Abstract
Neurons in the supraoptic nuclei (SON) produce oxytocin and vasopressin and express insulin receptors (InsR) and glucokinase. Since oxytocin is an anorexigenic agent and glucokinase and InsR are hallmarks of cells that function as glucose and/or metabolic sensors, we evaluated the effect of glucose, insulin, and their downstream effector ATP-sensitive potassium (KATP) channels on calcium signaling in SON neurons and on oxytocin and vasopressin release from explants of the rat hypothalamo-neurohypophyseal system. We also evaluated the effect of blocking glucokinase and phosphatidylinositol 3 kinase (PI3K; mediates insulin-induced mobilization of glucose transporter, GLUT4) on responses to glucose and insulin. Glucose and insulin increased intracellular calcium ([Ca2+]i). The responses were glucokinase and PI3K dependent, respectively. Insulin and glucose alone increased vasopressin release (P < 0.002). Oxytocin release was increased by glucose in the presence of insulin. The oxytocin (OT) and vasopressin (VP) responses to insulin+glucose were blocked by the glucokinase inhibitor alloxan (4 mM; P ≤ 0.002) and the PI3K inhibitor wortmannin (50 nM; OT: P = 0.03; VP: P ≤ 0.002). Inactivating KATP channels with 200 nM glibenclamide increased oxytocin and vasopressin release (OT: P < 0.003; VP: P < 0.05). These results suggest that insulin activation of PI3K increases glucokinase-mediated ATP production inducing closure of KATP channels, opening of voltage-sensitive calcium channels, and stimulation of oxytocin and vasopressin release. The findings are consistent with SON oxytocin and vasopressin neurons functioning as glucose and “metabolic” sensors to participate in appetite regulation.
Keywords: insulin, glucokinase, calcium imaging, hormone release, phosphatidylinositol 3 kinase, glucose-sensing
obesity has reached epidemic proportions in the United States and currently gastric bypass is the only effective long-term treatment. In these studies, we investigated a largely overlooked hypothalamic mechanism for appetite regulation that might prove useful for weight intervention. Oxytocin (OT) is an anorexigenic agent that inhibits food intake and reduces body weight following central or peripheral administration (1, 2, 6, 13, 36, 43, 49, 50, 85), and deficits in OT or mutations in the OT receptor (OTR) are associated with obesity in humans (11, 22, 81) and mice (8, 46, 70, 71). Studies on the role of OT in appetite regulation have largely focused on the paraventricular nucleus (PVN) and its hindbrain projections (7, 31, 43, 52, 53, 55, 62). PVN contains both parvocellular and magnocellular OT neurons. Parvocellular OT neurons project to preganglionic neurons of the autonomic nervous system in the brain stem and spinal cord regulating autonomic functions including gastric motility (16). In contrast, the magnocellular neurons (MCNs) project to the neural lobe of the pituitary and release OT into the peripheral circulation in response to anorexigenic and other stimuli (38, 69). However, the OT MNCs in the supraoptic nucleus (SON) and PVN are also a major source of OT in the brain. They have been shown to project to areas of the brain involved in motivated behaviors [e.g., amygdala and nucleus accumbens (29, 58)]. Since OT neurons have not been described in these regions except in the naked mole rat (57), it is likely that the MNCs provide the ligand for OT receptors in these regions, and could participate in appetite regulation. This possibility has not received attention by investigators studying hypothalamic mechanisms of appetite regulation.
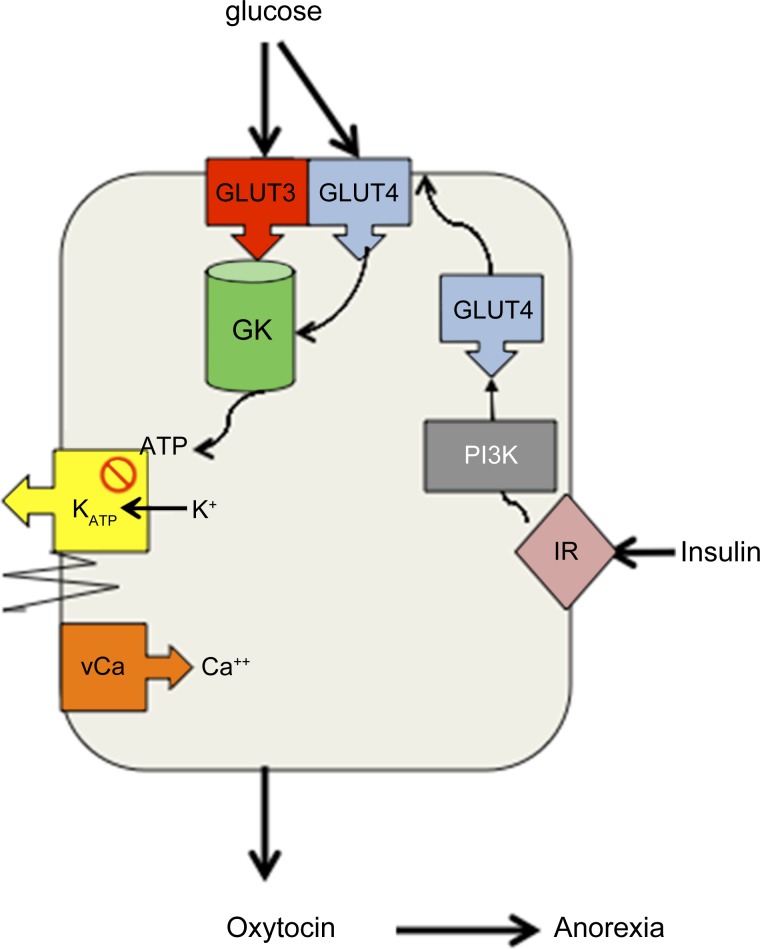
Autoradiographic binding of insulin and prominent insulin receptor (InsR) immunohistochemistry was described in SON in the late 1980s (19, 76). Since SON contains only OT and vasopressin (VP) MCNs projecting to neural lobe, and since insulin serves as an appetite-regulating signal in other parts of the hypothalamus, expression of InsR in MNCs raises the possibility that, consistent with an anorexigenic role for OT, MNCs may have the ability to monitor nutrient stores and in turn regulate peptide release to appropriately adjust food intake to maintain body nutrient stores. We assessed the ability of SON MNCs to function as glucose and metabolic sensors as diagrammed in Fig. 1. First, we used quantitative real-time PCR (qRT-PCR) to confirm expression of InsR in SON and to evaluate expression of glucokinase (GK), a hallmark of glucose sensors, to determine whether MNCs expressed molecules characteristic of metabolic sensors. We then evaluated the effect of glucose and insulin on intracellular calcium signaling in SON MNCs and OT and VP release from explants of the rat hypothalamo-neurohypophyseal system (HNS). HNS explants include the SON with their projections to neural lobe. Since SON contains only MNCs, HNS explants are an excellent preparation for studying responses of MNCs independent of parvocellular OT neurons. Finally, we examined the roles of ATP-sensitive potassium (KATP) channels, GK, and phosphoinositol 3-kinase (PI3K), the kinase that mediates insulin-induced insertion of GLUT4 glucose transporters into the membrane in adipocytes and arcuate neurons (9, 47, 48), in response to glucose and insulin.
Fig. 1.
Do magnocellular neurons (MNCs) function as glucose and metabolic sensors? Expression of insulin receptors (InsR) and glucokinase (GK) by supraoptic MNCs suggests that MNCs can modulate release of the anorexigenic agent, oxytocin (OT), in response to changes in circulating insulin and the extracellular glucose concentration. We postulate that since glucose transporter 3 (GLUT3), the “brain glucose transporter,” is saturated at physiological glucose concentrations, glycolysis of glucose by GK, a hexokinase that is not inhibited by its own product (34), provides a glucose concentration-dependent source of ATP. GLUT 3 and GK are present in supraoptic nuclei (SON) (45, 75). In other glucose-sensing cells [e.g., pancreatic β-cells and ventromedial nucleus (VMN) neurons], ATP closes ATP-sensitive K+ channels (KATP) channels resulting in membrane depolarization, opening of voltage-sensitive calcium channels (vCa), and initiation of exocytosis or generation of actions potentials. Kir6.1 and 6.2 (KATP channels) are present in SON (14, 74). Thus we postulate that GK-mediated ATP production inactivates (closes) KATP channels inducing depolarization, opening of vCa, and propagation of action potentials to the nerve terminals in posterior pituitary and other central nervous system regions to initiate exocytosis of OT. We also postulate that insulin mediated membrane insertion of GLUT4, which is PI3K dependent (9, 47, 48), provides additional substrate for GK-mediated glycolysis thus further promoting KATP-mediated depolarization and OT release.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats [CRL: CD(SD)Br; Charles Rivers Laboratories, Wilmington, MA], 150–175 g, were used in all experiments. All protocols were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver.
Detection of mRNA for GK and InsR by qRT-PCR
SON was microdissected using the optic chiasm as a landmark. A rectangular block of tissue immediately rostral to optic chiasm approximately 2 mm wide × 1 mm deep × 3 mm long was removed from each side of the brain using irridectomy scissors (64). Microdissected samples of SON were collected into RNA-Later (QIAGEN) and shipped to B. Levin's laboratory for processing and analysis as described previously (10, 32). Primer sets for cyclophilin, the housekeeping reference gene, GK, and insulin receptor mRNA were designed by reference to published sequences, and their specificity was verified using GenBank and by comparing the sequenced PCR product for cyclophilin, GK, and InsR to these references. For each mRNA species, a pair of conventional primers was used in combination with a sequence-specific 6-carboxyfluoroscein (FAM)-labeled probe to allow real-time PCR quantitation using an Applied Biosystems 7700 Sequence detector set for 40 PCR cycles. The primers for cyclophilin [constitutive gene (39)] were GenBank (NM_017101): forward beginning at 253 bp, CCAATACGTCATTCACAACAACAAG; reverse beginning at 330 bp, AAGTTGCTGGAATTCATGGTATAGC; FAM-labeled probe TCTACGGAGAGAAATT. The primers for the insulin receptor were Genebank (NM_017071): forward beginning at 1250 bp: AACCTGTGAGGATGAGTGTCAGAGT; reverse beginning at 1321 bp: CCTTGCTCTTCATCAGTTTCCA; and FAM-labeled probe TGCATCCCCGAGTGC. The primers for GK were Genebank (NM012565): forward primer: CGAGGA GGCCA GTGTAAAGATG; reverse primer: TCTCCGACTTCTGAGCCTTCTG; and probe AACGCACGTAGGTGGG. Reference standards were created for cyclophilin and InsR from pooled aliquots of arcuate nucleus samples, and these were used to generate standard curves from which quantitative data were read. Data were then expressed as the ratio of GK and InsR to cyclophilin mRNA.
Hypothalamo-Neurohypophyseal Explant Preparation
HNS explants were used for calcium imaging and hormone release studies. Explants were prepared as described previously from male rats (67). HNS explants include the SON neurons, their axons, and axon terminals in the neural lobe as well as organum vasculosum of the lamina terminalis and suprachiasmatic and arcuate nuclei. They do not include the PVN.
Calcium Imaging
HNS explants were loaded with the calcium-sensitive dye Fura-2 AM as described previously (67). They were placed in a recording chamber with the ventral surface up allowing easy visualization of SON neurons using the optic chiasm as an anatomical landmark (67). SON neurons were identified by the size of the cell body (>25 μm in diameter) and their location adjacent to the optic chiasm. Explants were perifused at a rate of 3 ml/min with gassed (95% O2-5% CO2) specially formulated F12 nutrient mixture modified to contain 0.5 mM glucose, 13 mM KCl, and 1.7 mM CaCl2. Fura-2-loaded MNCs were alternately excited with 340 nm and 380 nm UV light from a Xenon Source (Sutter Instruments, Novato, CA). The 380-nm exposure time was between 200 and 500 ms and was tripled for the 340-nm exposure. Emitted light was passed through a ×60 fluorwater immersion lens attached to an Olympus upright microscope and collected at 510 nm by an intensified charge-coupled device camera (Hamamatsu, Japan). Paired 340- and 380-nm images were acquired every 3 s using Slide-Book software (Intelligent Imaging, Denver, CO) for a period of 100 frames. The 340-to-380 ratio (R) was used as an index of the change in intracellular Ca2+ concentration ([Ca2+]i). Rmax was previously determined in ionomycin-treated explants and far exceeded the highest R obtained with agents studied in these experiment (67). R data are presented as percentage of the basal 340:380 R for each cell determined from the average R of 10 frames preceding drug exposure. Explants were allowed to equilibrate for 1 h.
Data analysis.
Means ± SE of the percentage values from individual neurons were calculated and plotted. Parametric one-way ANOVA (F value) followed by Student-Newman-Keuls individual mean analysis or Kruskal-Wallis one-way ANOVA on ranks (H value) followed with Dunn's individual mean analysis were used to determine significant group differences and paired t-tests were used to compare peak responses to the basal [Ca2+]i.
Hormone Release From HNS Explants
Explants were positioned individually in perifusion chambers having a 500-μl volume and perifused at 2 ml/h as described previously (27) with specially formulated F12 nutrient mixture containing a final glucose concentration of 1 mM. After a 4- to 5-h equilibration period to allow hormone release to stabilize at basal level, explants were either maintained under control conditions or exposed to an increase in glucose (5 mM) or insulin (3 ng/ml) for 1 h followed by the addition of glucose to achieve 5 mM glucose. Since the perifusion medium contained 20% fetal bovine serum (FBS), the amount of glucose and insulin added was adjusted to account for the glucose and insulin concentration in each lot of FBS. Where appropriate, drugs were added to inhibit GK or PI3K, and glibenclamide was used to evaluate the effect of inactivating KATP channels on hormone release. Effluent was collected individually at 20-min intervals using a refrigerated fraction collector maintained at 4°C. VP and OT concentration in the perifusate was determined by radioimmunoassay as described previously (82). Changes in VP and OT release from HNS explants reflects altered release from nerve terminals in the neural lobe, because although VP and OT are released from dendrites in SON and VP from suprachiasmatic nucleus (15), the amount from neural lobe far exceeds these other sources (17).
Data analysis.
Basal VP/OT release was determined during the hour immediately preceding exposure to insulin, glucose, or drugs. Hormone release in response to experimental manipulations is expressed as a percentage of this initial basal release for each explant. Basal release for the explants included in these studies was 159 ± 27 pg/ml for VP and 190 ± 10 pg/ml for OT (means ± SE). ANOVA with repeated measures followed by post hoc simple main effects analysis was performed to evaluate changes in hormone release and to compare responses between groups.
RESULTS
Detection of mRNA for GK and InsR by qRT-PCR
With the use of primers described previously (10, 32), the transcripts for both InsR and GK were found to be abundant in SON. The average number of cycles to detection compared with cyclophilin (2-ΔCt), the reference housekeeping gene, was 4.9 for InsR and 6.1 for GK, indicating that InsR mRNA is in the order of 3.4% and GK 1.5% as abundant as cyclophilin (assuming equal efficiencies in the PCR reactions). This is consistent with the prominent expression of InsR observed with immunohistochemistry (76). The expression of GK is significant, because it raises the possibility that these neurons may function as glucose sensors (e.g., cells that alter their firing pattern proportionally to the extracellular glucose concentration). Since the insulin-producing β-cells in the pancreas as well as glucose-sensing neurons express GK, GK is considered to be the “gate keeper” for glucosensing (34). The abundant presence of GK in SON supports the hypothesis that these neurons can monitor extracellular glucose. Since the ventromedial nucleus (VMN) glucose-sensing neurons also express GK and InsR (26), the prominent expression of GK and InsR in SON supports the hypothesis that MNCs may function as glucose sensors similar to VMN neurons. Thus these observations provided the crucial evidence to pursue the subsequent studies on the effects of glucose and insulin on SON neurons and the role of GK and PI3K.
Effect of 5 mM Glucose on [Ca2+]i
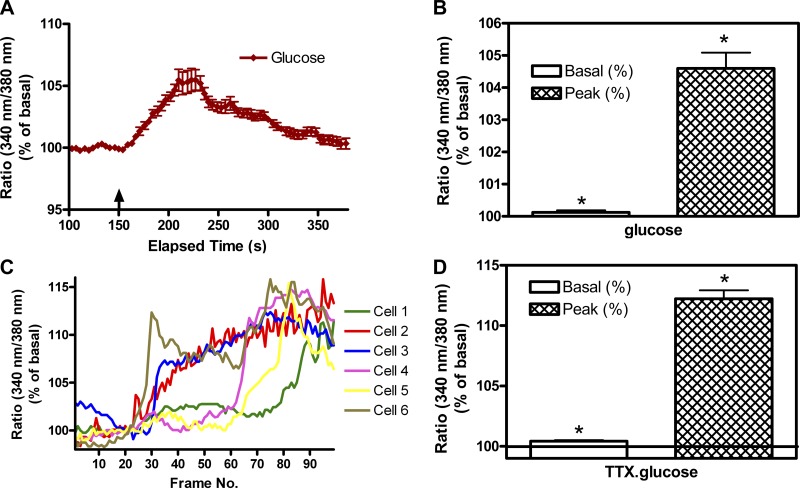
Increasing the glucose concentration to 5 mM in F12 containing 3 mM K+ increased [Ca2+]i in some, but not all, SON neurons (data not shown). With the use of responsiveness to VP or OT as criteria for OT or VP phenotypic identification of the neurons (12), 44% of OT neurons and 74% of VP neurons showed responses. However, when the K+ concentration in the perifusate was increased to 13 mM to move neurons closer to their depolarization threshold (80), increasing the glucose concentration from 0.5 to 5 mM reliably increased [Ca2+]i in all of the MNCs recorded (Fig. 2, A and B). The response was sustained in the presence of tetrodotoxin (TTX, Fig. 2, C and D) indicating that the glucose response is not synaptically mediated. Since all the MNCs responded to an increase in glucose with an increase in [Ca2+]i, they would be classified as “glucose-excited.” It should be noted, however, that these experiments were not optimally designed to reveal “glucose-inhibited” neurons.
Fig. 2.
Effect of increasing glucose on intracellular Ca2+ concentration ([Ca2+]i) in SON neurons. A: time course of change in 340-to-380 (340:380) ratio indicative of change in [Ca2+]i induced by increasing the glucose concentration from 0.5 to 5 mM. Values shown are means ± SE of 18 SON neurons imaged simultaneously in a single hypothalamo-neurohypophyseal system (HNS) explant. B: peak increase in the 340:380 ratio in response to the addition of glucose in 34 neurons imaged in 2 HNS explants. Values are means ± SE, *P ≤ 0.001 by signed rank test between basal and peak response. C: time course of individual neuronal responses to increasing glucose (from 0.5 to 5 mM) in the presence of tetrodotoxin (TTX, 3 μM). In this preparation, the responses were not tightly temporally synchronized as in A. This could reflect neurons at different depths within the preparation. D: peak increase in the 340:380 ratio in response to the addition of glucose in 40 neurons imaged in 2 HNS explants. Values are means ± SE, *t = 17.500, P ≤0.001, basal to peak response.
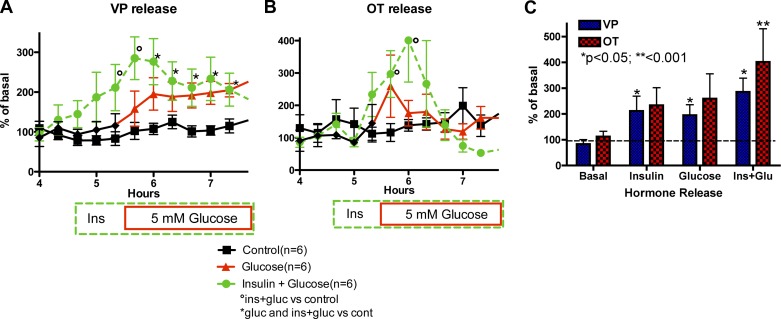
Effect of 5 mM Glucose and Insulin on OT and VP Release
As seen in Fig. 3A, VP release from HNS explants was stimulated by increasing glucose alone (glucose group from hour 4.6 to end; Ftreatment = 25.74, P < 0.0005; Ftime = 4.91, P < 0.0001), insulin alone (first hour in the Ins+glucose group; Ftreatment = 14.47, P = 0.0035; Ftime = 2.67, P = 0.032; Finteraction = 2.81; P = 0.026), and the response to insulin was sustained when glucose was increased in the presence of insulin (Ins+glucose group from hour 4.6 to end; Ftreatment = 11.26, P = 0.001; Ftime = 5.13, P < 0.0001). OT release was significantly increased during combined exposure to insulin and 5 mM glucose (Ftime = 5.17, P < 0.0001; Finteraction = 3.57, P < 0.0001). Thus glucose and insulin stimulated VP release from the posterior pituitary, and OT release was increased by increasing the glucose concentration in the presence of insulin.
Fig. 3.
Effect of glucose (Glu), insulin (Ins), and insulin plus glucose (Ins + Glucose) on vasopressin (VP) and oxytocin (OT) release from HNS explants. A and B: time course of VP and OT responses, respectively, to increasing the perifusate Ins concentration to 3 ng/ml for 1 h followed by increasing the Gluc concentration to 5 mM (Ins + Gluc), increasing the Gluc concentration alone, or maintaining the basal Ins (70 pg/ml from fetal bovine serum) and Gluc (1 mM) concentrations throughout the experiment (Basal). °P < 0.05 Ins alone or Ins + Glu vs. basal; *P < 0.05 Ins + Glu and Gluc alone vs. basal (n = 6/group). C: peak increase in OT or VP release from the same explants in A and B during exposure to insulin alone, Gluc alone, or Ins + Glu. *P < 0.05, **P < 0.001.
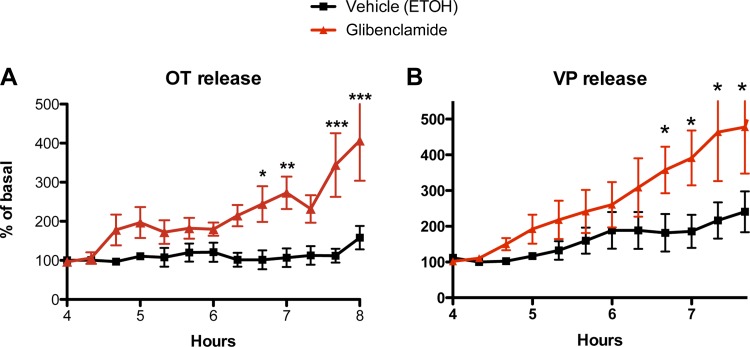
Role of KATP Channels in OT and VP Neurons
To assess the effect of inactivating KATP channels on OT and VP release, HNS explants were incubated in glibenclamide (200 nM), a blocker of KATP channels, for 3 h. Glibenclamide was solubilized in ethanol and a comparable amount of ethanol was added to the control explants. As shown in Fig. 4, the addition of glibenclamide resulted in a gradual and sustained increase in OT and VP release during the duration of exposure exposure to glibenclamide that reached statistical significance after 2 h (OT: Ftreatment = 17.24, P = 0.002; Ftime = 6.32, P < 0.0001; Finteraction = 3.87, P < 0.0001; VP: Ftreatment = 3.86, P = 0.07; Ftime = 10.98, P < 0.0001; Finteraction = 2.64, P = 0.005). Thus the presumptive depolarization resulting from inactivation of KATP channels is sufficient to increase OT and VP release.
Fig. 4.
Effect of glibenclamide to inhibit KATP channels on OT (A) and VP (B) release from HNS explants in 1 mM glucose. Glibenclamide increased OT and VP release (OT: *, **, ***P < 0.05, 0.01, and 0.001, respectively, by Bonferroni post hoc analysis; VP: *P < 0.05 by post hoc t-test).
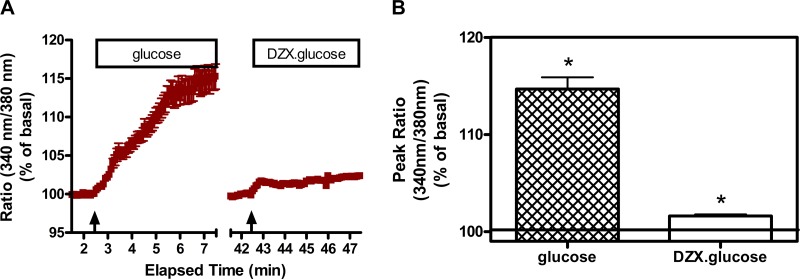
To assess the role of KATP channels in the glucose-induced increase in [Ca2+]i in SON neurons, the effect of glucose was evaluated in the absence and presence of diazoxide (0.4 mM). As shown in Fig. 5, increasing the glucose concentration from 0.5 to 5 mM resulted in a robust and sustained elevation in [Ca2+]i. After a washout period, the same increase in glucose in the presence of diazoxide was ineffective. Thus the glucose-induced increase in [Ca2+]i is dependent on closure of KATP channels. This suggests that the glucose-induced increase in calcium is most likely due to depolarization-induced opening of voltage-dependent calcium channels, but our studies do not test that directly.
Fig. 5.
Effect of activating (opening) KATP channels with diazoxide (DZX, 0.4 mM) on the glucose-induced increase in [Ca2+]i in SON neurons. A: time course of Ca2+ response to glucose (step rise from 0.5 to 5 mM) in the presence of 13 mM KCl (glucose) and subsequently in the presence of DZX (DZX.glucose). Arrows indicate when glucose was administered. B: comparison of the peak responses under these conditions. Glucose (5 mM) induced an increase in [Ca2+]i in the majority of SON neurons in the presence of 13 mM KCl (to increase resting membrane potential). The response was abolished when cells were pretreated with the KATP channel opener DZX (*H = 47.264, P ≤ 0.001). Data were combined from neurons imaged in 2 explants. Total number of neurons = 32.
Role of GK in OT and VP Neurons
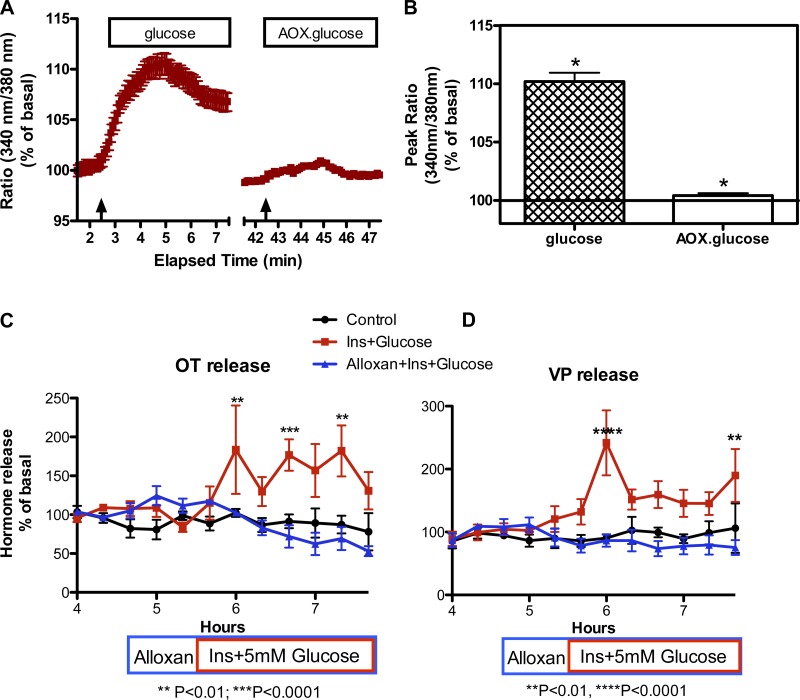
To determine whether the glucose-induced increase in [Ca2+]i and the stimulation of OT and VP release by glucose and insulin are GK dependent, we used alloxan (4 mM) to block GK. As shown in Fig. 6, the glucose-induced increase in [Ca2+]i was essentially abolished in the presence of alloxan, and alloxan prevented the glucose plus insulin-induced increase in OT (Ftreatment = 18.04, P = 0.0017; Ftime = 2.63, P = 0.0051; Finteraction = 2.667, P = 0.0045) and VP release (Ftreatment = 17.75, P = 0.0001; Ftime = 2.97, P = 0.0013; Finteraction = 4.331, P < 0.0001). Thus GK mediates the glucose-induced increase in [Ca2+]i and the stimulation of OT and VP release induced by the combined increase in glucose and insulin.
Fig. 6.
GK dependence of the effects of glucose on [Ca2+]i and Ins + glucose on OT and VP release. A: time course of Ca2+ response to glucose (5 mM) in the presence of 13 mM KCl in the absence (glucose) or presence of alloxan (AOX.glucose; 4 mM AOX). The basal medium contains 0.5 mM glucose. Arrows indicate when glucose was administered. B: comparison of the peak responses under these conditions. Glucose (5 mM) induced an increase in [Ca2+]i in the majority of SON neurons in the presence of 13 mM KCl (to increase resting membrane potential). The response was abolished when cells were pretreated with GK inhibitor AOX (*H = 59.260, P ≤ 0.001). Data were combined from neurons imaged in 2 explants. Total number of neurons = 40. C and D: effect of alloxan on OT and VP release, respectively. Addition of alloxan (4 mM) 1 h before increasing the perifusate glucose and insulin concentrations to 5 mM and 3 ng, respectively, did not alter basal release of OT (198 ± 25 pg/ml) or VP (169 ± 46 pg/ml), but it prevented the increase in OT and VP release induced by the addition of glucose and insulin. **P < 0.01, ***P < 0.0001. n = 6 explants per group.
Role of PI3K in OT and VP Neurons
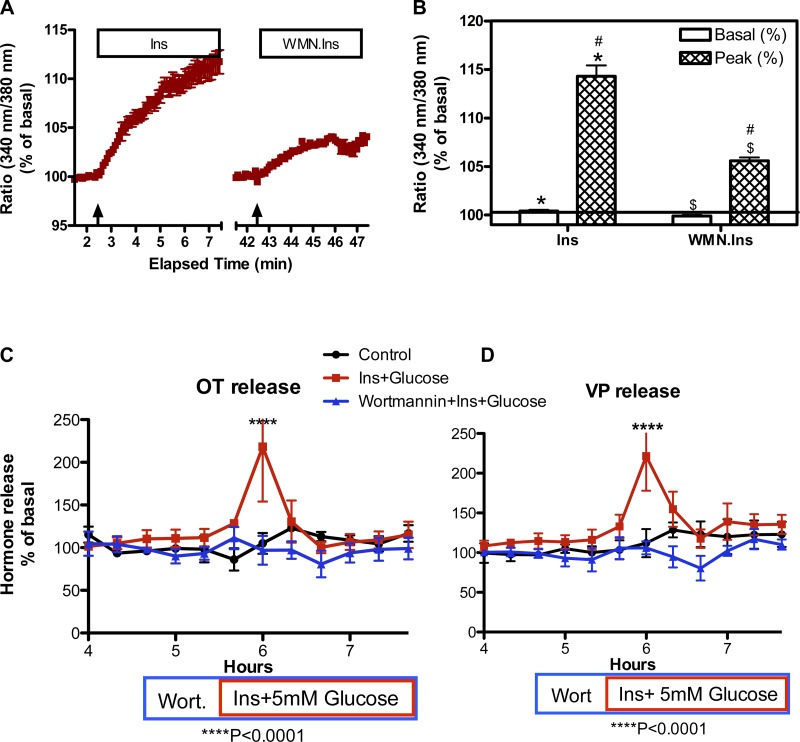
Since insulin activation of PI3K is required for insulin-induced translocation of the GLUT4 glucose transporter to the plasma membrane (60), we used wortmannin (50 nM), a PI3K inhibitor, to determine whether the insulin-induced increase in [Ca2+]i and the stimulation of OT and VP release by insulin and glucose are PI3K dependent. As shown in Fig. 7, in the presence of 5 mM glucose, the insulin-induced increase in [Ca2+]i was significantly reduced by 10 or 50 nM wortmannin, and no increase in OT and VP release was detected in the presence of 50 nM wortmannin in response to simultaneously increasing the glucose and insulin concentrations of the perifusate to 5 mM and 3 ng/ml, respectively (OT: Ftreatment = 6.289, P = 0.031; Ftime = 2.887, P = 0.0023; Finteraction = 2.808, P = 0.0029; VP: Ftreatment = 9.75, P = 0.0019; Ftime = 5.416, P < 0.0001; Finteraction = 3.141, P < 0.0001). Thus the responses to insulin reflect PI3K-mediated intracellular events that could include insulin-induced insertion of GLUT4 transporters into the membrane. However, PI3K-dependent insulin actions that are independent of GLUT4 translocation exist and are not excluded in these experiments (61).
Fig. 7.
Effect of the phosphatidylinositol 3 kinase (PI3K) inhibitor wortmannin (WMN) on the Ins-induced increase in [Ca2+]i and insulin with glucose (Ins+glucose)-induced OT and VP release. A: time course of Ca2+ response to Ins (3 ng/ml) in the presence of 13 mM KCl in the absence (Ins) or presence of WMN (50 nM, WMN.Ins) The medium contained 5 mM glucose. Arrows indicate when Ins was administered. B: comparison of the peak responses under these conditions. Ins (3 ng/ml) induced an increase in [Ca2+]i in the majority of SON neurons in the presence of 5 mM glucose and 13 mM KCl (to increase resting membrane potential) (*t = 820.0, P ≤ 0.001;). The peak response was greatly reduced in the experiments when cells were pretreated with WMN (#H = 42.879, P ≤ 0.001; $t = 18.299, P ≤ 0.001). Data were combined from neurons imaged in 2 explants. Total number of neurons = 40 in each experiment. C and D: effect of WMN on OT and VP release, respectively. Addition of WMN alone 1 h before increasing the perifusate glucose and insulin concentrations to 5 mM and 3 ng/ml, respectively, did not alter basal release of OT (175 ± 22 pg/ml) or VP (231 ± 20 pg/ml), but it prevented the increase in OT and VP release induced by the addition of glucose and insulin. ****P < 0.0001. n = 6 explants per group.
DISCUSSION
The ability of glucose and insulin to alter VP and OT release from the neurohypophysis is consistent with the possibility that the MNC neurons can function as metabolic sensors. Although dendritic release of VP and OT also may be affected, the intact HNS explant does not allow us to explore that possibility. An important aspect of these findings is that the reported effects of glucose and insulin reflect responses to physiologically relevant concentrations of glucose and insulin. Glucose concentrations measured in the hypothalamus range from 0.5 mM in fasting rats to 5 mM in severely hyperglycemic rats with 2.5 mM representing postingestive normoglycemia (63). Thus the changes in glucose utilized in these experiments represented the full physiological range of hypothalamic glucose. Insulin is transported into the brain by a saturable transport mechanism that is physiologically and regionally regulated (3). Hypothalamic insulin levels are among the highest in the brain at 0.4 ng/g (4). Thus it is reasonable, given the diffusion barriers inherent in in vitro preparations, that the glucose and insulin concentrations employed in these experiments resulted in local fluctuations that SON neurons might encounter in vivo.
Using qRT-PCR, we found that InsR and GK are prominently expressed in rat SON. This confirmed the earlier immunohistochemical report showing dense InsR expression and the in situ hybridization study showing GK expression in SON neurons (45, 76). Since some of the glucose-sensing neurons in VMN also express GK and InsR, expression of these molecules in SON neurons is consistent with the hypothesis that MNCs function as glucose sensors. In VMN neurons, glucose induces depolarization via GK-mediated inactivation of KATP. Specifically, GK-mediated glycolysis is likely to result in ATP production, closure of KATP channels, and depolarization (34). We found that similar mechanisms regulate OT and VP secretion from the neurohypophysis. Specifically, inhibition of KATP channel activity with glibenclamide was sufficient to increase OT and VP secretion, and the ability of glucose to increase [Ca2+]i is dependent on closure of KATP channels. This is consistent with the reported expression of the KATP channels Kir6.1 and 6.2 in SON (14, 73). Furthermore, the increase in [Ca2+]i induced by glucose and the increase in OT and VP release induced by combined exposure to increases in glucose and insulin are GK dependent. Finally, since insulin-mediated membrane insertion of GLUT4 is PI3K dependent (9, 47, 48), the ability of wortmannin to significantly reduce the calcium response to insulin and to prevent combined glucose and insulin-stimulated OT and VP release, is consistent with the hypothesis that one role of InsR in SON neurons is to increase glucose transport potentially via inducing translocation of glucose transporters to the membrane (34, 75). However, since we have not demonstrated that insulin increases glucose uptake, it remains possible that the insulin actions, although PI3K dependent, are independent of changes in glucose uptake. Insulin has been shown to activate KATP channels in VMN and arcuate neurons via PI3K (68). However, this results in membrane hyperpolarization, which is not consistent with our findings that insulin increases [Ca2+]i and hormone release. Thus, although future studies should evaluate the effect of insulin on glucose uptake, our findings are consistent with the model shown in Fig. 1. Compartmentalization of InsR with other membrane proteins may allow for selective actions in various cell types (60).
Our studies did not demonstrate a differential effect of glucose or insulin on OT and VP neurons. How is this compatible with the evidence that OT is the neurohypophyseal hormone associated with satiety in rats (49–51)? It is consistent with our prior findings that most physiological stimuli and neurotransmitters elicit similar responses from MNC OT and VP neurons. In previous experiments with HNS explants, the two hormones show similar responses to osmolality as well as glutamatergic, adrenergic, purinergic, and peptidergic agents (21, 27, 28, 41, 42, 65, 66). Similarly, in vivo, both hormones are released by hypertonicity and hypovolemia with only suckling, gastric distention, and peripherally administered cholecystokinin (CCK) identified as stimuli that selectively activate OT neurons or specifically stimulate OT secretion in rats (54, 56). These OT-specific stimuli discriminate between OT and VP neurons via efferent pathways that selectively activate the OT MNCs not as a result of expression of a particular receptor or ion channel being limited to the OT neuron (23, 24). The selectivity of the anorexigenic efferents for OT versus VP neurons is species dependent: While peripheral administration of CCK induces OT release in rats, in monkeys and humans, peripheral CCK stimulates VP release but not OT release (39, 78). Species variation in OT and VP receptor expression in target brain areas may also contribute to the anorexigenic specificity of OT in rats versus VP in primates.
The hypothesis that, in addition to anorexigenic effects generated by peripheral OT (1, 2, 6, 13, 36, 43, 49, 50, 85), OT-induced anorexia partially reflects MNC-derived activation of OT receptors in the motivated behavior circuit is plausible based on the evidence for differential localization of OT and VP in receptors in forebrain regions that participate in appetite regulation (44, 59) as well as the recent evidence that MNC OT neurons innervate these regions (29, 58). OT receptors are present in multiple regions of the motivated behavior circuitry including the amygdala, nucleus accumbens, prefrontal cortex, and ventral pallidum (caudate/putamen) (59), and all of these areas have been implicated in the motivated and hedonic components of appetite regulation (5, 33). Evidence for innervation of the amygdala and nucleus accumbens by OT neurons that also project to the posterior pituitary (e.g., MNCs) has been obtained in elegant studies utilizing fluorogold and pseudorabies virus to retrogradely label neurons projecting to nucleus accumbens and amygdala (29, 58). Both studies identified neurons in SON (which only contains MNCs) that project both to the limbic region and the posterior pituitary. Thus it is likely that axon collaterals from OT MNCs provide the substrate for OT receptors in these limbic regions. This suggests that glucose and insulin may have similar effects on OT release in the motivated behavior circuitry to that reported here on neurohypophyseal hormone release.
While our studies specifically targeted the metabolic sensor capability of the MNCs by examining the responses to glucose and insulin, these neurons also are activated in response to feeding (25, 35, 40), gastric distention (56), activation of vagal afferents (72), and refeeding after an overnight (83) or 48 h fast (35). They also respond to other molecules involved in appetite regulation including leptin (20), ghrelin (18, 84), cholecystokinin (23, 24), nesfatin (30, 37), and prolactin-releasing peptide (83). This further supports a role for these neurons in appetite regulation.
Perspectives and Physiological Significance
Although involvement of insulin in mediating glucose uptake by the neurohypophyseal system has been recognized since the late 1980s when Gary Robertson and colleagues reported that in untreated, insulin-dependent diabetics glucose became an osmotic stimulus for VP release (79) and Unger et al. (77) reported prominent expression of InsR in the SON, the possibility that the neurohypophyseal system monitors metabolic status and contributes to appetite regulation was not investigated and the anorexigenic actions of OT were attributed primarily to parvocellular OT neurons and hindbrain mechanisms (7). However, the intractability of the recent obesity epidemic has led to the realization that a highly redundant and distributed system is involved in appetite regulation. Thus, while the evidence presented here that neurohypophyseal neurons can monitor nutrient and metabolic status and potentially act on OT receptors in the motivated behavior circuitry may simply represent an additional layer of redundancy, it also might represent a previously under appreciated opportunity to enhance anorexigenic actions, because an integrated peripheral and central release of OT has the potential to influence peripheral metabolism (6) as well as both motivated/hedonic and homeostatic pathways mediating food intake.
GRANTS
This work was supported by grants from the National Institutes of Health to C. D. Sladek (R21-HD072428) and to B. E. Levin (RO1-DK53181).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S., B.E.L., and C.D.S. conception and design of research; Z.S., B.E.L., and W.S. performed experiments; Z.S., B.E.L., W.S., and C.D.S. analyzed data; Z.S., B.E.L., and C.D.S. interpreted results of experiments; Z.S. and C.D.S. prepared figures; Z.S., B.E.L., W.S., and C.D.S. edited and revised manuscript; Z.S., B.E.L., W.S., and C.D.S. approved final version of manuscript; C.D.S. drafted manuscript.
REFERENCES
- 1.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides 10: 89–93, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48: 825–830, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Banks WA. The source of cerebral insulin. Eur J Pharmacol 490: 5–12, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baskin DG, Porte D, Jr, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocrinology 112: 898–903, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol 300: R1266–R1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins JE, Ho JM. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord 14: 311–329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 17: 980–984, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol 14: 4902–4911, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Coiro V, Passeri M, Davoli C, d'Amato L, Gelmini G, Fagnoni F, Schianchi L, Bentivoglio M, Volpi R, Chiodera P. Oxytocin response to insulin-induced hypoglycemia in obese subjects before and after weight loss. J Endocrinol Invest 11: 125–128, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Dayanithi G, Widmer H, Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol 490: 713–727, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deblon N, Veyrat-Durebex C, Bourgoin L, Caillon A, Bussier AL, Petrosino S, Piscitelli F, Legros JJ, Geenen V, Foti M, Wahli W, Di Marzo V, Rohner-Jeanrenaud F. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLos One 6: e25565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res 814: 41–54, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Earnest DJ, Sladek CD. Circadian vasopressin release from perifused rat suprachiasmatic explants in vitro: effects of acute stimulation. Brain Res 422: 398–402, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res 578: 256–260, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Gregg CM, Sladek CD. A compartmentalized, organ-cultured hypothalamo-neurohypophysial system for the study of vasopressin release. Neuroendocrinology 38: 397–402, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, Takei Y, Ueta Y. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rat. J Physiol Sci 60: 19–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17: 1127–1138, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Honda K, Narita K, Murata T, Higuchi T. Leptin affects the electrical activity of neurones in the hypothalamic supraoptic nucleus. Brain Res Bull 57: 721–725, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci 24: 10103–10110, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoybye C, Barkeling B, Espelund U, Petersson M, Thoren M. Peptides associated with hyperphagia in adults with Prader-Willi syndrome before and during GH treatment. Growth Horm IGF Res 13: 322–327, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jarvis CR, Bourque CW, Renaud LP. Depolarizing action of cholecystokinin on rat supraoptic neurones in vitro. J Physiol 458: 621–632, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis CR, Van de Heijning BJ, Renaud LP. Cholecystokinin evokes vasopressin release from perfused hypothalamic-neurohypophyseal explants. Regul Pept 56: 131–137, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab 4: 313–321, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci 20: 8868–8875, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor JR, Sladek CD. Substance P and NPY differentially potentiate ATP and adrenergic stimulated vasopressin and oxytocin release. Am J Physiol Regul Integr Comp Physiol 280: R69–R78, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149: 1295–1301, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22: 1723–1734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297: R655–R664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 16, Suppl 3: S11–S22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes 53: 2521–2528, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lucio-Oliveira F, Franci CR. Effect of the interaction between food state and the action of estrogen on oxytocinergic system activity. J Endocrinol 212: 129–138, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Milano) 3: 1169–1177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009 [DOI] [PubMed] [Google Scholar]
- 38.McCann MJ, Verbalis JG, Stricker EM. LiCl and CCK inhibit gastric emptying and feeding and stimulate OT secretion in rats. Am J Physiol Regul Integr Comp Physiol 256: R463–R468, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Miaskiewicz SL, Stricker EM, Verbalis JG. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distention in humans. J Clin Endocrinol Metab 68: 837–843, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Mitra A, Gosnell BA, Schioth HB, Grace MK, Klockars A, Olszewski PK, Levine AS. Chronic sugar intake dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides 31: 1346–1352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morsette DJ, Sidorowicz HE, Sladek CD. Role of metabotropic glutamate receptors in the regulation of vasopressin and oxytocin release from the rat hypothalamo-neurohypophyseal explants. Am J Physiol Regul Integr Comp Physiol 281: R452–R458, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Morsette DJ, Sidorowicz HE, Sladek CD. Role of non-NMDA receptors in vasopressin and oxytocin release from rat hypothalamo-neurohypophyseal explants. Am J Physiol Regul Integr Comp Physiol 280: R313–R322, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302: E134–E144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair HP, Gutman AR, Davis M, Young LJ. Central oxytocin, vasopressin, and corticotropin-releasing factor receptor densities in the basal forebrain predict isolation potentiated startle in rats. J Neurosci 25: 11479–11488, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blazquez E. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 67: 1982–1991, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Prog Brain Res 170: 79–90, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem 269: 3568–3573, 1994 [PubMed] [Google Scholar]
- 49.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12: 113–118, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol Regul Integr Comp Physiol 260: R448–R452, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin's role as inhibitor of carbohydrate but not fat intake. Endocrinology 151: 4736–4744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olszewski PK, Klockars A, Schioth HB, Levine AS. Oxytocin as feeding inhibitor: maintaining homeostasis in consummatory behavior. Pharm Biochem Behavior 97: 47–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poulain DA, Wakerley JB, Dyball REJ. Electrophysiological differentiation of oxytocin- and vasporessin-secreting neurones. Proc R Soc Lond B 196: 367–384, 1977 [DOI] [PubMed] [Google Scholar]
- 55.Qi Y, Henry BA, Oldfield BJ, Clarke IJ. The action of leptin on appetite-regulating cells in the ovine hypothalamus: demonstration of direct action in the absence of the arcuate nucleus. Endocrinology 151: 2106–2116, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distention activate oxytocinergic cells in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol 253: R661–R665, 1987 [DOI] [PubMed] [Google Scholar]
- 57.Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience 155: 809–817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162: 892–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 30: 534–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol 12: 65–71, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Lasheras C, Konner AC, Bruning JC. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front Neuroendocrinol 31: 4–15, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol 402: 435–441, 1998 [PubMed] [Google Scholar]
- 63.Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14: 5068–5076, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sladek CD, Stevens W, Levinson SR, Song Z, Jensen DD, Flynn FW. Characterization of nuclear neurokinin 3 receptor expression in rat brain. Neuroscience 196: 35–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sladek CD, Swenson KL, Kapoor R, Sidorowicz HE. The role of steroid hormones in the regulation of vasopressin and oxytocin release and mRNA expression in hypothalamo-neurohypophysial explants from the rat. Exp Physiol 85S: 171S–177S, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Somponpun S, Sladek CD. Role of estrogen receptor β in regulation of vasopressin and oxytocin release in vitro. Endocrinology 143: 2899–2904, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Song Z, Vijayaraghavan S, Sladek CD. Simultaneous exposure to ATP and phenylephrine induces a sustained elevation in the intracellular calcium concentration in supraoptic neurons. Am J Physiol Regul Integr Comp Physiol 291: R37–R45, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol Regul Integr Comp Physiol 250: R267–R275, 1986 [DOI] [PubMed] [Google Scholar]
- 70.Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19: 951–955, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA 102: 16096–16101, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang M, Zhang J, Xu L, Chen JD. Implantable gastric stimulation alters expression of oxytocin- and orexin-containing neurons in the hypothalamus of rats. Obes Surg 16: 762–769, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Thomzig A, Laube G, Pruss H, Veh RW. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir62, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol 484: 313–330, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Thomzig A, Pruss H, Veh RW. The Kir6.1-protein, a pore-forming subunit of ATP-sensitive potassium channels, is prominently expressed by giant cholinergic interneurons in the striatum of the rat brain. Brain Res 986: 132–138, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Uemura E, Greenlee HW. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp Neurol 198: 48–53, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Unger J, McNeill TH, Moxley3rd RT, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31: 143–157, 1989 [DOI] [PubMed] [Google Scholar]
- 77.Unger JW, Moss AM, Livingston JN. Immunohistochemical localization of insulin receptors and phosphotyrosine in the brainstem of the adult rat. Neuroscience 42: 853–861, 1991 [DOI] [PubMed] [Google Scholar]
- 78.Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am J Physiol Regul Integr Comp Physiol 252: R749–R753, 1987 [DOI] [PubMed] [Google Scholar]
- 79.Vokes TP, Aycinena PR, Robertson GL. Effect of insulin on osmoregulation of vasopressin. Am J Physiol Endocrinol Metab 252: E538–E548, 1987 [DOI] [PubMed] [Google Scholar]
- 80.Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol 183: 849–863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, Henning E, Blackburn H, Loos RJ, Wareham NJ, O'Rahilly S, Hurles ME, Barroso I, Farooqi IS. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nature Genetics 45: 513–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yagil C, Sladek CD. Osmotic regulation of vasopressin and oxytocin release is rate sensitive in hypothalamoneurohypophysial explants. Am J Physiol Regul Integr Comp Physiol 258: R492–R500, 1990 [DOI] [PubMed] [Google Scholar]
- 83.Yamashita M, Takayanagi Y, Yoshida M, Nishimori K, Kusama M, Onaka T. Involvement of prolactin-releasing peptide in the activation of oxytocin neurones in response to food intake. J Neuroendocrinol 25: 455–465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokoyama T, Saito T, Ohbuchi T, Suzuki H, Otsubo H, Okamoto T, Fujihara H, Nagatomo T, Ueta Y. Ghrelin potentiates miniature excitatory postsynaptic currents in supraoptic magnocellular neurones. J Neuroendocrinol 21: 910–920, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Zhang G, Cai D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am J Physiol Endocrinol Metab 301: E1004–E1012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]