Abstract
Angiogenic expansion of the vasa vasorum (VV) is an important contributor to pulmonary vascular remodeling in the pathogenesis of pulmonary hypertension (PH). High proliferative potential endothelial progenitor-like cells have been described in vascular remodeling and angiogenesis in both systemic and pulmonary circulations. However, their role in hypoxia-induced pulmonary artery (PA) VV expansion in PH is not known. We hypothesized that profound PA VV neovascularization observed in a neonatal calf model of hypoxia-induced PH is due to increased numbers of subsets of high proliferative cells within the PA adventitial VV endothelial cells (VVEC). Using a single cell clonogenic assay, we found that high proliferative potential colony-forming cells (HPP-CFC) comprise a markedly higher percentage in VVEC populations isolated from the PA of hypoxic (VVEC-Hx) compared with control (VVEC-Co) calves. VVEC-Hx populations that comprised higher numbers of HPP-CFC also demonstrated markedly higher expression levels of CD31, CD105, and c-kit than VVEC-Co. In addition, significantly higher expression of CD31, CD105, and c-kit was observed in HPP-CFC vs. the VVEC of the control but not of hypoxic animals. HPP-CFC exhibited migratory and tube formation capabilities, two important attributes of angiogenic phenotype. Furthermore, HPP-CFC-Co and some HPP-CFC-Hx exhibited elevated telomerase activity, consistent with their high replicative potential, whereas a number of HPP-CFC-Hx exhibited impaired telomerase activity, suggestive of their senescence state. In conclusion, our data suggest that hypoxia-induced VV expansion involves an emergence of HPP-CFC populations of a distinct phenotype with increased angiogenic capabilities. These cells may serve as a potential target for regulating VVEC neovascularization.
Keywords: high proliferative potential endothelial colony-forming cells, endothelial progenitor cells, vascular remodeling, pulmonary hypertension, endothelial clusters
vascular remodeling is a fundamental pathological hallmark of a number of cardiovascular diseases (22, 38). Chronic hypoxia is an important contributing factor to the vascular remodeling process and is a potent stimulus for neovessel growth in a number of pathological settings. It has recently been shown that pathological remodeling includes not only intimal, medial, and adventitial thickening but also significant expansion of the VV network (36, 40). Increased VV density is a characteristic feature of a number of diseases in both the systemic and pulmonary circulations, including atherosclerosis, type II diabetes, restenosis, vasculitis, and pulmonary hypertension (PH) (15, 28, 31, 32, 36, 40). In the pulmonary circulation, increases in VV density have been observed in patients with severe idiopathic fibrosis, as well as idiopathic pulmonary arterial hypertension (IPAH), where a dramatic increase in VV density occurred around remodeled pulmonary arteries (PA) and plexiform lesions (31, 33). In animal models of PH, a marked expansion of the vasa vasorum (VV) network has also been observed in the adventitia and the media (3, 30). Moreover, in a neonatal bovine model of PH, it was demonstrated that chronic hypoxia markedly increases VV neovascularization along the entire longitudinal axis of the PA and is accompanied by infiltration and homing of circulating progenitor and inflammatory cells to the PA adventitia and around the expanding VV network (3, 4, 12, 39). However, despite the obvious important role of VV neovascularization in the pathophysiology of PH and the mechanisms of its expansion, the nature and characteristics of different cellular populations involved in the remodeling process remain unexplored.
Recently, endothelial progenitor cells (EPC) have been implicated in the remodeling process in vascular physiological and pathological responses (7, 8). However, since their discovery by Asahara et al. in 1997 (2), there has been considerable controversy with regard to their origin, sources, and identities. In particular, the role of resident EPC vs. recruiting circulating EPC in the pathogenesis of most vascular wall abnormalities has not been defined. Thus far, at least three ways have been identified to isolate and define EPC as reviewed by Yoder (44). In most studies, EPC identification has been carried out based on the expression of CD34 and/or CD133. Vessel-forming resident EPC that have a high proliferative potential were termed high proliferative potential colony-forming cells (HPP-CFC) (46).
The microvasculature has previously been suggested to be a rich source of EPC (1) and probably endothelial colony-forming cells (ECFC), which have been shown to participate in pathological vascular remodeling and have been described to be comprised within cultured endothelial cells derived from human PA of patients with pulmonary arterial hypertension (6). Therefore, we hypothesized that higher numbers of EPC/CFC would be present in the VV isolated from the PA adventitia of chronically hypoxic neonatal calves (VVEC-Hx) compared with control (VVEC-Co). In this study we isolated and characterized high proliferative populations from VVEC-Co and VVEC-Hx via clonogenic assays, expression of endothelial and progenitor markers, and angiogenic responses. The results from our study suggest that a HPP-CFC population may contribute to hypoxia-induced VV neovascularization and can be considered a potential target for regulating vascular remodeling in PH.
MATERIALS AND METHODS
Animals.
Lung tissue and PA were collected from normoxic [2 wk kept at ambient Denver altitude; barometric pressure (PB) = 640 mmHg] and chronically hypoxic (2 wk exposed to hypobaric hypoxia; PB = 430 mmHg) male Holstein calves (n = 6–7 for both groups). Standard veterinary care was used following institutional guidelines, and the procedure was approved by the Institutional Animal Care and Use Committee (Department of Physiology, School of Veterinary Medicine, Colorado State University, Ft. Collins, CO). Animals were killed by an intravenous overdose of pentobarbital. The protocol was approved by the Institutional Animal Care and Use Committee at Colorado State University. Older (5–6 mo old) calves with naturally occurring PH (in cattle, so-called “brisket disease”) were also analyzed (n = 3). These calves (of mixed British-based Aberdeen Angus and Hereford breeds) were born at a high-altitude (2,438 m) cattle ranch in Southwest Colorado and pastured at 2,438–3,505 m altitude for several months until their death. Postmortem, lung lesions consistent with PH and right heart failure in the absence of bronchopneumonia were validated.
Histological and immunofluorescence analysis of PA VV.
Hematoxylin and eosin (H&E) or Pentachrome staining of tissue sections was performed according to standard protocol to visualize VV in the PA wall of Holstein and British-based Aberdeen Angus and Hereford breeds, respectively. To determine the expression of CD31, CD34, and CD133 in PA adventitial VV, bovine main PA (MPA) sections were fixed with 4% paraformaldehyde (PFA) for 10 min and blocked with 10% donkey or goat serum for 15 min, at room temperature. Tissue sections were incubated with mouse monoclonal anti-CD31 (1:50 dilution; Novus Biological) and rabbit polyclonal anti-CD34 or anti-CD133 antibodies (1:50 dilution; Santa Cruz Biotechnology) overnight at 4°C. Sections were washed with PBS and incubated with goat anti-mouse AlexaFluor488 and donkey anti-rabbit AlexaFluor594 antibodies (1:250 dilution; Invitrogen) for 1 h at room temperature. Finally, slides were washed with PBS and mounted with VECTASHIELD with DAPI (Vector Laboratories) to observe the nuclei. Images were captured using a fluorescence microscope (Nikon) with AxioVision40 Software.
VVEC isolation and culture.
VVEC were isolated from PA adventitia of both VVEC-Co and VVEC-Hx animals and cultured according to our previously published methods (14). Cells were cultured routinely in DMEM/10% FBS supplemented with Endothelial Growth Supplement (Upstate Biotechnology) and incubated at 37°C, 5% CO2. All studies were performed on cells between passages 2 and 7.
Clonogenic assay.
Single-cell clonogenic assay was carried out on VVEC-Co and VVEC-Hx as described (19, 21). Trypsinized cells were sorted (1 cell/well) using a Legacy MoFlow FACS and cultured in DMEM/10% FBS supplemented with Endothelial Growth Supplement. After 14 days, cells were fixed with 4% PFA and stained with 0.5 μg/ml propidium iodide. Each well was examined using a Nikon TI inverted microscope and analyzed using Nikon NIS elements software. Visual inspection and cell counting were performed using ImageJ 1.43u. The colonies were defined as: HPP-CFC for >2,000 cells; low proliferative potential (LPP-CFC) for 50–2,000 cells; and endothelial clusters (EC) for <50 cells. Selected HPP-CFC-Co and HPP-CFC-Hx were expanded in culture for PCR analysis.
Quantitative real-time PCR.
To determine CD31, CD34, CD105, CD133, and c-kit expression, quantitative real-time PCR (qRT-PCR) was carried out on total RNA isolated from VVEC-Co, VVEC-Hx, HPP-CFC-Co, and HPP-CFC-Hx using an RNeasy Mini kit (QIAGEN). cDNA was synthesized using 1 μg RNA with an iScript cDNA Synthesis Kit (Bio-Rad). For qRT-PCR, cDNA samples were then amplified in duplicates in a final volume of 12.5 μl using iQ SYBR Green Supermix (Bio-Rad) and 500 nM gene-specific primers using the Bio-Rad iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). Starting quantity was calculated from the standard curve for each primer and normalized to bovine 18S. Custom primer (Life Technologies) sequences were as follows: CD31, forward: TTGCAGTGGTGGTCATCGGAGT, reverse: TGCTTGGCCTTGGCTTTCTTCA; CD34, forward: ATGGTCTTGCAGCTTCCACGCA, reverse: ACAGTCCAGAAAAGCCCCTGCT; CD105, forward: ACATCTACTCGCACACGCGTCA, reverse: TGCTGTGGTTGGTGCTACTGCT; CD133, forward: TGTGTGGCACGTTGGGCTATGA, reverse: AGTCCAACCCCAACCATGAGGA; and c-kit, forward: TCCTGATTGACCTTCCCT, reverse: TGTCAAATCCTTGGGGAG (23, 24).
Telomerase assay.
Telomerase activity was measured using the TRAPEZE RT telomerase kit (Millipore) according to the manufacturer's instructions. Briefly, cell pellets were washed with PBS, lysed in CHAPS buffer for 30 min on ice, and centrifuged at 12,000 g for 20 min at 4°C. Protein was measured using Bradford assay. 25 Reaction mix was prepared using the Amplifuor primers, DNA Taq polymerase, and 100 ng/μl cell extract (or TSR8 standard dilutions). PCR amplification was carried out in a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) using the following parameters: 1 cycle (30°C for 30 min), 1 cycle (95°C for 2 min), and 45 cycles (94°C for 15 s, 59°C for 60 s, and 45°C for 10 s). Telomerase units were extracted relative to the TSR8 amplification curve (linear equation).
Cell migration.
To test the angiogenic capacity of VVEC and HPP-CFC populations, migration and tube formation in vitro assays were performed as previously described (14, 24). Migration was carried out using Boyden chamber assay in 24-well plates. Growth-arrested cells (DMEM without serum, 72 h) were seeded on top of the transwell permeable support (8 μm pore size; Corning) precoated with 1% gelatin at the amount of 1 × 105 cells. ADP (500 μM) was added to the lower compartment to initiate migration. After 24 h, nonmigrated cells were scraped off from the top of the filters; migrated cells were fixed in methanol for 15 min at room temperature and stained with 0.2% crystal violet in 2% ethanol for 15 min. Filters with migrated cells were photographed under ×20 magnifications in a phase-contrast microscope (Nikon) at three random fields per well and counted using ImageJ software.
Tube formation.
Tube formation assay was carried out in ibidi angiogenesis slides (ibidi) precoated with 10 μl of Growth Factor Reduced Matrigel Matrix (BD Bioscience). Growth-arrested VVEC and HPP-CFC were seeded on polymerized Matrigel in triplicate at a density of 1.7 × 104 cells/well and incubated for 6 h with or without ADP (100 μM). Images were captured using a digital camera connected to a phase-contrast microscope (Nikon) at three to five random fields. The average tube length, the number of tubes, and triple nodes (branch points) were quantified using a macro developed in the Image Pro Premier 9.0 software (Media Cybernetics).
Statistical analysis.
For the analysis of variances between groups of data, two-way ANOVA with a Bonferroni posttest or unpaired t-test with Mann-Whitney posttest was performed using GraphPad Prism 4.0. Data are expressed as the means ± SE. A P value of <0.05 was considered statistically significant. Three or four individual experiments were carried out for each assay, each in duplicate.
RESULTS
PA adventitial VV comprises progenitor-like cells.
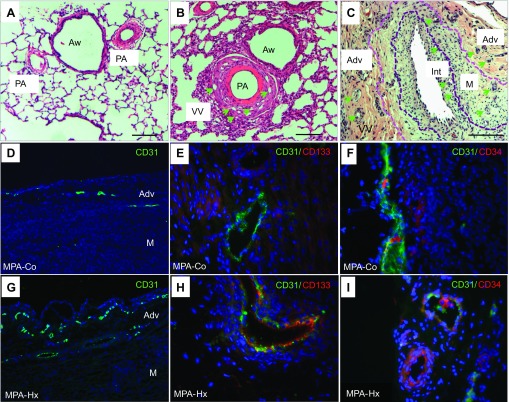
To analyze hypoxia-induced VV neovascularization, we performed histological evaluation of the VV in the PA of neonatal chronically hypoxic calves with severe PH and age-matched controls. H&E-stained tissue sections demonstrated marked thickening of the PA wall in chronically hypoxic hypertensive animals compared with arteries from controls. This vascular remodeling was associated with a prominent increase in the density of the VV network that was localized within the adventitial compartment and at the adventitial-medial interface (Fig. 1, A and B), consistent with our previous observations (3). Interestingly, in older (5–6 mo-old) calves that were raised at altitudes ≥9,000 ft and that developed terminal right heart failure (so-called brisket disease), dramatic increases in the density of VV were observed not only in adventitial but also in medial and even intimal compartments (Fig. 1C) (34).
Fig. 1.
Vasa vasorum (VV) neovascularization in pulmonary arteries (PA) of normal and pulmonary hypertensive animals. Hematoxylin and eosin (H&E) staining of lung sections of control (A) and hypoxic (B) calves demonstrates that thickening of the PA wall is associated with an apparent increase in the density of VV (green arrows) in vessels from hypoxic animals. Aw, airways; scale bar, 20 μm. In more advanced stages of the pulmonary hypertensive process in the “brisket disease” calf model of PH (see materials and methods), further expansion of VV [in adventitia (Adv), media (M), and even neointima (Int)] is observed (C). Demarcation lines identify the border between the neointimal and medial layers (as defined by the basal elastic lamellae). Scale bar, 100 μm. Immunofluorescence analysis of endothelial and progenitor cell marker expression in main pulmonary artery (MPA) VV shows the expression of CD31+ cells (D and G; ×10 magnification), CD31+CD133+ cells (E and H; ×40 magnification), and CD31+ CD34+ cells (F and I; ×40 magnification) in VV in sections from control (Co) and chronically hypoxic (Hx) animals.
Because EPC are known to contribute to vessel wall neovascularization, we evaluated whether EPC contributed to hypoxia-induced VV expansion. Immunofluorescent analysis of endothelial and progenitor marker (CD31, CD34, and CD133) expression in the adventitial VV demonstrated the presence of increased numbers of CD31+ cells in hypoxic animals compared with controls (Fig. 1, D and G), which is consistent with previously reported evidence of hypoxia-induced angiogenic VV expansion (3). However, not all cells comprising VV were CD31+. Our data also showed the presence of CD31+CD133+ (Fig. 1, E and H) and CD31+CD34+ (Fig. 1, F and I) cells in the VV of both control and hypoxic animals. Not all CD34+ or CD133+ cells were positive for CD31, indicating that the VV is likely to consist of various progenitor and endothelial cell populations that exist in the vessel at different stages of differentiation. Notably, we did not find CD34+ or CD133+ elsewhere in the PA adventitia.
VVEC-Hx cultures express higher levels of CD31, CD105, and c-kit.
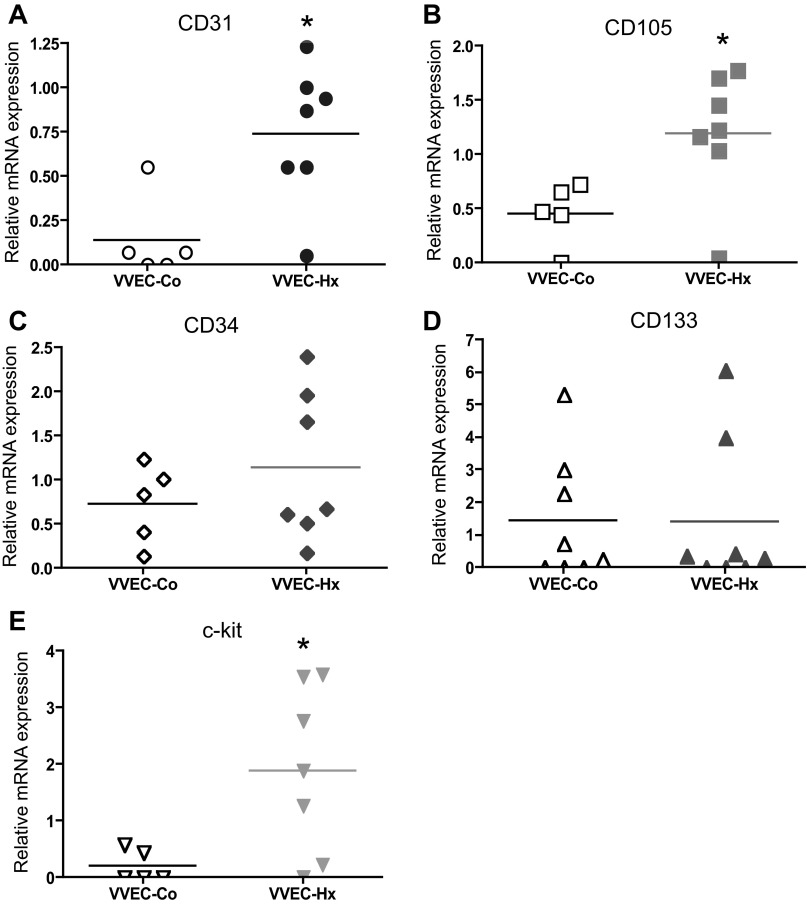
To further characterize VVEC phenotype, qRT-PCR analysis was carried out on VVEC-Co and VVEC-Hx. Data showed that both cell populations expressed CD31, CD34, CD105, CD133, and c-kit (Fig. 2). The median mRNA expression levels of endothelial marker CD31 were threefold higher in VVEC-Hx compared with VVEC-Co, consistent with an elevated level of CD31 expression observed in PA sections. The median mRNA expression of progenitor marker CD34 was 1.3-fold higher in VVEC-Hx. The median mRNA expression levels of progenitor marker CD133 were similar in both VVEC-Co and VVEC-Hx. The mRNA expression of the proliferation and vascular repair-associated marker CD105 was twofold higher in VVEC-Hx vs. VVEC-Co. The median mRNA expression of the multipotent stem cell antigen c-kit was markedly (9-fold) higher in VVEC-Hx compared with VVEC-Co. These data demonstrate that there were higher expression levels of CD31, CD105, and especially c-kit antigens in VVEC cultures from hypoxic hypertensive calves compared with controls, whereas other markers showed only moderate increases. The expression of the leukocyte common antigen CD45 was not found in VVEC populations (data not shown).
Fig. 2.
Endothelial and progenitor cell marker expression in VV endothelial cells (VVEC). Quantitative RT-PCR analysis was performed on total cellular mRNA isolated from VVEC-Co and VVEC-Hx populations. Expression level of CD31, CD34, CD133, CD105, and c-kit was calculated relative to bovine 18S RNA in VVEC-Co and VVEC-Hx. Data represent mean relative mRNA expression in VVEC-Co and VVEC-Hx; P = 0.0303 (CD31); P = 0.5303 (CD34); P = 0.8763 (CD133); P = 0.0303 (CD105); P = 0.0408 (c-kit); *P < 0.05 (Student's t-test, followed by a Mann-Whitney posttest).
VVEC consist of a hierarchy of cells with different proliferative potential.
Given that EPC have been implicated in the vascular remodeling process (8) and that ECFC possess increased proliferative capacity, we aimed to investigate the presence of a different hierarchy of proliferative cells in VVEC-Co and VVEC-Hx. An established method for ECFC isolation and identification is the single-cell clonogenic assay, where single EC would give rise to populations with different proliferative potential (21). A hierarchy of CFC in both VVEC-Co and VVEC-Hx was observed 14 days after single cell sorting. Figure 3A demonstrates representative images of HPP-CFC, LPP-CFC, and EC colonies formed after a 14-day clonogenic assay. A significantly higher percentage of proliferating cells (HPP-CFC and LPP-CFC) was observed in VVEC-Hx than in VVEC-Co (Fig. 3B), emphasizing the presence of a highly proliferative population in VVEC-Hx. Moreover, VVEC-Hx contained 26.85 ± 2.14% HPP-CFC (considered ECFC, a type of EPC), which was significantly higher than that in VVEC-Co (17.47 ± 1.79%, P = 0.0082). In contrast, there was a significantly higher percentage of mature EC (EC clusters) in VVEC-Co compared with VVEC-Hx (56.54 ± 3.96 vs. 7.59 ± 1.43 respectively, P = 0.0001). Thus, VVEC contained a highly proliferative population (HPP-CFC) that showed increased numbers in VVEC-Hx compared with VVEC-Co. To compare a hierarchy of proliferative cells in VVEC and endothelial cells from large vessels, we performed clogenic assay on aortic (AOEC) and main pulmonary artery (MPAEC) endothelial cells isolated from hypoxic animals (Fig. 3C). We found that AOEC and MPAEC contained a slightly lesser percentage on HPP-CFC and a markedly lesser percentage of LPP-CFC compared with the percentages of these cell populations in VVEC-Hx. In contrast, the percentage of differentiated cell clusters possessing almost no proliferative activity was higher in AOEC and MPAEC.
Fig. 3.
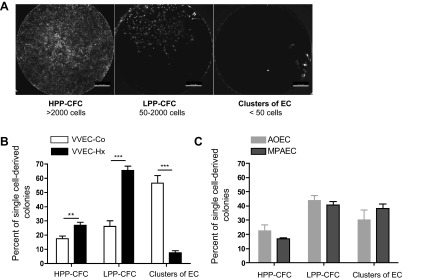
Quantitation of clonogenic and proliferative potential of single cell-derived colonies from VVEC, aortic endothelial cells (AOEC), and main pulmonary artery endothelial cells (MPAEC). Data represent the percentage of single cell-derived colonies from VVEC-Co (n = 3), VVEC-Hx (n = 3), AOEC-Hx (n = 3), and MPAEC-Hx (n = 3) on day 14 after single cell sorting. Colonies of each cell population were analyzed from 4–5 96-well plates (384–480 wells in total). HPP-CFC, high proliferative potential colony-forming cells; LPP-CFC, low proliferative potential colony-forming cells; EC, endothelial clusters. **P = 0.008 and *** P = 0.0001 (Student's t-test, followed by a Mann-Whitney posttest).
VVEC and HPP-CFC demonstrate alterations in telomerase activity.
HPP-CFC populations have been previously reported to retain high telomerase activity contributing to their high replicative capacity (19–21, 46). Using the TRAPeze RT telomerase kit, we showed that six from seven tested HPP-CFC-Co had higher relative telomerase activity compared with VVEC-Co (Fig. 4A). However, 9 from 11 tested HPP-CFC-Hx showed lower telomerase activity compared with VVEC-Hx (Fig. 4B). There were no visible differences in the morphological endothelial-like appearance of HPP-CFC and their parent VVEC cultures, as was demonstrated by bright-field microscopy (Fig. 4B).
Fig. 4.
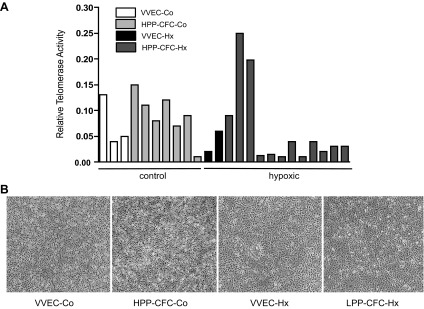
Characterization of high proliferative potential colony-forming cells (HPP-CFC) morphology and telomerase activity. A: the graph shows relative telomerase activity measured using a TRAPEZE RT telomerase kit. Shown are the data from individual cell populations isolated from control (control) and chronically hypoxic (hypoxic) animals. VVEC (n = 3), HPP-CFC-Co (n = 7), and HPP-CFC-Hx (n = 11). B: bright-field images represent VVEC and HPP-CFC cultures isolated from control and chronically hypoxic animals.
HPP-CFC express elevated levels of endothelial and progenitor cell markers.
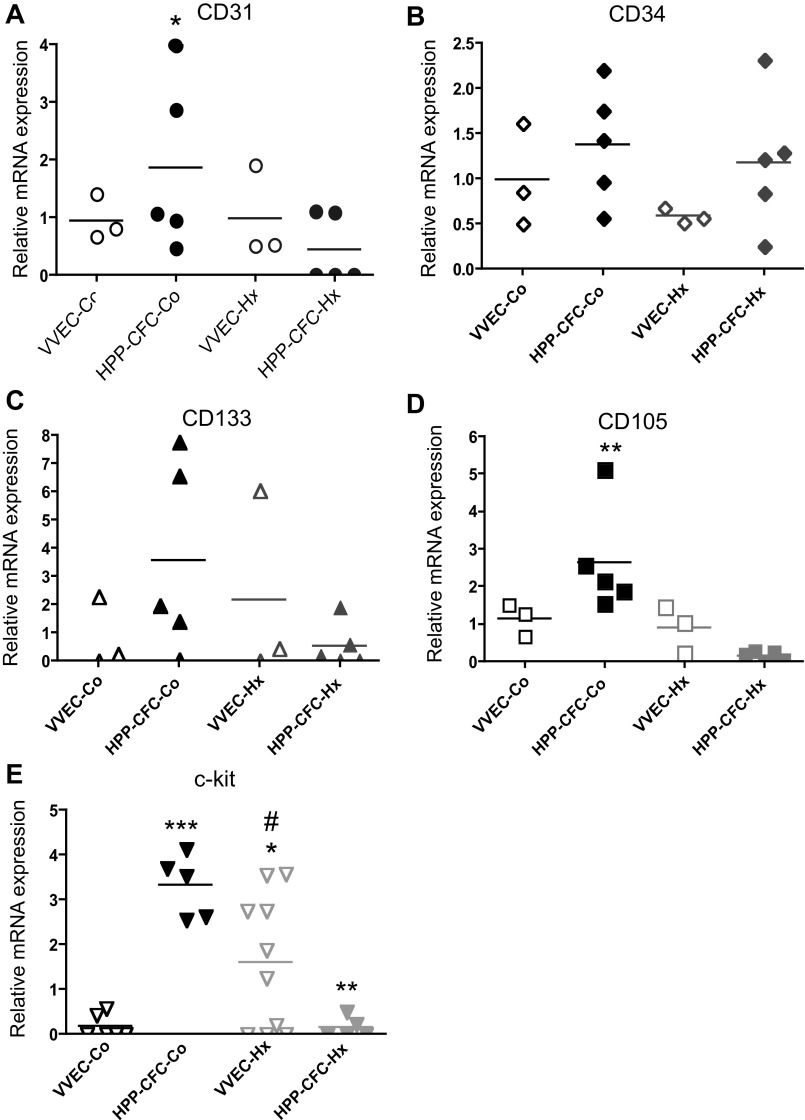
Vascular endothelium is known to exhibit a variable degree of heterogeneity due to the presence of cell populations with distinct proliferative and cell surface phenotypes. To characterize HPP-CFC derived from VVEC-Co and VVEC-Hx, qRT-PCR analysis of endothelial and progenitor cell markers was carried out. HPP-CFC-Co showed higher (compared with VVEC-Co) mRNA for CD31, CD133, CD105, and c-kit, indicating that the HPP-CFC-Co population is comprised of more progenitor-like and/or activated endothelial cells (Fig. 5, A-E). In HPP-CFC-Hx, mRNA levels of CD31, CD133, and CD105, but not of CD34, appeared to be lower, yet not statistically significant, than in parent VVEC-Co populations. Expression of c-kit was significantly lower in HPP-CFC-Hx vs. VVEC-Hx, and it was much lower in HPP-CFC-Hx compared with HPP-CFC-Co.
Fig. 5.
Quantitative RT-PCR analysis of endothelial and progenitor cell marker expression in HPP-CFC compared with VVEC. Quantitative RT-PCR analysis was performed on total cellular mRNA isolated from VVEC-Co and VVEC-Hx populations. Expression levels of CD31, CD34, CD105, and CD133 mRNA were calculated relative to bovine 18S RNA in cell populations of control and hypoxic animals. Data represent mean relative mRNA expression; CD31: *P < 0.05, VVEC-Co vs. VVEC-Hx. c-kit: *P < 0.05, VVEC-Co vs. VVEC-Hx; ***P < 0.001, VVEC-Co vs. HPP-CFC-Co; #P < 0.05, HPP-CFC-Co vs. HPP-CFC-Hx; and **P < 0.01, HPP-CFC-Co vs. HPP-CFC-Hx. CD105: **P < 0.01, HPP-CFC-Co vs. HPP-CFC-Hx (CD105) (1-way ANOVA, followed by a Bonferroni posttest).
VVEC and HPP-CFC are characterized by similar but not identical proangiogenic capabilities.
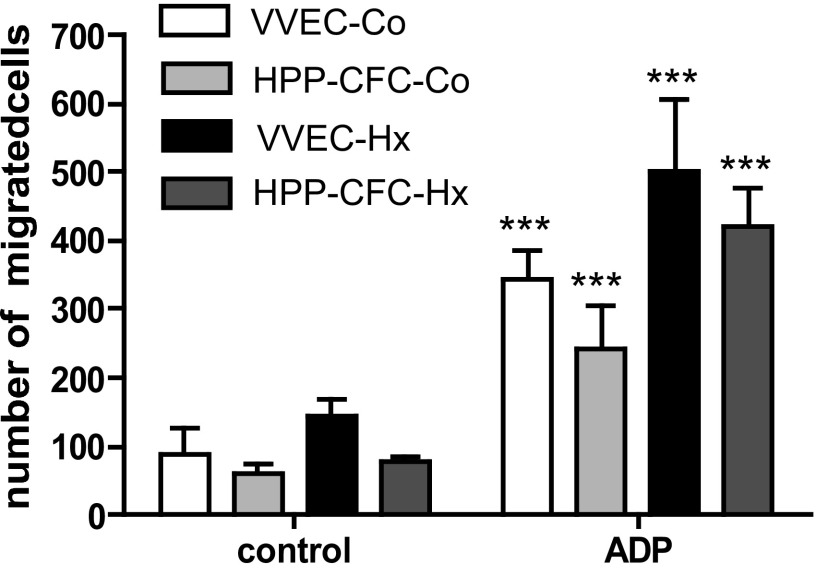
Next, VVEC and HPP-CFC were examined for their migratory and tube formation responses, two angiogenic attributes featured in vascular endothelial and EPC. Earlier we have demonstrated that extracellular nucleotides ATP and ADP exhibit a potent angiogenic effect on VVEC (14, 27). Using ADP as an angiogenic ligand for P2Y1 and P2Y13 receptors (27) we observed a significantly enhanced migration in all cell populations (Fig. 6). The number of HPP-CFCs migrating in response to ADP was similar in cells isolated from both control and hypoxic animals. Tube formation assay on Growth Factor Reduced Matrigel demonstrate that the formation of tubular-like networks in the HPP-CFC and their parent VVEC cultures was more pronounced in cells isolated from hypoxic compared with control animals (Fig. 7, A and B). Stimulation with extracellular ADP increased average tube length in all cell populations that was accompanied by a decreased number of tubes and triple node counts. These data suggest that morphogenetic response represents a distinct angiogenic characteristic that may not necessarily correlate with cell proliferative and migratory potential.
Fig. 6.
Characterization of migratory properties of VVEC and HPP-CFC. Migration was carried out using Boyden chamber assay as described in materials and methods. Growth-arrested cells were seeded on top of the transwell permeable support, and ADP (500 μM) was added to the lower compartment to initiate migration. Migrated cells were fixed in methanol and stained with 0.2% crystal violet. Filters with migrated cells were photographed under ×20 magnifications in a phase-contrast microscope (Nikon) at 3 random fields and counted using ImageJ software. Graph represents the mean ± SE of migrated cells treated or untreated with ADP; ***P < 0.0001, control vs. ADP-stimulated cells.
Fig. 7.
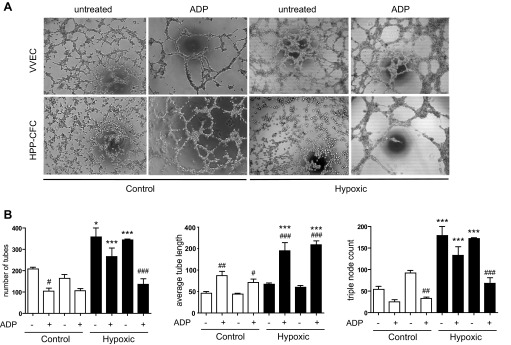
Tube formation responses in VVEC and HPP-VVEC. A: tube formation was performed in angiogenesis slides (ibidi) as described in materials and methods. Growth-arrested VVEC and HPP-CFC (17,000/well) were seeded on polymerized Growth Factor Reduced Matrigel and incubated for 6 h with or without ADP (100 μM). B: evaluation of tube formation responses in VVEC and HPP-CFC were carried out using the Image-Pro Premier 9.0 software. VVEC-Co and VVEC-Hx (n = 3), HPP-CFC-Co (n = 3) and HPP-CFC-Hx (n = 6), each in triplicate. *P < 0.05 and ***P < 0.0001, control vs. hypoxic cells; #P < 0.05, ##P < 0.01, and ###P < 0.001, control vs. ADP-stimulated cells.
DISCUSSION
Previous studies have shown that VV neovascularization is critically involved in the vascular remodeling process in a number of diseases, including atherosclerosis, aortic aneurism, idiopathic fibrosis, and PH (3, 31, 32, 36, 40). However, information on the mechanisms of VV expansion and its regulation in hypoxia-induced vascular remodeling, as well as the cell populations involved in this process, remain unexplored. Because VV expansion is thought to play a critical role in pulmonary vascular remodeling in PH, this study was aimed at identifying, isolating, and characterizing highly proliferative potential cell populations residing in the VV, which might contribute to the angiogenic expansion of the VV observed in PH.
The presence of vascular wall resident ECFC possessing high proliferative potential has been demonstrated within the endothelium of several vascular beds, including the endothelium of the pulmonary circulation (1, 6, 18, 20). Given that ECFC may contribute to angiogenesis (8, 9) and also exhibit a hierarchy of functional cell surface markers, we carried out immunofluorescence analysis on sections of the MPA to examine the expression of endothelial and progenitor markers such as CD31, CD34, and CD133 in the adventitial VV to characterize the nature of the cells in the normal (control) and expanding (hypoxic) VV. Increased VV density was demonstrated by the increased numbers CD31+ cells in the MPA adventitia of chronically hypoxic compared with control animals, consistent with previously reported evidence of hypoxia-induced VV expansion (4) and with VV expansion in the large PA of patients with IPAH (31). Double staining showed that some cells in VV were CD31+CD34+ or CD31+CD133+ in both control and hypoxic animals, with a higher number of these cells in the hypoxic animals, indicating a recruitment of endothelial progenitors in the expanding VV in hypoxia and subsequent differentiation of these cells to endothelial phenotype.
To evaluate the expression of endothelial and progenitor markers at the cellular level, we used isolated VVEC of PA of control and hypoxic animals. The true identity of an EPC and the cell surface markers used to identify those cells remains controversial since EPC have been isolated from different sources and different species. Generally, cell surface antigens including CD34 and CD133 (8) have been used to identify endothelial progenitor phenotype, whereas CD31 and CD105 have been used to identify endothelial-specific phenotype (1). CD31 (PECAM-1) is known to be involved in endothelial cell proliferation, intercellular junction formation, and inflammatory cell trafficking through the endothelial monolayer (24, 35, 42). Importantly, CD31+ cells isolated from peripheral blood and bone marrow possessed a high vasculogenic activity (23), indicating that CD31 also represents a marker of endothelial lineage commitment. In the present study, we found that relative mRNA expression of CD31 in VVEC-Hx was threefold higher than in VVEC-Co, supporting a role of CD31 in VV neovascularization in hypoxia. In addition, it is possible that increased expression of CD31 in VVEC of hypoxic animals also contributes to the considerable infiltration and accumulation of circulating inflammatory and progenitor cells in the PA adventitia and around the VV network (12).
CD105 (endoglin) is known as a proliferation-associated and hypoxia-inducible protein in angiogenically active endothelial cells (5, 10), including coronary artery VV (26). Our data demonstrated that CD105 expression was twofold higher in VVEC-Hx compared with VVEC-Co, consistent with the idea that the VV network expanding under hypoxic conditions consists of endothelial cells with a more activated phenotype compared with the cells of control VV. We also found that CD34 and CD133 were both expressed in VVEC, indicating the presence of a population with progenitor-like phenotype in VV of control and hypoxic animals. Finally, VVEC were evaluated for the expression of c-kit, a transmembrane protein tyrosine kinase, which plays a role in hematopoietic, immune, and other cell type proliferation and development (42). We found significantly higher c-kit expression in VVEC-Hx compared with VVEC-Co, suggesting that bone marrow-derived hematopoietic stem cells may be recruited to VV and differentiate to endothelial cells. The presence of c-kit+ in VV is complementary to previously reported findings demonstrating the presence of c-kit+ cells in PA adventitia of chronically hypoxic calves (3). In addition, in patients with IPAH, c-kit+ cells were localized around PA adventitial VV and within the plexiform lesions (31).
The presence of a population of cells expressing progenitor-like markers in VVEC-Hx suggested that these populations may exhibit elevated proliferative capacity. EPC have been previously implicated in the neovascularization process associated with several diseases. A type of EPC isolated through clonogenic assay, termed ECFC, are structural cells of the endothelium, possess an enhanced angiogenic capacity, and are rare in the circulation (7). Thus far, they have been isolated from rat PA and microvessels (1), human umbilical vein, and aorta (21). ECFC have also been shown to reside in PAEC where they were more proliferative in patients with PAH compared with control subjects (6). Furthermore, ECFC dysfunction has been shown in pathological angiogenesis associated with PAH (8, 41). To our knowledge, this study is the first report on the analysis of a hierarchy of clonal cell populations in VV. To determine if ECFC/HPP-CFC are present in VVEC and if this population exhibits EPC characteristics, we employed clonogenic assay on VVEC isolated from the PA of control and PH calves. This assay has been used previously to isolate ECFC/HPP-CFC from several vascular beds (21, 46). Our results demonstrated a significantly higher percentage of HPP-CFC in VVEC-Hx compared with VVEC-Co, indicating a potential role for those cells in VV angiogenic expansion observed in the PA adventitia of hypoxic animals.
HPP-CFC were cultured and evaluated for their endothelial morphology, telomerase activity, expression of endothelial and progenitor markers, and angiogenic responses. HPP-CFC possessed endothelial morphology similar to parent VVEC in culture. Notably, HPP-CFC-Co had similar characteristics to HPP-CFC-Hx, but fewer colonies were present after the clonogenic assay, and thus fewer cells were available for investigation.
Most ECFC have been shown to retain high telomerase activity, which contributes to their high proliferative state and regenerative characteristic (19, 46). Indeed, in our studies, HPP-CFC-Co showed higher telomerase activity than VVEC-Co, indicating that this population is the highly proliferative progenitor population. Interestingly, however, most HPP-CFC-Hx showed low telomerase activity compared with HPP-CFC-Co, although VVEC-Hx had a higher percentage of HPP-CFC. Low or absent telomerase activity has been found in somatic tissues and primary cells where telomere dysfunction is likely an indicator of chromosomal aberrations, genomic stability, proliferative arrest, and apoptosis (37). It has also been shown that critically short telomeres contribute to endothelial dysfunction and impaired angiogenesis, both involved in vascular remodeling in a number of pulmonary diseases (11). Moreover, vascular endothelial cells with senescence-associated phenotype induced by telomere shortening have been shown in human atherosclerotic lesions (29). Low levels of telomerase activity have also been seen in adult stem cells, including hematopoietic and nonhematopoietic stem cells (17), contributing to genomic instability and thus disease progression. Our data showing low telomerase activity in HPP-CFC-Hx potentially may point to cellular senescence and genomic instability. Therefore, this population could be responsible for dysfunctional VV and PH pathogenesis. Marked variations in telomerase activity in HPP-CFC-Hx are consistent with a previous report on murine bone marrow HPP-CFC (45) that demonstrated HPP-CFC heterogeneity with respect to their proliferative capacity and in vitro responsiveness to hematopoietic growth factors. It can be speculated that, in hypoxic microenvironments, some VV HPP-CFC-Hx escape replicative senescence, become apoptosis resistant, and can be responsible for VVEC repopulation and VV expansion. A better understanding of telomerase activity regulation in progenitor cells could be of critical therapeutic potential in vascular remodeling in PH.
As previously mentioned, elevated expression of CD31, CD105, and c-kit antigens can be functionally linked to increased proliferative responses, however, there are limited data to support this idea. In this study we compared the expression of the selected endothelial and progenitor markers in HPP-CFC and parent VVEC cultures. We showed significantly higher expression of CD31, CD105, and c-kit in HPP-CFC-Co compared with VVEC-Co, however, the expression of these antigens was lower in HPP-CFC-Hx compared with VVEC-Hx. These observations suggest that, despite similar proliferative characteristics, HPP-CFC-Co and HPP-CFC-Hx may exhibit distinct cell surface phenotype, and there is apparently no direct correlation between proliferative responses and elevated expression of CD31, CD105, and c-kit. These observations are unexpected, since a receptor tyrosine kinase c-kit can mediate proliferative signals in tumor-related diseases (25), and CD31 can undergo tyrosine phosphorylation and contribute to integrin-mediated Grb2/Sos/Ras signaling (35, 42). Further exploration of HPP-CFC phenotypes and their modulation by hypoxia would be necessary for better understanding a specific contribution of these cell populations to VV angiogenesis. Development of commercially available bovine-reactive antibodies to CD31, CD34, CD133, CD105, and c-kit would facilitate FACS analysis of these antigens on the cell surface.
To characterize the angiogenic capacity of HPP-CFC, we investigated the migratory and tube formation responses of these cell populations in vitro. Migration is very important for progenitor cells to be recruited to the sites of injury (13), whereas tube formation is important for vessel morphogenesis and is a critical attribute of EPC and EC (16). Previously, our group has reported that VVEC isolated from the PA adventitia of chronically hypoxic calves possess an activated proangiogenic phenotype, characterized by elevated mitogenic and migratory responses to extracellular nucleotides (ATP and ADP) through the activation of P2Y1 and P2Y13 purinergic receptors (4, 14, 27, 43). In this study, VVEC and HPP-CFC were treated with ADP, the most potent ligand of P2Y1 and P2Y13 receptors (27), to evaluate migration and tube formation responses. We observed a significant migratory response to ADP from all populations of VVEC and HPP-CFC. The responses in ADP-treated HPP-CFC were slightly lower compared with those in VVEC, suggesting a possible difference in purinergic receptor expression and/or downstream cellular pathways leading to cell migration. Differences in purinergic receptor signaling may also explain increased migratory responses in VVEC and HPP-CFC isolated from hypoxic animals compared with controls. In a Matrigel tube formation assay, we observed tubular rearrangement in VVEC and HPP-CFC in response to ADP. Notably, ADP induced marked morphogenetic response in these cell populations and increased numbers of branching points (triple node counts), again pointing to HPP-CFC as potentially being an important contributor to VV expansion and morphogenesis.
In conclusion, this is the first study to show the presence of HPP-CFC (ECFC) in PA adventitial VV. We demonstrated that the abundance of HPP-CFC in hypoxic VVEC may be an important contributor to the extensive neovascularization of the PA VV observed in the hypertensive animals via their high proliferative potential and vasculogenic capacity. Therefore, this cell population could be a potential target in regulating VV neovascularization in hypoxia-induced pulmonary vascular remodeling. Meanwhile, low telomerase activity observed in some populations of HPP-CFC-Hx may indicate functional impairment of those cells as a result of chronic hypoxic exposure and/or functional and phenotypical changes that result from isolation of those cell population from tissue microenvironment. Further characterizing and defining a role of HPP-CFC in VV neovascularization and understanding the involvement of this population in the progression of vascular diseases may unravel the mechanisms behind these phenomena.
GRANTS
This work was funded by National Heart, Lung, and Blood Grants R01-HL-086783 (to E. V. Gerasimovskaya), PPG-HL-14985 (to K. R. Stenmark).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.N., N.B., and E.V.G. performed experiments; H.N. and A.A. analyzed data; H.N. and E.V.G. prepared figures; H.N. drafted manuscript; H.N., V.B., N.B., A.A., K.R.S., and E.V.G. approved final version of manuscript; V.B., K.R.S., and E.V.G. conception and design of research; A.A., K.R.S., and E.V.G. edited and revised manuscript; E.V.G. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Dr. Maria Frid for critical evaluation of this manuscript.
REFERENCES
- 1. Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Davie NJ, Crossno JT, Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668–L678, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol 168: 1793–1807, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J 17: 984–992, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Duong HT, Comhair SA, Aldred MA, Mavrakis L, Savasky BM, Erzurum SC, Asosingh K. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ 1: 475–486, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duong HT, Erzurum SC, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis 14: 411–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J 35: 418–425, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res 110: 624–637, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 22: 6557–6563, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Franco S, Segura I, Riese HH, Blasco MaA. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res 62: 552–559, 2002 [PubMed] [Google Scholar]
- 12. Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. George AL, Bangalore-Prakash P, Rajoria S, Suriano R, Shanmugam A, Mittelman A, Tiwari RK. Endothelial progenitor cell biology in disease and tissue regeneration (Abstract). J Hematol Oncol 4: 24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11: 169–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation (Abstract). Cardiovasc Diabetol 3: 1, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer 96: 1020–1024, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang L, Harkenrider M, Thompson M, Zeng P, Tanaka H, Gilley D, Ingram DA, Bonanno JA, Yoder MC. A hierarchy of endothelial colony-forming cell activity displayed by bovine corneal endothelial cells. Invest Ophthalmol Vis Sci 51: 3943–3949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang L, Harkenrider M, Thompson M, Zeng P, Tanaka H, Gilley D, Ingram DA, Bonanno JA, Yoder MC. A hierarchy of endothelial colony-forming cell activity displayed by bovine corneal endothelial cells. Invest Ophthalmol Visual Sci 51: 3943–3949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105: 2783–2786, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173–202, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon Ys. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol 56: 593–607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev 92: 1619–1649, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci 9: 435–443, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luque A, Slevin M, Turu MM, Juan-Babot O, Badimon L, Krupinski J. CD105 positive neovessels are prevalent in early stage carotid lesions, and correlate with the grade in more advanced carotid and coronary plaques (Abstract). J Angiogenes Res 1: 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lyubchenko T, Woodward H, Veo KD, Burns N, Nijmeh H, Liubchenko GA, Stenmark KR, Gerasimovskaya EV. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Cell Physiol 300: C266–C275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31: 1530–1539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541–1544, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Mitzner W, Wagner EM. Vascular remodeling in the circulations of the lung. J Appl Physiol 97: 1999–2004, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, Launay JM, Cohen-Kaminsky S, Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 116–123, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation 113: 2245–2252, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 175: 875–880, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Neary JM, Gould DH, Garry FB, Knight AP, Dargatz DA, Holt TN. An investigation into beef calf mortality on five high-altitude ranches that selected sires with low pulmonary arterial pressures for over 20 years. J Vet Diagn Invest 25: 210–218, 2013 [DOI] [PubMed] [Google Scholar]
- 35. Newman PJ. The biology of PECAM-1. J Clin Invest 99: 3–8, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res 75: 649–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ Res 94: 575–584, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75: 23–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75: 23–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 180: 780–787, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27: 2514–2523, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol 297: L954–L964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoder MC. Human endothelial progenitor cells. Cold Spring Harbor Perspectives in Medicine 2: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoder MC, Du XX, Williams DA. High proliferative potential colony-forming cell heterogeneity identified using counterflow centrifugal elutriation. Blood 82: 385–391, 1993 [PubMed] [Google Scholar]
- 46. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]