Abstract
The phospholipase A2 activity of peroxiredoxin 6 is inhibited by the transition state analog, 1-hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol (MJ33). This activity is required for the activation of NADPH oxidase, type 2. The present study evaluated the effect of MJ33 on manifestations of acute lung injury. Mice were injected intratracheally (IT) with LPS from Escherichia coli 0111:B4 (LPS, 1 or 5 mg/kg), either concurrently with LPS or 2 h later, and evaluated for lung injury 24 h later. MJ33 inhibited reactive oxygen species (ROS) generation by lungs when measured at 24 h after LPS. LPS at either a low or high dose significantly increased lung infiltration with inflammatory cells, secretion of proinflammatory cytokines (IL-6, TNF-α, and the chemokine macrophage inflammatory protein-2), expression of lung vascular cell adhesion molecule, lung permeability (protein in bronchoalveolar lavage fluid, leakage of FITC-dextran, lung wet-to-dry weight ratio), tissue lipid peroxidation (thiobarbituric acid reactive substances, 8-isoprostanes), tissue protein oxidation (protein carbonyls), and activation of NF-κB. MJ33, given either concurrently or 2 h subsequent to LPS, significantly reduced all of these measured parameters. Previous studies of toxicity showed a high margin of safety for MJ33 in the intact mouse. Thus we have identified MJ33 as a potent, nontoxic, and specific mechanism-based inhibitor of NADPH oxidase type 2-mediated ROS generation that protects mice against lung injury associated with inflammation.
Keywords: reactive oxygen species, lipid peroxidation, protein oxidation, lung permeability, phospholipase A2, transition state inhibitor
we have recently demonstrated the role of oxidative stress in lung injury associated with ischemia followed by reperfusion (I/R) in a perfused mouse lung model by showing increased production of reactive oxygen species (ROS), increased tissue lipid peroxidation, increased tissue protein oxidation, and increased lung permeability (22). Similar studies with NADPH oxidase type 2 (NOX2)-null mouse lungs indicated that ∼80% of the ROS production and most of the oxidative lung injury with I/R in the isolated lung is due to NOX2 activation. These manifestations of I/R-induced lung injury in wild-type (WT) lungs were markedly reduced by pretreatment of mice with 1-hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol (MJ33). This agent inhibits the phospholipase A2 (PLA2) activity of peroxiredoxin 6 (Prdx6) that is required for activation of NOX2 and subsequent generation of ROS in normal cells (13).
Cellular generation of ROS leading to oxidative stress plays a crucial role in the pathogenesis of the acute lung injury (ALI) syndrome associated with lung inflammation (21). As described above for I/R, the primary source of ROS generation with inflammation of the lung is activation of NOX2, mostly in polymorphonuclear leukocytes (PMN), but also in other cell types (10, 12, 17). In the present study, we evaluated MJ33 for its therapeutic efficacy in a model of lung inflammation produced by the intratracheal (IT) instillation of LPS. This model is widely used to reflect lung inflammation associated with Gram-negative bacterial infection (28, 37). MJ33 was administered either concurrently with or 2 h postadministration of LPS. As a first goal, we determined that LPS-induced ROS generation in the lungs of mice is inhibited by MJ33. We then evaluated the effect of MJ33 on LPS-induced lung injury using as parameters lung infiltration with leukocytes, the release of chemokines/cytokines, the expression of vascular cell adhesion molecule (VCAM) and NF-κB, the oxidation of lung tissue lipids and protein, and alterations of alveolar permeability.
MATERIALS AND METHODS
Reagents.
LPS (0111:B4 from Escherichia coli), MJ33, O-dianisidine dihydrochloride, hydrogen peroxide, human leukocyte myeloperoxidase (MPO), protease inhibitor cocktail (P8340), and authentic lipids for preparation of liposomes were purchased from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase (HRP), Dulbecco's phosphate buffered saline (DPBS), and the fluorescent dyes 2′,7′-dichlorofluorescin (H2DCF) diacetate, Amplex Red, Alexa acetylated low-density lipoprotein (AcLDL), and Nile Red were obtained from Life Technologies (Grand Island, NY). DuoSet Elisa kits for mouse IL-6, TNF-α, or CXCL2/macrophage inflammatory protein 2 (MIP-2) were purchased from R & D Systems (Minneapolis, MN). Anti-VCAM-1 monoclonal antibody (S-14R) was raised against an extracellular domain of mouse VCAM-1 and was purchased from Santa Cruz Biotechnology (Dallas, TX); anti-tubulin monoclonal antibody was purchased from Sigma-Aldrich. The ELISA-based kit to detect and quantify transcription factor activation of Trans AM-NF-κB p65 was purchased from Active Motif (Carlsbad, CA).
Preparation of liposomes.
To prepare a stock solution, MJ33 (10 mg/ml in PBS) was warmed to 60°C, and the container was gassed with N2, sealed tightly with paraffin film, and kept at 4°C. Unilamellar liposomes were prepared with the following composition (mole fraction): 0.5 dipalmitoylphosphatidylcholine, 0.25 egg phosphatidylcholine, 0.15 cholesterol, and 0.1 phosphatidylglycerol to reflect the approximate composition of lung surfactant (16). Liposomes were prepared by evaporating the mixture of lipids under N2 gas overnight to become a film that was resuspended in PBS ± MJ33 (at 1 mol % of lipids) with vigorous mixing, then frozen and thawed three times by exposing the mixture to liquid N2 followed by a 50°C water bath. The mixture was then extruded at 50°C for 20 cycles through a 100-nm pore filter (15) and stored at 4°C until use within 24 h.
Mice.
Three strains of mice were studied: C57Bl/6J (WT), Prdx6-null, and NOX2 (gp91phox)-null. Ten-wk-old WT and NOX2-null mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The generation of Prdx6-null mice has been described previously (30); these mice have been fully back-crossed to the C57Bl/6J background (24). Animal care was in compliance with all regulations set by and with protocols approved by the University of Pennsylvania Animal Care and Use Committee (IACUC) and was in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Animals were kept in HEPA-filtered vivariums maintained at 25 ± 3°C. The number of mice used for each experiment is indicated in the figure legends.
Administration of LPS and MJ33.
Mice were anesthetized with pentobarbital sodium (50 mg/kg body wt) via an intraperitoneal (IP) injection. LPS (1 or 5 mg/kg) followed by liposomes (0.05 ml) with or without MJ33 (4 nmol) was instilled into the lung through an endotracheal catheter placed at the level of the tracheal carina. Mice were allowed to recover and maintained in the vivarium with access to food and water for 24 h and then killed by exsanguination.
ROS production in the isolated lung.
After death, the thorax was incised, the lungs were cleared of blood by perfusion through the pulmonary artery, removed from the thorax, and placed in a chamber for continuous perfusion as described previously (15, 16). Lungs were continuously ventilated through a tracheal cannula with air containing 5% CO2 and perfused with recirculating Krebs-Ringer bicarbonate solution supplemented with 10 mM glucose and 3% bovine serum albumin. Amplex red and horseradish peroxidase were added to the perfusate to determine ROS production (6). Aliquots of the perfusate were removed at intervals, and fluorescence intensity of the Amplex Red was measured (excitation/emission, 545/610 nm) using a spectrofluorometer (Photon Technology International, Birmingham, NJ). At the end of the perfusion period, the lung was removed from the chamber and dried in an oven to constant weight. ROS generation, determined by oxidation of Amplex Red, was calculated as nanomoles per gram of dry weight of lung using an extinction coefficient of 54,000 M−1·cm−1. The slope of the line for oxidation of Amplex Red vs. time was calculated by the least mean squares method.
Cellular production of ROS was imaged in the intact isolated lung perfused with fluorescent dyes. For these studies, bovine serum albumin in the perfusate was replaced with 3% dextran 70 (5, 7, 47, 48). Separate lungs were used for detection of ROS in epithelial and endothelial cells. Lungs were labeled with H2DCF added to the perfusate plus either IT instillation of the epithelial fluorophore (Nile Red) or perfusion with the endothelial fluorophore (Alexa AcLDL). Lungs were placed on the stage of an inverted microscope and imaged by confocal microscopy (Radiance 2000; Bio-Rad, Hercules, CA). Cell types were identified with the red fluorophore (Alexa 594 or Nile Red) and ROS production with the green dye (H2DCF). Images were acquired in a sequential scan mode to eliminate possible overlap of the fluorescence signals between the two channels. All images were obtained at the same settings (scale of 0–4,095) and exposure time. For each field, the red epithelial or endothelial cells were outlined, and this mask was transferred onto the green field indicating ROS; the green intensity of all regions that were labeled red was integrated, and this was normalized per unit area (825 pixels).
The effect of MJ33 on LPS-induced ALI.
LPS-induced ALI was evaluated by examining parameters from bronchoalveolar lavage fluid (BALf), lung tissue, and lung homogenate. Lungs were analyzed for the following parameters: 1) LPS-induced lung inflammation was analyzed for total cells in BALf, MPO activity in cell pellets from BALf, cytokines (IL-6 and TNF-α) and a chemokine (MIP-2) in BALf, VCAM in lung homogenate, and activated NF-κB. 2) LPS-induced oxidative stress in lung homogenates was analyzed for thiobarbituric acid reactive substances (TBARS) and 8-isoprostanes for lipid peroxidation and protein carbonyls for protein oxidation. 3) LPS-induced lung permeability was analyzed for total protein in BALf, permeability to fluorescein isothiocyanate (FITC)-dextran 70 measured in lung homogenate, and lung wet-to-dry weight ratio.
Harvest and assay of BALf.
After intubation of the mouse trachea with a 22-gauge cannula, the lungs of intact mice were lavaged three times, each with 1 ml of iced PBS containing 0.1 M EDTA. The BALf (3 ml) was centrifuged for 5 min at 375 g. The supernatant was kept at −80°C until assay with the Bio-Rad protein assay kit using Coomassie Blue with bovine IgG as the standard. The pellet was resuspended in 1 ml saline, recentrifuged, and resuspended in 0.4 ml DPBS. The total number of cells in the reconstituted supernatant was counted with a hemocytometer. The remaining supernatant was recentrifuged, and the cell pellet was kept at −80°C. For MPO assay, 600 μl of 1× MPO buffer (containing 100 mM potassium phosphate, pH 6.0, 1 mM EDTA, and 0.5% hexadecyltrimethyl ammonium bromide) was added to the thawed samples, transferred to microcentrifuge tubes, and lysed by freeze-thawing followed by sonication for ∼15 s (38). The sample was centrifuged at 9,300 g for 5 min, and the supernatant was assayed for MPO. Samples of 100 μl were appropriately diluted and added to each well in a 96-well plate. MPO buffer (100 μl) that contained 0.167 mg/ml of O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide was added into each well. A standard curve of human leukocyte MPO activity from 1,000 mU/ml to 1 mU/ml in serial dilutions was set up. Absorbance was measured at 450 nm. Total cells and protein in the BALf and MPO activity in the cell pellet are expressed per gram of body weight.
Cytokines (IL-6, TNF-α) and chemokine (MIP-2) in BALf.
Cytokines and the chemokine were measured in the BALf by ELISA assay. Capture antibodies for IL-6, TNF-α, or MIP-2 (also known as CXCR2) were diluted in PBS, and 100 μl was placed into 96-well plates, sealed with Elisa plate sealer tape, and incubated overnight at room temperature. Each well was repeatedly aspirated and washed thoroughly with wash buffer. After the last wash, the remaining wash buffer was removed either by aspiration or by blotting the inverted plate against a clean paper towel. Reagent diluent (DY995, 300 μl) was added to each well; plates were incubated for 2 h at room temperature and rewashed followed by addition of BALf (100 μl) or the standard solution. After incubation for 2 h at room temperature and rewashing, detection antibody (100 μl) was added to the plate, incubated for 2 h at room temperature, and washed. Streptavidin-HRP (100 μl) was added for 20 min at room temperature (avoiding direct light), and substrate solution (100 μl) was added for 20 min followed by 50 μl stop solution. Absorbance was determined immediately using a microplate reader at 450 nm with wavelength correction by subtracting the reading at 540 nm. Cytokines/chemokine content was normalized to mouse body weight.
VCAM expression in lung.
Lungs were cleared of blood, excised from the thorax, washed in cold PBS, and homogenized in PBS containing protease inhibitor cocktail. The homogenate was centrifuged for 15 min at 100 g at 4°C, the supernatant was collected, protein concentration estimated, and 50 μg protein was loaded on a 10% Tris-glycine SDS polyacrylamide gel and immunoblotted using anti-VCAM-1 antibody. Anti-tubulin antibody was used as a loading control. Immunoblots were quantified using NIH Image J software. Lanes were outlined, and the intensity of pixels in the entire lane was obtained. Within each lane, the software was used for quantification of the intensity of the bands. The value for intensity of the VCAM band was expressed relative to that for the tubulin band.
Tissue oxidation, lipid peroxidation, and protein carbonyls.
Following death of the mouse and clearance of blood, lungs were rapidly frozen by clamping with aluminum tongs precooled in liquid N2 and stored in liquid N2. For assay, an aliquot of frozen lung was homogenized under N2 in PBS containing 0.01% butylated hydroxytoluene. TBARS were assayed in the lung homogenate by reaction with thiobarbituric acid in trichloroacetic acid, and absorbance was measured at 535 nm (14, 24). 8-Isoprostanes were measured by immunoassay using an 8-isoprostane kit (Cayman, Ann Harbor, MI) (24). TBARS and 8-isoprostanes were normalized to lung homogenate protein.
For analysis of protein carbonyls, an aliquot of frozen lung tissue was homogenized under N2 in buffer containing protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) and centrifuged at 10,000 g for 15 min. The supernatant was reacted with dinitrophenylhydrazine (DNPH). The DNPH derivative of the oxidized protein was separated from unbound DNPH by Sephadex G-25 column chromatography, and absorbance was measured at 360 nm (2, 24). Protein carbonyl content was calculated using an extinction coefficient of 21 cm−1·mM−1 and normalized to lung homogenate protein.
Permeability to FITC-dextran.
Permeability of lungs was determined by retention of FITC-dextran in the lung tissue indicating extravasation from the vascular space. FITC-dextran (70 or 2,000) was prepared at 1 mg/ml, and 0.1 ml was administered IV at 22 h post-LPS. Mice were killed 2 h later, the lung was cleared of blood by perfusion via the pulmonary artery, and the lungs and heart were removed en bloc, carefully washed, and weighed. Samples on ice were finely cut with scissors and homogenized in a 15-ml centrifuge tube containing 5 ml RPMI1640 without phenol, maintaining volume levels above the hole of the homogenizer to minimize foaming, and then centrifuged at 15,000 g for 30 min. The OD of the supernatant was measured at 494 nm. A standard curve for absorbance vs. FITC-dextran 70 concentration was constructed for each experiment. The pellet was assayed for protein, and microgram of FITC per milligram of protein was calculated.
Lung wet/dry weight ratio.
After termination of the experiment, the lungs were removed from the thorax en bloc, and the heart was trimmed away. The lungs were weighed (wet weight) and placed in an oven at 65°C for 7 days and then weighed again to determine dry weight. The ratio of the two weight measurements was calculated.
NF-κB bound to lung DNA.
The lung homogenate was prepared as described above for FITC-dextran, and 20 μg was used per assay. We followed the instructions for the Trans AM-NF-κB p65 ELISA-based kit to detect and quantify the content of the p65 subunit of NF-κB. This kit has 96-well plates to which an oligonucleotide containing the NF-κB consensus site has been immobilized and which specifically binds the active form of NF-κB. The primary antibodies used to detect NF-κB recognize an epitope of p65 that is accessible only when NF-κB is bound to its target DNA (www.activemotif.com/catalog/180/transam-transcription-factor-elisas).
Statistical analysis.
All measured values are presented as the means ± SE for each group. Group differences were evaluated by one-way ANOVA followed by a post hoc t-test with Bonferroni correction. Statistical significance was set at 95% (P < 0.05).
RESULTS
Selection of a control group.
As described in our previous report (22), MJ33 is a lipid-soluble agent (critical micelle concentration ∼10 μM) that was administered as a component of unilamellar liposomes formulated to mimic the lipid composition of lung surfactant. Lipid formulations of this composition (without MJ33) have been administered to a variety of animals for many years without adverse effects, and indeed they are used therapeutically in human babies for surfactant deficiency (16). For essentially all parameters studied, we have evaluated the effects of both saline alone and liposomes in saline as control adjuvants and found no differences. Because the agent (MJ33) was administered in liposomes (at 1 mol %), we have selected liposomes suspended in saline (without MJ33) as the most appropriate control for reporting in this manuscript.
The effect of MJ33 on NOX2-mediated ROS production in the intact lung after LPS treatment.
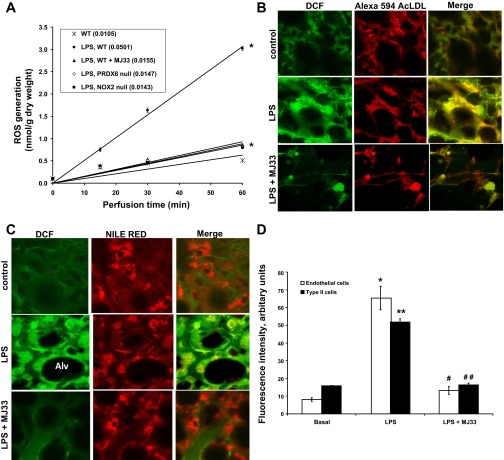
The potential utility of MJ33 as an agent to prevent ROS generation following LPS was evaluated in the isolated perfused lung model. LPS (1 mg/kg body wt) was instilled IT in anesthetized mice. MJ33 (1 mol % in unilamellar liposomes) was instilled immediately after the LPS. After 24 h, the lungs were removed from the chest for lung perfusion with medium containing Amplex Red plus HRP. Amplex Red detects H2O2 that is extracellular because this fluorophore does not permeate cell membranes. The control condition was isolated lungs from WT mice that were treated with liposomes in the absence of MJ33. Control lungs (WT) showed very low rates of ROS production (Fig. 1A). The lungs from mice that were administered LPS showed a 4.8-fold increase in the rate of ROS production (LPS, WT) that was largely abolished by pretreatment with MJ33 (LPS, WT + MJ33) (Fig. 1A). ROS production by LPS-treated NOX2-null and Prdx6-null lungs was minimal with levels similar to WT lungs treated with MJ33; the slight differences among these three models (WT + MJ33, NOX2-null, Prdx6-null) were not statistically significant (P > 0.05). These results indicate that the major fraction of ROS production at 24 h after LPS is NOX2 dependent and is inhibited by pretreatment with MJ33. Approximately 10% of ROS production appears to be NOX2 independent in this model of lung injury and could arise from other NOX enzymes, mitochondrial reactions, or other pathways. Thus MJ33 is an effective inhibitor of LPS-induced ROS production in this isolated lung perfusion model.
Fig. 1.
Reactive oxygen species (ROS) production in isolated perfused mouse lungs and lung cells in situ after LPS ± 1-hexadecyl-3-(trifluoroethyl)-sn-glycero-2-phosphomethanol (MJ33). LPS at 1 mg/kg and liposomes containing 4 nmol MJ33 were concurrently administered intratracheally (IT) to the intact mouse. A: ROS generation by isolated perfused wild-type (WT), NADPH oxidase type 2 (NOX2)-null, and peroxiredoxin 6 (Prdx6)-null mouse lungs was measured by Amplex Red oxidation. Perfusate samples were taken at intervals and analyzed for oxidized Amplex Red; results are plotted vs. time of perfusion. A perfusate sample from time zero was used as the baseline. The numbers in parentheses indicate the slope of the line (nmol/g dry wt per min) calculated by the least mean squares method. Values represent mean ± SE for n = 3; in some cases the SE bars are within plotted points. *P < 0.01 compared with WT at 60 min perfusion time. Effect of MJ33 on LPS-induced ROS in endothelial cells (B) and type 2 epithelial cells (C) of mouse lungs by confocal microscopy. Fluorescent dyes were used to detect ROS (dichloroflorescein, DCF; green color) and to identify cells (red color), either endothelial cells (Alexa 594AcLDL) or epithelial cells (Nile Red). The yellow areas indicate colocalization of DCF fluorescence with the cell marker. The dark areas are the alveolar spaces (Alv). D: quantification of the effects of MJ33 on LPS-induced ROS in endothelial cells and lung alveolar type II cells from the isolated perfused mouse lungs. Data represent the means ± SE for n = 3 independent experiments. Each experiment utilized a separate perfused lung, and for each 3 randomly selected fields were imaged, the integrated fluorescence was determined, and the values were averaged. Studies evaluated 3 treatment groups (basal, LPS, and LPS + MJ33). *P < 0.05, **P < 0.01 compared with basal and #P < 0.05, ##P < 0.01 compared with LPS.
Although the isolated perfused lung experiments with Amplex Red determined global ROS production after LPS, the cells responsible were not identified. We were especially interested in an epithelial vs. endothelial site for ROS generation because this could determine whether IT or IV might be the better route for delivery of the inhibitor. To identify which cell types are activated by LPS to produce ROS, we used a confocal microscopic technique to independently image capillary endothelial and type II epithelial cells in the intact lung. Oxidation of H2DCF to the fluorescent dichloroflorescein (DCF) was used as an index of ROS production. Unlike Amplex Red, H2DCF (administered as the diacetate) does permeate the lung cells so that this fluorophore indicates intracellular H2O2 or possibly some other oxidant. Endothelial cells were identified by uptake of Alexa-tagged AcLDL that was administered in the lung perfusate; lung epithelial type II cells were identified by uptake of Nile Red administered into the trachea (25). In control lungs, AcLDL and Nile Red showed distinct distribution patterns, as expected, confirming cell specificity of the labels (Fig. 1, B and C). With addition of H2O2 to the perfusate, both of these cell types showed increased DCF, indicating diffusion of H2O2 from the vascular space to both endothelium and epithelium (data not shown).
Both pulmonary microvascular endothelium (Fig. 1B) and alveolar type II cells (Fig. 1C) that were imaged at 24 h following IT LPS showed a marked increase in DCF fluorescence compared with control. ROS production was quantitated by measuring the intensity of green fluorescence in those areas with red fluorescence in endothelial or epithelial cells (Fig. 1D). This result suggests that both cell types (endothelial and type 2 epithelial) are activated to generate ROS after treatment with LPS, and the presence of NOX2 has been demonstrated in both cell types (18, 29, 41, 48). However, it also is possible that only one of the cell types was responsible for generating H2O2 that then diffused to the other type of cells. Fluorescence of both cell types was markedly reduced in the presence of MJ33, indicating that this treatment effectively inhibited LPS-induced ROS production (Fig. 1, B–D).
The effect of MJ33 on LPS-induced lung inflammation.
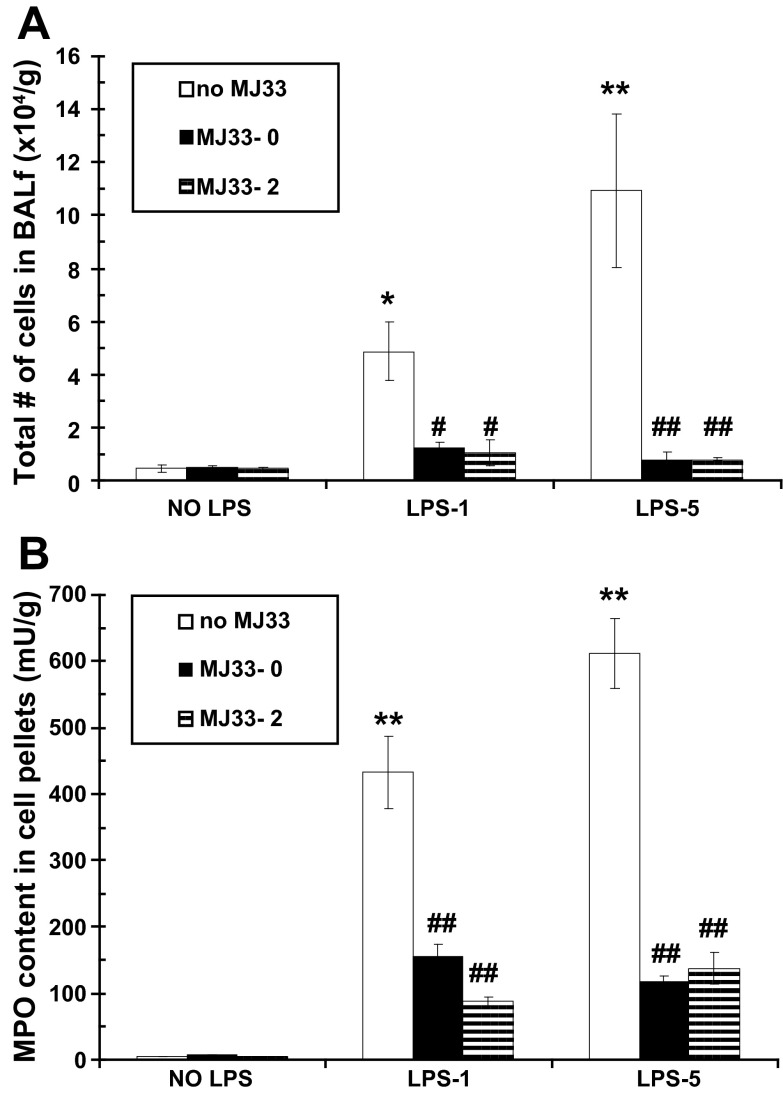
Lung inflammation following LPS insult is characterized by influx of leukocytes, primarily PMN (3, 20). We evaluated the effectiveness of MJ33 in modulating LPS-induced lung inflammation by measuring total nucleated cells in the BALf and MPO activity in the BALf cell pellet at 24 h post-LPS instillation. The total number of nucleated cells in the BALf was 0.39 ± 0.06 × 104 cells/g body wt in the control group. The total number of cells obtained in the BALf (Fig. 2A) and the MPO activity of the pelleted cells (Fig. 2B) were significantly increased after an IT instillation of LPS at 1 mg/kg (LPS-1), indicating an inflammatory response. The cellular influx was significantly greater with administration of LPS at 5 mg/kg (LPS-5) (Fig. 2, A and B). The increased MPO activity in the cell pellet (expressed per gram of body weight) reflects its PMN content (38). This influx of cells as reflected by cell count or MPO assay was dramatically reduced by administration of MJ33 concurrently with LPS. Importantly, MJ33 was equally effective when given 2 h post-LPS. Thus MJ33 can prevent lung inflammation associated with the administration of LPS.
Fig. 2.
Effect of MJ33 administered concurrently with LPS (MJ33-0) or 2 h after LPS (MJ33-2) on cellular infiltration of lungs at 24 h after LPS. LPS was administered at 1 (LPS-1) or 5 (LPS-5) mg/kg and MJ33 at 0.1 mg/kg. Total cells in bronchoalveolar lavage fluid (BALf) (A) and myeloperoxidase (MPO) activity in cell pellets (B) are expressed per gram of body weight. Results are means ± SE for n = 5–7. *P < 0.05, **P < 0.01 compared with control (no MJ33 and no LPS) and #P < 0.05, ##P < 0.01 compared with no MJ33 for LPS-1 or LPS-5.
The effect of MJ33 on release of cytokines/chemokines and VCAM expression following LPS.
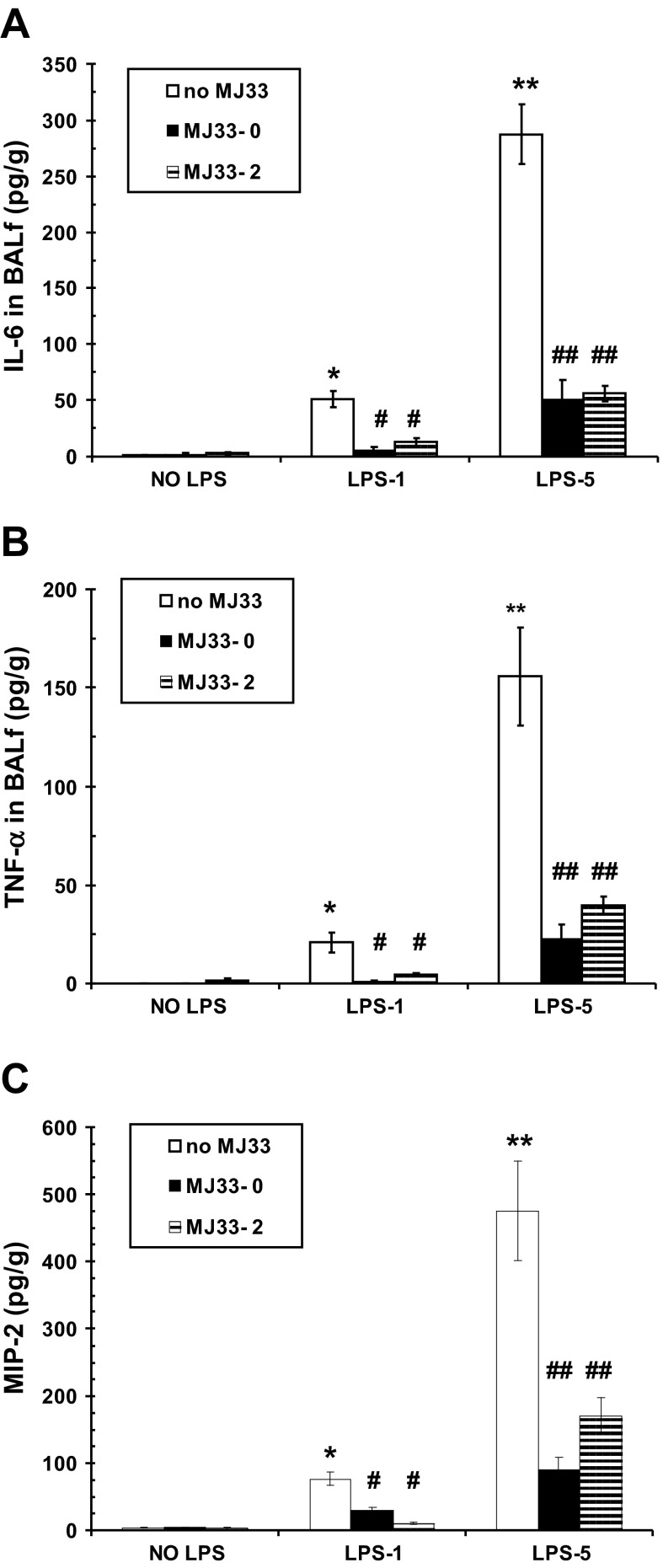
We measured cytokines (IL-6, TNF-α) and a chemokine (MIP-2) in the BALf at 24 h after administration of LPS. Neither IL-6 nor TNF-α was detected under control conditions (Fig. 3, A and B). Levels of these cytokines increased slightly with LPS-1 and to a much greater degree with LPS-5. Treatment with MJ33 either concurrently or 2 h post-LPS resulted in a dramatic decline in the levels of both cytokines although their content in BALf remained slightly above control. The response to MJ33 for MIP-2 was similar to the cytokine response.
Fig. 3.
Effect of MJ33 administered with LPS (MJ33-0) or 2 h after LPS (MJ33-2) on cytokine (IL-6, TNF-α) and chemokine (macrophage inflammatory protein 2, MIP-2) release into the BALf at 24 h after LPS. LPS was administered at 1 (LPS-1) or 5 (LPS-5) mg/kg and MJ33 at 0.1 mg/kg. IL-6 (A), TNF-α (B), and MIP-2 (C) are expressed per gram of body weight. Values are means ± SE for n = 5–7. *P < 0.05, **P < 0.01 compared with control (no MJ33 and no LPS) and #P < 0.05, ##P < 0.01 compared with no MJ33 for LPS-1 or LPS-5.
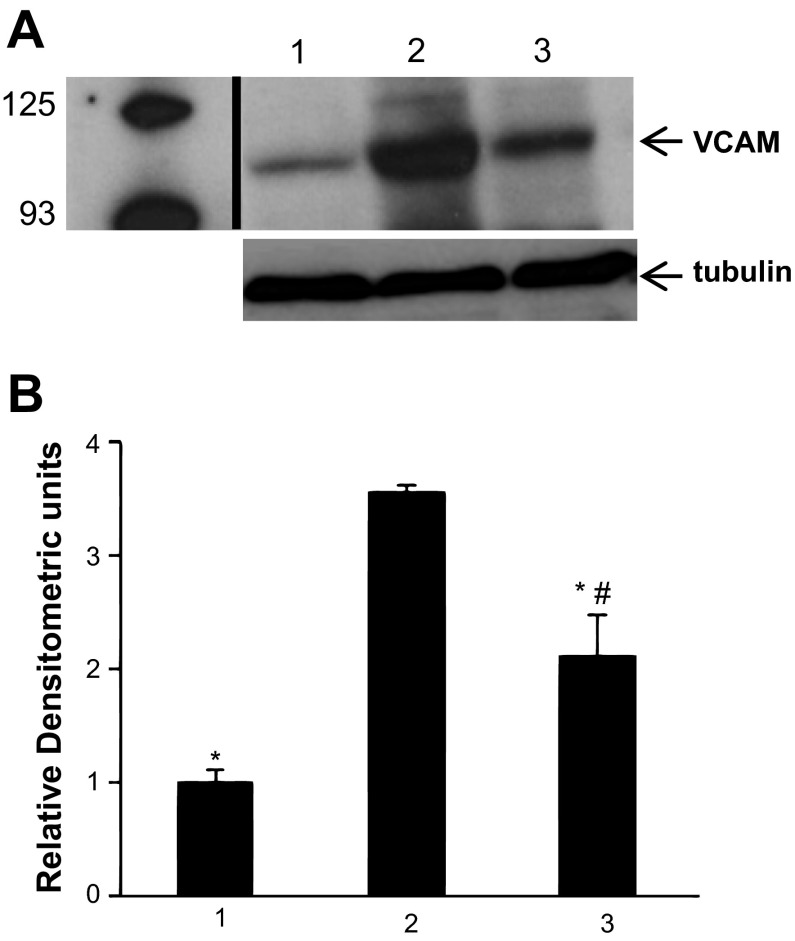
The expression of VCAM was studied at 24 h after administration of LPS (1 mg/kg) by Western blot analysis of lung homogenate. Several bands are visible on the gels with the most prominent at ∼110 kDa (Fig. 4A). This latter band corresponds to the VCAM molecular weight according to the specifications provided by the supplier of the antibody (www.scbt.com/table-vcam.html). LPS resulted in almost fourfold increase in VCAM expression that was decreased significantly, although not quite back to control levels, in the lungs of mice treated with MJ33 (Fig. 4B).
Fig. 4.
Effect of MJ33 (0.1 mg/kg) administered concurrently with LPS (1 mg/kg) on vascular endothelial cell adhesion molecule (VCAM) expression in the lung homogenate at 24 h after LPS. A: band at 110 kDa on Western blot indicates VCAM-1 expression for control (no LPS, no MJ33, lane 1), LPS (lane 2), and LPS + MJ33 (lane 3). The tubulin band, used as a loading control, was ∼55 kDa. The vertical dark line indicates the removal of several nonrelevant lanes. Anti-VCAM antibody was used at 1:1,000 and anti-tubulin antibody at 1:500. B: immunoblots were quantified by ImageJ software; values are expressed in arbitrary units for VCAM relative to the corresponding tubulin expression. Results are means ± SE for n = 3. *P < 0.01 compared with lane 2; #P < 0.05 compared with lane 1.
The effect of MJ33 on transcription factor activation following LPS.
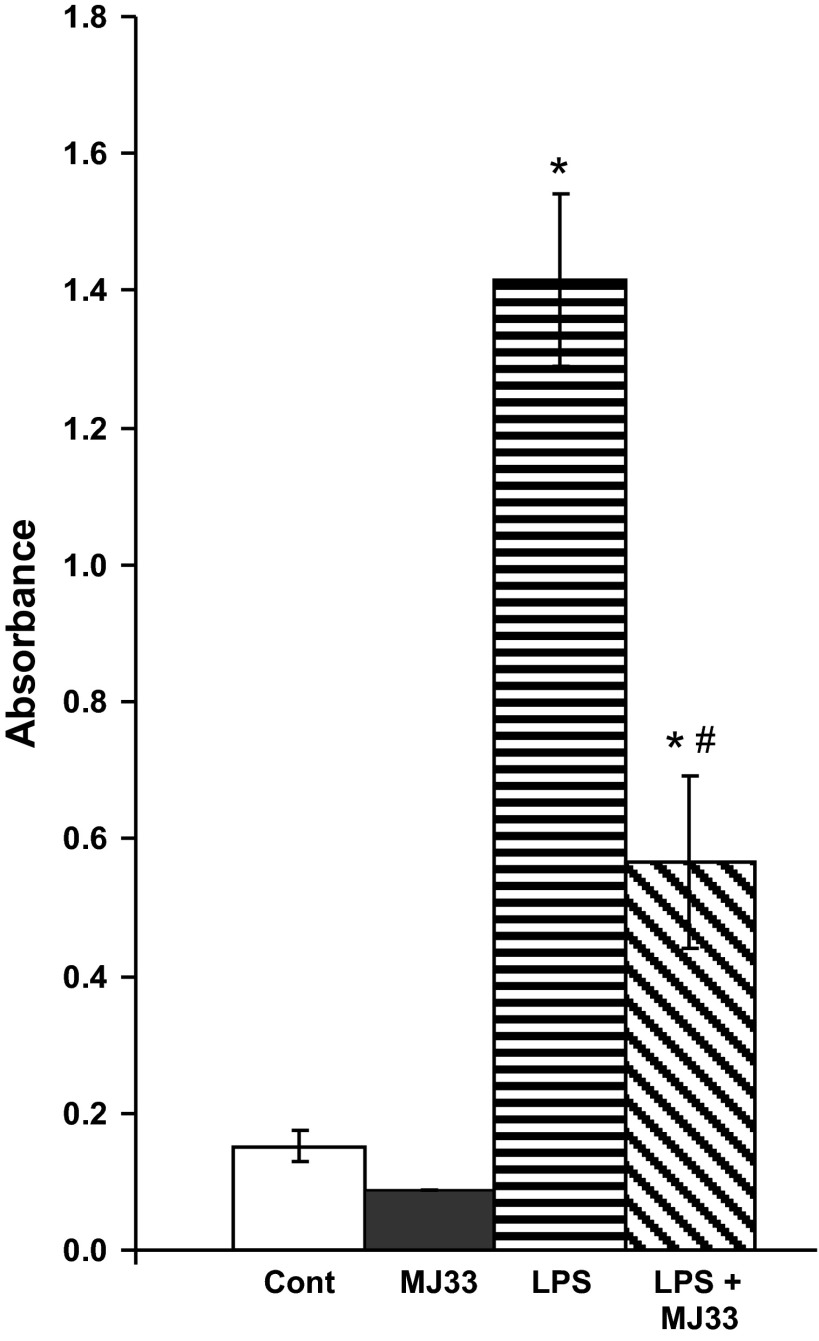
The activation of NF-κB in the lung homogenate (as determined by assay of the p65 subunit that is bound to DNA) was measured at 4 h after administration of LPS (1 mg/kg). The content of DNA-bound NF-κB in the lung homogenate increased markedly after LPS and was inhibited by 65% in mice treated with MJ33 (Fig. 5).
Fig. 5.
Effect of MJ33 (0.1 mg/kg) on NF-κB activation as indicated by assay of the p65 subunit bound to DNA at 4 h after LPS (1 mg/kg). The expression of DNA-bound p65 was measured by ELISA. The units indicated are the total absorbance (measured at 450 nm) per 20 μg lung homogenate protein. Data represent means ± SE for N = 3–5. *P < 0.05 compared with MJ33 and #P < 0.05 compared with LPS.
The effect of MJ33 on oxidation of lung lipid and protein following LPS.
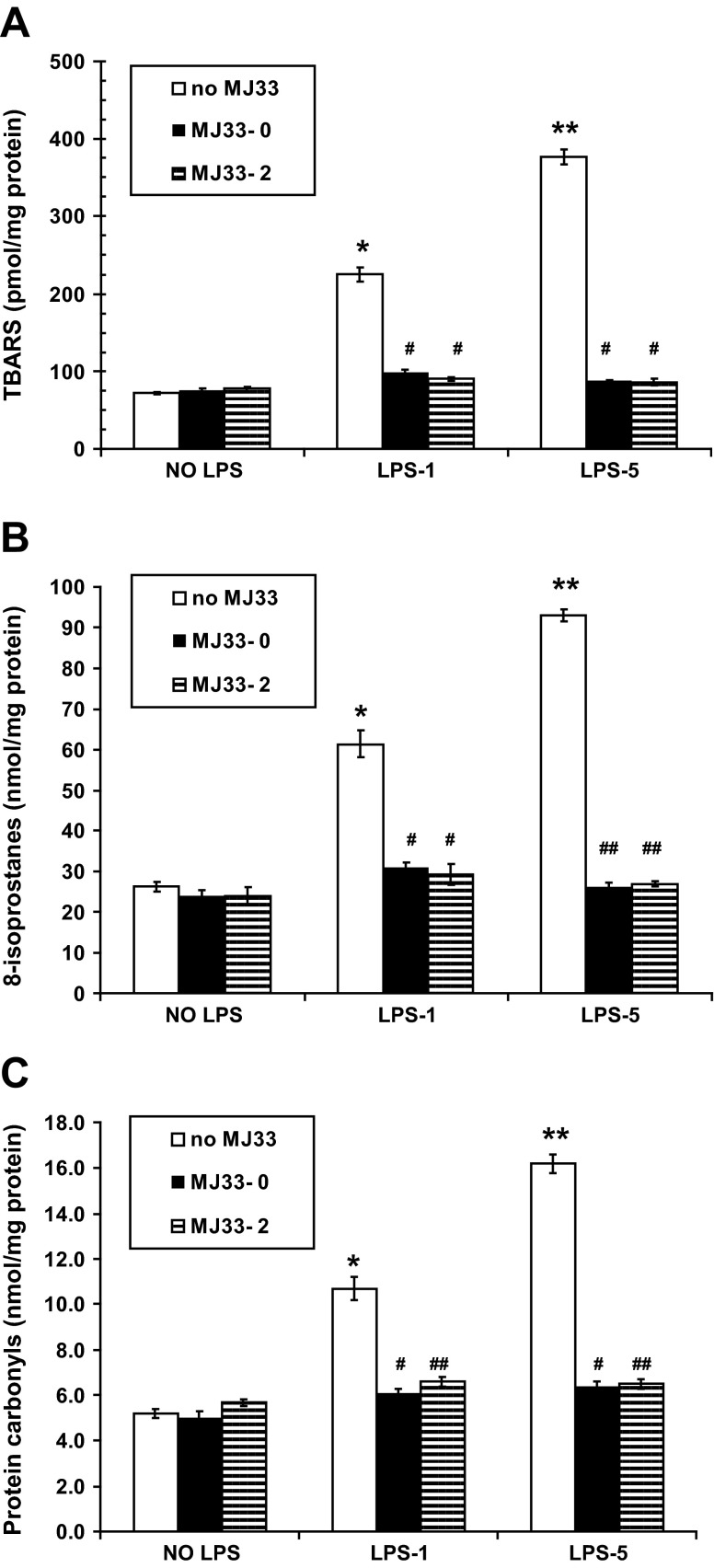
Oxidative stress to the lung tissue was evaluated at 24 h after LPS by measurement of TBARS and 8-isoprostanes for lipid peroxidation and protein carbonyls for protein oxidation. TBARS increased by 3.1- or 5.3-fold following LPS-1 or LPS-5, respectively (Fig. 6A). A similar pattern was seen with 8-isoprostanes; there was an increase with LPS-1 and a greater increase with LPS-5 (Fig. 6B). Both indices of lipid peroxidation returned to nearly control levels with MJ33 given either concurrently or 2 h post-LPS (Fig. 6, A and B). Likewise, protein carbonyls in lung homogenates showed ∼2.1- or 3.1-fold increase following LPS-1 or LPS-5, and the increase was nearly abolished by MJ33, administered either concurrently or 2 h post-LPS (Fig. 6C). Thus oxidation of lung tissue components was observed at 24 h after LPS administration and was largely prevented by MJ33.
Fig. 6.
Effect of MJ33 administered with LPS (MJ33-0) or 2 h after LPS (MJ33-2) on indices of lung tissue oxidation at 24 h after LPS. LPS was administered at 1 (LPS-1) or 5 (LPS-5) mg/kg and MJ33 at 0.1 mg/kg. Thiobarbituric acid reactive substances (TBARS) (A), 8-isoprostanes (B), and protein carbonyls (C) in the lung homogenate normalized to homogenate protein. Values are means ± SE for N = 3. *P < 0.05, **P < 0.01 compared with control (no MJ33 and no LPS) and #P < 0.05, ##P < 0.01 compared with no MJ33 for LPS-1 or LPS-5.
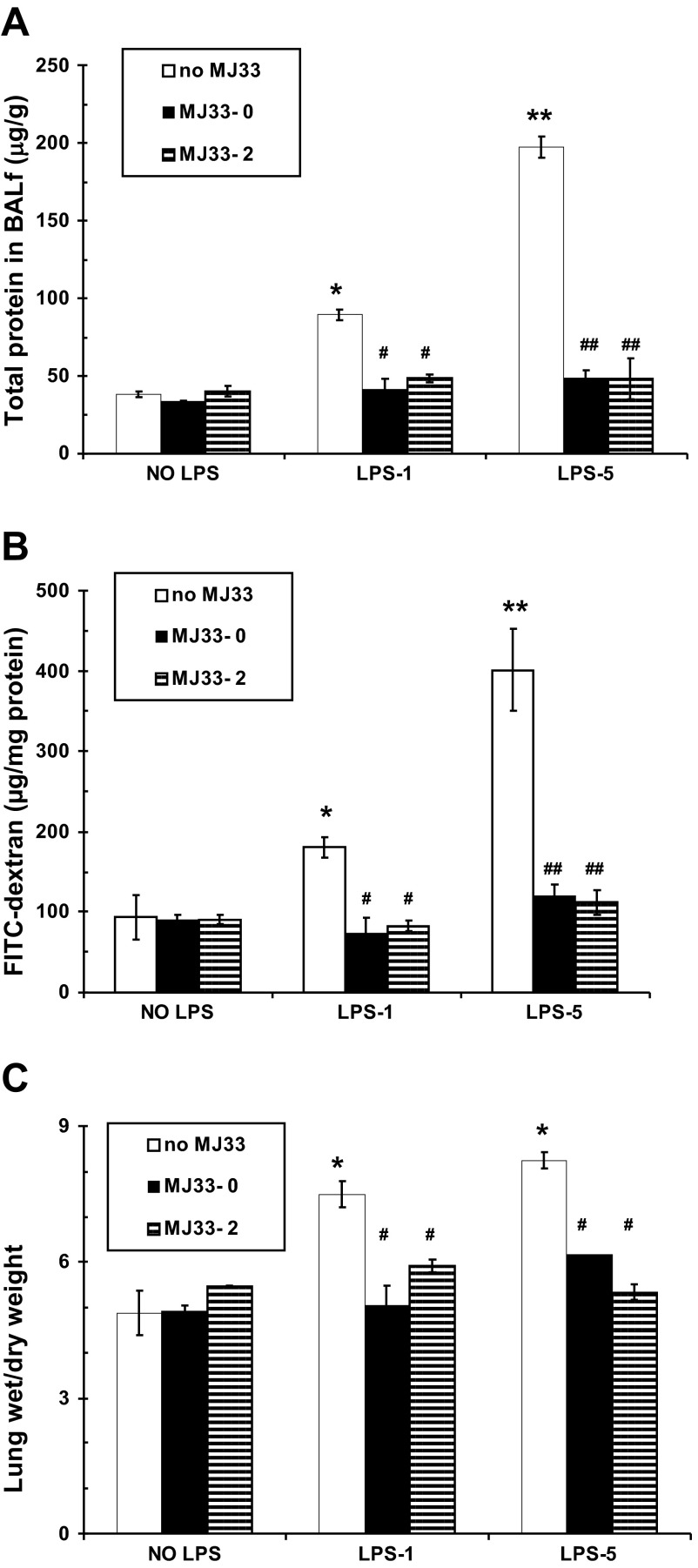
The effect of MJ33 on lung permeability following LPS.
LPS is known to increase the alveolar capillary permeability in the mouse lung as a manifestation of the inflammatory response. We evaluated alveolar epithelial and capillary permeability at 24 h after LPS by measuring the “leakage” of protein into the alveolar space (BALf), extravasation of FITC dextran into the lung tissue, and the lung wet-to-dry weight ratio. BALf protein increased 2.3-fold vs. control with the low dose LPS and 5.1-fold with the higher dose (Fig. 7A). Protein in the BALf was dramatically reduced to values not significantly different from control by administration of MJ33 concurrently with or 2 h post-LPS. Increased protein in the BALf reflects increased permeability to endogenous proteins, predominantly serum albumin.
Fig. 7.
Effect of MJ33 administered with LPS (MJ33-0) or 2 h after LPS (MJ33-2) on indices of lung permeability at 24 h after LPS. LPS was administered at 1 (LPS-1) or 5 (LPS-5) mg/kg and MJ33 at 0.1 mg/kg. Total protein in BALf normalized to body weight (A), FITC-dextran 70 measured in the supernatant of lung homogenate and normalized to homogenate protein (B), and lung wet/dry weight ratio (C). Data represent means ± SE for N = 5–7. *P < 0.05, **P < 0.01 compared with control (no MJ33 or LPS) and #P < 0.05, ##P < 0.01 compared with no MJ33 for LPS-1 or LPS-5.
An increase of FITC-dextran in the lung homogenate represents increased permeability of the pulmonary vasculature to an exogenous macromolecule. We used FITC-dextran of two different sizes with molecular masses of 2,000 kDa and 70 kDa in these permeability studies. No extravasation of FITC-dextran 2000 was observed under control conditions or after LPS, consistent with its large size (Stokes radius 27 nm). FITC-dextran 70 (Stokes radius 6 nm) was recovered at a low level in the lung homogenate under control conditions (Fig. 7B) but was significantly elevated by 1.7-fold after LPS-1 and 4.3-fold after LPS-5. The effect of LPS on permeability to FITC-dextran 70 was reversed (to a level not significantly different from control values) by treatment with MJ33 administered concurrently or at 2 h post-LPS.
Alveolar permeability also was evaluated by measuring the ratio of lung wet weight to lung dry weight. Similar to the other indices of permeability, there was a significant increase in the ratio, indicating fluid accumulation in the lung after LPS although, unlike the other indices, the increase was similar for the 1 and 5 mg/kg doses of LPS (Fig. 7C). Treatment with MJ33 reversed the LPS-induced increase in the wet-to-dry weight ratio.
DISCUSSION
LPS instilled into the lung provides a model for ALI associated with inflammation. Inflammation of the lung is characterized by the migration of neutrophils from the endothelial surface through the endothelial and epithelial layers to the epithelial surface in response to secreted chemoattractants (11, 46). These migratory cells as well as some of the constitutive lung cells, including the resident macrophages, are stimulated by the presence of LPS to generate ROS (17, 37). It is the cellular ROS generation that appears to be largely responsible for the subsequent lung injury. The specific metabolic pathway responsible for producing ROS following LPS appears to vary with the cell type in both lung and non-lung cells. Pathways include a mitochondrial origin in mouse embryonic fibroblasts (4), NOX type 1 in macrophages (26), and NOX type 4 in HEK293 cells (33). NOX2 has been identified as the enzymatic source of ROS following LPS in whole lung extracts (37), PMN (10, 12, 17), renal mesangial cells (23), microglia (9), RAW 264.7 cells (36), and alveolar macrophages (37). More than one pathway may be involved in ROS generation after LPS in some cell types. The increased generation of ROS by NOX1 and NOX4 was dependent on induction of the protein (26, 33), whereas generation of ROS by NOX2 primarily reflects activation of the enzyme.
In the present study, we evaluated ROS production by the isolated mouse lung following intratracheal LPS. We confirmed by using NOX2-null lungs that this enzyme was responsible for ∼90% of LPS-stimulated ROS production as measured by Amplex Red oxidation in the lung perfusate. Using imaging techniques, increased ROS production after LPS was demonstrated in both lung alveolar epithelial and endothelial cells. Minimal ROS production was seen in the Prdx6-null and MJ33-treated lungs consistent with our previous observations that the PLA2 activity of Prdx6 is required for NOX2 activation in PMN and lung cells (6, 22). Although MJ33 can inhibit other PLA2s, this activity associated with Prdx6 is the only significant target of the inhibitor in the lung (22). The activation of NOX2 to generate ROS is relatively rapid when cells are treated with specific agonists such as angiotensin II for endothelial cells or formyl Met-Leu-Phe for PMN. However, ROS production was not detected in the lung or isolated endothelial cells for several hours after LPS administration (data not shown). This delayed response is compatible with the requirement to mobilize the inflammatory response to detect a significant increase in lung ROS production.
MJ33 when given to mice at the relatively low dose of 0.2 μmol/kg body wt (0.1 mg/kg body wt) resulted in a marked inhibition of the effects of LPS when assayed at 24 h. MJ33 resulted in decreased cellular infiltration, decreased cytokine/chemokine release, decreased VCAM expression, decreased NF-κB activation, decreased oxidation of tissue lipid and protein, and maintenance of normal alveolar-capillary permeability. Protection by MJ33 against LPS-mediated lung injury presumably reflects the ability of this agent to inhibit the PLA2 activity of Prdx6 and prevent NOX2 activation. Interestingly, Prdx6 also can function as a ROS scavenger through its peroxidase activity; thus the absence of Prdx6 results in increased ROS-mediated cellular injury in models where oxidants are delivered exogenously or where endogenous oxidants are generated through non-NOX2 sources (42–44). The peroxidase activity of Prdx6 is not inhibited by MJ33 (13, 27).
On the basis of the proposed paradigm for ALI, ROS generation subsequent to LPS administration is due in part to the activation of PMN that accumulate in the lung (17, 37). An important question concerns the mechanism through which MJ33 inhibits the accumulation of lung inflammatory cells after LPS. One possibility is that the LPS-mediated activation of NOX2 in endothelium and possibly other cells is responsible for PMN recruitment to the lung and that this pathway is inhibited by MJ33. Expression of the cell adhesion molecule VCAM-1 has been shown in various cell types to be dependent at least in part on ROS generated by NOX2 (23, 32, 39). VCAM-1 mediates recruitment and anchoring of PMN and other phagocytic cells (31). Thus inhibition of the ROS-mediated induction of VCAM expression by MJ33 as seen in the present study could (Fig. 4) explain the lack of an increase in lung-associated inflammatory cells following LPS.
There have been many disappointments in the search for therapeutic strategies to prevent ALI associated with inflammation. In some circumstances, apparently promising molecular targets or pharmacological agents have not fulfilled their promise. For example, drotrecogin-α, a recombinant human activated protein C and the only drug ever approved by the FDA for the treatment of sepsis, was withdrawn from the market due to its lack of efficacy (35, 45). The lack of a specific therapy for treatment of sepsis results in a continuing high mortality rate, much of it associated with ALI (1). The generation of ROS via NOX2 as a manifestation of lung inflammation is recognized as an important target for therapy, but appropriate inhibitors have not been available for clinical use (19). There are several inhibitors of this pathway that are effective in vitro but are not suitable for translational application (8). Examples are diphenyleneiodonium (DPI), a nonspecific inhibitor that is toxic to cells in vitro (34), and apocynin, an inhibitor that appears to be more specific than DPI but has an ill-defined mechanism for its effect and is toxic to the central nervous system when administered systemically (40).
The present study suggests that MJ33 may uniquely fulfill the role of a specific therapy for ameliorating ALI associated with oxidant stress. Importantly, MJ33 was equally effective when administered concurrently with or at 2 h after treatment with LPS, indicating the potential for postinsult treatment to prevent the development of ALI. We have not yet evaluated the effect of MJ33 on the course of established lung injury. On the basis of the present findings, we speculate that MJ33 could be useful in the prevention of ALI in those conditions where NOX 2-generated ROS play an important pathophysiological role. Those conditions might include lung inflammation associated with pneumonia or sepsis and I/R injury associated with pulmonary embolism or lung transplantation. Although the agent (MJ33) appears to be relatively nontoxic for acute use (22), its chronic use requires more study based on the possibility of inducing chronic granulomatous disease as seen with the genetic deficiency of NOX2.
In conclusion, MJ33 dramatically reduced several critical parameters associated with oxidative lung injury following LPS administration to mice. Inhibiting the PLA2 activity of Prdx6 with MJ33 limited ROS production, the influx of inflammatory cells, cytokine/chemokine release, the increased VCAM expression and NF-κB activation, and the tissue injury that accompanies LPS-mediated lung inflammation. We have shown previously a large (>103-fold) margin of safety between the effective inhibitory and the toxic concentrations of MJ33 (22). Thus MJ33 represents a potentially useful agent to protect against acute inflammatory lung injury.
GRANTS
This work was supported by R01-HL105509 from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.L., C.D., and S.C. performed experiments; I.L. and A.B.F. analyzed data; I.L. prepared figures; I.L. and A.B.F. drafted manuscript; I.L., S.I.F., and A.B.F. edited and revised manuscript; I.L., C.D., S.C., S.I.F., and A.B.F. approved final version of manuscript; A.B.F., I.L., S.C., and S.I.F. conception and design of research; I.L., S.C., and A.B.F. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Dr. Diane Marotta and Ms. Hui Wang for assistance with assays. This work was presented, in part, at the Experimental Biology meeting in 2013 Boston, MA.
REFERENCES
- 1. Anger KE, Degrado JR, Greenwood BC, Cohen SA, Szumita PM. Evaluation of recombinant activated protein C for severe sepsis at a tertiary academic medical center. Ther Clin Risk Manag 9: 277–284, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayene IS, al-Mehdi AB, Fisher AB. Inhibition of lung tissue oxidation during ischemia/reperfusion by 2-mercaptopropionylglycine. Arch Biochem Biophys 303: 307–312, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Boots AW, Gerloff K, Bartholome R, van Berlo D, Ledermann K, Haenen GR, Bast A, van Schooten FJ, Albrecht C, Schins RP. Neutrophils augment LPS-mediated pro-inflammatory signaling in human lung epithelial cells. Biochim Biophys Acta 1823: 1151–1162, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208: 519–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, Birnbaum MJ, Fisher AB. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol 302: H105–H114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, Debolt K, Speicher D, Fisher AB. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem 286: 11696–11706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13: 633–644, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cifuentes-Pagano E, Csanyi G, Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clement HW, Vazquez JF, Sommer O, Heiser P, Morawietz H, Hopt U, Schulz E, von Dobschutz E. Lipopolysaccharide-induced radical formation in the striatum is abolished in Nox2 gp91phox-deficient mice. J Neural Transm 117: 13–22, 2010 [DOI] [PubMed] [Google Scholar]
- 10. DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest 101: 455–463, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol 30: 547–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med 9: 315–321, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Fisher AB. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal 15: 831–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher AB, Dodia C, Chander A. Alveolar uptake of lipid and protein components of surfactant. Am J Physiol Lung Cell Mol Physiol 261: L334–L340, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Fisher AB, Dodia C, Chander A, Jain M. A competitive inhibitor of phospholipase A2 decreases surfactant phosphatidylcholine degradation by the rat lung. Biochem J 288: 407–411, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res 46: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123: 887–902, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. NADPH oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O2− signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Kantrow SP, Shen Z, Jagneaux T, Zhang P, Nelson S. Neutrophil-mediated lung permeability and host defense proteins. Am J Physiol Lung Cell Mol Physiol 297: L738–L745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, Birukova AA. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol 47: 688–697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee I, Dodia C, Chatterjee S, Zagorski J, Mesaros C, Blair IA, Feinstein SI, Jain M, Fisher AB. A novel nontoxic inhibitor of the activation of NADPH oxidase reduces reactive oxygen species production in mouse lung. J Pharmacol Exp Ther 345: 284–296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun Signal 10: 33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu G, Feinstein SI, Wang Y, Dodia C, Fisher D, Yu K, Ho YS, Fisher AB. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic Biol Med 49: 1172–1181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu L, Wang M, Fisher AB, Zimmerman UJ. Involvement of annexin II in exocytosis of lamellar bodies from alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 270: L668–L676, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Maitra U, Singh N, Gan L, Ringwood L, Li L. IRAK-1 contributes to lipopolysaccharide-induced reactive oxygen species generation in macrophages by inducing NOX-1 transcription and Rac1 activation and suppressing the expression of antioxidative enzymes. J Biol Chem 284: 35403–35411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mo Y, Feinstein SI, Manevich Y, Zhang Q, Lu L, Ho YS, Fisher AB. 1-Cys peroxiredoxin knock-out mice express mRNA but not protein for a highly related intronless gene. FEBS Lett 555: 192–198, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Mori N, Horie Y, Gerritsen ME, Anderson DC, Granger DN. Anti-inflammatory drugs and endothelial cell adhesion molecule expression in murine vascular beds. Gut 44: 186–195, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orndorff RL, Hong N, Yu KJ, Feinstein SI, Zern BJ, Fisher AB, Muzykantov VR, Chatterjee S. NOX2 in lung inflammation: Quantum dot based in situ imaging of NOX2 mediated expression of vascular cell adhesion molecule-1 (VCAM). Am J Physiol Lung Cell Mol Physiol 306: L260–L268, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173: 3589–3593, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Riganti C, Gazzano E, Polimeni M, Costamagna C, Bosia A, Ghigo D. Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidative stress. J Biol Chem 279: 47726–47731, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Rosenberg-Yunger ZR, Thorsteinsdottir H, Daar AS, Martin DK. Stakeholder involvement in expensive drug recommendation decisions: an international perspective. Health Policy 105: 226–235, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem 276: 30188–30198, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16: 1713–1720, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Schmekel B, Hornblad Y, Linden M, Sundstrom C, Venge P. Myeloperoxidase in human lung lavage. II. Internalization of myeloperoxidase by alveolar macrophages. Inflammation 14: 455–461, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Song HY, Ju SM, Seo WY, Goh AR, Lee JK, Bae YS, Choi SY, Park J. Nox2-based NADPH oxidase mediates HIV-1 Tat-induced up-regulation of VCAM-1/ICAM-1 and subsequent monocyte adhesion in human astrocytes. Free Radic Biol Med 50: 576–584, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 154: 556–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tickner J, Fan LM, Du J, Meijles D, Li JM. Nox2-derived ROS in PPARgamma signaling and cell-cycle progression of lung alveolar epithelial cells. Free Radic Biol Med 51: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Feinstein SI, Fisher AB. Peroxiredoxin 6 as an antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J Cell Biochem 104: 1274–1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Radic Biol Med 37: 1736–1743, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid Redox Signal 8: 229–237, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Wenzel RP, Edmond MB. Septic shock—evaluating another failed treatment. N Engl J Med 366: 2122–2124, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 40: 519–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Q, Chatterjee S, Wei Z, Liu WD, Fisher AB. Rac and PI3 kinase mediate endothelial cell-reactive oxygen species generation during normoxic lung ischemia. Antioxid Redox Signal 10: 679–689, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol 289: L954–L961, 2005 [DOI] [PubMed] [Google Scholar]