Abstract
Prenatal and early childhood exposures are implicated as causes of allergy, but the effects of intrauterine growth restriction on immune function and allergy are poorly defined. We therefore evaluated effects of experimental restriction of fetal growth on immune function and allergic sensitization in adolescent sheep. Immune function (circulating total red and white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and basophils, and the antibody response to Clostridial vaccination) and responses to house dust mite (HDM) allergen and ovalbumin (OVA) antigen sensitization (specific total Ig, IgG1, and IgE antibodies, and cutaneous hypersensitivity) were investigated in adolescent sheep from placentally restricted (PR, n = 23) and control (n = 40) pregnancies. Increases in circulating HDM-specific IgE (P = 0.007) and OVA-specific IgE (P = 0.038) were greater in PR than control progeny. PR did not alter total Ig, IgG1, or IgM responses to either antigen. PR increased OVA-specific but not HDM-specific IgA responses in females only (P = 0.023). Multiple birth increased Ig responses to OVA in a sex-specific manner. PR decreased the proportion of positive cutaneous hypersensitivity responders to OVA at 24 h (P = 0.030) but had no effect on cutaneous responses to HDM. Acute wheal responses to intradermal histamine correlated positively with birth weight in singletons (P = 0.023). Intrauterine growth restriction may suppress inflammatory responses in skin downstream of IgE induction, without impairment in antibody responses to a nonpolysaccharide vaccine. Discord between cutaneous and IgE responses following sensitization suggests new mechanisms for prenatal allergy programming.
Keywords: allergy, animal model, intrauterine growth restriction, developmental programming, sheep
increasing evidence suggests that environmental factors, including prenatal or early childhood exposures, modulate later allergy susceptibility (29). One such prenatal event is intrauterine growth restriction (IUGR), which affects 6–12% of babies in developed countries (15) and usually results in small size at birth for gestational age where the birth weight of babies falls below the tenth percentile for sex at a given gestational age (SGA, 42). IUGR and SGA babies have increased risks of perinatal mortality and morbidity and of adult metabolic and cardiovascular disease (9). Less is known about the effects of IUGR on postnatal immune function, including allergy. Low-birth-weight individuals are at greater risk of pneumococcal infection as infants (34) and may have impaired B-cell function as adults (22, 23), but these studies did not separate low birth weight due to IUGR and prematurity. Risks of allergic sensitization and atopic diseases including eczema and hay fever in children and adults are positively related to size at birth, independent of gestational age, in some (4, 10, 14), but not all studies (12, 28). Similarly, in a twin study, the heavier birth weight twin had a higher risk of childhood atopic eczema (16). Although SGA is associated with increased, rather than decreased, risks of childhood and adult asthma (26, 40), these studies did not differentiate allergic and nonallergic asthma and possibly reflect impaired lung development following IUGR (36). Together, these findings suggest IUGR suppresses immune function and decreases the risk of postnatal allergy, although these human studies may be confounded by environmental factors that affect both fetal growth and immune function (25, 30).
Poor placental function is a major cause of IUGR due to reduced fetal substrate supply (32). Restricted placental growth and function (PR) can be induced surgically in sheep, with similar prenatal and postnatal consequences as human IUGR (1, 31). Similar to humans, sheep sensitized to allergens develop IgE responses, cutaneous hypersensitivity, and airway hyperresponsiveness following allergen challenge (19). This provides a repeatable, well-characterized protocol for induction of allergy in this species (2, 3, 19), allowing susceptibility to allergic challenge to be tested independent of prenatal environment. We therefore used the sheep to directly test the hypothesis that restricted fetal growth impairs immune function and reduces allergy susceptibility in adolescence.
METHODS
All procedures were approved by the University of Adelaide Animal Ethics Committee (M-2010-139) and conducted in accordance with Australian guidelines (24).
Animal model.
Placental growth of primiparous Merino × Border Leicester ewes was restricted by surgical removal of all but four visible endometrial placental attachment sites (caruncles) from each uterine horn (1, 31) at least 10 wk before timed mating (27). No maternal surgery occurred during pregnancy, and control ewes were unoperated. Pregnant control (CON, unoperated) and PR ewes were housed indoors from day 110 of gestation until their spontaneously born lambs were weaned at 13 wk of age. Ewes were fed 1 kg Rumevite pellets daily (Ridley AgriProducts, Melbourne, Australia), with ad libitum access to lucerne chaff and water. Gestational ages, birth weights, and litter sizes were recorded. After being weaned, progeny were housed in outside paddocks in same sex groups of similar ages and fed 0.5 kg Rumevite pellets per sheep daily, with ad libitum access to oaten hay, pasture, and water. Sheep were housed indoors in individual pens for ≥6 days before and 3 days during cutaneous hypersensitivity testing, with 0.5 kg pellets/day and ad libitum access to lucerne chaff and water. Immune function was studied in 17 CON males (2 singletons, 13 twins, 2 triplets), 23 CON females (5 singletons, 18 twins), 10 PR males (5 singletons, 5 twins), and 13 PR females (9 singletons, 3 twins, 1 triplet).
Immunization, sensitization, and cutaneous hypersensitivity testing.
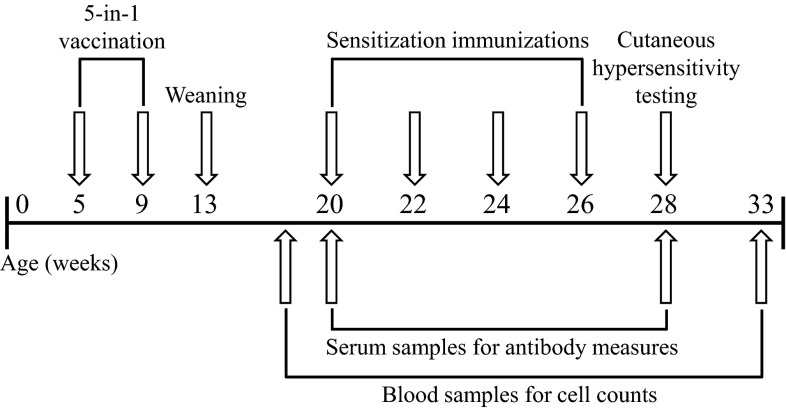
Sheep were immunized with an anti-Clostridial vaccine (Ultravac 5-in-1; Pfizer Animal Health, West Ryde, Australia) at 5 and 9 wk of age (Fig. 1). Sheep were then sensitized to house dust mite allergen (HDM; CSL, Parkville, Australia) and ovalbumin (OVA; A2512, Sigma, MO), each administered mixed with aluminium hydroxide as adjuvant (1:1) by subcutaneous injections (2, 39) at 20, 22, 24, and 26 wk of age. Immediate and delayed cutaneous responses (cutaneous hypersensitivity) to intradermal injections of 50 μl saline (negative control), histamine (10 μg/ml, H7375, Sigma), HDM (100 μg/ml), and OVA (10 μg/ml) were assessed at 28 wk of age (3). No adjuvants were given with intradermal injections. Skin wheal responses were measured with calipers at 0.5, 4, 2, and 48 h, and an average diameter across two perpendicular readings of ≥3 mm was classified as a positive reaction.
Fig. 1.
In vivo study timeline.
Serum antibody concentrations.
Peripheral blood was collected at 20 wk of age and immediately before cutaneous hypersensitivity tests at 28 wk of age (Fig. 1), and serum was stored at −80°C. Serum clostridial-specific total Ig was assayed on ELISA plates precoated with 10 μg/ml Chauvoei antigen (Pfizer Animal Health, West Ryde, Australia), with samples taken at 28 wk diluted 1/500 in Blue Diluent (AsureQuality, Tullamarine, Australia). Sheep serum was used for standards (serially diluted to 1/32,000) and positive controls. Horseradish peroxidase (HRP)-conjugated rabbit anti-sheep IgG was diluted 1/2,000 in Blue Diluent and used as the detection antibody. Plates were developed with 3′,3′,5′,5′-tetramethyl-benzidine dihydrochloride hydrate (TMB, Sigma, Castle Hill, Australia), and optical density was read at 450 nm.
HDM- and OVA-specific total Ig, IgG1, IgE (2, 3, 33, 39), IgM, and IgA antibodies pre- (20 wk) and post- (28 wk) immunization were determined in duplicate by ELISA, with optical density read at 450 nm. IgM and IgA were assayed by ELISA as for total antigen-specific Ig (3, 39), but with rabbit anti-ovine IgA (Bio-Rad AbD Serotec, Kidlington, UK), or rabbit anti-ovine IgM (diluted 1/5,000, Bio-Rad AbD Serotec, Kidlington, UK) as primary antibody, and HRP-conjugated swine anti-rabbit Ig (diluted 1/1,000, Dako, Glostrup, Denmark) as secondary antibody. Antibody responses to sensitization were classified as positive when they increased by greater than two fold relative to basal concentrations.
Cell counts.
Peripheral blood was collected into EDTA-coated tubes at 18 (subset of ∼75% of cohort) and 33 wk of age. Samples were stained with Wright's Giemsa stain (Siemens, Munich, Germany). Total red blood cells (RBC) and white blood cells (WBC) were quantified using an automated cell counter (Cell Dyn 3700, Abbott Diagnostics, IL), then 100 WBC per sample were classified manually under light microscopy to differentiate WBC subtypes (neutrophils, lymphocytes, monocytes, eosinophils, and basophils).
Statistical analysis.
Continuous and binary outcomes were analyzed using a Generalized Linear Mixed Models framework that examined the effects of PR, litter size (singleton vs. multiple birth), and sex, treating the dam as the experimental unit and data from siblings as repeated measures on each dam. The distributions of continuous variables were assessed for normality, and a log, square root or inverse transformation was applied as necessary. Binary outcomes were analyzed within this framework, assuming a binomial distribution and logit link function. Interaction effects were non-significant for all binomial outcomes, and the final model used for these included main effects only. Relationships between continuous variables were examined through the calculation of Pearson's correlation coefficient, restricted to singletons to remove effects of clustering due to ewes. Data were analyzed using SPSS software, version 20.0 (SPSS, Chicago, IL) and are shown as estimated means ± SE. P < 0.05 was accepted as statistically significant. Interactions are not mentioned unless significant.
RESULTS
Birth weight and gestational age.
PR reduced birth weight by 20% (CON: 5.70 ± 0.22 kg, PR: 4.55 ± 0.21 kg, P < 0.01), and multiple birth reduced birth weight by 14% (singleton birth: 5.52 ± 0.23, multiple birth: 4.73 ± 0.20, P = 0.010). Sex did not affect birth weight. Gestational age at birth was 139–150 days and was reduced by 2.2 days in PR pregnancies (CON: 147.1 ± 0.5 days, PR: 144.9 ± 0.5 days, P = 0.004). Neither litter size nor sex affected gestational age. Inclusion of gestational age as a covariate did not change effects of PR on continuous outcomes; therefore, it was not included as a factor in final analyses.
Circulating immune cells.
At 18 wk of age, PR, litter size and sex did not affect concentrations of RBC, WBC, and WBC subtypes. At 33 wk of age, PR and litter size did not affect concentrations of RBC, WBC, and WBC subtypes except eosinophils. Effects of PR on eosinophil concentrations at 33 wk differed between sexes (PR × sex interaction, P = 0.019) but did not differ between PR and CON in either males or females and were unaffected by litter size. Neutrophil concentrations at 33 wk were higher in males than females (males: 3.70 ± 0.29 × 109 cells/l, females: 2.79 ± 0.22 × 109 cells/l, P = 0.025), whereas the reverse was true for lymphocyte concentrations (males: 3.30 ± 0.37 × 109 cells/l, females: 4.55 ± 0.29 × 109 cells/l, P = 0.020). Sex did not affect concentrations of RBC, WBC, monocytes, eosinophils, and basophils.
Antibody responses to HDM allergen and OVA sensitization.
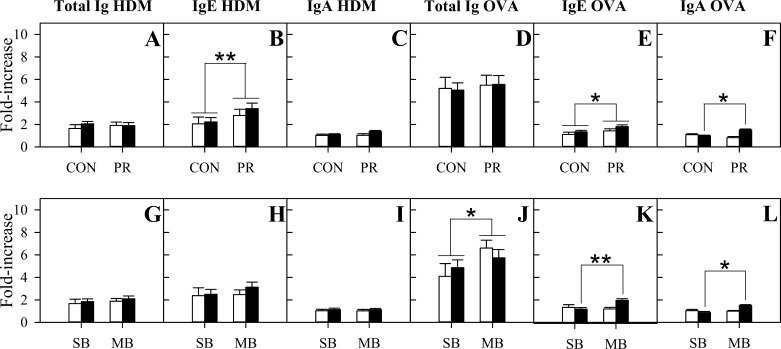
PR and sex did not affect levels of HDM-specific (Fig. 2A) or OVA-specific (Fig. 2D) total Ig. Litter size did not affect HDM-specific total Ig responses (Fig. 2G), but the OVA-specific total Ig response was greater in multiple birth than singleton birth sheep (Fig. 2J, P = 0.047). The increases in HDM-specific IgE (Fig. 2B, P = 0.007) and OVA-specific IgE (Fig. 2E, P = 0.038) postsensitization were higher in PR than CON sheep. Litter size and sex did not affect HDM-specific IgE responses (Fig. 2H). There were no main effects of litter size or sex on OVA-specific IgE responses (Fig. 2K), although effects of litter size differed between sexes (litter size × sex interaction, P = 0.015). OVA-specific IgE responses were greater in multiple birth than singleton birth females (P = 0.003) but did not differ with litter size in males. PR and litter size did not affect HDM-specific IgG1 (overall: 1.53 ± 0.07-fold increase) or OVA-specific IgG1 (overall: 3.97 ± 0.31-fold increase) responses to sensitization. The HDM-specific IgG1 response to sensitization was greater in females than males (males: 1.34 ± 0.15-fold increase, females: 1.72 ± 0.12-fold increase, P = 0.017), and sex did not affect OVA-specific IgG1 responses. The HDM-specific IgM (overall: 1.38 ± 0.06-fold increase), OVA-specific IgM (overall: 1.46 ± 0.12-fold increase), and HDM-specific IgA (overall: 1.14 ± 0.06-fold increase) responses were not affected by PR (Fig. 2C), litter size (Fig. 2I), or sex. Females (1.25 ± 0.07-fold increase) had greater OVA-specific IgA responses than males (0.96 ± 0.05-fold increase, P = 0.038). Effects of PR on the OVA-specific IgA response differed between sexes (PR × sex interaction, P = 0.006), with a greater OVA-specific IgA response (Fig. 2F) in PR females than CON females (P = 0.023) and no PR effect on OVA-specific IgA response in males. Similarly, effects of litter size on OVA-specific IgA responses differed between sexes (litter size × sex interaction, P = 0.014), with a greater OVA-specific IgA response (Fig. 2L) in multiple birth females than singleton birth females (P = 0.015) and no litter size effect on OVA-specific IgA response in males.
Fig. 2.
Serum antibody responses to house dust mite (HDM) allergen and ovalbumin (OVA) (HDM/OVA) sensitization in control (CON) and placentally restricted (PR) progeny overall (A–F, CON: n = 17 males and 23 females, PR: n = 9 males and 13 females) and in singleton birth (SB) and multiple birth (MB) sheep (G–L, SB: n = 7 males and 14 females, MB: n = 20 males and 22 females). Data are shown as fold changes in HDM-specific total Ig (A, G), IgE (B, H), and IgA (C, I), and in OVA-specific total Ig (D, J), IgE (E, K), and IgA (F, L) from presensitization to 2 wk postsensitization. Open bars, males; solid bars, females. Values are estimated means ± SE; *P < 0.05; **P < 0.01.
More PR than CON animals had a positive HDM-specific IgE response (CON: 32.1 ± 0.1% positive, PR: 89.1 ± 0.07% positive, P = 0.003). Similarly, more multiple birth than singleton birth animals had a positive HDM-specific IgE response (singleton birth: 44.2 ± 14.3% positive responders, multiple birth: 83.0 ± 7.7% positive responders, P = 0.038). Proportions of HDM-specific IgE responders did not differ between sexes. PR, litter size, and sex did not affect the proportion of sheep that were positive responders in terms of HDM-specific total Ig (overall: 26.8 ± 6.6% positive), IgG1 (overall: 10.1 ± 4.8% positive), IgM (overall: 6.3 ± 3.5% positive), and IgA (overall: no positive responders, IgA increased by less than 2-fold in all sheep). Similarly, PR, litter size, and sex did not affect the proportion of sheep that were positive responders in terms of OVA-specific total Ig (overall: 94.0 ± 23.5% positive), IgE (overall: 7.0 ± 4.0% positive), IgG1 (overall: 69.5 ± 8.4% positive), IgM (overall: 5.0 ± 2.9% positive), and IgA (overall: 3.4 ± 2.6% positive).
Antibody responses to Clostridial vaccination.
Antibody responses to vaccination against Clostridium spp. were highly variable, ranging from titers of 1.46 IU to 169.02 IU, with a mean of 11.75 ± 3.71 IU. Antibody responses to vaccination were not altered by PR, litter size, or sex (data not shown).
Cutaneous hypersensitivity responses.
All sheep had a positive cutaneous hypersensitivity response to HDM at 30 min, and this response was sustained to 4 h in most sheep (overall: 100.0 ± 4.0% positive). Similarly, most sheep had a positive acute response to OVA at 30 min (overall: 87.2 ± 4.8% positive) and 4 h (overall: 72.6 ± 6.2% positive responders). The proportions of HDM and OVA cutaneous hypersensitivity responders at 30 min and 4 h, and the proportion of HDM cutaneous hypersensitivity responders at 24 h (overall: 42.7 ± 7.0% positive) were not affected by PR, litter size, or sex. A lower proportion of PR than CON sheep had positive cutaneous hypersensitivity responses to OVA at 24 h (CON: 49.8 ± 10.0% positive, PR: 15.7 ± 7.8% positive, P = 0.030), and the proportion of responders was unaffected by litter size or sex. At 48 h after challenge, the proportion of HDM-positive responders was greater in singleton birth than multiple birth sheep (singleton birth: 53.0 ± 11.5% positive, multiple birth: 19.3 ± 6.9% positive, P = 0.027) but was not affected by PR or sex. Males were more likely than females to have positive cutaneous hypersensitivity responses to OVA at 48 h (males: 36.7 ± 10.5% positive, females: 12.6 ± 5.8% positive), and the proportion of responders was unaffected by PR or litter size.
Correlation between birth weight and cutaneous histamine responses.
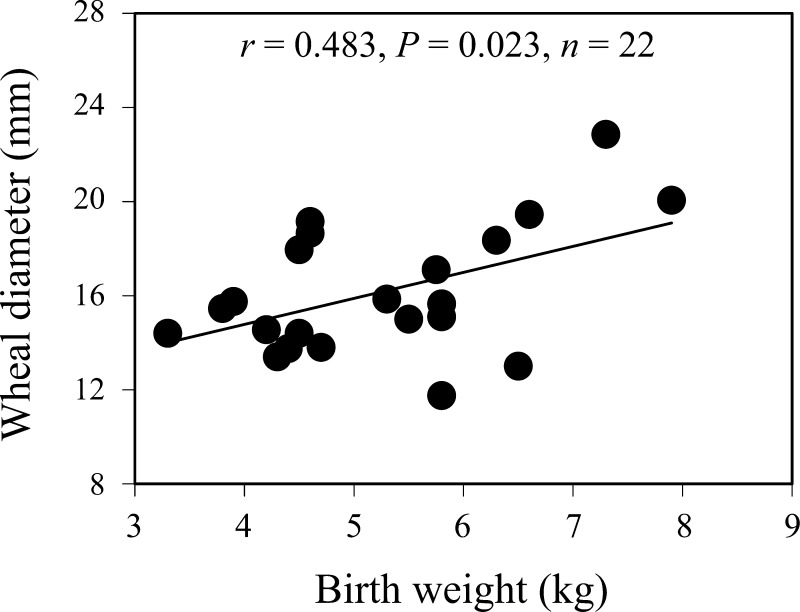
Skin wheal diameter at 30 min after intradermal injection of histamine correlated positively with birth weight in singletons (Fig. 3, P = 0.023). There was no correlation, either overall or in singletons, between birth weight and skin wheal diameter at 4, 24, and 48 h after injection of histamine.
Fig. 3.
Relationship between birth weight and skin wheal response to histamine at 30 min after injection in singletons.
DISCUSSION
Here we have shown directly that IUGR, induced by surgical restriction of placental implantation and function, alters later allergic responses in adolescent sheep, with fewer positive cutaneous hypersensitivity responses than would be expected given changes in IgE. This is the first demonstration of altered allergy susceptibility after experimental IUGR, where IUGR and control progeny share a common postnatal environment. These outcomes reflect IUGR rather than prematurity, with >95% of PR lambs born within 7 days of normal term (147 days gestation in this breed). These results are consistent with reports from human epidemiological studies suggesting decreased susceptibility to allergy after SGA (4, 10, 14, 16). Furthermore, our results directly confirm an independent effect of the constrained prenatal environment on allergy.
The IgE responses to both HDM and OVA antigens were increased in PR compared with CON progeny, although the HDM-specific increases were of greater magnitude than those induced by OVA. This probably explains why PR increased the proportion of IgE responders to HDM but not OVA, because only 7% of sheep reached the threshold of a positive (≥2-fold increase) IgE response to OVA. The different magnitudes of IgE responses may reflect different antigenic potential of the two preparations, since both antigens were given under the same conditions and timing to sheep in the present study. We have previously reported that the concentration of sensitizing antigen influences IgE responses in sheep (2); however, testing effects of PR on responses to multiple antigen concentrations was beyond the scope of the present study. The approximately threefold overall increase in HDM-specific IgE is consistent with the increases we have reported previously in sensitized sheep (3). There is mixed evidence for effects of IUGR on IgE responses in humans, which may at least in part reflect confounding due to common pre- and postnatal exposures to an adverse environment. Studies of circulating antibody concentrations in SGA humans have largely focussed on IgE in response to environmental allergen exposure, with increased total IgE dependent on exposure levels in one study (18) but lower circulating IgE specific for common allergens in 5 to 7 year old children (4). Similar responses to vaccination (with bacterial antigens) in CON and PR lambs in the present study are consistent with previous findings that low birth weight and exposure to maternal seasonal undernutrition during gestation did not alter antibody production following vaccination with nonpolysaccharide vaccines in humans (21–23). This evidence of enhanced or normal immunoglobulin responses to antigens and vaccination after SGA contrasts with the evidence that low-birth-weight infants have greater susceptibility to infectious diseases in early life (34) and exhibit markers of impaired B-cell function as adults (22, 23), although this evidence for greater susceptibility probably also reflects effects of prematurity. Together, these results suggest that some, but not all, immune responses are impaired by IUGR.
Effects of natural IUGR induced by twinning in the present study had similarities to effects of PR, with greater immunoglobulin responses to sensitization in multiple birth (mostly twins) than in singleton birth progeny. These effects of multiple birth, however, were only evident for OVA-specific responses, whereas PR increased responses to both antigens. Twinning decreases placental function and reduces fetal growth in sheep (38) and in human twin pregnancies from 32 wk gestation (30). In the present study, multiple birth reduced birth weight to a lesser extent than PR, suggesting that this natural growth restriction was less severe than the surgically induced PR, possibly accounting for the smaller programming effect on immune function in later life. We also saw evidence of sex-specific developmental programming of immune function, with enhanced IgA responses to OVA after PR or multiple birth and greater IgE responses to OVA in multiple birth than singleton birth progeny evident in females only. This contrasts with evidence that preimplantation methyl donor deficiency enhanced acute (haptoglobulin) responses to antigens in male, but not female, young adult sheep (35). Different sex-specific susceptibility of immune function to perturbation during development between these two studies might reflect the different prenatal exposures or different interactions between sex and exposure for acute non-specific versus antigen-specific responses. Studies in humans and rats have shown that allergic disease rates and processes differ between sexes and are modulated by sex steroids, including potentiation of IgE responses to antigens by estradiol (reviewed in Ref. 5). Since IUGR decreases circulating estradiol in adolescent girls after puberty (13), however, changes in estradiol seem unlikely to explain sex-specific differences in effects of litter size on IgE responses to antigens.
Cutaneous hypersensitivity responses were lower than might be expected given changes in circulating IgE. Despite increased IgE responses in PR progeny, cutaneous hypersensitivity responses to HDM were normal in PR progeny. More strikingly, cutaneous hypersensitivity responses to OVA were lower in PR progeny, despite elevated OVA-specific IgE responses in PR progeny. This suppressed cutaneous reactivity to antigens in the PR sheep is consistent with reduced cutaneous hypersensitivity reactions to phytohemagglutinin in SGA children born at ≥35 wk gestational age with known placental insufficiency or maternal hypertension compared with controls (8). Early life and adult environmental factors may interact in determining inflammatory responses to antigens. For example, perinatal exposure to short-day photoperiod in the Siberian hamster, which delays postnatal growth and reproductive development, programs increased adult hypersensitivity responses only when these animals were also housed in short-day photoperiod as adults (41). The contrasting effects of PR on antibody and inflammatory responses to sensitization in the present study suggest an alteration in the inflammatory pathway downstream of IgE production, which may reduce inflammatory responses to antigens after IUGR. Consistent with this hypothesis, in the present cohort of sheep, acute cutaneous hypersensitivity responses to histamine correlated positively with birth weight. Acute responses to histamine include local inflammation, expression of eotaxin, and recruitment of eosinophils to the site of allergic skin reactions (20), with amplification by activation of the histamine H4 receptor on mast cells (43). Decreased acute responses to histamine in sheep with lower birth weights might therefore inhibit subsequent late-phase reactions to antigens. Although circulating eosinophils were not measured concurrently with antibody abundance or acute reactions to sensitization in the present study, eosinophil abundance was similar in CON and PR sheep 2 wk before the first sensitization and 5 wk after cutaneous hypersensitivity testing, suggesting that a deficiency in peripheral blood eosinophils is not the primary mechanism causing suppressed late-phase reactions to HDM and OVA in PR sheep. Similarly, although elevated IgG concentrations can suppress IgE-mediated mast cell degranulation (37), increases in IgG after sensitization were not altered by PR or litter size and are unlikely to explain differences in cutaneous responses between these groups. Further studies are needed to determine effects of IUGR on mast cell numbers and function, and the underlying mechanisms.
The mechanisms underlying the effects of IUGR on postnatal immune function are currently poorly understood. Reduced nutrient availability in utero might directly reduce cell proliferation in immune tissues. Thymus weight was reduced in IUGR humans (7, 11) and newborn PR rats, and the spleen and thymus of PR rats had fewer lymphocytes at weaning (6). In adolescent humans, circulating concentrations of thymopoietin, a hormone produced by the thymus that regulates T-cell differentiation and function, were lower in SGA than adequate size for gestational age individuals who had been exclusively breast-fed for at least 50 days after birth (17). Whether IUGR-induced changes in lymphocyte numbers in the thymus or spleen persist after weaning is unclear.
Perspectives and Significance
The incidence of allergy is increasing, and understanding the factors that determine individual susceptibility may help to identify potential preventative interventions. Studies of effects of prenatal environment on immune function in human populations are often confounded by use of birth weight as a marker, which reflects gestational age as well as prenatal environment, and by common prenatal and postnatal adverse exposures. The present study establishes an animal model in which to investigate effects of restricted growth in utero on postnatal immune function, independent of gestational age, and where all progeny share a common postnatal environment. Consistent with a lack of effect of birth weight in human studies, antibody responses to a protein-based vaccine were unaffected by PR in sheep, indicating that IUGR programs specific cell types and/or immune pathways without global suppression of immune function. Our finding of enhanced IgE responses, but decreased cutaneous hypersensitivity responses to antigens after sensitization, suggests a role for mast cells in programming of susceptibility to allergy. Which specific aspects of a restricted fetal environment induce changes in postnatal immune function, and the underlying mechanisms for this, require further investigation.
GRANTS
This work was supported by project grants from the National Health and Medical Research Council of Australia. Amy Wooldridge was supported by the Commonwealth Hill Honours Scholarship from the University of Adelaide.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.W., R.J.B., H.L., G.K.H., D.S.H., and K.L.G. performed experiments; A.L.W., R.J.B., L.C.G., K.L.K., and K.L.G. analyzed data; A.L.W., R.J.B., V.L.C., and K.L.G. interpreted results of experiments; A.L.W. and K.L.G. prepared figures; A.L.W., V.L.C., and K.L.G. drafted manuscript; A.L.W., R.J.B., E.N.M., H.L., G.K.H., D.S.H., L.C.G., K.L.K., J.A.O., V.L.C., and K.L.G. edited and revised manuscript; A.L.W., R.J.B., E.N.M., H.L., G.K.H., D.S.H., L.C.G., K.L.K., J.A.O., V.L.C., and K.L.G. approved final version of manuscript; R.J.B., E.N.M., J.A.O., V.L.C., and K.L.G. conception and design of research.
ACKNOWLEDGMENTS
The authors thank the staff of Laboratory Animal Services, University of Adelaide, for their excellence in animal care, and Dr Martin Elhay, Pfizer Animal Health, Parkville, Australia for provision of Clostridial Ig ELISA reagents.
REFERENCES
- 1.Alexander GR. Studies on the placenta of the sheep (Ovis aries L). Effect of surgical reduction in the number of caruncles. J Reprod Fertil 7: 307–322, 1964 [DOI] [PubMed] [Google Scholar]
- 2.Bischof RJ, Snibson K, Shaw R, Meeusen EN. Induction of allergic inflammation in the lungs of sensitized sheep after local challenge with house dust mite. Clin Exp Allergy 33: 367–375, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bischof RJ, Snibson KJ, Velden JV, Meeusen EN. Immune response to allergens in sheep sensitized to house dust mite. J Inflamm 5: 16–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolte G, Schmidt M, Maziak W, Keil U, Nasca P, Von Mutius E, Weiland SK. The relation of markers of fetal growth with asthma, allergies and serum immunoglobulin E levels in children at age 5–7 years. Clin Exp Allergy 34: 381–388, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 63: 1418–1427, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Contreras YM, Yu X, Hale MA, Callaway CW, Bareyan D, McKnight RA, Joss-Moore LA, Enioutina EY, Lane RH. Intrauterine growth restriction alters T-lymphocyte cell number and dual specificity phosphatase 1 levels in the thymus of newborn and juvenile rats. Pediatr Res 70: 123–129, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Cromi A, Ghezzi F, Raffaelli R, Bergamini V, Siesto G, Bolis P. Ultrasonographic measurement of thymus size in IUGR fetuses: a marker of the fetal immunoendocrine response to malnutrition. Ultrasound Obstet Gynecol 33: 421–426, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Ferguson AC. Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J Pediatr 93: 52–56, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 15: 183–187, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Godfrey KM, Barker DJP, Osmond C. Disproportionate fetal growth and raised IgE concentration in adult life. Clin Exp Allergy 24: 641–648, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Hartge R, Jenkins DM, Kohler HG. Low thymic weight in small-for-dates babies. Eur J Obstet Gynaecol Reprod Biol 8: 153–155, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Hesselmar B, Dahlgren J, Wennergren G, Aberg N, Albertsson-Wikland K. Born small for gestational age: relation to future allergy and asthma. Acta Paediatr 91: 992–994, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Ibáñez L, Potau N, Zegher FD. Ovarian hyporesponsiveness to follicle stimulating hormone in adolescent girls born small for gestational age. J Clin Endocrinol Metab 85: 2624–2626, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Katz KA, Pocock SJ, Strachan DP. Neonatal head circumference, neonatal weight, and risk of hayfever, asthma and eczema in a large cohort of adolescents from Sheffield, England. Clin Exp Allergy 33: 737–745, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr 133: 1592S–1596S, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Lundholm C, Ortqvist AK, Lichtenstein P, Cnattingius S, Almqvist C. Impaired fetal growth decreases the risk of childhood atopic eczema: a Swedish twin study. Clin Exp Allergy 40: 1044–1053, 2010 [DOI] [PubMed] [Google Scholar]
- 17.McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr 74: 543–548, 2001 [DOI] [PubMed] [Google Scholar]
- 18.McDade TW, Kuzawa CW, Adair LS, Beck MA. Prenatal and early postnatal environments are significant predictors of total immunoglobulin E concentration in Filipino adolescents. Clin Exp Allergy 34: 44–50, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Meeusen EN, Snibson KJ, Hirst SJ, Bischof RJ. Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov Today 6: 101–106, 2009 [Google Scholar]
- 20.Menzies-Gow A, Ying S, Phipps S, Kay AB. Interactions between eotaxin, histamine and mast cells in early microvascular events associated with eosinophil recruitment to the site of allergic skin reactions in humans. Clin Exp Allergy 34: 1276–1282, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Moore SE, Collinson AC, Prentice AM. Immune function in rural Gambian children is not related to season of birth, birth size, or maternal supplementation status. Am J Clin Nutr 74: 840–847, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Moore SE, Jalil F, Ashraf R, Szu SC, Prentice AM, Hanson LA. Birth weight predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. Am J Clin Nutr 80: 453–459, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Moore SE, Jalil F, Szu SC, Hahn-Zoric M, Prentice AM, Hanson LA. Revaccination does not improve an observed deficit in antibody responses in Pakistani adults born of a lower birth weight. Vaccine 26: 158–165, 2008 [DOI] [PubMed] [Google Scholar]
- 24.National Health, and Medical Research Council of Australia Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Canberra: Australian Government Publishing Service, 2004 [Google Scholar]
- 25.Neuman A, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, Lau S, Nieuwenhuijsen M, Sunyer J, Tischer C, Torrent M, Wahn U, Wijga AH, Wickman M, Keil T, Bergstrom A. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 186: 1037–1043, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Örtqvist AK, Lundholm C, Carlström E, Lichtenstein P, Cnattingius S, Almqvist C. Familial factors do not confound the association between birth weight and childhood asthma. Pediatrics 124: e737-e743, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Owens JA, Thavaneswaran P, De Blasio MJ, McMillen IC, Robinson JS, Gatford KL. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am J Physiol Endocrinol Metab 292: E1879–E1889, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Pekkanen J, Xu B, Järvelin MR. Gestational age and occurrence of atopy at age 31-a prospective birth cohort study in Finland. Clin Exp Allergy 31: 95–102, 2001 [PubMed] [Google Scholar]
- 29.Prescott SL. Allergic disease: understanding how in utero events set the scene. Proc Nutr Soc 69: 366–372, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Resnik R. Intrauterine growth restriction. Obstet Gynecol 99: 490–496, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of endometrial caruncles on fetal size and metabolism. J Dev Physiol 1: 379–398, 1979 [PubMed] [Google Scholar]
- 32.Sankaran S, Phillipa KM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol 23: 765–777, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shaw RJ, McNeill MM, Gatehouse TK, Douch PG. Quantification of total sheep IgE concentration using anti-ovine IgE monoclonal antibodies in an enzyme immunoassay. Vet Immunol Immunopathol 57: 253–265, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J 21: 182–186, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, Lea RG, Craigon J, McEvoy TG, Young LE. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 104: 19351–19356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis 7: 161–173, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest 116: 833–841, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Linden DS, Sciascia Q, Sales F, McCoard SA. Placental nutrient transport is affected by pregnancy rank in sheep. J Anim Sci 91: 644–653, 2013 [DOI] [PubMed] [Google Scholar]
- 39.van Gramberg JL, de Veer MJ, O'Hehir RE, Meeusen EN, Bischof RJ. Induction of allergic responses to peanut allergen in sheep. PLos One 7: e51386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villamor E, Iliadou A, Cnattingius S. Is the association between low birth weight and asthma independent of genetic and shared environmental factors? Am J Epidemiol 169: 1337–1343, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Weil ZM, Pyter LM, Martin LB, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus). Am J Physiol Regul Integr Comp Physiol 290: R1714–R1719, 2006 [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization A WHO collaborative study of maternal anthropometry and pregnancy outcomes. Int J Gynaecol Obstet 57: 1–15, 1997 [PubMed] [Google Scholar]
- 43.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol 157: 24–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]