Abstract
Removal of plasma proteins from perfusates increases vascular permeability. The common interpretation of the action of albumin is that it forms part of the permeability barrier by electrostatic binding to the endothelial glycocalyx. We tested the alternate hypothesis that removal of perfusate albumin in rat venular microvessels decreased the availability of sphingosine-1-phosphate (S1P), which is normally carried in plasma bound to albumin and lipoproteins and is required to maintain stable baseline endothelial barriers (Am J Physiol Heart Circ Physiol 303: H825–H834, 2012). Red blood cells (RBCs) are a primary source of S1P in the normal circulation. We compared apparent albumin permeability coefficients [solute permeability (Ps)] measured using perfusates containing albumin (10 mg/ml, control) and conditioned by 20-min exposure to rat RBCs with Ps when test perfusates were in RBC-conditioned protein-free Ringer solution. The control perfusate S1P concentration (439 ± 46 nM) was near the normal plasma value at 37°C and established a stable baseline Ps (0.9 ± 0.4 × 10−6 cm/s). Ringer solution perfusate contained 52 ± 8 nM S1P and increased Ps more than 10-fold (16.1 ± 3.9 × 10−6 cm/s). Consistent with albumin-dependent transport of S1P from RBCs, S1P concentrations in RBC-conditioned solutions decreased as albumin concentration, hematocrit, and temperature decreased. Protein-free Ringer solution perfusates that used liposomes instead of RBCs as flow markers failed to maintain normal permeability, reproducing the “albumin effect” in these mammalian microvessels. We conclude that the albumin effect depends on the action of albumin to facilitate the release and transport of S1P from RBCs that normally provide a significant amount of S1P to the endothelium.
Keywords: albumin, endothelium, permeability, sphingosine-1-phosphate, vascular
we and others have demonstrated that red blood cells (RBCs) are an important source of the plasma phospholipid sphingosine-1-phosphate (S1P), which acts continuously to maintain normal vascular permeability (7, 9, 24, 34, 43, 50). The aim of the present experiments was to investigate the mechanisms that control the delivery of S1P from RBCs to the endothelium in intact microvessels. Our specific focus was the action of albumin as a carrier of S1P and as a modulator of vascular permeability. It has been known for many decades that the presence of albumin in vascular perfusates stabilizes the endothelial barrier (13, 14, 18, 23, 25, 28, 46). Removal of plasma proteins from perfusates increases vascular permeability and results in loss of the endothelial glycocalyx (11, 29, 32, 33). The common interpretation of the action of albumin is that it forms part of the permeability barrier by electrostatic binding to the endothelial glycocalyx and possibly occupying space within all or part of the intercellular junction (5, 8, 18, 30, 41, 47, 49). However, in the light of the new understanding of albumin as a carrier of S1P (4, 6, 16, 27), we tested the hypothesis that at least part of the action of albumin to stabilize vascular permeability is its ability to bind S1P released from RBCs and thereby enhance its delivery to endothelial cells, where it attenuates increased permeability. Furthermore, in collaboration with Tarbell and colleagues, we have demonstrated that the presence of albumin contributes to the maintenance of the glycocalyx via a S1P-dependent mechanism (9, 49). Thus, the present experiments were designed to evaluate how the presence and absence of albumin in vascular perfusates modulates the availability of S1P and the action of S1P to account for the well-known “protein effect” on vascular permeability. To enable direct comparison with previous observations of the action of albumin to maintain a stable permeability in individually perfused microvessels [measured as the hydraulic conductivity (Lp) of the microvessel wall], we began these experiments using concentrations of RBCs [hematocrits (Hct)] in perfusates with and without albumin that were characteristic of those used in those previous experiments.
METHODS
Animal preparation.
The animal protocol (no. 16158) was approved by the Institutional Animal Care and Use Committee of the University of California (Davis, CA). Male rats (Sprague-Dawley, 350–450 g, age: 70–90 days, Hilltop Laboratory Animals) were anesthetized with pentobarbital (100 mg/kg body wt sc) and subsequently maintained with additional pentobarbital (30 mg/kg sc) as determined using the toe pinch reflex. At the end of the experiments, rats were euthanized with saturated KCl. During experiments, rats were positioned on a tray with a heating pad to maintain normal temperature. Using a midline abdominal incision (2–3 cm), the mesentery was gently arranged over a coverglass or quartz pillar for microscopic observations. The mesentery was continuously suffused with Ringer solution (35–37°C). Diameters of the venular microvessels chosen for study ranged from 22 to 36 μm.
Solutions and reagents.
Mammalian Ringer solution included (in mM) 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, and 20 HEPES and Na-HEPES. The ratio of acid-HEPES to Na-HEPES was adjusted to achieve pH 7.40–7.45. All mammalian Ringer solution perfusates additionally contained fatty acid-free BSA (A0281, Sigma) at 10 mg/ml and test reagents or vehicle. A stock solution of S1P (1 mM) was prepared in ethanol. A stock solution of the S1P1 receptor antagonist W-146 (Avanti Polar Lipids, Alabaster, AL) was prepared (5 mM) with 2% 2-hydroxypropyl-β-cyclodextrin in Ringer solution; W-146 was diluted to a working concentration (10 μM and 0.004% 2-hydroxypropyl-β-cyclodextrin).
To measure the permeability to tracer albumin, fatty acid-free BSA (A0281, Sigma, 66 kDa, 3.6-nm Stokes radius) was labeled with Alexa fluor 555 (emission maximum: 570 nm) according to the manufacturer's (Invitrogen) instructions to produce BSA-555 with a labeling efficiency of 3–5 mol dye/mol protein and then processed to reduce the free dye concentration as previously described (9, 26). Fluorescently labeled albumin was frozen in small aliquots and thawed before use. Concentrations of labeled solutes in the final perfusates typically varied from 0.5 to 1 mg/ml.
The preparation of RBCs and RBC-conditioned perfusates has been previously detailed (9). Briefly, RBCs from each rat collected by tail vein puncture were washed in Ringer solution to remove plasma, white blood cells, and platelets. Conditioned perfusates were made by adding measured volumes of packed RBCs to test solutions for 20 min; cells were then pelleted by centrifugation, and the supernatant was removed for perfusion.
Preparation of liposomes.
l-α-Phosphatidylcholine, hydrogenated soy (HSPC), 1,2 distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy polyethylene glycol-2000 (DSPE-PEG2k), and cholesterol were purchased from Avanti Polar Lipids. Multilamellar lipid vesicles (MLVs) were prepared from HSPC-cholesterol-DSPE-PEG2k at molar ratios of 56:39:5 by the lipid film hydration method (17, 21, 42). Briefly, lipids (15 mg) were dissolved in chloroform, dried into a thin film by evaporation under a gentle stream of nitrogen, and lyophilized overnight to remove the remaining chloroform. The dried lipid (15 mg) was hydrated with 0.5 ml PBS (pH 7.4) at 55–60°C for 30 min. The resulting multilamellar liposomes were subjected to five freeze-thaw cycles, alternating between liquid nitrogen and a water bath set at 40°C, to create MLVs. The nominal mean diameter of the liposomes was 1.9 ± 1.5 μm, as measured using a NICOMP 380 ZLS submicron particle analyzer (Particle Sizing System, Santa Barbara, CA). However, due to some coalescing of the liposomes during handling, the size of the liposomes tracked as flow markers on the video recordings ranged from ∼5 to 15 μm.
S1P measurements.
The S1P concentration was measured with an ELISA kit using the manufacturer's instructions modified to match the ratio of serum to saline in standards and samples (K-1900 Echelon Bioscience).
Measurement of vessel wall solute apparent permeability.
A detailed description of the methods and assumptions used to measure fluorescent solute flux (Js) crossing the walls of single perfused vessels and to calculate apparent permeability coefficients [solute permeability (Ps)] has been previously published (9, 10, 19). Ps includes both diffusive and convective components of Js between blood and tissue. Present methods, with the same assumptions, use analysis of charge-coupled device images of the fluorescent solute as the solute fills a perfused vessel, permeates the vessel wall, and accumulates in the immediately surrounding tissue. We calculated the intensity of fluorescence (If) within a region of interest (ROI) defined in the images to include a segment of vessel and tissue (usually 100–200 μm along the vessel axis and ∼100 μm to either side of the vessel) as the vessel initially fills with fluorescent solute (ΔIf0) and during accumulation over 30–60 s [(dIf/dt)i]. For a vessel of radius r and surface area S, and assuming a constant concentration difference across the wall (ΔC), we calculated apparent Ps from the following expressions:
| (1) |
| (2) |
Further details of the methods as applied in rat venular microvessels for the present experiments have been previously published (9). As in previous experiments, the measurements of apparent permeability were made at microvessel pressures close to 20 cmH2O.
Lp measurements.
Lp was measured to characterize vessel wall permeability. Experiments using RBCs as flow markers were based on the modified Landis technique, which measures the volume flux of water crossing the wall of a microvessel perfused via a glass micropipette after downstream occlusion of the vessel (3, 20, 30, 31). The initial transcapillary water flow per unit area of the capillary wall [(Jv/S)0] was measured at predetermined capillary pressures of 30–60 cmH2O. Microvessel Lp was calculated as the slope of the relation between (Jv/S)0 and applied hydraulic pressure. Details of recent applications of the method have been previously published (9). The average RBC Hct in the pipette was 1.3%. For measurements involving liposomes, the assumption that a small liposome tracks the same centerline flow as an RBC leads to an overestimate of Lp by about only 3% when the liposome is 2 μm in diameter (31). Because liposomes that were tracked to measure Jv ranged from ∼5 to 15 μm, they were assumed to have the same diameter as RBCs (7 μm), and no further corrections were made.
Analysis and statistics.
Nonparametric ANOVA tests were used to analyze groups of three measurements and the Wilcoxon test was used for pairs of groups. The indicated statistical tests were assumed significant for P values of <0.05.
RESULTS
Exogenous S1P maintains normal permeability when the absence of albumin increases Ps.
In agreement with our previous observations (9), perfusate containing albumin (10 mg/ml) when conditioned by exposure to RBCs (Hct: 1.3%) for a period of 20 min maintained normal Ps of individually perfused microvessels (0.92 ± 0.38 × 10−6 cm/s, n = 9; Fig. 1). The previous study also showed that perfusate containing albumin (10 mg/ml), but not conditioned by RBCs, failed to maintain Ps (15.3 ± 6.3× 10−6 cm/s). In contrast, a Ringer solution perfusate without BSA, but conditioned with RBCs, did not maintain normal permeability (16.1 ± 3.9 × 10−6 cm/s, P < 0.05). In six of the experiments (as shown in Fig. 1A), we found that the addition of exogenous S1P (1,000 nM) rapidly restored normal permeability in microvessels perfused with Ringer solution or RBC-conditioned Ringer solution in the absence of BSA (0.83 ± 0.18 × 10−6 cm/s).
Fig. 1.
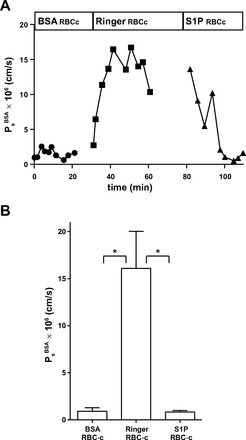
A: representative data showing that Ringer solution conditioned with red blood cells (RBCs) does not maintain normal permeability to albumin relative to BSA (10 mg/ml) containing solution conditioned by RBCs (RBCc; n = 9). In a subset of the experiments, we additionally perfused the vessel with sphingosine-1-phosphate (S1P; 1,000 nM) in Ringer solution, which returned solute permeability (Ps) values toward normal values (n = 6). Solutions were conditioned (20–22°C) with RBCs at a concentration typical of that used as flow markers in microperfusion experiments [hematocrit (Hct): 1.3% Hct]. Tracer BSA (Alexa fluor 555 labeled, concentration: 0.5–1 mg/ml) alone did not maintain low permeability. B: summary data from vessels as in A. *P < 0.05 (by Kruskal-Wallis test with Dunn's posttest).
The striking difference between the stability of Ps when albumin is present and the increased permeability when albumin is not present is consistent with a role for albumin to modulate the availability of S1P. However, the observation that exogenous S1P reverses the increased Ps of Ringer solution-perfused vessels suggests that, when sufficient S1P is available, albumin is not essential to maintain the low permeability state. These observations are consistent with the hypothesis that S1P is the primary modulator of the well-described protein effect to lower permeability.
Direct measurements of S1P in perfusates with and without albumin.
The average concentration of S1P in the Ringer solution/BSA perfusate conditioned with RBCs (Hct: 1.3%) for 20 min at room temperature, as used in the experiments shown in Fig. 1, was 439 ± 46 nM (n = 12; Fig. 2). In the absence of albumin, the mean concentration of S1P measured in the Ringer solution perfusate after 20-min exposure to RBCs was more than an order of magnitude lower (28 ± 5 nM, n = 13).
Fig. 2.
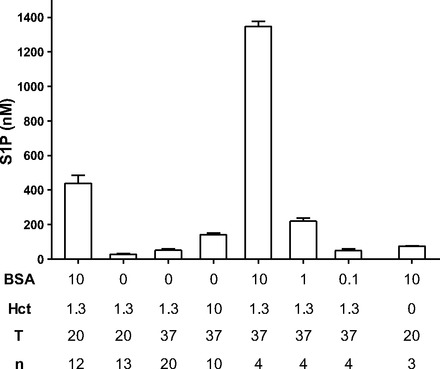
S1P measured in RBC-conditioned solutions. BSA is shown in mg/ml, and Hct is %RBC by volume, T is temperature (in °C), and n is the number of independent measurements.
The differences in S1P concentrations in conditioned media with and without albumin were even more striking at 37°C. Ringer solution perfusates conditioned with RBCs (Hct: 1.3%) at 37°C had an average S1P concentration of 52 ± 8 nM (n = 20), whereas in the presence of BSA, the RBC-conditioned solution had 1,350 nM S1P. These values in protein-free Ringer solutions are close to an order of magnitude less than the corresponding S1P concentrations in solutions with BSA (10 mg/ml). All values are shown in Fig. 2. Figure 2 also shows that S1P release from RBCs at 37°C is strongly dependent on albumin concentrations, falling to 220 ± 19 nM for S1P conditioned in the presence of 1 mg/ml albumin and 50 ± 10 nM at 0.1 mg/ml albumin (Hct: 1.3%, 37°C). We measured S1P in our BSA (10 mg/ml) solution without preconditioning and found that it contained S1P at 75 ± 2 nM. When combined with the results shown in Fig. 1, these measurements show that concentrations of S1P in conditioned media below 100 nM fail to maintain normal permeability, whereas concentrations of 440 nM and above maintain permeability. This conclusion is based on the assumption that results from in vitro preconditioning are representative of S1P levels in the perfusates in a micropipette immersed in the superfusate at 37°C before perfusion of the test microvessel.
Further evaluation of exogenous S1P regulation of Ps.
A direct test of the dependence of Ps on S1P concentration is shown in Fig. 3A. These results extend the observations shown in Fig. 1 to demonstrate Ps measured with Ringer solution perfusates with exogenous S1P added at concentrations of 1,000 nM (as in Fig. 1), 100 nM, and 50 nM. There was a clear concentration dependence of albumin permeability on exogenous S1P concentrations consistent with a threshold (above 100 nM and <440 nM) to maintain normal permeability.
Fig. 3.
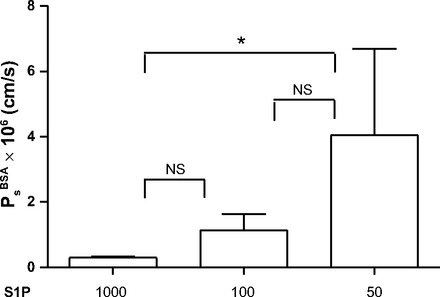
S1P added to Ringer solution perfusates maintains low permeability. Ps was measured with Ringer solution perfusates (no RBCs and not conditioned) with exogenous S1P added at the indicated concentrations to each vessel. Ps increased as S1P concentration decreased (n = 8). NS, not significant. *P < 0.05 (by Friedman test with Dunn's posttests).
Measurement of permeability when RBCs are present in the Ringer solution perfusate.
Mason et al. (28) demonstrated that, in frog microvessels (at room temperature and as low as 15°C), simply removing albumin from the perfusate increased Lp of the microvessel walls measured with the modified Landis technique using RBC flow markers. Based on the above results, we hypothesized that, at these temperatures, S1P from RBCs in a Ringer solution perfusate (<50 nM) was not sufficient to maintain stable permeability. To further evaluate the role of albumin to modulate S1P delivery from RBCs to the endothelium in mammalian vessels at 37°C when albumin was removed from the perfusate but RBCs were present in the perfusate (as opposed to a RBC-conditioned perfusate, as investigated above), we measured Lp of perfused microvessels using the same modified Landis technique and a Ringer solution perfusate containing RBCs at a similar Hct (Hct: 1.3%). Under these conditions (RBCs are continuously in contact with the perfusate and the perfusate in the pipette tip was at a temperature of 37°C), there was no increase in Lp relative to control (albumin present). To test if the stable Lp was due to S1P released into the perfusate, we added a S1P1 antagonist to the Ringer solution/RBC perfusate. Figure 4 shows that the stable permeability state with Ringer solution and continuously present RBCs was reversed using the S1P1 antagonist W-146. Thus, at 37°C, a Ringer solution perfusate in constant contact with RBCs in a pipette contains sufficient S1P to maintain permeability, whereas at lower temperatures there appears to be insufficient S1P available for the stable state to be maintained. The results also indicate that at 37°C, S1P concentrations measured after conditioning Ringer solution with RBCs in vitro before filling the pipette may underestimate the S1P concentration in a Ringer solution that remains in contract with RBCs in the pipette.
Fig. 4.
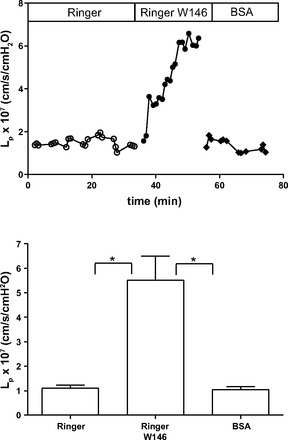
A: we measured hydraulic conductivity (Lp) to test for the “protein effect” by comparing Lp measured with BSA and that measured with protein-free Ringer solution. This representative experiment shows that Lp with Ringer solution was not different from that measured with BSA. The addition of W-146 to the Ringer solution/RBC perfusates caused an increase in Lp, demonstrating that RBCs are able to deliver S1P to the endothelium in the absence of BSA. All perfusates include RBCs as flow markers (Hct: 1.3%). B: summary data from experiments as in A (n = 12). *P < 0.05 (by Friedman test with Dunn's posttests).
Absence of RBCs and albumin increases Lp.
To further evaluate the role of RBCs as an exogenous source of S1P when the perfusate was protein-free Ringer solution, we replaced RBCs with relatively large liposomes, which acted as flow markers but produce no S1P. Consistent with results described above (Fig. 1), Fig. 5 shows that Lp first measured in the presence of BSA and RBCs with a control value of 0.8 ± 0.1 × 10−7 cm·s−1·cmH2O−1 increased significantly to the test value of 2.6 ± 0.3 × 10−7 cm·s−1·cmH2O−1 measured during protein-free Ringer solution perfusion using liposomes as flow markers (n = 9, P < 0.05). As a further test, in five experiments, we compared Lp during RBC-conditioned BSA perfusion (no RBCs during perfusion) versus Ringer solution only, both using liposomes as flow markers, and found that RBC conditioning significantly decreased Lp from 2.7 ± 0.5 × 10−7 to 1.4 ± 0.1 × 10−7 cm−1·s·cmH2O−1 (P < 0.05 by Wilcoxon test). Because we found, as shown in Fig. 4, that, when RBCs were used as flow markers, Ringer solution perfusion does not measurably increase Lp relative to perfusion with BSA, the increased Lp when measured with Ringer solution and liposomes clearly implicates removal of RBCs with the increased Lp.
Fig. 5.
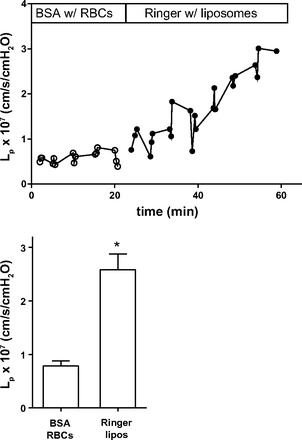
A: representative experiment showing that when liposomes were used as flow markers, the Lp measured with Ringer solution as the perfusate increased relative to Lp with BSA when RBCs were used as flow markers. B: summary of data from experiments conducted as in A (n = 9). *P < 0.05, different from control (by Wilcoxon test).
DISCUSSION
Our results conform to the hypothesis that the primary action of albumin to maintain vascular permeability is to facilitate the release of S1P from erythrocytes, rather than to act directly by binding to the endothelial surface to form part of the barrier to water and solute as previously suggested. Albumin is not required to maintain normal permeability when S1P is present at sufficient amounts in the perfusate. The lowest S1P concentration that maintains a stable permeability is above 100 nM. For perfusates that have been conditioned by exposure to RBCs for 20 min with 10 mg/ml albumin, S1P concentrations between 300 and 500 nM (mean: 440 nM) are sufficient to maintain normal permeability. These results extend our previous observation that removal of S1P or inhibition of its action by use of a S1P1 receptor antagonist (W-146) leads to an increase in microvascular permeability (9). In those experiments, we also demonstrated that a S1P1 receptor agonist (SEW-2871) was effective to return Ps to normal when permeability had increased after RBCs were removed from the perfusate (9). To place these new results in the context of the current understanding of mechanisms maintaining normal microvascular permeability, this discussion is divided into three main sections: first, cellular mechanisms whereby S1P maintains normal permeability are described, and these are evaluated as an alternative to the model of physical interactions between albumin and the endothelial cell glycocalyx to increase resistance to water and solute flow. Second, the results are evaluated in relation to the growing understanding of the role of albumin and other S1P carriers [e.g., high-density lipoprotein (HDL)] to facilitate the release of S1P into plasma. Finally, the importance of these results to the interpretation of experiments conducted in vivo and in vitro under different perfusion conditions is discussed. These include the use of solutions with and without plasma proteins, including FCS and albumin, at various concentrations in bathing media and in the presence and absence of RBCs as a source of S1P.
Cellular mechanisms.
The mechanisms of action of S1P have been reviewed in recent publications (12, 45). Ligation of the G protein-coupled S1P1 receptor rapidly activates the Rho family small GTPase Rac1, leading to peripheral localization of cytoskeletal effectors (e.g., cortactin and nonmuscle myosin light chain kinase). This localization promotes adherens junction (including vascular endothelial-cadherin and associated catenins) and tight junction (occludin, zonula occludens proteins, and claudins) formation. Cell-substrate adhesion (via paxillin and focal adhesion kinase) as well as cell-cell adhesion are increased. These changes all serve to improve barrier function. A second mechanism of action is to stabilize the endothelial glycocalyx. We have recently demonstrated that heparin sulfate, chondroitin sulfate, and syndecan-1 ectodomain, which are shed from cultured endothelial cells after removal of plasma proteins, were retained in the presence of S1P. S1P has been found to reduce matrix metalloproteinase activation, thereby attenuating the loss of endothelial cell surface glycocalyx components (49). Both actions appear to involve signaling via the S1P1 receptor. The actions to modulate the glycocalyx by intracellular signaling via the activity of matrix metalloproteinases is contrasted with the idea that albumin directly modifies the resistance of water and solute flows by electrostatic interactions between the positively charged arginine groups on the protein and negatively charged heparin sulfate side chains of core glycoproteins such as syndecan-1 and glypican-1 (33).
The primary action of S1P via the S1P1 receptor accounts for some of the observations in frog microvessels after albumin is removed from the perfusate that were not accounted for by a simple model of albumin occupying space within a primary water channel. One is that the increased permeability with Ringer solution perfusion was associated with an increase in intracellular Ca2+ concentration in endothelial cells. Furthermore, Ca2+-free Ringer solution attenuated the increase in permeability. We and others (2, 34) have shown that S1P acting via Rac1 attenuates Ca2+-dependent increases in microvessel permeability. It is likely that S1P also attenuates the Ca2+-dependent mechanism that increases permeability after albumin is removed from the perfusate. Another observation was that albumin added to the superfusate attenuated the increase in permeability caused by removal of albumin from the perfusate. Although it has been suggested that such extracellular albumin might diffuse into the intercellular junction to occupy space, the more likely mechanism is that S1P carried by the extracellular albumin was sufficient to stimulate S1P receptors on endothelial cells. Another key observation consistent with the idea that albumin modulates the availability of S1P, which acts tonically to maintain permeability, is that the removal of albumin from the microvessel perfusate results in a sustained increase in permeability, as shown in Figs. 1 and 4, as opposed to the transient increase in permeability that is seen with most inflammatory agents (12). Furthermore, S1P acts rapidly to modulate microvessel permeability. As shown in Figs. 1 and 4, as well as in previous publications from this laboratory and others (34), reduced levels of S1P, or inhibition of the S1P1 receptor, results in a rapid increase in permeability. The increase is also rapidly reversed when S1P is added back to the perfusate (2).
We note that our observations do not rule out other actions of a large macromolecule binding to the endothelial surface to modulate permeability or endothelial cell uptake of circulating macromolecules. The observations by Michel and colleagues (32) demonstrating that cationized ferritin bound to the surface of endothelial cells and reduced permeability were used as an example of how bound macromolecules could modify the endothelial surface. It was assumed that positively charged arginine groups in albumin could facilitate albumin binding to the endothelium (33). Such albumin binding to the endothelial surface has been demonstrated (39, 40, 48). Furthermore, the removal of albumin from the perfusate was shown to increase the loading of macromolecules into vesicles on the luminal surface of endothelium (33, 38). Giantsos and colleagues (15) have described the development of cationized polymers that bind to the endothelial surface and reduce the permeability of endothelial monolayers. Thus, the role of both S1P and absorbed macromolecules as modulators of transvascular transport and endothelial cell uptake (including vascular targeted gene therapy agents) requires further investigations (37).
S1P delivery to plasma.
Although platelets are an important source of S1P during injury and inflammation, S1P found in plasma under normal conditions is derived largely from erythrocytes and from endothelial cells (27, 44). The mechanism of transport from the intracellular space to the plasma space is not well understood but is known to involve ATP-binding cassette-type transporters in some cell types (22). The primary acceptors and carriers of S1P in plasma are albumin (∼30%), HDL (∼60%), and very-low-density lipoprotein (<10%). In the absence of these acceptors, very little S1P accumulates in bathing media (6). Our observations that S1P delivery is reduced as temperature, albumin concentration, and Hct are reduced are consistent with these mechanisms. The primary role of albumin as a modulator and carrier of S1P possibly accounts for the observation that modified albumins are less effective to maintain permeability. Thus, when arginine groups are shielded on albumin, the present observations suggest that the resultant reduction in the effectiveness of albumin to maintain permeability (33) is at least as likely to be the result of reduced capacity to facilitate S1P release and transport as reduced electrostatic binding to the endothelium. While complete removal of albumin from a perfusate is not physiological, the above observations indicate that conditions that reduce the capacity of albumin to bind S1P may compromise permeability regulation. Such conditions possibly include glycation of albumin (36) and apolipoprotein M in HDL as well as competition for S1P-binding sites by other drugs or toxins.
A key observation is that even with the lowest conditioning Hct (<2%), albumin promotes S1P concentrations of 1,350 nM in vitro compared with 52 nM in the absence of albumin. This implies that albumin increases the release of S1P by up to 25 times. Even higher rates of release are expected at higher Hct. Yet normal S1P concentrations in blood plasma are 300–400 nM (35). This observation suggests that, in the normal circulation, S1P is rapidly removed from plasma and an important physiological role for albumin is to promote the delivery of S1P from RBCs to maintain S1P levels. While further investigations of the modulation of the kinetics of S1P supply and removal are needed in the whole circulation, it is reasonable to argue that the actions of albumin as a promoter of S1P release and as a carrier (along with HDL) contribute to the balance between the rate of supply of S1P from RBCs and the rate of removal. Our observations that S1P can be supplied to individually perfused microvessels without albumin suggest that the mechanisms removing S1P from the whole circulation are not present in microvessels under the conditions of our microperfusion experiments.
Reevaluation of the protein effect as a determinant of normal vascular permeability.
In a separate collaborative study (49) between our laboratory and the Tarbell laboratory, we have demonstrated that S1P maintains the stability of the endothelial glycocalyx in cultured endothelial cell monolayers. The role of albumin and plasma proteins as modulators of S1P delivery from RBCs likely accounts for observations of changes in the glycocalyx in protein-free perfusates. Specifically, when microvessels that had previously been labeled with cationized ferritin (CF) to reveal the surface layer of the endothelial luminal glycocalyx were reperfused with plasma, the CF-labeled layer was found to be separated from the endothelial surface by ∼30 nm (1). In contrast, when vessels were reperfused with protein-free Ringer solution, the CF-labeled layer remained very close (3-nm separation) to the endothelial surface. Treatment with solutions containing BSA (10–50 mg/ml) resulted in a separation of only ∼8 nm. It is tempting to speculate that the differential effect of the plasma reperfusion was due to S1P in the plasma. However, the S1P concentrations in those experiments are unknown, as are the details of amphibian pharmacology regarding S1P.
We examined possible sources of S1P in the experimental solutions and found that commercial FCS and various BSA preparations contribute significantly to S1P in solution (49). We found the fatty acid-free BSA used in the present study contributes 75 nM (at 10 mg/ml in Ringer solution) to the perfusates. We note that while some studies indicate that endothelium can provide S1P to incubation media (44), removal of sources of S1P from perfusion solutions in the present study uniformly resulted in increased permeability, indicating that any S1P released directly from the endothelium of microvessels was insufficient to maintain local normal Ps. Many experiments on cultured endothelial cells or on isolated vascular segments involve the use of artificial bathing fluids. Our observations indicate that the amount of S1P that is available to the endothelium under these conditions depends of several factors, including temperature, albumin concentration, FCS concentration, and RBC Hct. Even when albumin and FCS are present, our experiments demonstrate that there may be insufficient S1P available to maintain the normal vascular barrier when RBCs or RBC-conditioned solutions are not present. Clearly, the factors modulating S1P availability and the amount of S1P present in bathing solutions during experiments on model endothelial barriers must be examined to investigate the stability of the barrier. Such information is not available for the majority of such investigations to date.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant HL-28607 (to F. E. Curry) and by the University of California-Davis Research Investments in Science and Engineering program and NIH Grant CA-134659 (to K. W. Ferrara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.H.A., J.F.C., M.Y.R., A.K., K.W.F., and F.-R.E.C. conception and design of research; R.H.A., J.F.C., M.Y.R., Y.J., and A.K. performed experiments; R.H.A., J.F.C., M.Y.R., Y.J., A.K., and F.-R.E.C. analyzed data; R.H.A., J.F.C., M.Y.R., Y.J., A.K., K.W.F., and F.-R.E.C. interpreted results of experiments; R.H.A. and J.F.C. prepared figures; R.H.A. and F.-R.E.C. drafted manuscript; R.H.A., M.Y.R., and F.-R.E.C. edited and revised manuscript; R.H.A., J.F.C., M.Y.R., Y.J., A.K., K.W.F., and F.-R.E.C. approved final version of manuscript.
REFERENCES
- 1.Adamson RH, Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol 445: 473–486, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Sarai RK, Clark JF, Altangerel A, Thirkill TL, Curry FE. Attenuation by sphingosine-1-phosphate of rat microvessel acute permeability response to bradykinin is rapidly reversible. Am J Physiol Heart Circ Physiol 302: H1929–H1935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406–H417, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem 283: 25074–25081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arkill KP, Neal CR, Mantell JM, Michel CC, Qvortrup K, Rostgaard J, Bates DO, Knupp C, Squire JM. 3D reconstruction of the glycocalyx structure in mammalian capillaries using electron tomography. Microcirculation 19: 343–351, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode C, Sensken SC, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman R, Huang T, Tolle M, van der Giet M, Graler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem 109: 1232–1243, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119: 1871–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curry FE, Adamson RH, Clark JF. Sphingosine-1-phosphate and the “albumin effect” on rat venular microvessels. FASEB J 27: 896, 2013 [Google Scholar]
- 9.Curry FE, Clark JF, Adamson RH. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am J Physiol Heart Circ Physiol 303: H825–H834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry FE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology, edited by Linden T. New York: Elsevier, 1983, p. 1–34 [Google Scholar]
- 11.Curry FE, Michel CC, Phillips ME. Effect of albumin on the osmotic pressure exerted by myoglobin across capillary walls in frog mesentery. J Physiol 387: 69–82, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry FR, Adamson RH. Tonic regulation of vascular permeability. Acta Physiol 207: 628–649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danielli JF. Capillary permeability and oedema in the perfused frog. J Physiol 98: 109–129, 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drinker CK. The permeability and diameter of the capillaries in the web of the brown frog (R. temporaria) when perfused with solutions containing pituitary extract and horse serum. J Physiol 63: 249–269, 1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giantsos KM, Kopeckova P, Dull RO. The use of an endothelium-targeted cationic copolymer to enhance the barrier function of lung capillary endothelial monolayers. Biomaterials 30: 5885–5891, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J 21: 1202–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta 812: 55–65, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Huxley VH, Curry FE. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol Heart Circ Physiol 248: H264–H273, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: α-lactalbumin transport. Am J Physiol Heart Circ Physiol 252: H188–H197, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol 80: 359–372, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kheirolomoom A, Mahakian LM, Lai CY, Lindfors HA, Seo JW, Paoli EE, Watson KD, Haynam EM, Ingham ES, Xing L, Cheng RH, Borowsky AD, Cardiff RD, Ferrara KW. Copper-doxorubicin as a nanoparticle cargo retains efficacy with minimal toxicity. Mol Pharm 7: 1948–1958, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem 284: 21192–21200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis EM, Pappenheimer JR. Exchange of substances through capillary walls. In: Handbook of Physiology. Circulation. Washington, DC: Am. Physiol. Soc., 1963, sect. 2, vol. II, chapt. 29, p. 961–1034 [Google Scholar]
- 24.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol 296: H33–H42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levick JR, Michel CC. The effect of bovine albumin on the permeability of frog mesenteric capillaries. Q J Exp Physiol Cogn Med Sci 58: 87–97, 1973 [DOI] [PubMed] [Google Scholar]
- 26.Lin YC, Samardzic H, Adamson RH, Renkin EM, Clark JF, Reed RK, Curry FR. Phosphodiesterase 4 inhibition attenuates atrial natriuretic peptide-induced vascular hyperpermeability and loss of plasma volume. J Physiol 589: 341–353, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucke S, Levkau B. Endothelial functions of sphingosine-1-phosphate. Cell Physiol Biochem 26: 87–96, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Mason JC, Curry FE, Michel CC. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res 13: 185–202, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Michel CC. Capillary permeability and how it may change. J Physiol 404: 1–29, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Michel CC, Mason JC, Curry FE, Tooke JE, Hunter PJ. A development of the Landis technique fo of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci 59: 283–309, 1974 [DOI] [PubMed] [Google Scholar]
- 32.Michel CC, Phillips ME. The effects of bovine serum albumin and a form of cationised ferritin upon the molecular selectivity of the walls of single frog capillaries. Microvasc Res 29: 190–203, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Michel CC, Phillips ME, Turner MR. The effects of native and modified bovine serum albumin on the permeability of frog mesenteric capillaries. J Physiol 360: 333–346, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minnear FL, Zhu L, He P. Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive. Am J Physiol Heart Circ Physiol 289: H840–H844, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, Osima N, Yokota H, Ikeda H, Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem 45: 356–363, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Powers MR, Huxley VH. Action of glucosylated albumin GA on capillary hydraulic conductivity Lp (Abstract). FASEB J 4: A1266, 1990 [Google Scholar]
- 37.Qin S, Fite BZ, Gagnon MK, Seo JW, Curry FR, Thorsen F, Ferrara KW. A physiological perspective on the use of imaging to assess the in vivo delivery of therapeutics. Ann Biomed Eng. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneeberger EE, Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol Heart Circ Physiol 247: H206–H217, 1984 [DOI] [PubMed] [Google Scholar]
- 39.Schneeberger EE, Lynch RD, Neary BA. Interaction of native and chemically modified albumin with pulmonary microvascular endothelium. Am J Physiol Lung Cell Mol Physiol 258: L89–L98, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Schnitzer JE, Carley WW, Palade GE. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci USA 85: 6773–6777, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squire JM, Chew M, Nneji G, Neal C, Barry J, Michel C. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol 136: 239–255, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sriwongsitanont S, Ueno M. Effect of a PEG lipid (DSPE-PEG2000) and freeze-thawing process on phospholipid vesicle size and lamellarity. Colloid Polym Sci 282: 753–760, 2004 [Google Scholar]
- 43.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res 103: 1164–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res 77: 39–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson PD. Effect of plasma and red blood cells on water permeability in cat hindlimb. Am J Physiol Heart Circ Physiol 246: H818–H823, 1984 [DOI] [PubMed] [Google Scholar]
- 47.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Yen WY, Cai B, Zeng M, Tarbell JM, Fu BM. Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res 83: 337–346, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Y, Adamson RH, Curry FE, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol 306: H363–H372, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang G, Xu S, Qian Y, He P. Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules. Am J Physiol Heart Circ Physiol 299: H1494–H1504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


