Abstract
Juxtacrine cell-cell signaling mediated by the direct interaction of adjoining mammalian cells is arguably the mode of cell communication that is most recalcitrant to engineering. Overcoming this challenge is crucial for progress in biomedical applications, such as tissue engineering, regenerative medicine, immune system engineering and therapeutic design. Here, we describe the significant advances that have been made in developing synthetic platforms (materials and devices) and synthetic cells (cell surface engineering and synthetic gene circuits) to modulate juxtacrine cell-cell signaling. In addition, significant progress has been made in elucidating design rules and strategies to modulate juxtacrine signaling based on quantitative, engineering analysis of the mechanical and regulatory role of juxtacrine signals in the context of other cues and physical constraints in the microenvironment. These advances in engineering juxtacrine signaling lay a strong foundation for an integrative approach to utilizing synthetic cells, advanced ‘chassis’ and predictive modeling to engineer the form and function of living tissues.
Advances in tissue engineering and regenerative medicine rely on the design of instructive microenvironments that promote desired cell behaviors and multicellular organization. Significant attention has been given to engineering the soluble microenvironment and adhesive scaffolds that emulate the extracellular matrix (ECM). In contrast, the development of engineering tools and strategies to modulate juxtacrine cell-cell interactions is at a nascent stage.
Juxtacrine signals are intrinsic to the cell whereas soluble factors and ECM cues can be supplied more readily as extrinsic factors in a synthetic microenvironment (Figure 1). This presents a significant challenge to developing an engineering toolbox for directly tuning cell-associated juxtacrine cues. Furthermore, the juxtacrine contribution to regulating cell functions is difficult to parse from the backdrop of regulatory inputs provided by soluble autocrine/paracrine signals and the ECM, making quantitative and direct analysis of juxtacrine cell-cell signaling particularly challenging.
Figure 1.
Engineering cell-cell signaling. Juxtacrine signals, such as cadherins, ephrins and Notch-Delta, are cues intrinsic to the cells in contrast to paracrine soluble signals and ECM proteins that provide extrinsic stimuli. The focus of this review (highlighted in red) is on engineering approaches to manipulate juxtacrine cues and associated intracellular regulatory signals and on the emerging design strategies to tune juxtacrine signals in the context of other microenvironmental cues that cumulatively affect cell functions with implications for biomedical applications.
In this review, we describe some of the promising advances in overcoming these challenges, including both the development of platforms to directly and specifically modulate cell-cell interactions and the utilization of systems-level analysis to parse the contribution of cell-cell interactions in the context of a complex microenvironment.
The Engineering Toolbox
Synthetic microenvironments: materials and devices
The importance of engineering cell-cell interactions has sparked the development of promising platforms for modulating multi-cell and multi-cell type interactions by controlling the size and shape of cell clusters and the relative positioning of cell populations [1-3]. Such platforms affect juxtacrine cell-cell signaling along with likely concomitant effects on the transmission of soluble autocrine/paracrine signals and cell accessibility to the ECM.
An approach to directly tune cell-cell signals utilizes material scaffolds that are normally used to present adhesive matrix cues, such as the short peptide RGD. Proteins or peptides involved in juxtacrine signaling are immobilized on the scaffold to mimic cues that would otherwise be presented by adjacent cells. This approach has been used to emulate E-cadherin-mediated adhesion [4] and Notch/Delta-mediated [5] signaling and more recently to induce ephrin signals in a synthetic polyethylene glycol (PEG)-based scaffold to promote pancreatic cell survival (Figure 2a) [6]. Furthermore, high throughput methods such as protein printing with a DNA spotter have been used to display cell-cell adhesion and signaling proteins in PEG hydrogel microwell arrays and to study the effect of these juxtacrine signals on stem cell proliferation [7]. This technology enables the use of functionalized scaffolds as tools for large scale, combinatorial screens.
Figure 2.
Devices and materials for modulating juxtacrine cell-cell signaling. (a) Juxtacrine cues are affixed to a scaffold, such as a PEG-based polymer network. (b) Chromium barriers restrict the movement of ephrin A1 on the supported membrane (bottom surface), thereby restricting the movement of EphA2-ephrin A1 complexes. (c) Controlling the direction of flow and using cell traps, isolated heterotypic cell pairs are induced. (d) Bowtie-shaped alginate-walled wells either accommodate one cell (half bowtie) or two cells (complete bowtie), thereby simulating cells with or without juxtracrine cell-cell interactions. (e) By controlling the gap size, juxtacrine signaling and the length scale for paracrine signaling are tunable in a dynamic manner.
This scaffold-based strategy to present juxtacrine cues is appealing for tissue engineering applications where scaffolds are already widely used to present ECM cues. A caveat, however, is that natural juxtacrine signals are laterally mobile in the plasma membrane. This lateral mobility can, in fact, be crucial to the signaling and structural roles of juxtacrine factors, such as ephrins and E-cadherin, respectively. Engineered supported membranes with nanoscale chromium barriers that disrupt and restrict the lateral mobility of the juxtacrine ligand ephrin A1 affected ligand-induced cytoskeleton reorganization, effector molecule recruitment and downstream expression profiles in EphA2 receptor-expressing breast cancer cells (Figure 2b). Furthermore, quantitative analysis of EphA2 radial transport in a panel of breast cancer cell lines showed a correlation with invasive ability, revealing the functional significance of the lateral mobility of juxtacrine signals [8].
While synthetic materials provide the advantage of selectively introducing juxtacrine signals onto a “clean slate”, natural cell membranes contain numerous juxtacrine receptor-ligand systems that are concomitantly triggered. Crosstalk and interactions among these juxtacrine signals are likely to play a significant role in the net effect on cell behavior. Furthermore, Notch/Delta and Eph/ephrins trigger feedback loops that regulate their expression levels [9,10]. Finally, membrane-associated juxtacrine signals can be internalized through transcytosis by the adjacent cell [11]. These and other regulatory processes underscore the need for directly and controllably inducing natural cell-cell interactions.
Bringing two cells “in touch” to induce contact-mediated signaling was recently demonstrated by combining a microfluidic device consisting of PDMS cups big enough to accommodate a pair of cells and a hydrodynamic flow-focused, three-step cell loading process (Figure 2c) [12]. This platform selectively brings two cells in a physical contact and allows monitoring of contact-mediated interactions in real time.
Meanwhile, for controlling juxtacrine interactions in cells that are adhered to an ECM, butterfly-shaped agarose-walled wells have been used to isolate the effect of cell-cell contact on the regulation of proliferation in homotypic cell systems (Figure 2d) [13]. A comb-inspired micromechanical platform provides dynamic control over cell-cell interactions (Figure 2e) [14]. By moving interdigitated mechanical combs closer together or further apart, the length scale for diffusion of paracrine signals can be tuned at arbitrary time points. Furthermore, for the subset of cells that sit at the edge of each comb, juxtacrine interactions can be decoupled from paracrine signaling. When the combs are touching, both cell-cell contacts and short range paracrine signaling are permitted; when the combs are brought slightly apart, the juxtacrine interaction is eliminated while the paracrine signaling continues. The application of dielectric forces [15] and laser-guided direct writing in 3D matrices [16], such as Matrigel, provide additional promising avenues for positioning cells and tuning cell-cell interactions.
Synthetic cells
Since juxtacrine signals are intrinsic to the interacting cells, a powerful complementary strategy to designing the microenvironment is to directly engineer the cells themselves. The classical demonstration of this approach involves tuning the level or type of cadherins on the cell surface to affect cell sorting within multicellular aggregates [17]. The cell population with the higher cell-cell adhesivity sorts to the middle of the spheroid; meanwhile, the less adhesive population rearranges to the spheroid surface.
In the context of juxtacrine signals in the immune system, recent approaches revolve around strategies to manipulate the cell surface either through genetic modification or by introducing moieties onto the cell surface without genetic manipulation (reviewed in [18]). A non-genetic approach is to induce interactions between T cells and their target cells by exogenously introducing cell-cell bridging molecules, such as antibodies or its fragments. Another approach is to “paint” the cell surface with immunomodulatory molecules that contain hydrophobic moieties, thereby anchoring these molecules to the cell surface. These and other approaches in cell surface engineering circumvent ex vivo genetic processing of T cells and promise to speed up the pace of translating engineering to clinical applications [18].
In addition to surface level interactions between cells, the intracellular signaling system triggered by juxtacrine communication is amenable to engineering design using molecular genetics and synthetic gene circuits approaches. Gene circuits have been assembled to exhibit interesting dynamics in mammalian cells (reviewed in [19]). Recently, in the context of juxtacrine cell-cell signaling in mammalian cell systems, an engineered transcriptional cascade was used to amplify an exogenous Delta-Notch signaling system in order to achieve signal propagation through a multicellular population of MDCK and CHO cells [20].
Engineering Principles of Juxtacrine Signaling
Alongside the development of platforms and methods to modulate juxtacrine signaling, significant advances have been made in the engineering analysis of cell-cell interactions and signaling to delineate design strategies for deploying this toolbox. These advances are aimed at the question of how juxtacrine signals ought to be tuned in order to achieve desired engineering objectives. What are the key design parameters and considerations, and how do these design parameters influence the form and function of the mammalian cell system we seek to engineer?
Juxtacrine signals are bidirectional and asymmetric. Both the receptor and the ligand initiate intracellular signaling cascades, as in the case of ephrin ligands and their Eph receptors. This asymmetry has long been appreciated at a phenotypic level in that adjoining cells take on distinct fates: thus, it plays a significant role in creating sharp boundaries and small length scale patterns in developing tissues [21] and directing cell-mediated target cell activation in the immune system [18]. However, the implications of this asymmetry on intracellular regulatory network at a systems scale is challenging to elucidate as typical “-omic” methods deal with population-wide samples from which it is difficult to distinguish molecular events within a single cell type in an heterotypic culture. A clever solution to this challenge is to differentially label cells engineered to express the receptor and counterpart cells equipped with the ligand [22]. Differential isotopic labeling and proteomics analysis revealed a striking level of asymmetric bidirectional post translational signaling in juxtacrine EphB2-Ephrin B1 signaling, and this asymmetry was demonstrated to be critical to the functional role that ephrin signaling plays in cell sorting.
Knowledge of this asymmetry in juxtacrine signaling opens a potential strategy to selectively modify one sub-population (the receptor-expressing cells or 'receivers', for example). Selective modification of the intracellular regulatory network, perhaps employing small molecule inhibitors, RNA interference or synthetic gene circuits, complements other elements in the engineering toolbox that can be used to manipulate external juxtacrine inputs using materials, devices and/or cell surface engineering.
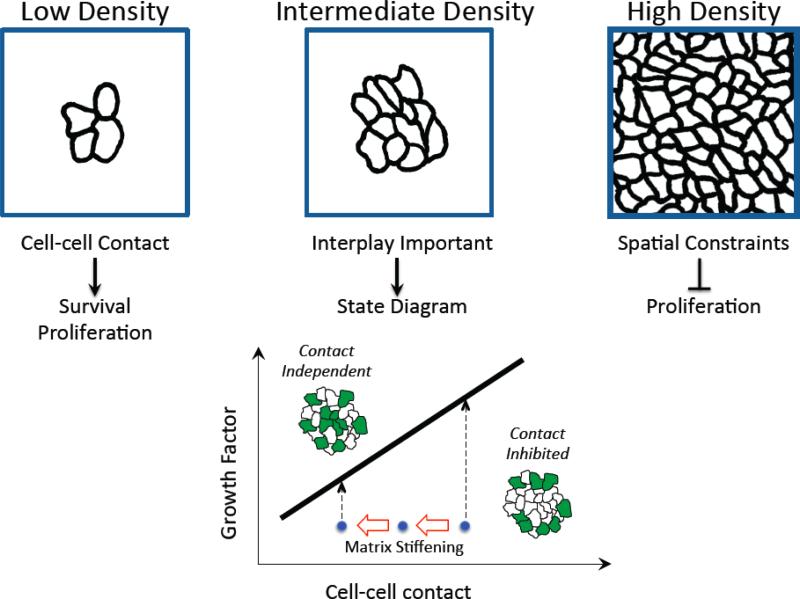
Design strategies to modulate juxtacrine signals will need to consider the cellular context, including other cues and physical constraints of the microenvironment. The effect of cell-cell contact on proliferation, for example, depends significantly on the microenvironmental context (Figure 3). At cell densities where cells interact in small clusters of 2-5 cells, juxtacrine signaling may in fact promote proliferation through Rac1 signaling [23]. This positive effect of cell-cell contact on proliferation could be a byproduct of contact-induced cell survival. Cell-cell contact promotes cell survival [24], and in doing so, intercellular contact may enable more viable cells to participate in other downstream cell functions, such as cell cycle progression. In contrast, at high cell density, cellular crowding may limit the available space for proliferation [25], a spatial constraint that the cells may sense through diminished availability of the extracellular matrix [23].
Figure 3.
The influence of juxtacrine cell-cell interactions on proliferation in epithelial cell systems depends on the microenvironmental context, including cell density (top three panels), soluble growth factors and a compliant ECM. The state diagram portrays the design space for shifting the cell system between a contact-inhibited state in which proliferation (green) occurs at the periphery of clusters and a contact-independent state in which proliferation occurs throughout the cluster. Matrix stiffening (leftward red arrows) perturbs cell-cell contacts, shifting the cell system (blue dots) closer to the transition line (solid black line) and enabling a switch from contact-inhibited to contact-independent proliferation at lower growth factor concentration.
Between these extremes lies an interesting regime where cells have ample room to proliferate. Here, the effect of cell-cell contact on cell cycle activity is determined by a quantitative competition between the growth-inhibitory effect of juxtacrine cadherin signaling and the mitogenic effect of growth factors [26], potentially involving the Nf2/Merlin tumor suppressor [27]. Only when the growth factor level is below a threshold amount, cadherin-mediated contact inhibits proliferation. Moreover, this crossover point at a threshold dose of growth factor is sensitive to the level of contact. Elevating cadherin expression increases the threshold growth factor dose so that a greater amount of pro-mitogenic soluble factor is needed to shift the system into contact-independent proliferation.
Elucidating the contact-growth factor state diagram for regulating proliferation provides a starting framework for understanding the effect of additional key environmental cues. Matrix stiffening, for example, compromises intercellular contacts and measurably reduces the threshold level of growth factor needed to switch cell clusters from contact-inhibited, circumferentially-localized proliferation to a contact-independent, uniform mode of proliferation [28].
It is appealing to consider whether a similar state diagram framework for the interplay between cell-cell contact, soluble factors and ECM stiffness extends to other cell behaviors and systems. Particularly, stem cell fate commitment clearly depends on all three microenvironmental cues [29,30]. While we can expect the quantitative details of such state diagrams to vary among cell types and behaviors, knowing even the qualitative contours as a function of important cell system parameters will help to guide design strategies, akin to the design and engineering of physical systems such as materials.
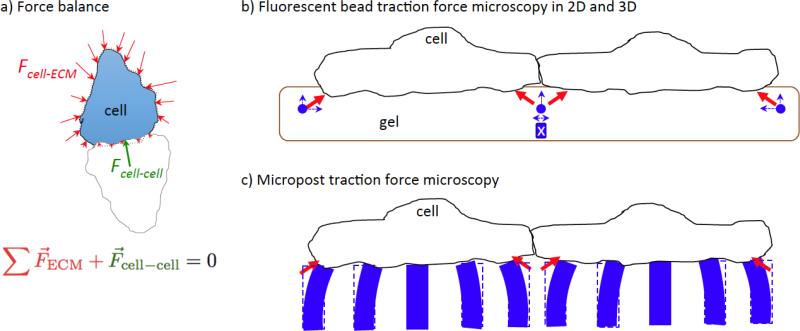
An influential process that contributes to the features of cell state diagrams involves mechanotransduction. Significant progress has been made in measuring the forces mediated by cell-cell interactions (Figure 4). Traction force microscopy is used to directly measure the displacement of fluorescent beads in the underlying gel substratum from which the cell-ECM traction forces are calculated and a Newtonian force balance is used to infer the cell-cell forces that must balance the traction forces. In some scenarios where cells are close to each other, the measured bead displacement may conceal the force cells actually exert on the matrix. For example, if neighboring cells are tugging on the matrix between them but in opposite directions, the bead displacements, at least viewed in the plane of the cell-substratum interface, would underrepresent the actual cell-ECM forces. Complementary methods, such as three-dimensional traction force microscopy [31] or micropillar-based traction force microscopy [32], circumvent this issue by measuring forces in the out-of-plane direction as well as in-plane forces or by measuring the local traction force specific to each cell using the bending of isolated pillars.
Figure 4.
Analysis of cell-cell forces. (a) Traction forces (red arrows) generated by the cell on the ECM are inferred from the measured displacements of fluorescent beads or microposts. The cell-cell force (green arrow) is calculated from a Newtonian force balance that requires the sum of the forces acting on a single cell to be zero. (b) Traction forces (red) cause displacement of fluorescent beads (blue arrows). In-plane (2D) characterization of bead displacement likely underestimates traction forces in the regions between cells because of counterbalancing forces (indicated by blue-boxed ‘X’). Bead displacements in the out-of-plane direction are reinforcing and can be captured using 3D confocal microscopy. (c) The extent of bending of microposts is proportional to the in-plane force applied at the top of the post. Because the posts bend independently from each other, they are more likely to capture the effect of in-plane traction forces in regions near the cell-cell interface.
Measurements of cell-cell physical coupling are revealing several design considerations for building tissue structures. First, in contrast to chemical signaling wherein juxtacrine signals promote asymmetric response in adjoining cells and enable the definition of sharp boundaries, physical coupling propagates over longer length scales. Significant cell-cell forces have been observed far from the leading edge in motile sheets [33]. Some of these forces possess distinct spatiotemporal patterns suggesting that distant cells have a substantial, non-constant impact on the cohesivity of the sheet and the progress of the leading edge [34]. In practical terms, this means that devices and materials that impose boundary conditions must consider the length scale of the synthetic platform relative to the length scale of cell-cell mechanotransduction. For example, cell sheets on lines shifted from inefficient swirling motions on broad lines to rapidly progressing caterpillar-like collective movement when the line width was narrowed to one or two cell diameters [35].
Second, cell-cell forces are a significant fraction of the overall adhesive force that cells experience [36]. Maruthamutu et al. conducted single and dual cell traction force experiments and found that the cell-cell forces are consistently 50% of cell-ECM forces over a range of ECM materials and stiffness. In a different system where the cell-cell contact area was constrained to bowtie patterns, cell-ECM forces were 20% the value of cell-cell forces [32]. If an engineer views the challenge of engineering tissues purely from a mechanical perspective, akin to building structural models of physical systems, these measurements reveal the extent to which cell-cell interactions contribute to forming and stabilizing multicellular architectures.
Finally, since the two principal avenues through which a cell exerts forces on its microenvironment involve cell-cell and cell-ECM interactions, the interplay between these two force transducers has important implications for designing tissues. Examination of cell pairs on micropatterns revealed that the location of cadherin-based cell-cell junctions and cell-substratum adhesions are mutually exclusive [37]. Hence, it is plausible to envision adhesive micropattern designs that not only control where individual cells adhere, but also in which locations these cells make strong connections with their neighbors.
In addition to its effect on the position of cell-cell adhesion, cell-ECM interactions can have a significant effect on the dynamics of cell-cell interactions. When two migrating cells collide, the stability of the binary pair, i.e., the duration the pair of cells remains together until dissociating, ranges from 200 min to 600 min for surfaces coated with high and low density of laminin, respectively [38]. Furthermore, on substrates of intermediate stiffness on which the cell can generate adequate traction and the material can deform due to that strain, cells that are nearby (~33 micron) but not yet touching sense each other through their mutual tugging on the matrix [39]. The stability of pairwise cell interactions and the length scale over which cells are drawn to each other influence the rates at which cell “dimers” come apart (e.g., koff in a coarse kinetic model of cell-cell interactions) and the encounter radius (ro) over which cells interact. The encounter radius is also sensitive to local soluble signals as in the case of the macrophage-tumor cell paracrine loop that mediates coordinated streaming motility [40]. Revealing the dependence of cell-cell interaction properties (ro and koff) on material design parameters, such as adhesion ligand density and stiffness, provides a promising avenue for engineering materials that tune juxtacrine interactions and thereby modulate multicellular aggregation dynamics [38].
Forward with an integrative strategy
Juxtacrine cell-cell signaling poses engineering challenges, both in terms of developing methods for manipulation and for conducting quantitative analysis. Significant advances have been realized on multiple fronts through the development of synthetic microenvironments (materials and devices) and synthetic cells (cell surface engineering and synthetic gene circuits) and through quantitative systems-level analysis of juxtacrine signaling in the context of other cues and physical constraints of the microenvironment.
These advances in engineering juxtacrine signaling, a mode of cell communication that is arguably the most recalcitrant to manipulation and analysis, pave the way to engineering robust multicellular structures and machines. Realizing this potential will likely require an integrative approach along at least two dimensions. First, the sophisticated engineering of intrinsic cellular machinery, as enabled by molecular genetics and synthetic gene circuits, must be integrated with an advanced ‘chassis’ that provides the microenvironmental context necessary for the synthetic cell community to execute its self-organization. Second, integrative models that simulate physiochemical mechanisms across a field of interacting cells will provide frameworks for predicting how the interplay of cell-cell, cell-ECM and soluble signals affect multicellular patterning and organization. The recent advances in engineering juxtacrine signaling provide a strong foundation to undertake this integrative approach to engineering the form and function of living tissues.
Highlights.
➢ Juxtacrine signaling between adjacent cells is difficult to analyze and manipulate.
➢ Synthetic environments and cells offer a toolbox for modulating juxtacrine signals.
➢ Engineering analysis is elucidating the design space for tuning juxtacrine signals.
Acknowledgements
We thank the members of the Asthagiri group, both past and present, for helpful discussions. This work was supported by grants from the National Institutes of Health (NIH R01CA138899), the National Cancer Institute USC Physical Sciences of Oncology Center (NIH U54CA143907) and Northeastern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• Of Special Interest: Refs. 9, 10, 17, 18, 20, 26.
•• Of Outstanding Interest: Refs. 8, 22, 23, 28, 33, 35, 39.
- 1.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nature Protocols. 2012;7:1247–1259. doi: 10.1038/nprot.2012.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumarkin E, Tzadu L, Csaszar E, Seo M, Zhang H, Lee A, Peerani R, Purpura K, Zandstra PW, Kumacheva E. High-throughput combinatorial cell co-culture using microfluidics. Integrative Biology. 2011;3:653–662. doi: 10.1039/c1ib00002k. [DOI] [PubMed] [Google Scholar]
- 4.Nagaoka M, Ise H, Akaike T. Immobilized E-cadherin model can enhance cell attachment and differentiation of primary hepatocytes but not proliferation. Biotechnology Letters. 2002;24:1857–1862. [Google Scholar]
- 5.Liu CY, Apuzzo ML, Tirrell DA. Engineering of the extracellular matrix: working toward neural stem cell programming and neurorestoration--concept and progress report. Neurosurgery. 2003;52:1154–1165. discussion 1165-1157. [PubMed] [Google Scholar]
- 6.Lin CC, Anseth KS. Cell-cell communication mimicry with poly(ethylene glycol) hydrogels for enhancing β cell function. Proceedings of the National Academy of Sciences. 2011;108:6380–6385. doi: 10.1073/pnas.1014026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nature Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 8.Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Developmental Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [An outstanding review of the largest family of receptor tyrosine kinases.] [DOI] [PubMed] [Google Scholar]
- 11.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nature Cell Biology. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 12.Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Microfluidic control of cell pairing and fusion. Nature Methods. 2009;6:147–152. doi: 10.1038/nmeth.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson CM, Chen CS. Cell-cell signaling by direct contact increases cell proliferation via a PI3K-dependent signal. FEBS Letters. 2002;514:238–242. doi: 10.1016/s0014-5793(02)02370-0. [DOI] [PubMed] [Google Scholar]
- 14.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proceedings of the National Academy of Sciences. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nature Methods. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 16.Nahmias Y, Odde DJ. Micropatterning of living cells by laser-guided direct writing: application to fabrication of hepatic-endothelial sinusoid-like structures. Nature Protocols. 2006;1:2288–2296. doi: 10.1038/nprot.2006.386. [DOI] [PubMed] [Google Scholar]
- 17.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Developmental Biology. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [The classical Differential Adhesion Paradigm for cell sorting and aggregation.] [DOI] [PubMed] [Google Scholar]
- 18.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Science Translational Medicine. 2012;4:148rv149. doi: 10.1126/scitranslmed.3003763. [An excellent review of emerging areas in the field of immunoengineering, including cell surface engineering approaches to manipulate juxtacrine interactions between immune cells and their targets.] [DOI] [PubMed] [Google Scholar]
- 19.Ausländer S, Fussenegger M. From gene switches to mammalian designer cells: present and future prospects. Trends in Biotechnology. 2012 doi: 10.1016/j.tibtech.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Koga M, Nishida E, Ebisuya M. Synthetic signal propagation through direct cell-cell interaction. Science Signaling. 2012;5:ra31. doi: 10.1126/scisignal.2002764. [An early example of a synthetic gene circuits approach to achieve mammalian cell patterning by engineering juxtacrine cell-cell signaling.] [DOI] [PubMed] [Google Scholar]
- 21.Giurumescu CA, Asthagiri AR. Signal processing during developmental multicellular patterning. Biotechnology progress. 2008;24:80–88. doi: 10.1021/bp070127t. [DOI] [PubMed] [Google Scholar]
- 22.Jørgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Cell-specific information processing in segregating populations of Eph receptor ephrin–expressing cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [A proteomic approach is used to provide a systems-scale characterization of the remarkable asymmetric tyrosine phosphorylation induced by Eph B2 receptor and ephrin B1 ligand.] [DOI] [PubMed] [Google Scholar]
- 23.Liu WF, Nelson CM, Pirone DM, Chen CS. E-cadherin engagement stimulates proliferation via Rac1. The Journal of Cell Biology. 2006;173:431–441. doi: 10.1083/jcb.200510087. [Application of geometric confinement of cells to demonstrate that E-cadherin promotes cell proliferation, contrary to the widely held notion of contact-inhibition of proliferation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejana E. Endothelial cell-cell junctions: happy together. Nature Reviews Molecular Cell Biology. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 25.Puliafito A, Hufnagel L, Neveu P, Streichan S, Sigal A, Fygenson DK, Shraiman BI. Collective and single cell behavior in epithelial contact inhibition. Proceedings of the National Academy of Sciences. 2012;109:739–744. doi: 10.1073/pnas.1007809109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Kushiro K, Graham NA, Asthagiri AR. Tunable interplay between epidermal growth factor and cell-cell contact governs the spatial dynamics of epithelial growth. Proceedings of the National Academy of Sciences. 2009;106:11149–11153. doi: 10.1073/pnas.0812651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curto M, Cole BK, Lallemand D, Liu C-H, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. The Journal of Cell Biology. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. Journal of Cell Science. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [Engineering analysis of the combined effect of growth factor concentration, cell-cell contact and matrix stiffness on proliferation in epithelial cell clusters yields a state diagram model for contact-inhibition of proliferation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toh YC, Blagovic K, Voldman J. Advancing stem cell research with microtechnologies: opportunities and challenges. Integrative Biology. 2010;2:305–325. doi: 10.1039/c0ib00004c. [DOI] [PubMed] [Google Scholar]
- 31.Notbohm J, Kim JH, Asthagiri AR, Ravichandran G. Three-dimensional analysis of the effect of epidermal growth factor on cell-cell adhesion in epithelial cell clusters. Biophysical journal. 2012;102:1323–1330. doi: 10.1016/j.bpj.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell–cell junctions. Proceedings of the National Academy of Sciences. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nature Physics. 2009;5:426–430. [Traction forces are observed deep into a sheet of cells in a wound healing model counter to previous assumptions, and a Newtonian force balance suggests substantial cell-cell forces in regions distant from the wound edge.] [Google Scholar]
- 34.Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Butler JP, Fredberg JJ, Trepat X. Mechanical waves during tissue expansion. Nature Physics. 2012;8:628–634. [Google Scholar]
- 35.Vedula SRK, Leong MC, Lai TL, Hersen P, Kabla AJ, Lim CT, Ladoux B. Emerging modes of collective cell migration induced by geometrical constraints. Proceedings of the National Academy of Sciences. 2012;109:12974–12979. doi: 10.1073/pnas.1119313109. [The mode of coordinated cell movement in cell sheet is sensitive to the boundary conditions of the microenvironment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proceedings of the National Academy of Sciences. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng Q, Duchemin-Pelletier E, Deshiere A, Balland M, Guillou H, Filhol O, Thery M. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proceedings of the National Academy of Sciences. 2012;109:1506–1511. doi: 10.1073/pnas.1106377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pope MD, Asthagiri AR. Short-lived, transitory cell-cell interactions foster migration-dependent aggregation. PLoS ONE. 2012;7:e43237. doi: 10.1371/journal.pone.0043237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophysical journal. 2008;95:6044–6051. doi: 10.1529/biophysj.107.127662. [Cells sense and are drawn to each other by exerting traction forces on a compliant substratum.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roussos ET, Balsamo M, Alford S, Wyckoff J, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, et al. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. Journal of Cell Science. 2011;124:2120–2131. doi: 10.1242/jcs.086231. [DOI] [PMC free article] [PubMed] [Google Scholar]