Abstract
Recent studies have indicated that the myogenic response (MR) in cerebral arteries is impaired in Fawn Hooded Hypertensive (FHH) rats and that transfer of a 2.4 megabase pair region of chromosome 1 (RNO1) containing 15 genes from the Brown Norway rat into the FHH genetic background restores MR in a FHH.1BN congenic strain. However, the mechanisms involved remain to be determined. The present study examined the role of the large conductance calcium-activated potassium (BK) channel in impairing the MR in FHH rats. Whole-cell patch-clamp studies of cerebral vascular smooth muscle cells (VSMCs) revealed that iberiotoxin (IBTX; BK inhibitor)-sensitive outward potassium (K+) channel current densities are four- to fivefold greater in FHH than in FHH.1BN congenic strain. Inside-out patches indicated that the BK channel open probability (NPo) is 10-fold higher and IBTX reduced NPo to a greater extent in VSMCs isolated from FHH than in FHH.1BN rats. Voltage sensitivity of the BK channel is enhanced in FHH as compared with FHH.1BN rats. The frequency and amplitude of spontaneous transient outward currents are significantly greater in VSMCs isolated from FHH than in FHH.1BN rats. However, the expression of the BK-α and -β-subunit proteins in cerebral vessels as determined by Western blot is similar between the two groups. Middle cerebral arteries (MCAs) isolated from FHH rats exhibited an impaired MR, and administration of IBTX restored this response. These results indicate that there is a gene on RNO1 that impairs MR in the MCAs of FHH rats by enhancing BK channel activity.
Keywords: cerebral blood flow, cerebral vascular disease, genes
autoregulation of cerebral blood flow (CBF) is a vital homeostatic mechanism that protects the brain from alterations in systemic pressure (37, 45). The myogenic response contributes to the autoregulation of CBF following elevations in arterial pressure. It is impaired following ischemic and hemorrhagic stroke or traumatic brain injury, and the autoregulatory range is shifted to higher pressures in hypertension (19, 20, 37, 45). The myogenic response is an intrinsic property of vascular smooth muscle cells (VSMCs) that initiates contraction of arterioles in response to elevations in transmural pressure (15). The subsequent rise in intracellular Ca2+ and membrane depolarization, however, activates a cluster of large conductance Ca2+-activated potassium (BK) channels to form spontaneous transient outward K+ currents (STOCs) (5, 25). Generation of these STOCs hyperpolarizes the membrane and limits calcium entry through voltage-gated calcium channels (VGCC), thereby attenuating the vasoconstrictor response (15). The interplay between STOCs, changes in the levels of intracellular Ca2+ (calcium sparks), and activation of the BK channel modulates the myogenic response (15). BK channels are composed of α- and β-subunits, in which the α-subunits form K+-selective pore, whereas β-subunits regulate calcium and voltage sensitivity of the channel (34). Changes in the expression of BK channel subunits have been previously suggested to play a critical role in altering the myogenic response in a number of physiological and pathological conditions (1, 9, 16, 21).
The Fawn Hooded Hypertensive (FHH) rat is a genetic model of renal disease that develops systolic hypertension and end-stage renal disease (28, 42). Previous studies from our laboratory have reported that the myogenic response of the renal afferent arteriole and autoregulation of renal blood flow (RBF) are impaired in FHH rats and substitution of a region of chromosome 1 (RNO1) from Brown Norway (BN) to FHH rats restores renal autoregulation and opposes the development of renal injury in FHH.1BN congenic strains (8, 32). More recently, we have reported that the impaired myogenic response in FHH rats is not restricted to the renal circulation, but the myogenic response of the middle cerebral artery (MCA) is also impaired (35). The mechanism of the impaired myogenic response in the cerebral circulation of FHH rats remains to be determined. Given the importance of K+ channels in modulating myogenic tone, the present study examined whether K+ channel activity is altered in cerebral arteries of FHH rats relative to that seen in a closely related FHH.1BN congenic strain in which the myogenic response and autoregulation of CBF is restored.
METHODS
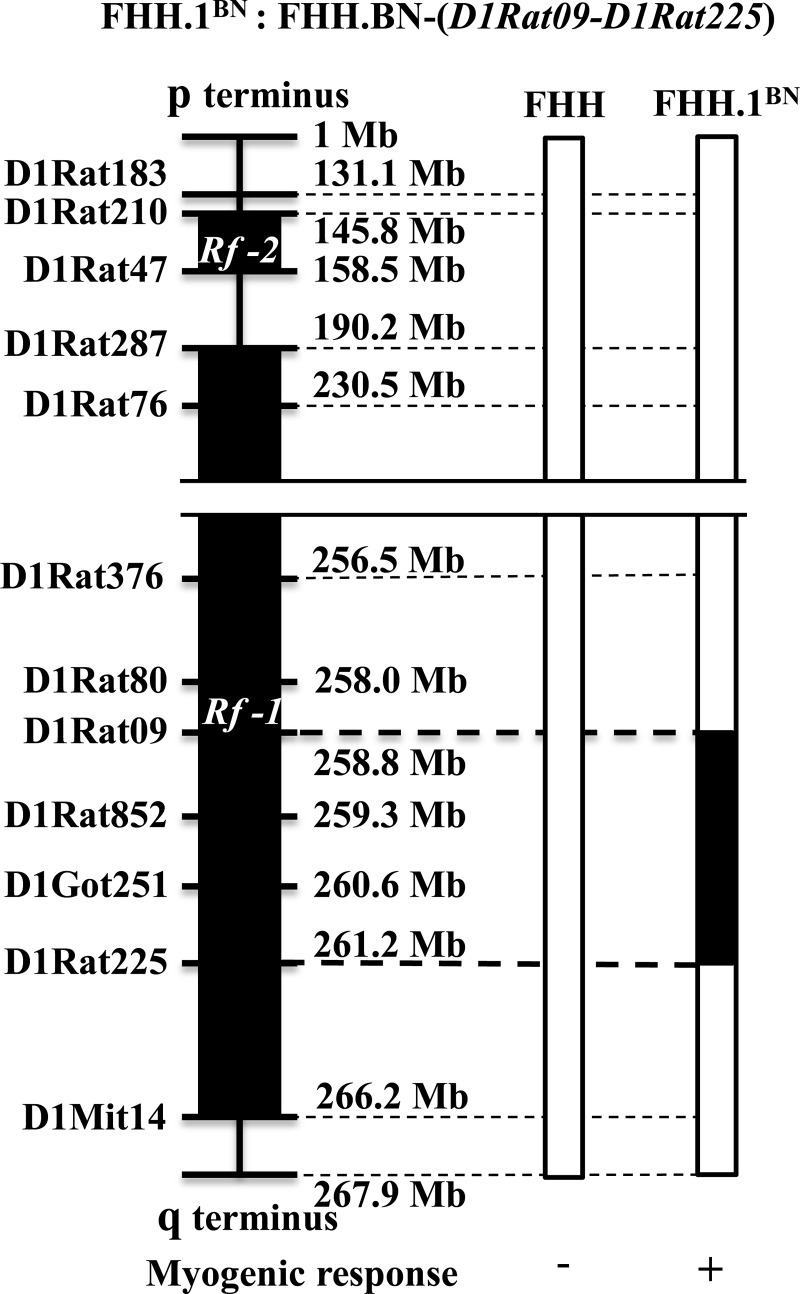
Experiments were performed on 72 9- to 12-wk-old male FHH rats and FHH.1BN congenic strain that were obtained from inbred colonies maintained at the University of Mississippi Medical Center (UMMC). Nine- to twelve-week-old Sprague Dawley (SD) rats were purchased from Charles River Laboratory (Wilmington, MA). A genetic map comparing the region of BN RNO1 introgressed into the FHH.1BN congenic strain is presented in Fig. 1. The congenic strain has a 2.4 megabase pair (Mbp) region of BN RNO1 between markers D1Rat09 and D1Rat225 introgressed into FHH rat genetic background. Transfer of this region containing 15 genes has been reported to restore autoregulation of renal and cerebral blood flow (8, 35). The rats were housed in the Animal Care Facility at UMMC, which is approved by the American Association for the Accreditation of Laboratory Animal Care. They had free access to food and water throughout the study. All protocols were approved by the Animal Care and Use Committee of UMMC. The rats were maintained on a rodent diet purchased from LabDiet (PMI Nutrition International, Brentwood, MO) containing 0.28% NaCl.
Fig. 1.
Genetic map of the introgressed region in FHH.1BN congenic rat. Genomic segments from the Fawn Hooded Hypertensive (FHH) and Brown Norway (BN) rat are presented as open and closed bars, respectively. Top: formal designation of the FHH.1BN congenic strain recommended by “Guidelines for Nomenclature of Mouse and Rat Strains” presented on the Rat Genome Database (RGD@mcw.edu). Left: some of the polymorphic genetic markers used to genotype the strain. Bottom: phenotype data for FHH rats and the FHH.1BN congenic strain. The ‘+’ and ‘−’ symbols refer to the presence and absence of the myogenic response in isolated middle cerebral arteries. The 2.4 Mbp region of BN chromosome 1 between D1Rat09 to D1Rat225 markers was introgressed into FHH rat genetic background. This region contains 15 genes.
Protocol 1: Patch-Clamp Experiments
Isolation of cerebral VSMCs.
The rats were euthanized using 4% isoflurane. The MCAs were microdissected and digested in a dissociation solution containing (in mM) 145 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 0.05 CaCl2, and 10 glucose (pH 7.4). The vessels were spun down at 1,000 rpm for 5 min and incubated in the dissociation solution containing papain (22.5 units/ml; Sigma, St. Louis, MO) and dithiothreitol (1 mg/ml) for 12 min at 37°C. The vessels were spun down again at 1,000 rpm for 5 min and resuspended in fresh dissociation solution containing collagenase (250 units/ml; Sigma), soybean trypsin inhibitor (10,000 units/ml), and elastase (2.4 units/ml). The vessels were incubated for 15 min at 37°C. Single cells were released from the vessels by gentle pipetting of the digested tissue. The VSMCs released into the media were collected by centrifugation, and the pellet was resuspended in fresh dissociation solution and maintained at 4°C until use in an experiment. All of the patch-clamp experiments were completed within 4 h after the isolation of the cells.
Whole cell patch-clamp experiments.
These experiments were performed on VSMCs freshly isolated from cerebral arterioles obtained from FHH and the FHH.1BN congenic strain. K+ channel currents and STOCs were recorded from VSMCs using a whole cell patch-clamp mode at room temperature. The bath solution contained (in mM) 130 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.2). The pipettes were filled with a solution containing 130 K gluconate, 30 KCl, 10 NaCl, 1 MgCl2, and 10 HEPES (pH 7.4). The concentrations of EGTA and Ca2+ in the pipette solution were adjusted to obtain cytosolic free Ca2+ concentrations of 100, 300, and 1,000 nM as determined using WinMAXC software written by C. Patton, Stanford University Pacific Grove, California (http://www.stanford.edu/∼cpatton/maxc.html). The pipettes were pulled from 1.5-mm borosilicate glass capillaries using a two-stage micropipette puller (model P-97; Sutter Instrument, San Rafael, CA) and heat-polished using a microforge. The pipettes had tip resistances of 2–8 MΩ. After the tip of a pipette was positioned on a cell, a 5–20 GΩ seal was formed and the membrane ruptured by gentle suction using a glass syringe. An Axopatch 200B amplifier (Axon Instruments, Foster City, CA) was used to clamp the pipette potential and to record whole cell currents. The amplifier output signal was filtered at 2 kHz using an eight-pole Bessel filter. The currents were acquired using p-CLAMP software (version 10; Axon Instruments) at a rate of 10 kHz and stored on the hard disk of a computer for offline analysis. Data analysis was performed using Clampfit software (version 10.0; Axon Instruments). Outward membrane K+ currents were elicited by a series of 20-mV voltage steps (from −60 to +120 mV) from a holding potential of −40 mV. Peak current amplitudes were determined from the average of 5–10 trials. Membrane capacitance, in pico farads (pF), was determined from the average of 30 currents measured in response to a 5-mV pulse. Peak currents (in pA) were expressed as current density (pA/pF) to normalize for differences in the size of the VSMCs. Iberiotoxin (IBTX; 100 nM) and 4-aminopyridine (4-AP; 1 mM)-sensitive K+ channel currents were dissected from the total current after administration of IBTX or IBTX + 4-AP to the bath solution. STOCs were determined by counting the number of events recorded over a 5-min interval greater than a current threshold set at 2.5 times the single BK channel current amplitude for a particular holding potential. The frequency and mean amplitude of the STOCs were determined offline using Clampfit (version 10.0; Axon Instruments) and Origin Pro 9 software (OriginLab, Northampton, MA).
Protocol 2: Inside-Out Single Channel Patch-Clamp Experiments.
Single BK channel currents were recorded from VSMCs using inside-out patch-clamp mode at room temperature. The bath solution contained (in mM) 145 KCl, 1.1 MgCl2, 10 HEPES, and 10 glucose (pH 7.2). The pipettes were filled with a solution containing (in mM) 145 KCl, 1.8 CaCl2, 1.1 MgCl2, and 5 HEPES (pH 7.4). The free cytosolic Ca2+ concentration in the bath solution was adjusted to 100 or 1,000 nM by varying the ratio of Ca2+ and EGTA as determined using WinMAXC software (C. Patton, Stanford University Pacific Grove, California, USA; http://www.stanford.edu/∼cpatton/maxc.html). The pipettes had a tip resistance of 8–10 MΩ. After the tip of a pipette was positioned on a cell, a gigaohm seal (5–20 GΩ) was formed by applying light suction and inside-out patch configuration was achieved by excising the membrane patch with a sudden upward movement of the pipette. An Axopatch 200B amplifier (Axon Instruments, Foster City, CA) was used to clamp pipette potential and record single-channel currents. Data acquisition and analysis were performed using p-CLAMP and Clampfit software (version 10; Axon Instruments). Open-state probability (NPo) for single-channel currents, expressed as a percentage of the total recording time in which a channel was open, was calculated using the equation NPo = (∑Tj X j)/T, where Tj is the sum of the open time at a given conductance level, j represents multiples of a given conductance, and T is the total recording time. Single channel current recordings were used to calculate NPo at membrane potentials between −60 and +80 mV at [Ca2+]i of 100 to 1,000 nM in the presence or absence of 100 nM IBTX.
Protocol 3: Determination of Single Channel Properties.
Single channel BK currents were recorded using the inside-out patch-clamp technique using a [Ca2+]i of 100, 300, and 1,000 nM, and mean single channel current amplitude was determined. Chord conductance was determined from the slope of the relationship between NPo and membrane patch potential. NPo/NPo max was calculated from 2- to 5-min recordings obtained at membrane potentials (20-mV steps) between −60 and +80 mV. NPo/NPo max values were fit to the Boltzmann function using the following equation: NPo = NPo max/[1 + exp (V − V1/2)/K], where N is the number of channels in the patch, Po is the single-channel open-state probability, and V1/2 is the voltage for half-maximal activation and K is the slope factor.
Protocol 4: Myogenic Response of Isolated MCAs.
These experiments were performed on MCAs isolated from 9- to 12-wk-old FHH and FHH.1BN rats that were euthanized with 4% isoflurane. These vessels were distinct from those used to isolate the VSMCs for the patch-clamp experiments. The brain was removed and placed in ice-cold physiologic salt solution (PSS) containing (in mM) 145 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 0.05 CaCl2, and 10 glucose (pH 7.4). The inner diameters of these vessels ranged from 100 to 140 μm. The vessels were mounted on glass micropipettes, pressurized to 40 mmHg at 37°C, and bathed in a physiological salt solution (PSS) containing (in mM) 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.8 CaCl2, 18 NaHCO3, 5 HEPES, 1.18 NaH2PO4, and 10 glucose (pH 7.4). Calcium-free PSS was prepared by replacing CaCl2 with an equimolar concentration of MgCl2 and the addition of 2 mM EGTA. The inflow pipette was connected to a reservoir to allow for control of intraluminal pressure that was monitored with a transducer (Cobe, Lakewood, CO). The cannulated MCAs were visualized using a videomicroscopy system consisting of a charge-coupled device camera and a stereomicroscope (model DRC; Zeiss, Oberkochen, Germany). The inner diameter of the vessels was measured using a videomicrometer (VIA-100; Boeckeler Instruments, Tucson, AZ). Magnification on the screen was ∼180×, and the measurement system was calibrated with a micrometer scale to a diameter within ± 2.0 μm. The bath solution was equilibrated with O2 (95%) and CO2 (5%) to provide adequate oxygenation and to maintain pH at 7.4. After mounting, the vessels were allowed to equilibrate for 60 min. They were then preconditioned three times by raising the intraluminal pressure from 40 to 140 mmHg for 5 min at 10-min intervals. After preconditioning, the inner diameter of the vessels was measured at intraluminal pressures from 40 to 140 mmHg in steps of 20 mmHg in the presence and absence of 100 nM IBTX. After the pressure-diameter relationships were determined, the bath solution was replaced with Ca2+-free PSS and the passive pressure-diameter relationship was determined. The percent myogenic tone was calculated from the difference in diameter measured in Ca2+-Free PSS and PSS divided by the diameter measured in Ca2+-free PSS times 100.
Protocol 5: Comparison of the Expression of BK Channel Protein in FHH and FHH.1BN Rats
FHH and FHH.1BN rats (FHH, 233 ± 55 g; FHH.1BN, 287 ± 43 g; n = 8 each) were euthanized with isoflurane and the MCAs from the brain were harvested and pooled together in ice-cold phosphate buffered saline (in mM) 100 NaH2PO4, 150 NaCl (pH 7.2). The vessels were spun down (500 g, 4°C, 2 min) and resuspended in 1 ml of a RIPA buffer containing (in mM) 25 Tris·HCl (pH 7.6), 150 NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS. The vessels were homogenized using a ground glass homogenizer, centrifuged at 10,000 g for 2 min, and the supernatant was collected.
The protein concentrations of the samples were determined using the Bradford protein assay (Bio-Rad). Aliquots of the samples (40 μg) were dissolved in a Laemmli Sample buffer containing 60 mM Tris·HCl, 2% SDS, 20% glycerol, 5% β-mercaptoethanol, and 0.01% bromophenol blue (pH 6.8), and the proteins were separated on a 4–15% Mini-PROTEAN TGX Gel (Bio-Rad). After transfer, the membrane was blocked with TBS-T buffer containing 20 mM Tris pH 7.5, 150 mM NaCl, 0.05% Tween, and a 5% blocking powder (Bio-Rad) at 4°C for 1 h. The blot was probed with primary antibody against BK α- and β-subunit [1:500 and 1:200, respectively; polyclonal rabbit Anti-KCa1.1, to amino acids 1184–1200 and Anti-sloβ1 (KCNMB1); Alomone Labs, Jerusalem, Israel] overnight at 4°C. After the membrane was washed, it was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:5,000; Santa Cruz Biotechnology) at 4°C for 1 h. The blot was subsequently probed with β-actin primary antibody (1:5,000; monoclonal mouse; Alomone Labs) for 2 h, followed by HRP-conjugated goat anti-mouse secondary antibody (1:10,000; Santa Cruz Biotechnology) at 4°C for 1 h. Specific binding was visualized by chemiluminescence (Immun-Star WesternC Kit; Bio-Rad) using a ChemiDoc XRS imager (Bio-Rad Life Science Research, Hercules, CA). The intensities of the bands corresponding to each protein were quantified using ImageLab software (Bio-Rad). The relative intensity for each band was normalized to the intensity of the β-actin staining.
Statistical Analysis
Mean values ± SE are presented. The significance of differences in mean values between groups was determined by paired or unpaired t-test or by a repeated-measure ANOVA followed by a Holm-Sidak test for preplanned comparisons.
RESULTS
FHH Rats Exhibit Increased Whole Cell Outward Currents Compared With FHH.1BN Rats
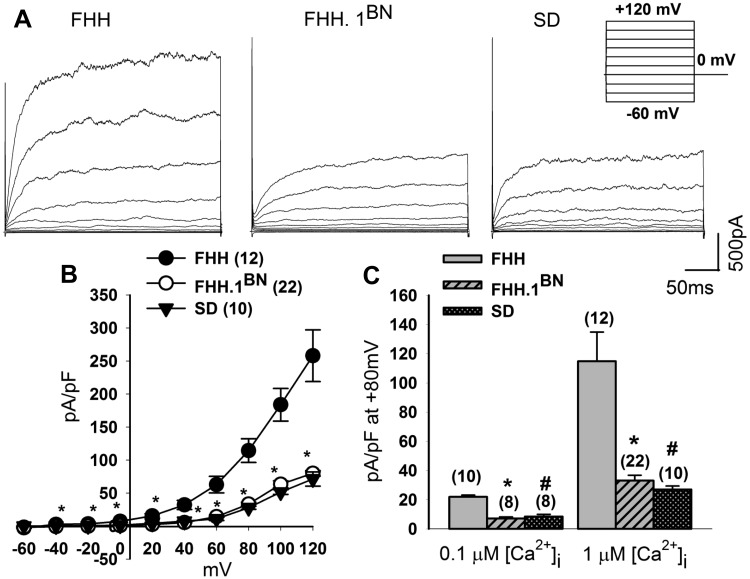
A representative tracing of whole cell outward currents recorded from VSMCs isolated from the MCAs of FHH, FHH.1BN, and SD rats are presented in Fig. 2A. Outward currents were elicited by a series of voltage steps (from −60 to +120 mV) from a holding potential of −40 mV (Fig. 2A, inset protocol). The cell capacitance was not significantly different between the groups and averaged ∼14 pF. Outward currents were 6.2-fold higher in VSMCs isolated from FHH rats than in those isolated from the FHH.1BN congenic strain or SD rats at a cytosolic calcium concentration of 1 μM (Fig. 2B). As summarized in Fig. 2C, peak current densities recorded from VSMCs at +80 mV were significantly higher in FHH rats as compared with FHH.1BN and SD rats at either 0.1 or 1 μM free cytosolic calcium concentrations.
Fig. 2.
Comparison of outward currents in FHH, FHH.1BN, and Sprague Dawley (SD) rats. Whole-cell outward currents were recorded from freshly dissociated cerebral vascular smooth muscle cells (VSMCs) of FHH, FHH.1BN, and SD rats during 300-ms voltage steps from −60 to +120 mV in 20-mV increments from a holding potential of −40 mV at 1 μM free cytosolic calcium [Ca2+]i (inset protocol). A: representative whole-cell outward current recordings from FHH, FHH.1BN, and SD rats. B: current-voltage curves that indicate that the peak current density (pA/pF) is greater in FHH rats (12 cells) than FHH.1BN congenic strain (22 cells) or SD rats (10 cells). *Significant difference P < 0.05 compared with corresponding value in FHH.1BN and SD rats. C: peak current density at +80 mV at 0.1 and 1 μM free cytosolic calcium in all the 3 groups. *#Significant difference in the current density in FHH.1BN and SD rats compared with the values measured in FHH rats. Values are means ± SE.
BK, but not Voltage-Gated K± Channel (Kv) Current, is Increased in FHH Rats as Compared With FHH.1BN Rats
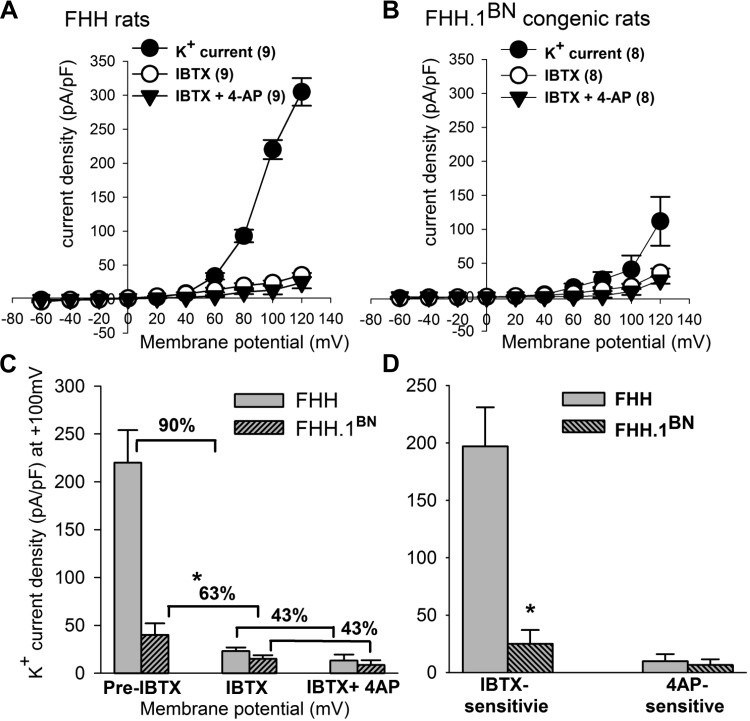
To determine the type of K+ channel contributing to the elevated whole cell outward K+ channel current in VSMCs isolated from FHH rats, we first examined the effects of the BK channel specific antagonist iberiotoxin (IBTX; 100 nM). To further determine the potential contribution of voltage-gated K+ channels (Kv) to the elevated current, we also studied the effects of the Kv channel inhibitor 4-aminopyridine (4-AP; 1 mM) along with IBTX. The total K+ channel current densities were plotted over a range of membrane potentials before and after application of these K+ channel inhibitors (Fig. 3). Administration of IBTX alone reduced outward K+ current by 90% in FHH rats, whereas it only reduced K+ current by 63% in the FHH.1BN congenic strain (Fig. 3) (FHH at +100 mV; pre-IBTX, 220 ± 34, after IBTX, 23 ± 3.9 pA/pF, n = 9; P < 0.05) (FHH.1BN; +40 mV; pre-IBTX 40 ± 12 pA/pF and after IBTX 15 ± 3.7 pA/pF; n = 8). Subsequent addition of 4-AP in the presence of IBTX reduced the residual peak outward K+ current by an additional 43%, and the magnitude of this response was similar in the VSMCs isolated from FHH rats and the FHH.1BN congenic strain (Fig. 3C and D). Addition of 4-AP alone decreased K+ channel currents by a similar amount in FHH and FHH.1BN rats (data not shown).
Fig. 3.
Comparison of outward K ± channel currents before and after administration of iberiotoxin (IBTX) and 4-aminopyridine (4-AP) in VSMCs isolated from cerebral arteries of FHH and FHH.1BN rats. Whole-cell K+ currents were recorded from freshly dissociated cerebral VSMCs from FHH and FHH.1BN rats at 1 μM free cytosolic calcium [Ca2+]i in the presence and absence of IBTX and IBTX + 4-AP. Current-voltage curves presenting peak K+ channel current density (pA/pF) in FHH rats (n = 9) and FHH.1BN rats (n = 8) are presented in A and B. C and D: summary graphs of the absolute, IBTX, and 4-AP-sensitive outward K+ channel current densities (pA/pF) in FHH and FHH.1BN rats at +100 mV. Note IBTX alone decreased a greater percentage of K+ current densities in FHH rats as compared with FHH.1BN congenic strain, whereas IBTX + 4-AP application decreased similar percentage of K+ current densities in FHH and FHH.1BN rats. *Significant difference in the corresponding values measured in FHH rats. Mean values ± SE are presented.
FHH Rats Exhibit Increased Outward K± Channel Open State Probability
The increased outward K+ current in FHH rats could result from an increase in the single channel activity and/or an increase in the expression of the number of channels in the membrane patch. To address these possibilities, we performed inside-out patch-clamp experiments to record single-channel K+ currents in cerebral VSMCs isolated from MCAs of FHH and FHH.1BN rats. Currents were recorded using symmetrical 145 mM K+ solutions at different [Ca2+]i in the bath and exhibited the expected reversal potentials of 0 mV in cells isolated from both FHH and the FHH.1BN congenic strain. Unitary current amplitudes for the BK single channel currents recorded between −60 and +80 mV pipette potential were similar in FHH and FHH.1BN cerebral VSMCs (data not shown). The single channel slope conductance averaged 208.9 ± 0.04 pS (n = 4) in FHH rats and 208.7 ± 0.007 pS (n = 4) in the FHH.1BN rats. A comparison of tracings of single K+ channel currents recorded from VSMCs isolated from the MCAs of FHH rats and the FHH. 1BN congenic strain at a membrane potential of +40 mV with 1 μM [Ca2+]i in the bath is presented in Fig. 4A. The number of channel openings was markedly greater and observed many multiple stacked openings in the patches obtained from VSMCs isolated from FHH rats than in those obtained from the FHH.1BN congenic strain. A current-voltage relationship for the outward K ± channel open state probability (NPo) in VSMCs isolated from FHH rats and the FHH.1BN congenic strain is presented in Fig. 4B. The NPo of BK channels increased with membrane depolarization in VSMCs isolated from both FHH and FHH.1BN rats. However, FHH rats exhibited a significantly higher NPo as compared with that seen in FHH.1BN rats in the presence of a normal (0.1 μM) or high (1 μM) calcium concentration in the bath (Fig. 4, B–D).
Fig. 4.
Comparison of large conductance calcium-activated potassium (BK) single-channel currents in FHH and FHH.1BN rats. BK single-channel currents were recorded from excised inside-out patches of VSMCs obtained from FHH rats and the FHH.1BN congenic strain. A: representative BK single-channel currents recorded from FHH and FHH.1BN rats at a +40 mV membrane potential and with 1 μM free calcium in bath solution. B: open-state probability (NPo) plotted as a function of membrane voltage (mV). The NPo is higher in VSMCs isolated from FHH rats as compared with the corresponding values obtained from FHH.1BN rats at 0.1 and 1 μM [Ca2+]i (FHH: n = 4 patches per data point; -at 0.1 and 1 μM free calcium; FHH.1BN: n = 3–5 patches per data point; -at 0.1 and 1 μM free calcium). C and D: a summary graph of NPo at −40 and +20 mV membrane potential in FHH and FHH.1BN rats at [Ca2+]i of 0.1 and 1 μM. Mean values ± SE are presented. *Significant difference from the corresponding value in FHH rat.
FHH Rats Exhibit Increased BK Channel NPo as Compared With FHH.1BN Rats
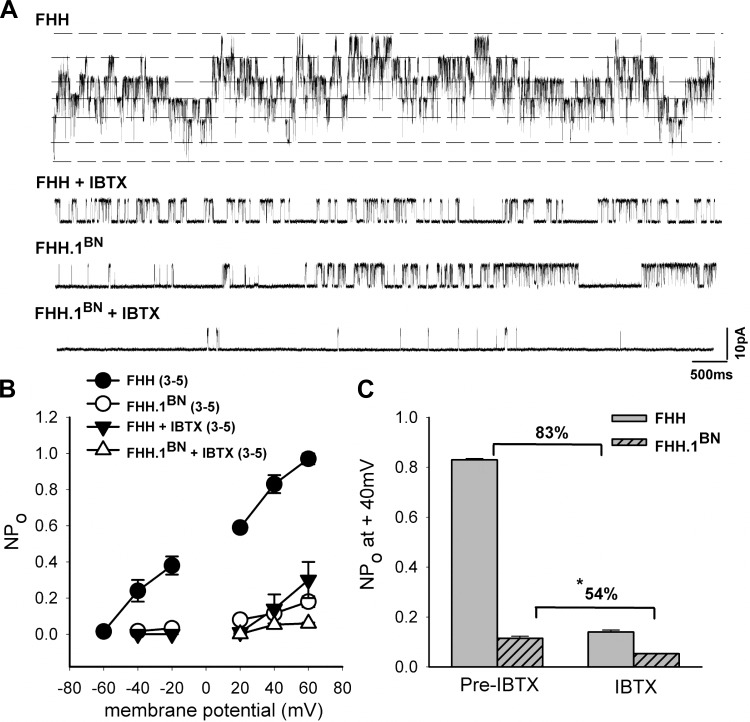
To examine the contribution of BK channel currents in altering the K+ channel function in FHH rats, we also studied the effects of the selective BK channel blocker IBTX (100 nM) on BK channel activity in FHH rats and the FHH.1BN congenic strain using inside-out patch-clamp method. Representative BK channel traces before and after administration of IBTX recorded from VSMCs isolated from FHH rats and FHH.1BN congenic strain are presented in Fig. 5A. BK channel activity was greater in inside-out patches of VSMCs isolated from FHH rats compared with those from FHH.1BN rats, and they exhibited multiple stacked openings. IBTX reduced K+ channel activity to a greater extent in FHH rats than in FHH.1BN rats. A comparison of inhibitory effects of IBTX on the NPo of the BK channel is presented in Fig. 5C. These results indicate that there is a 83% decrease in NPo after IBTX application in cells isolated from FHH rats as compared with a 54% fall in the NPo seen in cells isolated from FHH.1BN rats at +40 mV membrane potential. The unitary amplitudes of the single BK channel currents were similar in cerebral VSMCs isolated from FHH rats and the FHH.1BN congenic strain before and after IBTX application (data not shown).
Fig. 5.
Comparison of BK single-channel function in FHH and FHH.1BN rats before and after administration of the BK channel antagonist IBTX. BK single-channel currents recorded from inside-out patch-clamp of VSMCs obtained from FHH and FHH.1BN congenic strain are shown. A: representative BK single-channel currents recorded from FHH and FHH.1BN rats at +40 mV membrane potential and at 1 μM free calcium concentration in the bath in the presence and absence of IBTX. In the presence of IBTX, the NPo plotted as a function of membrane voltage (mV), presented in B, is significantly lower in FHH rats compared with FHH.1BN rats (n = 3–5 patches per data point). C: a summary graph that indicates that BK channel NPo is reduced by 83% in FHH rats after administration of IBTX compared with a 54% fall in NPo in FHH.1BN rats at +40 mV membrane potential. Mean values ± SE are presented. *Significant difference from the corresponding value in FHH rats.
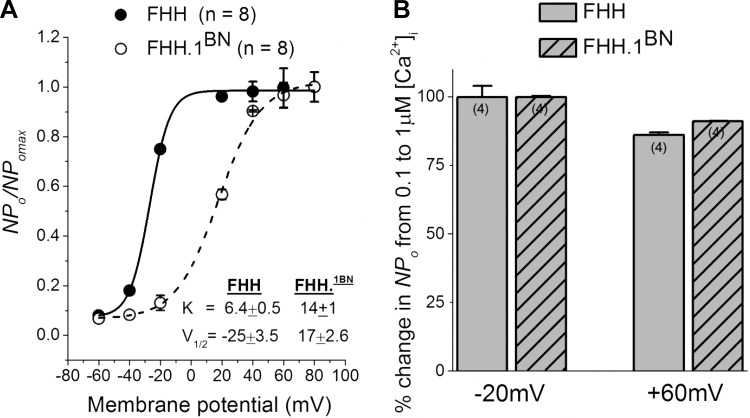
FHH Rats Exhibit Increased BK Channel Voltage Sensitivity Compared With FHH.1BN Rats
The observed increase in BK channel activity in FHH rats could be due to an increase in Ca2+ or voltage sensitivity of the channel. To address this issue, the NPo was normalized to the maximal NPo obtained from each patch and plotted as a function of membrane potential. The data were then fit to the Boltzmann equation (Fig. 6A). The steepness of the curve is greater in VSMCs isolated from FHH rats (slope factor; 6.4 ± 0.5) than in those isolated from the FHH.1BN congenic strain (slope factor; 14 ± 1; P < 0.05). The, half-maximal activation (V1/2) is shifted to hyperpolarized potentials in FHH rats (−25 ± 3.5 mV; n = 8) as compared with those observed in the FHH.1BN congenic strain (+17 ± 2.6 mV; n = 8). To test whether the Ca2+ sensitivity is altered, we calculated percent change in NPo when [Ca2+]i was increased from 0.1 to 1 μM at a membrane potentials of −20 and +60 mV. The percent increase in NPo in both strains was not significantly different when [Ca2+]i is increased from 0.1 to 1 μM in FHH rats at −20 and +60 mV (99.9% and 86%, respectively) or in the FHH.1BN congenic strain (99.9% and 91%, respectively) (Fig. 6B).
Fig. 6.
Comparison of the voltage and Ca2 ± sensitivity of BK single-channel currents in excised inside-out patches of cerebral VSMCs isolated from FHH and FHH.1BN rats. A: normalized open probability (NPo/NPo max) values plotted as a function of membrane potential and fitted to the Boltzmann equation NPo/NP o max = 1/1 + exp[(V½ − Vm)/K], where V½ is the membrane potential required for half-maximal activation of the channels and K is the slope factor representing voltage sensitivity at 1 μM free [Ca2+]i concentration. Each curve includes the NPo/NPo max values obtained from inside-out patches at 20-mV intervals between −40 and +80 mV. The solid and dotted lines represent the best curve fits of Boltzmann equation of data collected from FHH and FHH.1BN rats. The K and V½ values are presented in the figure inset. Note that FHH rats exhibit a steep curve (lower K value) compared with FHH.1BN rats. The V½ value is shifted to the left in FHH rats compared with FHH.1BN rats. B: bar graphs of the percent change in NPo of BK channel when [Ca2+]i was increased from 0.1 to 1 μM at −20 and +60 mV membrane potential in FHH (n = 4 cells) and FHH.1BN rats (n = 4 cells). Note that the percent NPo did not change in either strain with increase in [Ca2+]i.
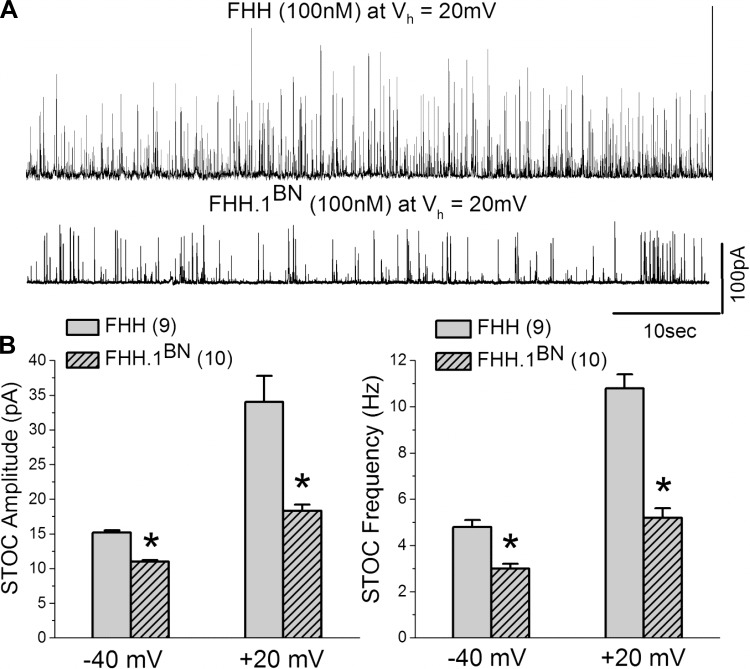
FHH Rats Exhibit Elevated Frequency and Amplitude of STOCs
Because BK channels are activated by calcium sparks to generate STOCs (5, 25), experiments were performed to compare the frequency and amplitude of STOCs recorded from VSMCs isolated from the MCAs of FHH and FHH.1BN rats. The results of these experiments are summarized in Fig. 7. The mean amplitude and frequency of the STOCs recorded from VSMCs isolated from FHH rats are significantly greater than those observed in the FHH.1BN congenic strain at membrane potentials of −40 and +20 mV (n = 9 and 10 cells, respectively). The differences in the characteristics of STOCs were not a function of differences in cell size as the cell capacitance was not significantly different and averaged ∼11.5 ± 0.8 pF in both the FHH rats and the FHH.1BN congenic strain.
Fig. 7.
Comparison of amplitude and frequency of spontaneous transient outward currents (STOCs) in cerebral VSMCs isolated from FHH and FHH.1BN rats. A: representative traces of STOCs recorded from FHH and FHH.1BN rat VSMCs held at +20 mV. B: comparison of the amplitude and frequency of STOCs at −40 and +20 mV membrane potentials in VSMCs isolated from FHH rats (n = 9) and FHH.1BN congenic rats (n = 10). *Significant difference from the corresponding value presented in FHH rats. Vh indicates holding voltage.
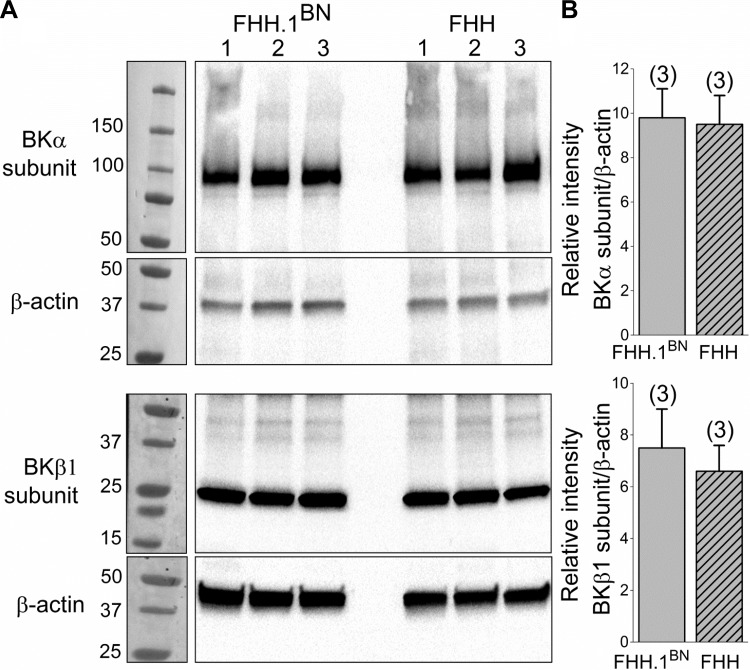
Cerebral Vessels Isolated from FHH and FHH.1BN Rats Have Similar Expression of BK-α and BK-β-subunit Proteins
A comparison of the expression of α- and β-subunits of the BK channel in MCAs isolated from FHH and FHH.1BN rats are presented in Fig. 8. The BKα and β-subunits were detected using Anti-KCa1.1 (amino acids 1184–1200) and anti-sloβ1 (KCNMB1) antibodies at the expected size of ∼100 and 25 KD in MCAs isolated from both FHH and FHH.1BN rats (n = 8 rats per strain). These results presented in Figure 8B indicate that the expression of the BKα and β-subunits is similar in the MCAs of FHH rats and the FHH.1BN congenic strain.
Fig. 8.
Comparison of BK-α- and BK-β-subunit expression in cerebral vessel homogenates isolated from FHH and FHH.1BN rats. BK-α- and BK-β-subunits were probed on different blots. The same blots were then reprobed for β-actin. A: representative bands corresponding to the molecular weight of the BK-α-subunit (∼100 kDa), BK-β-subunit (∼25 kDa), and β-actin (∼42 kDa) in cerebral vessels obtained from FHH and FHH.1BN rats (n = 8 animals each; 3 sets of experiments). B: a comparison of the expression of α- and β-subunits. Numbers in parentheses indicate the number of samples studied.
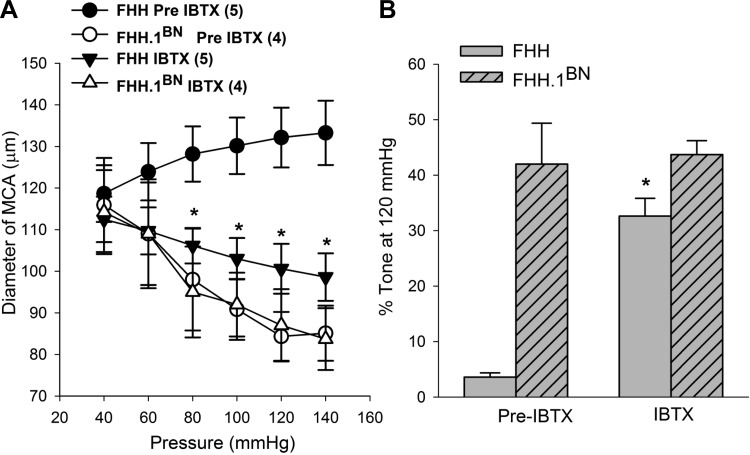
MCAs Isolated from FHH Rats Exhibit Restored Myogenic Response After Application of IBTX
Experiments were also performed to determine the contribution of elevated BK channel activity to the impaired myogenic response of the MCAs of FHH rats. The results of these experiments are presented in Fig. 9. MCAs isolated from FHH rats did not exhibit a myogenic response, and the diameter of these vessels increased significantly when transmural pressure was increased from 40 to 140 mmHg. In contrast, the diameter of MCAs isolated from the FHH.1BN congenic strain decreased significantly when transmural pressure was elevated over the same range. Blockade of BK channels with IBTX (100 nM) restored the myogenic response of the MCAs isolated from FHH rats but it had no significant effect on the response of the MCAs isolated from the FHH.1BN congenic strain. The percent active tone calculated at a transmural pressure of 120 mmHg in MCAs is presented in Fig. 9B. The degree of active tone in the MCAs was markedly reduced in FHH rats relative to the levels seen in the FHH.1BN congenic strain. Addition of IBTX restored the generation of active tone in the MCAs of FHH rats but it had no significant effect on the tone in FHH.1BN rats.
Fig. 9.
Comparison of the myogenic response in middle cerebral arteries (MCAs) of FHH and FHH.1BN rats before and after application of the BK channel antagonist IBTX. A: pressure-diameter relationships that were obtained from MCAs isolated from FHH and FHH.1BN congenic rat strain in the presence and absence of calcium and before and after addition of IBTX to the bath. MCAs isolated from FHH rats exhibit an impaired myogenic response relative to FHH.1BN congenic rats, and the response was restored after administration of IBTX. In contrast, IBTX did not alter the myogenic response of MCAs isolated from FHH.1BN congenic strain. B: summary graph presenting the percent active tone generated in the MCAs at 120 mmHg before and after IBTX application in MCAs isolated from FHH and FHH.1BN rats. Mean values ± SE are presented. *Significant difference from the corresponding values presented in FHH rats before administration of IBTX.
DISCUSSION
Previous studies have indicated that the myogenic response in cerebral arteries is impaired in FHH rats but the mechanism involved is unknown (35). The present study compared BK channel function in VSMCs isolated from the MCAs of FHH rats and a closely related FHH.1BN congenic strain in which autoregulation of CBF is restored. The results indicate that K+ channel activity is markedly elevated in VSMCs isolated from the MCAs of the FHH rats relative to that seen in FHH.1BN congenic strain. Moreover, this was associated with an elevation in IBTX-sensitive BK channel current and increased frequency and amplitude of STOCs. The increase in BK channel current in FHH rats is due to a marked increase in the open probability of the BK channel, which is partially due to an increase in the voltage sensitivity of this channel. Inhibition of BK channel activity with IBTX restored the myogenic response of the MCAs of FHH rats but it had no significant effect on the myogenic response in the MCAs of FHH.1BN rats. These results indicate that there is a gene or genes in the 2.4 Mbp introgressed region of RNO1 that can restore the myogenic response of the MCA by affecting BK channel function in FHH rats.
Role of BK Channel in the Vascular Myogenic Response of FHH Rats
The low open probability of the BK channel in VSMCs isolated from MCA of FHH.1BN rats studied at a resting membrane potential of −40 mV and with a physiological level of cytosolic calcium is consistent with previous reports that the BK channel has little effect on the resting membrane potential under basal conditions (33). Indeed, administration of IBTX had little effect on the myogenic response or the generation of active vascular tone in MCAs isolated from the FHH.1BN congenic strain. However, when BK channel activity is increased by elevations in [Ca2+]i in response to elevations in transmural pressure or vasoconstrictor agonists, the BK channel has a large influence on the membrane potential due to its large single channel conductance (∼200 pS). As such, administration of the BK channel blocker IBTX markedly reduced the elevated BK channel activity in FHH rats and restored the myogenic response of the MCA of FHH rats (Fig. 9). However, it had very little effect on the diameter of the MCAs of FHH.1BN rats. This later finding is not consistent with the results of previous studies indicating that the BK channel contributes to the maintenance of basal vascular tone shown in MCAs isolated from dogs and SD rats (2, 23). However, it is consistent with the results of other studies suggesting that blockade of the BK channel has little effect on basal vascular tone in renal, cremaster, or cerebral arterioles (24, 33, 36). Although the mechanism underlying the differences in response remains to be determined, it is likely that there are strain differences in the surface expression and activity of the BK channel, especially in vessels obtained from different vascular beds.
Previous studies have indicated that activation of BK channels in VSMCs by calcium sparks generates STOCs that play an important role in buffering vasoconstriction (5, 25). The present study indicates that amplitude and frequency of STOCs is markedly elevated in VSMCs isolated from FHH rats as compared with that seen in the FHH.1BN congenic strain (Fig. 7). These results are consistent with the increase in BK channel activity seen in the VSMCs isolated from FHH rats. The increase in STOCs in FHH rats would be expected to hyperpolarize the membrane potential and oppose opening of VGCC. The calcium that is released from SR via RyR's typically generates calcium sparks. Refilling of SR calcium stores is dependent in part on Ca2+ influx through VGCC (47). Thus these data suggest that other nonvoltage gated Ca2+ pathways in VSMCs isolated from FHH rats can maintain elevated STOC activity even with diminished VGCC activity.
The results of the present study indicate that an elevation in BK channel activity largely accounts for the elevated K+ channel current in FHH rats, since there was a significant difference in K+ channel current after administration of IBTX in VSMCs isolated from FHH rats compared with FHH.1BN rats. Moreover, 4-AP has a similar effect to reduce K+ channel current in both FHH and FHH.1BN rats (Fig. 3). On the other hand, IBTX did not restore the magnitude of the myogenic response in FHH to the same level as seen in FHH.1BN rats (Fig. 9). This finding suggests that although elevated BK channel activity is largely responsible for the impaired myogenic response in FHH rats, changes in the expression or activity of other ion-channels may also be involved. In this regard, previous studies have indicated that inward-rectifying K+ channels (Kir) and glibenclamide-sensitive K+ channels contribute to the regulation of membrane potential and vascular tone in a variety of vessels (12, 46). Other studies have suggested that the initiation of the myogenic response involves activation of stretch-activated cation channels, possibly TRPM4 and TRPC6 channels, via a PKC-dependent pathway (6, 17, 18). Thus further studies are needed to delineate the potential additional role of TRP, Kir, or KATP channels in the impaired myogenic response in the FHH rats.
Another question raised from the results of the present study is whether change in the gating properties of BK channels in FHH rats is due to changes in the subunit expression. BK channels are formed by the α- and β1-subunits. The four α-subunits form the basic channel pore, which in the absence of accessory β-subunits still acts as a voltage- and Ca2+-dependent K+ channel, the β1-subunit confers additional voltage and Ca2+ sensitivity (14). We noticed 10-fold increase (+40 mV) in single BK channel function in FHH rats, which was associated with an increase in the voltage sensitivity of the channel (Fig. 6A). The increase in the voltage sensitivity of BK channel could have been secondary to changes in the expression of the α- or β1-subunits. However, the results of the present study indicate that the expression of the α- or β-subunits of the BK channel is not significantly different at the whole tissue level in the cerebral vessels isolated from FHH rats relative to the FHH.1BN congenic strain (Fig. 8). However, this finding does not exclude the possibility that the gene or pathway involved might alter the trafficking of the BK channel.
Mechanisms Involved in Enhanced BK Channel Function in FHH Rats
The precise mechanism underlying the observed increase in BK channel function in cerebral vasculature of the FHH rat remains to be determined. An increase in BK channel current density could be due to increase in the surface expression of BK channel protein. Liu et al. (30, 31) have shown that surface expression of BK was increased in pial cerebral arteries and aorta from SHR rats compared with those from WKY rats, but the activation parameters of voltage and calcium sensitivity of BK were not different between the two strains. A second possibility is that the increase in the current density could be due to a decrease in the phosphorylation of the BK α-subunit already resident in the surface membrane by PKC or enhanced dephosphorylation by PP1 (22, 51). Both these proteins are intimately associated with BK-α (51). PKC phosphorylation of BK-α causes reversible silencing (unconditional inhibition) and decreased activation of the BK channel at all membrane voltages and calcium concentrations. Thus a decrease in the phosphorylation of the channel via a PKC-dependent pathway could potentially increase BK channel activity as well as permitting PKA/PKG protein kinase to phosphorylate and enhance activation of the channel (22, 51). A third possibility is that an increase in the voltage sensitivity of BK channel in VSMCs isolated from the MCAs of FHH rats versus the FHH.1BN congenic strain is consistent with enhanced phosphorylation of the BK-α-subunits by PKA/PKG in FHH (22, 29, 36, 39, 51) or increased ratio of the expression of the BK β1-subunit relative to the BK α-subunit (43, 48–50). Both PKA/PKG phosphorylation of the BK α-subunit and increased ratio of BK β1:α shift the voltage activation curve of the BK channel to the left and this would be expected to diminish the myogenic response (7, 38, 48, 49). Fourth, disruption of actin cytoskeleton and its components could alter membrane trafficking of ion-channels and other signal transduction proteins (10, 26). Likewise, BK channels could be trafficked to the membrane through its actin-binding domain that connects to actin cytoskeleton (52) and thus any changes in the actin cytoskeletal network might alter the number of channels expressed in the membrane.
The fact that FHH rats and the congenic FHH.1BN strain differ genetically by only a 2.4 Mbp consisting of 15 genes on RNO1 suggests that the difference in the myogenic response and BK channel activity must be due to a difference in the expression or activity of one or more of the genes in this region. The genes encoding for BK-α-subunit (MCNMa1) and BK-β (MCNMb1) reside on RNO15 and 10 and are not found in this region. As discussed by Burke et al. and Pabbidi et al. (8, 35), two genes in this region, Mxi1 (decreased) and Rbm20 (increased), are differentially expressed in the renal vasculature of FHH rats relative to the FHH.1BN congenic strain. However, these genes do not have any known influence on BK channel function or vascular tone. Three other genes in the region, Add3, Shoc2, and Rbm20, have sequence variants in the coding region in FHH rats that are predicted to alter amino acids structure of their respective proteins. Of these genes, Shoc2 is a positive regulator of the ERK pathway by acting as a scaffolding protein (27, 41), and the adducin family of gene products interact with F-actin/spectrin cytoskeleton (3). One could speculate that changes in the function of these genes might alter membrane trafficking or the activity of the BK channel by an unknown mechanism. However, it remains to be determined whether the sequence variants found in these genes actually alter the function of these genes or have any impact on BK channel function or vascular tone in FHH rats.
Perspectives
Hypertension is the major risk factor of stroke and it is not adequately controlled in more than 40% of the patients (13, 44). The causative factors and the altered signaling pathways that lead to stroke in these patients are not known. The present study provides evidence that impaired myogenic response in FHH rats is due to elevated BK channel function and increased STOCs. Our results also suggest a possible presence of altered gene(s) that lies in the 2.4 Mbp region between markers D1Rat09 and D1Rat225 in FHH rats that enhances BK channel function and contributes to the impaired myogenic response in FHH rats. We have previously reported (35) that loss of the myogenic response increases the magnitude and duration of hyperemia and increases infarct size following transient occlusion of the MCA. It should also increase transmission of pressure to the pial vessels and capillaries in the brain leading to vascular leakage following increases in arterial pressure. Long-term increase in transmission of pressure should cause vascular rarefaction and remodeling of the small vessels and increase the susceptibility to ischemic stroke and perhaps contribute to the vascular cognitive impairment in hypertension. Because BK channel activity is regulated by cGMP, PKG, and PKC, which are the most common downstream mediators of vasoactive stimuli (4, 11, 40), FHH rats would also be predicted to exhibit altered cerebrovascular responses to various vasoactive stimuli that are important in the metabolic regulation of CBF and functional hyperemia.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants PO1 HL-059996, RO1 HL-105997, R01 HL-092105, and HL-036279; a VA Research Career Scientist Award (to D. R. Harder); an Intramural support research program from UMMC; amd American Heart Association Scientist Development Grant 13SDG14000006 (awarded to M. R. Pabbidi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R.P., J.M.F., and R.J.R. conception and design of research; M.R.P. and O.L.M. performed experiments; M.R.P. analyzed data; M.R.P. and R.J.R. interpreted results of experiments; M.R.P. prepared figures; M.R.P., D.G., and R.J.R. drafted manuscript; M.R.P., J.M.F., D.G., D.R.H., and R.J.R. edited and revised manuscript; M.R.P., O.L.M., F.F., J.M.F., D.G., D.R.H., and R.J.R. approved final version of manuscript.
REFERENCES
- 1.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res 93: 965–971, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Asano M, Masuzawa-Ito K, Matsuda T, Suzuki Y, Oyama H, Shibuya M, Sugita K. Functional role of charybdotoxin-sensitive K+ channels in the resting state of cerebral, coronary and mesenteric arteries of the dog. J Pharmacol Exp Ther 267: 1277–1285, 1993 [PubMed] [Google Scholar]
- 3.Baines AJ. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma 244: 99–131, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 286: L1275–L1281, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol 381: 385–406, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol 35: 1116–1120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, Sarkis A, Lazar J, Jacob HJ, Roman RJ. Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304: F565–F577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 290: H1520–H1527, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Butterworth MB, Frizzell RA, Johnson JP, Peters KW, Edinger RS. PKA-dependent ENaC trafficking requires the SNARE-binding protein complexin. Am J Physiol Renal Physiol 289: F969–F977, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol Heart Circ Physiol 273: H76–H84, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K+ currents and Kir21 expression in renal afferent and efferent arterioles. Clin J Am Soc Nephrol 19: 69–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol 116: 411–432, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Dong L, Zheng YM, Van Riper D, Rathore R, Liu QH, Singer HA, Wang YX. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab 28: 377–386, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Baumbach GL, Heistad DD. Cerebral circulation: humoral regulation and effects of chronic hypertension. Clin J Am Soc Nephrol 1: 53–57, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Mayhan WG, Heistad DD. Segmental vascular responses to acute hypertension in cerebrum and brain stem. Am J Physiol Heart Circ Physiol 252: H738–H742, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Feng J, Liu Y, Khabbaz KR, Sodha NR, Osipov RM, Hagberg R, Alper SL, Sellke FW. Large conductance calcium-activated potassium channels contribute to the reduced myogenic tone of peripheral microvasculature after cardiopulmonary bypass. J Surg Res 157: 123–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall SK, Armstrong DL. Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem 275: 3749–3754, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Howitt L, Sandow SL, Grayson TH, Ellis ZE, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on BKCa β1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol 301: H29–H40, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Jackson WF, Blair KL. Characterization and function of Ca2+-activated K+ channels in arteriolar muscle cells. Am J Physiol Heart Circ Physiol 274: H27–H34, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276: 42436–42444, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Kaplan FM, Kugel CH, 3rd, Dadpey N, Shao Y, Abel EV, Aplin AE. SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J Biol Chem 287: 41797–41807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuijpers MH, Gruys E. Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65: 181–190, 1984 [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J 23: 2196–2205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res 82: 729–737, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension 30: 1403–1409, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Lopez B, Ryan RP, Moreno C, Sarkis A, Lazar J, Provoost AP, Jacob HJ, Roman RJ. Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol 290: F1213–F1221, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Magnusson L, Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Renovascular BKCa channels are not activated in vivo under resting conditions and during agonist stimulation. Am J Physiol Regul Integr Comp Physiol 292: R345–R353, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci 17: 156–161, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pabbidi MR, Juncos J, Juncos L, Renic M, Tullos HJ, Lazar J, Jacob HJ, Harder DR, Roman RJ. Identification of a region of rat chromosome 1 that impairs the myogenic response and autoregulation of cerebral blood flow in fawn-hooded hypertensive rats. Am J Physiol Heart Circ Physiol 304: H311–H317, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterno R, Faraci FM, Heistad DD. Role of Ca2+-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke 27: 1603–1607, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990 [PubMed] [Google Scholar]
- 38.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ Spark/STOC coupling and elevated blood pressure. Circ Res 87: e53–e60, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Reinhart PH, Chung S, Martin BL, Brautigan DL, Levitan IB. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci 11: 1627–1635, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 265: C299–C303, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell 22: 217–230, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Rudofsky UH, Magro AM. Spontaneous hypertension in fawn-hooded rats. Lab Anim Sci 32: 389–391, 1982 [PubMed] [Google Scholar]
- 43.Shi L, Liu B, Li N, Xue Z, Liu X. Aerobic exercise increases BKCa channel contribution to regulation of mesenteric arterial tone by upregulating beta1-subunit. Exp Physiol 98: 326–336, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Staessen JA, Kuznetsova T, Acceto R, Bacchieri A, Brand E, Burnier M, Celis H, Citterio L, de Leeuw PW, Filipovsky J, Fournier A, Kawecka-Jaszcz K, Manunta P, Nikitin Y, O′Brien ET, Redon J, Thijs L, Ferrari P, Valentini G, Bianchi G. OASIS-HT: design of a pharmacogenomic dose-finding study. Pharmacogenomics 6: 755–775, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Strandgaard S. Cerebral blood flow in the elderly: impact of hypertension and antihypertensive treatment. Cardiovasc Drugs Ther 6: 1217–1221, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 295: F171–F178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell calcium 34: 211–229, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol 587: 3025–3044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, Korthuis RJ, Davis MJ, Braun AP, Hill MA. Mechanisms underlying regional differences in the Ca2+ sensitivity of BKCa current in arteriolar smooth muscle. J Physiol 591: 1277–1293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao G, Zhao Y, Pan B, Liu J, Huang X, Zhang X, Cao C, Hou N, Wu C, Zhao KS, Cheng H. Hypersensitivity of BKCa to Ca2+ sparks underlies hyporeactivity of arterial smooth muscle in shock. Circ Res 101: 493–502, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci USA 107: 8005–8010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol 73: 359–368, 2008 [DOI] [PubMed] [Google Scholar]