerol (99 atom %); [13C3]lactate (98 atom %) and [1,2-13C2]octanoic, myristic, and palmitic acids (98 atom %) were purchased from Cambridge Isotope Laboratory (Andover, MA). O-(2,3,4,5,6-pentafluorbenyl) bromide (PFBBr) and tetrabutylammonium hydrogen sulfate (TBA) were obtained from Aldrich Chemicals (Milwaukee, WI). FA standards (C2-C22) were obtained from Sigma-Aldrich (St. Louis, MO). The uniformly deuterium-labeled (99 atom %), even-chain, saturated FA (C2:0, C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0) were obtained from Cambridge Isotope Laboratory (Andover, MA) as internal standards. Details of FA standards and their deuterated labeled internal standards has been described previously (27).
Analytical Methods
Substrate concentration.
Glucose, lactose, and lactate in milk and plasma were determined as previously reported (28). Free glycerol concentration in milk and plasma was measured using reverse isotope dilution methodology employing [2H5]glycerol as internal standard.
Isotopic enrichments.
GLUCOSE AND GLYCEROL.
Enrichments of 13C in the glucose isotopomers in plasma and milk were performed using the acetic anhydride derivative (43). Similarly, the triacetate derivative of glycerol was prepared using acetic anhydride. The isotopic enrichments of glycerol isotopomers (M+1 to M+3) and the internal standard [2H5]glycerol were measured by GC-MS (HP 5890/HP5970; Hewlett-Packard, Palo Alto, CA) and an HP-1701 column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, Wilmington, DE). Positive chemical ionization mode using methane was used and selected ion monitoring of m/z 159–164 applied.
FA IN MILK AND PLASMA.
The 13C enrichment and quantification of FA in milk and plasma were performed on samples obtained during the near steady state of tracer infusion (8–9 AM and 3–4 PM, representing the fasting and fed states, respectively) using the PFBBr derivatization and GC-MS negative chemical ionization (NCI) as previously reported (27) with slight modification. In this study, we utilized an Rtx-225 GC column (30 m × 0.25 mm × 0.25 μm; Restek, Bellefonte, PA), allowing better peak shape and separation. The conditions for the GC were as follows: injector: 250°C (splitless injection of samples); oven: 60°C for 1.0 min; ramp, 15°C/min to 240°C; hold at 240°C for 10 min. In addition to better retention on the GC column and higher resolutions and sensitivity, the PFBBr derivatives do not disrupt the natural isotopomer distribution in NCI, because only the FA moiety is measured in NCI (47). The numerous advantages of PFPBr derivative under NCI conditions, including sensitivity and fragmentation pattern, enabled us to trace the isotopic incorporation of 13C carbons from the [U-13C]glucose and to quantify a wide spectrum of FA ranging from C2 to C22 in both plasma and milk. Mass-to-charge ratios (m/z) of 89, 90, 91, and 92 were used to monitor M+0, M+1, M+2, and M+3 for lactate, respectively. Acetate enrichments and concentrations were performed similarly using PFBBr derivative as previously described (27), with the exception that the samples were not subjected to the saponification process. The same column and GC-MS condition modifications mentioned above were also applied. The fragment masses of 59, 60, and 62 were used to monitor M+0, M+1, M+2, and M+3. M+3 in this case represents the internal standard d4-acetate, in which the deuterium on the carboxyl group is being lost during derivatization. All measurements were made in the Stable Isotope Core Laboratory of the Children's Nutrition Research Center.
RNA isolation, cRNA amplification and expression microarray.
Total RNA was isolated from TRIzol-treated milk fat as previously described (26). The methods utilized for cRNA amplification and microarray expression analyses were identical to that previously validated using RT-PCR and published (24, 25), using human Ref-8 BeadChips and the BeadStation system from Illumina (San Diego, CA). After scanning of the probe array, the resulting image was analyzed using the GenomeStudio software (Illumina). Details about raw intensity data analysis and normalization were discussed in our previous publication (24). These data have been deposited in NCBI's Gene Expression Omnibus (6) and are accessible through GEO Series accession number GSE51874 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51874).
Calculations
The precursor product relationship has been utilized to calculate de novo synthesis of both FA and glycerol.
Total amount of labeled FA was calculated using the following formula:
where is the sum of total labeled FA (mg/l), i represents an even-chain FA of 2–16 carbons, M+2 is the enrichment of FAi (%), and C is the concentration of the FAi (mg/l). Total amount of labeled free glycerol was calculated by multiplying [M+3]glycerol enrichment with the free glycerol concentration.
Statistical Analysis
Data were averaged for each subject during each study occasion and the mean ± SE was calculated for each diet occasion. A paired Student's t-test was used to compare the effect of the two diets on the average of variables measured on day 4 during the fasting (8–9 AM) and feeding (3–4 PM) states. The Benjamini-Hochberg false discovery rate (B-H FDR) correction for multiple analyses following paired Student's t-test was used to compare the gene expression between diets and fasting/feeding conditions. Significance was set as P < 0.05.
RESULTS
Data related to dietary intakes, plasma substrate concentrations, milk volume and composition, infant intakes, glucose kinetics, energy expenditure, and substrate oxidations are presented in our previous publication (28).
FA Concentrations
Following overnight fasting, the concentration of C10:0 FA in plasma was lower (P < 0.01) in the H-CHO than in the H-FAT diet (Table 1). During feeding, plasma C2:0, C16:0, C18:0, C18:1, C18:2, and C20:4 and sum of total FA were higher (P < 0.05) during the H-FAT diet than in the H-CHO diet. The concentration of individual and sum of C4–C14 were all higher (P < 0.01) in milk in the H-CHO compared with the H-FAT diet during both feeding and fasting (Table 1). However, the concentrations of C18:1, C20:2, C20:3, C20:4, C20:2, and C22:6 were all higher during the H-FAT diet following the overnight fasting. Similarly, C16:0, C18:0, C18:1, C18:2, and C18:3 were higher during feeding in milk, reflecting the FA composition of the diet (Table 1).
Table 1.
Total and individual plasma (Pl) and milk (Mk) fatty acid composition during fasting and feeding of high carbohydrate (HC) and high fat (HF) diets in lactating women
| Overnight Fasting |
Feeding |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pl-HC | Pl-HF | Mk-HC | Mk-HF | Pl-HC | Pl-HF | Mk-HC | Mk-HF | |
| g/l | ||||||||
| Total | 2.828 | 2.815 | 41.908 | 40.833 | 2.919 | 3.411* | 42.239 | 46.893* |
| C2:0 | 0.010 | 0.011 | 0.007 | 0.008 | 0.008 | 0.010* | 0.008 | 0.009 |
| C4:0 | 0.001 | 0.003 | 0.102 | 0.071* | 0.003 | 0.002 | 0.148 | 0.087* |
| C6:0 | 0.001 | 0.003 | 0.202 | 0.131** | 0.003 | 0.002 | 0.333 | 0.183* |
| C8:0 | 0.002 | 0.004 | 0.357 | 0.246** | 0.004 | 0.004 | 0.530 | 0.381** |
| C10:0 | 0.002 | 0.005* | 1.313 | 1.112* | 0.008 | 0.010 | 1.920 | 1.547* |
| C12:0 | 0.006 | 0.009 | 2.618 | 2.383 | 0.016 | 0.015 | 3.569 | 3.078* |
| C14:0 | 0.046 | 0.044 | 2.632 | 2.320** | 0.055 | 0.053 | 3.915 | 3.427** |
| ∑C2:C14 | 0.069 | 0.078 | 7.230 | 6.269** | 0.097 | 0.097 | 10.423 | 8.712** |
| C16:0 | 0.417 | 0.409 | 5.326 | 5.456 | 0.411 | 0.483** | 4.846 | 5.620* |
| C18:0 | 0.230 | 0.235 | 3.157 | 3.308 | 0.232 | 0.285** | 2.944 | 4.034** |
| ∑SAT | 0.716 | 0.722 | 15.713 | 15.033 | 0.740 | 0.865* | 18.212 | 18.366 |
| C16:1 | 0.100 | 0.081 | 2.040 | 2.125 | 0.094 | 0.082 | 2.143 | 1.760 |
| C18:1 | 0.558 | 0.555 | 11.408 | 12.748 | 0.556 | 0.625* | 10.110 | 13.230** |
| C20:1 | 0.048 | 0.051 | 1.303 | 0.909 | 0.046 | 0.050 | 1.118 | 1.018 |
| ∑MUFA | 0.706 | 0.687 | 14.751 | 15.783 | 0.696 | 0.757 | 13.371 | 16.008** |
| C18:2 | 0.746 | 0.798 | 6.678 | 6.471 | 0.792 | 1.060** | 6.317 | 7.595** |
| C18:3 | 0.067 | 0.056 | 1.403 | 1.335 | 0.069 | 0.064 | 1.268 | 1.836** |
| C20:2 | 0.027 | 0.020 | 0.598 | 0.439* | 0.028 | 0.025 | 0.493 | 0.532 |
| C20:3 | 0.110 | 0.081 | 0.616 | 0.372* | 0.128 | 0.097 | 0.657 | 0.563 |
| C20:4 | 0.318 | 0.320 | 1.120 | 0.820* | 0.322 | 0.378* | 1.020 | 1.173 |
| C20:5 | 0.008 | 0.008 | 0.106 | 0.024* | 0.008 | 0.009 | 0.083 | 0.056 |
| C22:5 | 0.038 | 0.038 | 0.261 | 0.148 | 0.043 | 0.051 | 0.234 | 0.229 |
| C22:6 | 0.091 | 0.085 | 0.664 | 0.408* | 0.092 | 0.105 | 0.582 | 0.535 |
| ∑PUFA | 1.406 | 1.407 | 11.445 | 10.017 | 1.483 | 1.788** | 10.655 | 12.519* |
| %Total | ||||||||
| ∑C2:C14 | 2.421 | 2.744 | 17.096 | 15.054* | 3.280 | 2.822 | 24.699 | 18.613** |
| ∑SAT | 25.317 | 25.656 | 37.198 | 36.230 | 25.343 | 25.415 | 43.139 | 39.142* |
| ∑MUFA | 24.959 | 24.515 | 35.459 | 38.962* | 23.841 | 22.187* | 31.753 | 34.289* |
| ∑PUFA | 49.724 | 49.829 | 27.342 | 24.809 | 50.817 | 52.398 | 25.108 | 26.569 |
Values are means from 7 subjects. SAT, saturated FA, including C2:C14; MUFA, monounsaturated FA and PUFA polyunsaturated FA. Significantly different from H-CHO (paired t-test):
P < 0.05,
P < 0.01. Obviously in milk, except for C2, concentration of individual and sum of FA groups are severalfold higher (P < 0.01) than the respective values in plasma.
Isotopomer Enrichments
Following overnight fasting.
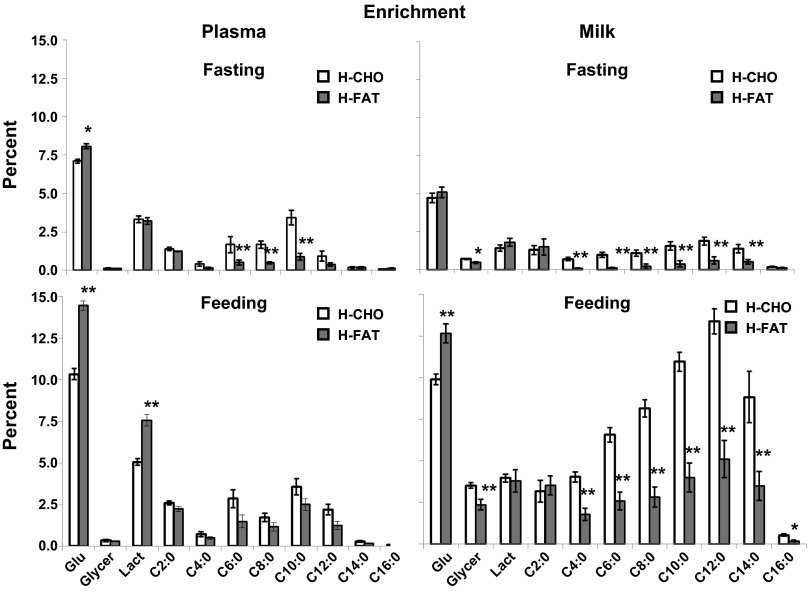
M+6 enrichment of glucose was slightly higher (P < 0.05) in both plasma and milk during the H-FAT diet compared with H-CHO diet and lower (P < 0.01) in milk compared with plasma during both diets (Fig. 1). M+3 enrichment of lactate was not different between the two diets in either plasma or milk but was higher (P < 0.01) in plasma compared with milk regardless of the diet (Fig. 1). The primary isotopomer detected in all FA (C2–C16) was M+2 (Fig. 1), with little contribution in M+1 or M+3 in FA ≥4C (data not shown). Enrichment of M+2 acetate was not different between the diets or between milk and plasma (Fig. 1). M+2 enrichments of C4–C10 FA in both plasma and milk were higher (P < 0.05) during the H-CHO diet than in the H-FAT diet. However, the M+2 enrichments of C4–C10 FA were lower (P < 0.05) in milk compared with plasma under both dietary conditions (Fig. 1). M+2 enrichment of C12 FA in plasma and milk was higher in the H-CHO than in the H-FAT diet and higher (P < 0.05) in milk compared with plasma during both diets (Fig. 1). M+2 enrichment of C14 FA in milk was higher in the H-CHO than in the H-FAT diet and higher (P < 0.05) in milk compared with plasma during both diets (Fig. 1). Little M+2 enrichment was observed in palmitate but was similar between the diets and between milk and plasma (Fig. 1).
Fig. 1.
Enrichment of Glu, [M+6]glucose; Glycer, [M+3]glycerol; Lact, [M+3]lactate, and [M+2] in different FA in plasma (left) and milk (right) during infusion of [U-13C]glucose following overnight fast (top) and during feeding (bottom) of high-carbohydrate/low-fat (H-CHO) and low-carbohydrate/high-fat (H-FAT) diets. Values are means ± SE; n = 7. Significantly different from H-CHO (paired t-test): *P < 0.05, **P < 0.01.
During feeding.
M+6 enrichment of glucose was higher (P < 0.01) during the H-FAT diet in both plasma and milk but lower (P < 0.01) in milk compared with plasma during both diets (Fig. 1). M+3 enrichment of lactate was higher in plasma during the H-FAT diet compared with H-CHO diet and was higher (P < 0.01) in plasma compared with milk during both diets. M+2 acetate was not different between the two diets and between milk and plasma. M+2 enrichments of the FA C4–C14 in plasma were similar between the two diets (P > 0.05), but all were higher in milk (P < 0.05) during the H-CHO diet compared with H-FAT diet and were higher (P < 0.05) in milk compared with plasma regardless of the diet. M+2 enrichment of C12 FA in plasma and milk was higher during the H-CHO compared with the H-FAT diet and higher (P < 0.05) in milk compared with plasma during both diets. M+2 enrichment of C14 FA in milk was higher during the H-CHO compared with the H-FAT diet and higher (P < 0.05) in milk compared with plasma during both diets. M+2 enrichment of palmitate was higher (P < 0.05) in milk during the H-CHO compared with the H-FAT diet during feeding and was higher (P < 0.05) in milk compared with plasma during the H-CHO diet only.
FA De Novo Synthesis from Glucose
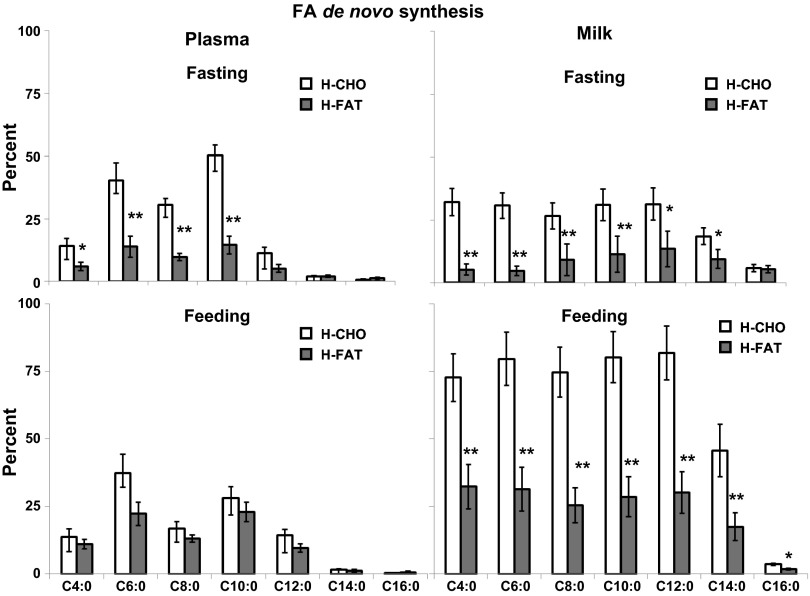
Following overnight fasting.
De novo synthesis of C4–C10 in plasma was higher (P < 0.01) during the H-CHO (10–45%) compared with H-FAT diet (7–15%; Fig. 2). In milk, %de novo synthesis of C4–C14 were higher (P < 0.01) during the H-CHO (20–35%) compared with the H-FAT (7–15%) diet (Fig. 2). During the H-CHO diet, %de novo synthesis of C6 and C10 was higher, but those of C12 and C14 were lower (P < 0.01) in plasma compared with milk (Fig. 2).
Fig. 2.
De novo synthesis of FA (%) in plasma (left) and milk (right) pools during infusion of [U-13C]glucose (and based on acetate M+2 enrichments as a surrogate for cytosolic acetyl-CoA enrichment) following overnight fast (top) and during feeding (bottom) of H-CHO and H-FAT diets. Values are means ± SE; n = 7. Significantly different from H-CHO (paired t-test): *P < 0.05, **P < 0.01.
During feeding.
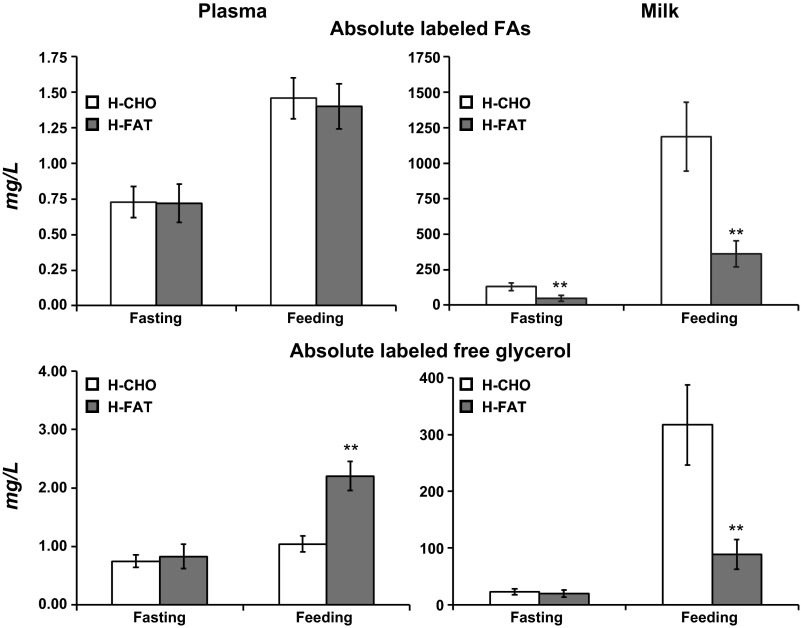
De novo synthesis of C4–C16 FA was not different between diets in plasma but in milk was ∼2.5 times higher (P < 0.05) in all FA (C4–C12) during the H-CHO (∼75%) compared with the H-FAT diet (∼30%). Similarly, de novo FA synthesis was higher (P < 0.01) in C14:0 FA (50 vs. 20%) and C16:0 FA (10 vs. 5%) in milk during the H-CHO diet compared with the H-FAT diet. In plasma, total amount of labeled FA was <1.5 mg/l, with no difference between diets regardless of whether fed or fasting (Fig. 4). The total labeled FAs in milk were higher in the H-CHO compared with the H-FAT diet during both fasting (317 ± 70 vs. 89 ± 26 mg/l, P < 0.01) and feeding (1,186 ± 240 vs. 326 ± 90 mg/l, P < 0.01) compared with the H-FAT diet.
Fig. 4.
Absolute amount of sum of labeled FA (top; as calculated by multiplying the enrichment of M+2 FAs times their concentrations) and free glycerol (bottom; as calculated by multiplying the enrichment of M+3 glycerol times its concentration) in plasma and milk following overnight fast and during feeding of H-CHO and H-FAT diets. Note the y-axis in the right is 1,000 (top) and 100 (bottom) times that in the left. Values are means ± SE; n = 7. Significantly different from H-CHO (paired t-test): **P < 0.01.
Glycerol Concentration, Enrichment, and De Novo Synthesis
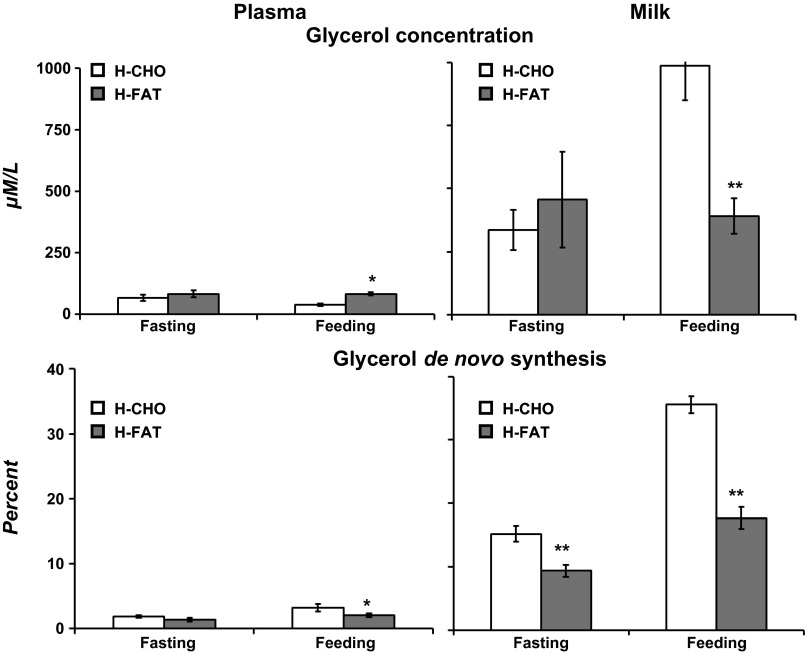
Generally, free glycerol concentrations were 5- to 10-fold higher (P < 0.01) in milk compared with plasma during either feeding or fasting (Fig. 3). Following an overnight fast, the concentration of free glycerol was not different between diets in either plasma or milk (Fig. 3). During feeding, glycerol concentrations were lower (P < 0.05) during the H-CHO diet in plasma but higher (P < 0.01) in milk compared with the H-FAT diet (Fig. 3). Plasma enrichment of M+3 glycerol in milk was higher (P < 0.01) than that of plasma during both the fast (5-fold higher) and fed (10-fold) conditions regardless of diet. Plasma enrichment (%) of M+3 glycerol was not different between H-CHO and H-FAT diets during either fasting or feeding (Fig. 1). However, milk M+3 glycerol was higher (P < 0.01) in the H-CHO compared with the H-FAT diet during both fasting and feeding. De novo synthesis of glycerol in plasma was higher (P < 0.05) in the H-CHO diet compared with the H-FAT diet during fed condition only (3.24 ± 0.59 vs. 2.05 ± 0.29, P < 0.05; Fig. 3). However, during the H-CHO diet compared with the H-FAT diet, de novo synthesis of glycerol in milk (%) was higher during both fasting (15.20 ± 1.24 vs. 9.36 ± 0.91, P < 0.01) and feeding (35.55 ± 1.36 vs. 17.66 ± 1.76, P < 0.01) (Fig. 3).
Fig. 3.
Concentration(μM; top) and de novo synthesis of free glycerol (%; bottom) in plasma and milk pools following overnight fast and during feeding of H-CHO and H-FAT diets and infusion of [U-13C] glucose. Values are means ± SE; n = 7. Significantly different from H-CHO (paired t-test): *P < 0.05, **P < 0.01.
Total amount of labeled free glycerol was <2.5 mg/l in plasma, but higher (P < 0.01) in the H-FAT compared with the H-CHO diet during feeding only (Fig. 4). Total labeled free glycerol in milk was not different between diets during fasting (23 ± 5 vs. 19 ± 6 mg/l, P > 0.05). However, during feeding, total labeled free glycerol was ∼4-fold higher in the H-CHO (317 ± 70 vs. 89 ± 26 mg/l, P < 0.01) compared with the H-FAT diet. Obviously, feeding increased (P < 0.01) the amount of labeled glycerol compared with fasting in both plasma and milk, but the magnitude of increase was greater in milk (Fig. 4).
Gene Expression Results
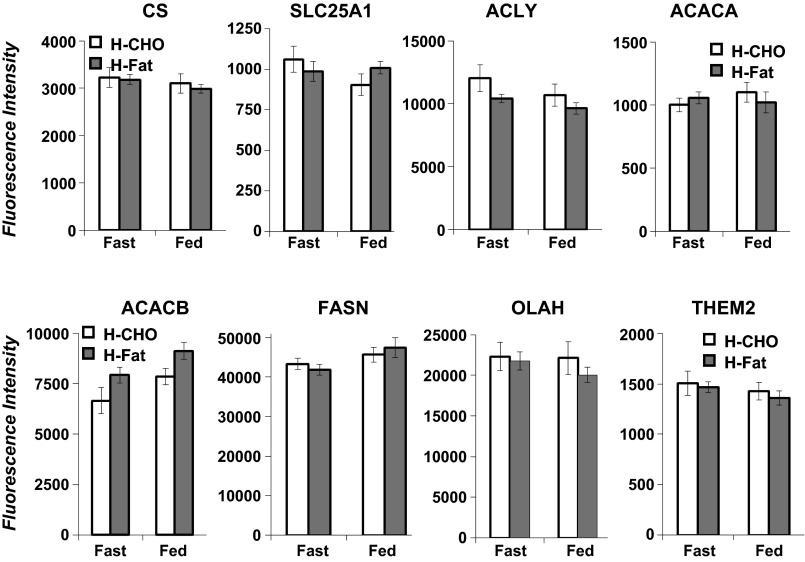
The mRNA expression of genes known to be involved in the de novo FA synthesis including ATP citrate lyase (ACLY), citrate synthase (CS), and transporter (SLC25A1), acetyl-CoA carboxylase-α (ACACA) and -β (ACACB), FA synthase (FASN), thioesterase 2 (OLAH), and thioesterase superfamily member 2 (THEM2) were not different between diets during either feeding or fasting or when compared between fed and fasted states within each diet (Fig. 5). Expression of the genes for sterol regulatory element-binding transcription factor 1 (SREBF1), estrogen receptor 1 (ESR1), thyroid hormone-responsive protein (THRSP), insulin-induced 2 (INSIG2), and peroxisome proliferator-activated receptor-γ (PPARG) were not different regardless of diet or absorptive state (data not presented). Similarly, expressions of genes for glycolysis or glycerol kinases associated with glycerol synthesis and its incorporation into the TG backbone were unchanged (data not presented).
Fig. 5.
Fluorescence intensity of genes involved in de novo FA synthesis in milk samples following overnight fast and during feeding of H-CHO and L-CHO diets. CS, citrate synthase; SLC25A1, solute carrier family 25 [mitochondrial carrier; citrate transporter 1; ACLY, ATP citrate lyase; ACACA, acetyl-CoA carboxylase-α; ACACB, acetyl-CoA carboxylase-β; FASN, FA synthase; OLAH, S-acyl FA synthase thioesterase, medium chain (thioesterase II or oleoyl-acyl-carrier protein hydrolase); THEM2, thioesterase superfamily member 2]. Values are means ± SE; n = 7. None of the genes (P, paired t-test; B-H FDR for multiple corrections, >0.05) was different between diets or fast/fed conditions.
DISCUSSION
We have previously reported (28) that the total milk fat concentration was ∼13% higher (4.8 ± 0.3 vs. 4.3 ± 0.3 g/dl, P < 0.05) and daily milk fat output was ∼15% higher (39 ± 2 vs. 34 ± 2 g/day, P < 0.05) during the H-FAT diet compared with the H-CHO diet. However, the present study showed that maternal dietary macronutrient composition, under isocaloric isonitrogenous conditions, influences milk lipid composition and that the human MG responds to dietary macronutrient manipulation by altering the de novo synthesis of MCFA and glycerol in milk. Whether expressed as concentration (g/dl) or percentage of total, the individual and the sum of FA (C6–C14) were higher during feeding of the H-CHO diet compared with the H-FAT diet. These findings are consistent with early reports in four ethnic groups in East Africa and the Middle East, in which the authors attributed the high concentrations of lauric (C12:0) and myristic (C14:0) acids in milk (30% of total) to their high-carbohydrate diet (35). Similar findings were also reported in humans by other investigators (8, 9, 13, 15). In other animal species, the MG is known to decrease FA synthesis in response to a high-fat diet (23, 32, 36).
The detection of 13C incorporation (as M+2) as the main isotopomer in both plasma and milk FA confirms the known role for acetyl-CoA as a precursor for FA synthesis. The detection of M+2 and its enrichment pattern in both plasma and milk indicate that human tissues are capable of significant de novo synthesis of SCFA and MCFA (C4–C14) but insignificant enrichment of FA ≥C16. Detection of M+2 enrichment in FA with 16 or fewer carbons in the plasma compartment may indicate that tissues other than the MG (most likely liver) contribute to the de novo FA synthesis process, assuming little or no FA escape the MEC and enter the circulation. The higher the M+2 enrichments of FA with 10 or fewer carbons in plasma compared with milk following an overnight fast may indicate a greater contribution from these tissues in this de novo synthetic process. Whether these FA are utilized by tissues as fuel and/or are specifically transported to the MG remains to be determined. Yet the concentrations of these FA in plasma are very low (<1/40) compared with those of milk, and consequently their higher enrichment may be due to the smaller pool size in plasma vs. milk. The higher M+2 enrichments of C12:0 and C14:0 in milk compared with plasma (regardless of diet or fasting/feeding conditions) and their relatively higher abundance in milk support the contention that the MG may be the primary site for synthesis of these two FAs. Generally, the higher M+2 enrichment in milk FA compared with plasma reflects the increase in the de novo synthesis during feeding condition. However, the higher M+2 enrichments and concentrations of these FA in both plasma and milk during the H-CHO diet indicate increased de novo synthesis induced by the carbohydrate diets. The inability to detect significant enrichments in FA longer than 16 carbons in either milk or plasma indicates little or no de novo synthesis of these FAs by the human tissues and/or tremendous dilution by the dietary and mobilized FAs under our study conditions.
The pattern of 13C labeling from our study is in general agreement with the findings from MG of lactating goats infused with substrates labeled with 14C. These studies demonstrated extensive 14C labeling of milk FA with chain length up to C(14) and to a smaller extent for the synthesis of palmitate (22). Similar findings have been reported in rabbits (5) in which the specific radioactivity of the individual FA (C6:0 to C14:0) and the proportions of these FAs synthesized were similar in mammary tissue and milk. Hexanoic acid (C6:0) had the highest specific radioactivity, and the C8:0 to C14:0 FAs had similar specific radioactivities, which were about five times those of C16 and C18 acids. No radioactivity was detected in FA of chain length <C14 in the liver, blood, or adipose tissue, and the specific radioactivities of FA of chain length >C14 in these tissues were similar to those of the LCFA in the milk and MG. The results demonstrated that the C4:0 to C14:0 FA are synthesized within the MG rather than the result of FA uptake from circulating blood or by partial or complete oxidation of LCFA within the MG. However, mammary slices from lactating guinea pigs synthesize LCFA (C16–C18, which are the predominant acids of guinea pig milk) saturated and unsaturated FA from acetate in the presence or absence of glucose (41). Cells from bovine lactating MG synthesized mostly SCFA and MCFA (C4–C12), whereas the rat mammary cells synthesized FA ranging from C6 to C18 (17).
Measurements of de novo lipogenesis are of great interest with the epidemic of obesity and its related comorbidities. Tracer techniques are required to distinguish between dietary and newly synthesized FA. To measure the fractional de novo synthesis of FA, the enrichment of the true precursor of FA synthesis (cytosolic acetyl-CoA) is required, and several methods have been attempted to do so, including sampling of the lipogenic hepatic acetyl-CoA pool in vivo in rats by using a xenobiotic probe (12) and prediction from isotopomeric distribution in circulating lipids and measurement of lipogenesis and acetyl-CoA dilution (11). In our study, we attempted to utilize the enrichment of acetate as a surrogate for intracellular acetyl-CoA. Although acetate (in milk or plasma) may not reflect the true cytosolic acetyl-CoA pool, we believe it gives an estimate of the intracellular acetate enrichment for the fractional de novo synthesis calculations to compare change in FA synthesis under our fasting and fed conditions using a paired study design. Nevertheless, our calculations showed that the de novo synthesis reached as high as 75% of FA with C4–C12 during the consumption of the H-CHO diet, which is almost 2.5 times greater than those obtained during the H-FAT diet (∼30%). In milk, the consistent values for de novo synthesis among these FAs (C4–C12) indicated that these FAs originated from a common precursor pool. However, in plasma, inconsistencies were observed for the values of de novo synthesis among C4–C12 FAs, indicating that they most likely had arisen from different precursor pools. Another possible explanation, as described above, is the trivial pool size of these circulating FAs. This might be further supported by the calculated absolute labeled FA in plasma compared with milk (Fig. 4). The decrease in the percent de novo synthesis in C14:0 in milk and longer-chain FAs may be explained by the increased contribution from the dietary and endogenously mobilized FA. Our data and those of Hachey et al. (9) are in good agreement with the in vitro studies of human MECs isolated from breast milk, indicating that the human MG has the ability to synthesize C10:0, C12:0, and C14:0 FAs but has a limited capacity to synthesize C16:0 and C18:0 (46).
During the secretory activation phase, changes in FA composition were mirrored in the expression of the genes for the enzymes involved in de novo FA synthesis within the MG (27). However, gene expression was unaltered by dietary macronutrient composition in the present study. This suggests that the substrate availability and/or change in circulating hormones (e.g., insulin) may be regulating de novo FA synthesis independently of the change in gene expression. In this regard, we previously reported (28) that circulating insulin concentrations were higher during the H-CHO diet compared with the H-FAT diet during both fasting and feeding. However, our recent studies elucidate that the major glucose transport systems in the human MECs are insulin-independent transporters (26), excluding a role for insulin in facilitating glucose transport into the MEC. Accordingly, insulin may be exerting an indirect effect by increasing the activity of acetyl-CoA carboxylase (ACC) by decreasing its phosphorylation (31), thus increasing de novo FA synthesis. This mechanism is based on two independent observations: 1) the ability of insulin to stimulate MG FA synthesis as a consequence of 24 h of food deprivation in rats (49), and 2) refeeding with and without inhibition of insulin release using streptozotocin (30). In addition, insulin's inhibition of lipolysis in lactating women (29) may support the notion that NEFA themselves regulate de novo MG FA synthesis by inhibition of the activity of ACC (32). Consistent with this speculation is the observation in these women that NEFA and ketones were higher during the H-FAT diet than during the H-CHO diet (28).
Prolactin has long been considered a primary regulator of the synthesis of milk components including lipids. However, we did not find any difference in circulating prolactin concentrations between the two diets under either fasting (99 ± 18 vs. 85 ± 16 μU/ml, P = 0.40) or fed (45 ± 15 vs. 62 ± 20 μU/ml, P = 0.11) conditions. Additionally, genes on the prolactin receptor and its downstream pathway, including SREBF1 and others related to de novo synthesis (26, 27), were not different regardless of diet or fast/fed states. Based on our limited sample size and microarray data, these observations exclude the role of prolactin in this process and may imply that the effects of this hormone may be related more to the establishment of lactogenesis and involution than to moment-to-moment regulation of the rate of synthesis of milk components (26, 27, 32). However, these findings may require further validation.
Data related to gene expression from our study are in agreement with those obtained from the MG of mice fed diets containing either 8 or 40% of their calories as lipid, substituting lipid for carbohydrate, from days L5 to L10, using Affymetrix gene chips (36). Those authors found that expression of only 85 genes (excluding those involved in de novo synthesis) was significantly altered by the high-fat diet in the MG compared with 760 in the liver (36). By comparison, the lactating MG is devoted to one process, the synthesis and secretion of milk (32). It worth noting that fat in rodent milk is fivefold higher in concentration than human milk (20 vs. 4% of total weight). However, our data are not consistent with those from dairy cows, in which a total of 972 genes were differentially expressed in the MG tissue when the grazing dairy cow diet was supplemented with unsaturated FA (UFA) compared with cows fed a control diet. This suggests a large degree of transcriptomic adaptation to the dietary UFAs (23) in the cow.
Our data show that the human MG can significantly synthesize glycerol from glucose (glyceroneogenesis), accounting for ∼15 and 30% of milk glycerol during fasting and fed conditions, respectively. Indeed, this feature of the MG would meet the higher demand of glycerol required for milk TG synthesis (∼30 mM). We speculate that the capability of MEC to efficiently synthesize glycerol from glucose is the result of expression of several genes in the glycolytic, pentose phosphate shunt, and glycerol activation pathways (26, 27), all of which are upregulated during the secretory activation phase of lactation. However, as observed with genes involved in FA synthesis, in the present study expression of genes associated with glycerol synthesis and its incorporation into the TG backbone was unaltered by dietary macronutrient composition. In contrast, non-MG tissues, compared with MG, appeared to have limited capabilities to de novo synthesize of glycerol (Fig. 3). The effect of the diet was prominent, however, in that the de novo synthesis of glycerol was high in milk during the H-CHO diet compared with the H-FAT during both fed and fasting conditions. Obviously, during the H-FAT diet lipolysis of the dietary fat during meal absorption and mobilization of stored TG following an overnight fast (possibly due to lower plasma insulin concentrations, and activated actions of lipases) contribute more to milk glycerol and TG than during the H-CHO diet.
The possibility of altering favorably the composition of milk lipid through dietary manipulation of maternal diet is interesting. Glycerol is a very important nutrient in breast milk, representing the second most abundant molecule (based on molar distribution, ∼40 mM) after lactose. In human newborns, ∼75% of transported glycerol was converted to glucose and represented 5.0% of hepatic glucose production (3); however, glycerol accounts for ∼60% of the substrate for total gluconeogenesis in very low birth weight infants receiving TPN (42). The FA in milk TG are metabolized differently based on their carbon chain length. MCFA differ from LCFA in that they are absorbed directly into the portal circulation and the vast majority are taken up by the liver on a first-pass basis and are rapidly oxidized (2). MCFA have been reported to induce satiety and, hence, decrease food intake and increase energy expenditure (40). Additionally, MCFA form precursors of important biological molecules, including lipoic acid (C8:0) (18) and ghrelin (C12:0) (33). Finally, premature infants fed MCT-containing formulae, when compared with controls, absorbed more calcium and magnesium (44) and improved fat and nitrogen absorption (45).
In summary our data show that alterations in the macronutrient composition of the maternal diet affect the production of selected FA and the lipid composition of the human milk and illustrate the complex relationships among diet, energy metabolism, and milk composition. The impact of these changes in milk lipid composition on infant nutrition and metabolism will require further investigation.
GRANTS
This project was supported by NIH Grants RO1 DK-55478, HD-37857, MO1 RR-00188, USDA/ARS 6250-5100. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX). The contents of this publication do not necessarily reflect the views of policies of the US Dept. of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.M., A.L.S., and M.W.H. conception and design of research; M.A.M. performed experiments; M.A.M. analyzed data; M.A.M., A.L.S., and M.W.H. interpreted results of experiments; M.A.M. and M.W.H. prepared figures; M.A.M. drafted manuscript; M.A.M., A.L.S., and M.W.H. edited and revised manuscript; M.A.M., A.L.S., and M.W.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank volunteers whose participation made this study possible. We gratefully acknowledge and thank the technicians in our laboratory (Dr. Susan Sharma, Marcia Ekworomadu, Dr. Shaji Chacko, and Dan Donaldson), our research coordinators (Amy Pontius, Cindy Bryant, and Linda Pleasant), and the staff in the Metabolic Research Unit and kitchen and the General Clinical Research Center who greatly facilitated the execution of these studies.
REFERENCES
- 1.Abraham S, Chaikoff IL. Glycolytic pathways and lipogenesis in mammary glands of lactating and nonlactating normal rats. J Biol Chem 234: 2246–2253, 1959 [PubMed] [Google Scholar]
- 2.Babayan VK. Medium chain triglycerides and structured lipids. Lipids 22: 417–420, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Bougneres PF, Karl IE, Hillman LS, Bier DM. Lipid transport in the human newborn. Palmitate and glycerol turnover and the contribution of glycerol to neonatal hepatic glucose output. J Clin Invest 70: 262–270, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breckenridge WC, Kuksis A. Molecular weight distributions of milk fat triglycerides from seven species. J Lipid Res 8: 473–478, 1967 [PubMed] [Google Scholar]
- 5.Carey EM, Dils R. The pattern of fatty acid synthesis in lactating rabbit mammary gland studied in vivo. Biochem J 126: 1005–1007, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emken EA, Adlof RO, Hachey DL, Garza C, Thomas MR, Brown-Booth L. Incorporation of deuterium-labeled fatty acids into human milk, plasma, and lipoprotein phospholipids and cholesteryl esters. J Lipid Res 30: 395–402, 1989 [PubMed] [Google Scholar]
- 8.Glew RH, Huang YS, Vander Jagt TA, Chuang LT, Bhatt SK, Magnussen MA, VanderJagt DJ. Fatty acid composition of the milk lipids of Nepalese women: correlation between fatty acid composition of serum phospholipids and melting point. Prostaglandins, Leukot Essent Fatty Acids 65: 147–156, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hachey DL, Silber GH, Wong WW, Garza C. Human lactation. II. Endogenous fatty acid synthesis by the mammary gland. Pediatr Res 25: 63–68, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Heesom KJ, Souza PF, Ilic V, Williamson DH. Chain-length dependency of interactions of medium-chain fatty acids with glucose metabolism in acini isolated from lactating rat mammary glands. A putative feed-back to control milk lipid synthesis from glucose. Biochem J 281: 273–278, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellerstein MK, Kletke C, Kaempfer S, Wu K, Shackleton CH. Use of mass isotopomer distributions in secreted lipids to sample lipogenic acetyl-CoA pool in vivo in humans. Am J Physiol Endocrinol Metab 261: E479–E486, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Hellerstein MK, Wu K, Kaempfer S, Kletke C, Shackleton CH. Sampling the lipogenic hepatic acetyl-CoA pool in vivo in the rat. Comparison of xenobiotic probe to values predicted from isotopomeric distribution in circulating lipids and measurement of lipogenesis and acetyl-CoA dilution. J Biol Chem 266: 10912–10919, 1991 [PubMed] [Google Scholar]
- 13.Insull W, Jr, Hirsch J, James T, Ahrens EH., Jr The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J Clin Invest 38: 443–450, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iverson SJ, Hamosh M, Bowen WD. Lipoprotein lipase activity and its relationship to high milk fat transfer during lactation in grey seals. J Comp Physiol B 165: 384–395, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Jenness R. The composition of human milk. Sem Perinatol 3: 225–239, 1979 [PubMed] [Google Scholar]
- 16.Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 117: e387–e395, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kinsella JE. Stearyl CoA as a precursor of oleic acid and glycerolipids in mammary microsomes from lactating bovine: possible regulatory step in milk triglyceride synthesis. Lipids 7: 349–355, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Kursu VA, Pietikainen LP, Fontanesi F, Aaltonen MJ, Suomi F, Raghavan Nair R, Schonauer MS, Dieckmann CL, Barrientos A, Hiltunen JK, Kastaniotis AJ. Defects in mitochondrial fatty acid synthesis result in failure of multiple aspects of mitochondrial biogenesis in Saccharomyces cerevisiae. Mol Microbiol 90: 824–840, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammi-Keefe CJ, Jensen RG. Lipids in human milk: a review. 2: Composition and fat-soluble vitamins. J Pediatr Gastroenterol Nutr 3: 172–198, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Libertini LJ, Smith S. Purification and properties of a thioesterase from lactating rat mammary gland which modifies the product specificity of fatty acid synthetase. J Biol Chem 253: 1393–1401, 1978 [PubMed] [Google Scholar]
- 22.Linzell JL, Annison EF, Fazakerley S, Leng RA. The incorporation of acetate, stearate and D(-)-beta-hydroxybutyrate into milk fat by the isolated perfused mammary gland of the goat. Biochem J 104: 34–42, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach N, Jacobs AA, Kruijt L, van Baal J, Smits MA. Alteration of gene expression in mammary gland tissue of dairy cows in response to dietary unsaturated fatty acids. Animal 5: 1217–1230, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Maningat PD, Sen P, Rijnkels M, Sunehag AL, Hadsell DL, Bray M, Haymond MW. Gene expression in the human mammary epithelium during lactation: the milk fat globule transcriptome. Physiol Genomics 37: 12–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maningat PD, Sen P, Sunehag AL, Hadsell DL, Haymond MW. Regulation of gene expression in human mammary epithelium: effect of breast pumping. J Endocrinol 195: 503–511, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Mohammad MA, Hadsell DL, Haymond MW. Gene regulation of UDP-galactose synthesis and transport: potential rate-limiting processes in initiation of milk production in humans. Am J Physiol Endocrinol Metab 303: E365–E376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad MA, Haymond MW. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cell during secretory activation. Am J Physiol Endocrinol Metab 305: E700–E716, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. Am J Clin Nutr 89: 1821–1827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammad MA, Sunehag AL, Rodriguez LA, Haymond MW. Galactose promotes fat mobilization in obese lactating and nonlactating women. Am J Clin Nutr 93: 374–381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munday MR, Hardie DG. The role of acetyl-CoA carboxylase phosphorylation in the control of mammary gland fatty acid synthesis during the starvation and re-feeding of lactating rats. Biochem J 237: 85–91, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munday MR, Williamson DH. Insulin activation of lipogenesis in isolated mammary acini from lactating rats fed on a high-fat diet. Evidence that acetyl-CoA carboxylase is a site of action. Biochem J 242: 905–911, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr 17: 159–183, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 146: 2255–2264, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Oftedal OT. The adaptation of milk secretion to the constraints of fasting in bears, seals, and baleen whales. J Dairy Sci 76: 3234–3246, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Read WW, Lutz PG, Tashjian A. Human milk lipids. II. The influence of dietary carbohydrates and fat on the fatty acids of mature milk A study in four ethnic groups. Am J Clin Nutr 17: 180–183, 1965 [DOI] [PubMed] [Google Scholar]
- 36.Rudolph MC, McManaman JL, Phang T, Russell T, Kominsky DJ, Serkova NJ, Stein T, Anderson SM, Neville MC. Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28: 323–336, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Smith S. Mechanism of chain length determination in biosynthesis of milk fatty acids. 1980. J Mammary Gland Biol Neoplasia 14: 245–260, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Smith S, Dils R. Factors affecting the chain length of fatty acids synthesised by lactating-rabbit mammary glands. Biochim Biophys Acta 116: 23–40, 1966 [DOI] [PubMed] [Google Scholar]
- 39.Smith S, Dils R. Factors effecting the chain-length of fatty acids synthesised by lactating-rabbit mammary glands. Biochim Biophys Acta 84: 776–778, 1964 [DOI] [PubMed] [Google Scholar]
- 40.St-Onge MP, Jones PJ. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr 132: 329–332, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Strong CR, Dils R. Fatty acids synthesized by mammary gland slices from lactating guinea pig and rabbit. Comp Biochem Physiol B Comp Biochem 43: 643–652, 1972 [DOI] [PubMed] [Google Scholar]
- 42.Sunehag AL, Haymond MW, Schanler RJ, Reeds PJ, Bier DM. Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes 48: 791–800, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Sunehag AL, Louie K, Bier JL, Tigas S, Haymond MW. Hexoneogenesis in the human breast during lactation. J Clin Endocrinol Metab 87: 297–301, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on calcium and magnesium absorption. Pediatrics 61: 537–545, 1978 [PubMed] [Google Scholar]
- 45.Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on fat and nitrogen absorption. Pediatrics 55: 359–370, 1975 [PubMed] [Google Scholar]
- 46.Thompson BJ, Smith S. Biosynthesis of fatty acids by lactating human breast epithelial cells: an evaluation of the contribution to the overall composition of human milk fat. Pediatr Res 19: 139–143, 1985 [DOI] [PubMed] [Google Scholar]
- 47.Tomcik K, Ibarra RA, Sadhukhan S, Han Y, Tochtrop GP, Zhang GF. Isotopomer enrichment assay for very short chain fatty acids and its metabolic applications. Anal Biochem 410: 110–117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 50 Suppl: S138–S143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williamson DH. The lactating mammary gland of the rat and the starved-refed transition: a model system for the study of the temporal regulation of substrate utilization. Biochem Soc Trans 18: 853–856, 1990 [DOI] [PubMed] [Google Scholar]