Abstract
Although the application of a 9-V battery to the epicardial surface is a simple method of ventricular fibrillation induction, the fundamental mechanisms underlying this process remain unstudied. We used a combined experimental and modelling approach to understand how the interaction of direct current (DC) from a battery may induce reentrant activity within rabbit ventricles and its dependence on battery application timing and duration. A rabbit ventricular computational model was used to simulate 9-V battery stimulation for different durations at varying onset times during sinus rhythm. Corresponding high-resolution optical mapping measurements were conducted on rabbit hearts with DC stimuli applied via a relay system. DC application to diastolic tissue induced anodal and cathodal make excitations in both simulations and experiments. Subsequently, similar static epicardial virtual electrode patterns were formed that interacted with sinus beats but did not induce reentry. Upon battery release during diastole, break excitations caused single ectopics, similar to application, before sinus rhythm resumed. Reentry induction was possible for short battery applications when break excitations were slowed and forced to take convoluted pathways upon interaction with refractory tissue from prior make excitations or sinus beats. Short-lived reentrant activity could be induced for battery release shortly after a sinus beat for longer battery applications. In conclusion, the application of a 9-V battery to the epicardial surface induces reentry through a complex interaction of break excitations after battery release with prior induced make excitations or sinus beats.
Keywords: cardiac modeling, ventricular fibrillation, optical mapping, bidomain, reentry
ventricular fibrillation (VF) remains a significant cause of sudden cardiac death worldwide. Despite decades of research, the processes involved in the initiation of VF from sinus rhythm are still subject to intense investigation. The induction of VF via direct current (DC) stimulation to the heart's surface with a simple 9-V battery is a widely used method. However, the precise mechanisms of arrhythmogenic interaction of DC from a battery with cardiac tissue, and potential interspecies differences, are currently unknown. Acquiring such mechanistic insights will help optimize VF induction via this method and, importantly, shed light on fundamental arrhythmia induction processes.
The induction of VF by simply touching a 9-V battery to the epicardial surface is most widely used in the experimental laboratory (8–11, 15–17, 22, 24), although it is also often used in a clinicial research environment (23, 27). In these cases, the exact location of the application, the timing relative to the cardiac cycle, and the duration of the application are widely varying parameters. Nonetheless, VF appears to be reliably induced. Despite its success, however, there appears to be little, if any, knowledge regarding the mechanisms by which a 9-V battery causes the ventricles to fibrillate. Mechanisms that may contribute to VF induction include battery-induced make and/or break excitations, virtual electrode polarisation, repetitive focal discharge, conduction block, involvement of the Purkinje system (PS), stretch-activated channels, and an interaction with sinus activation patterns.
All reports in the literature describing successful induction of VF via this method are almost exclusively in porcine (8, 9, 17, 22) and canine (11, 24) hearts, with some reports of its use in sheep (15) and guinea pigs (10), in addition to ex vivo human hearts (16). Intriguingly, though, there appear to be no reports of VF induction with a 9-V battery in the rabbit heart, potentially due to the noted difficulty in achieving self-sustained VF in healthy rabbit ventricles (3, 12, 14). It is therefore important to understand the fundamental mechanisms by which a 9-V battery may induce reentrant activity within the ventricles and the potential interspecies differences in the degeneration of these initial reentrant cycles into fully self-sustained VF.
In this study, using a combined computational modeling and experimental approach, we investigated the mechanism by which a 9-V battery induces reentry in rabbit ventricles. We examined how interactions of battery electrodes with tissue during application and release may induce reentrant activity and the dependence of the witnessed effects on application timing and stimulus duration as well as the interaction of these effects with sinus beats. Model simulations allowed a precise dissection of the mechanisms at play, with corresponding optical mapping experiments used to validate the findings at each stage.
MATERIALS AND METHODS
Computational Methods
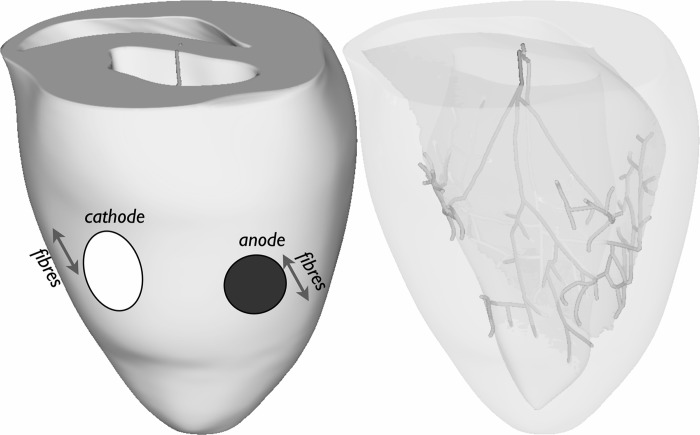
Electrical activity was simulated by the bidomain equations within a finite-element model of the rabbit ventricle (7) using the Cardiac Arrhythmia Research Package (CARP) (26). The model, shown in Fig. 1, left, included histologically derived fiber architecture and a representation of the PS (7). Ionic membrane dynamics within the myocardium were represented by a recent rabbit ventricular cell model (13) augmented to include two additional currents to faithfully simulate the membrane response to strong shocks (1), specifically an electroporation current and a hypothetical K+ current that activates at larger positive polarizations beyond +160 mV. Membrane kinetics within the PS were represented by a recent Purkinje-specific cell model (2). The model also included transmural and apicobasal electrophysiological heterogeneity in ionic currents, as previously described (4). Anisotropic tissue conductivities along the fiber and cross-fiber directions were based on previous experimentally derived values (6) within the intracellular (0.174 and 0.0193 S/m) and extracellular (0.625 and 0.236 S/m) domains, respectively, adjusted to reduce conduction velocity by 25% in each direction in accordance with previous studies (3, 4) and to provide a closer match to that witnessed in experiments.
Fig. 1.
Computational ventricular model showing positioning of battery electrodes (left) and the Purkinje system (PS) network (right). The approximate fiber orientation direction is also shown close to the electrodes.
Battery electrodes were defined by constant voltage (9-V anode and 0-V cathode) circular regions on the epicardial surface of the left ventricular (LV) free wall with diameters of 5 mm (anode) and 7 mm (cathode), separated by 13 mm, corresponding to a standard 9-V battery, as shown in Fig. 1, left.
Sinus activation paced the ventricles via the PS at a fixed cycle length of 350 ms throughout the entire simulation. After a variable time (50–300 ms) after the 10th paced beat, the battery electrodes were activated for a variable duration (50–700 ms), after which activity was allowed to evolve and vulnerability for reentry induction assessed. After the cessation of the battery stimulus, electrode regions were treated as normal myocardial tissue. The significant computational burden of a full bidomain run precluded a thorough parameter sweep of all shock application and duration timings. For example, simulation of battery application required a bidmoain solve with a very fine time step (2 μs) to resolve the rapid changes in potential associated with the strong extracellular field. Simulation of a 700-ms stimulus took ∼128 central processing unit hours (Xeon, 2.53 GHz), with further postshock simulation (500–1,000 ms) then required to assess reentry induction.
Simulations were performed with CARP on Oxford Supercomputing Centre clusters. Visualization of results was performed with custom-written Meshalyzer software.
Experimental Optical Mapping
Optical mapping recordings were obtained from ex vivo Langenndorff-perfused rabbit hearts following the same overall protocol and setup to the simulations for comparison and validation.
All experiments conformed with the “Guide for the Care and Use of Laboratory Animals” published by the Animals (Scientific Procedures) Act 1986 (United Kingdom). Hearts were swiftly isolated from young adult New Zealand White rabbits (∼1-kg weight) euthanized by an overdose of anesthetic (pentobarbitone) and then placed in ice-cold normal Tyrode solution [containing (in mM) 140 NaCl, 5.4 KCl, 1 MgSO4, 5 HEPES, 11 glucose, and 1.8 CaCl2; pH 7.4] before being mounted. Hearts were mounted to a Langendorff perfusion system, stabilized in a custom-built optical mapping chamber, perfused via gravity with warm (35–37°C) normal Tyrode solution for 5 min to resume normal contractions, and loaded with the membrane dye N-(4-sulfobutyl)-4-{6-[4-(dibutylamino)phenyl]hexatrienyl}pyridinium (rh237) as a bolus injection by coronary perfusion (5 μm over 5 min). Blebbistatin was used to immobilize the heart (10 μM, recirculated). The heart was illuminated with two LED light sources (530 nm, Cairn Research), passed through a 650-nm long-pass optical filter (Chroma Technology) attached to a 25-mm f0.95 imaging lens (Navitar). Signals were recorded with a Photometrics Evolve 128 EMCCD camera with a 1.5-ms frame period (64 × 64 pixels, 2 × 2 pixel binning). A series of 3,000 images was captured for each experimental run.
A custom chamber was constructed from Plexiglas with two platinum wire loop electrodes attached to the respective terminals of a 9-V Duracell Procell alkaline battery to prevent obstruction of the optical field of view by the battery itself. Wire electrode spacing (13 mm), diameter (5–7 mm), and wire loop thickness (1 mm) matched those of 9-V battery terminals. The LV was lightly pressed against the electrodes before being imaged. Current was controlled by a relay (sil05-1a72-71d). A second bipolar stimulator was pressed against the atria. A microcontroller (Arduino Uno, Arnuino.cc) was used to control the camera and stimulator and to drive the relay.
The heart was paced at its intrinsic sinus period minus 50 ms. For each run, the heart was paced for 40 beats before the application of the battery. The battery was applied for various time intervals so that the initiation and termination of the battery application fell at different time points, or phases, in the pacing cycle. Pacing protocols where the phase of the stimulus was progressively incremented by 25 ms were carried out for all preparations. Data were analyzed using a combination of custom-written software (available on request) based on ImageJ (http://rsb.info.nih.gov/ij/) and Scipy (www.scipy.org) software packages. Fluorescence versus time traces in all images were generated from unprocessed data. Isochronal activation maps were generated from data that was denoised by performing spatial (r = 3 pixels) and temporal (5 frames) averaging. Activation times were found by first performing a running correlation operation on each trace with a 150-ms segment centered on an action potential upstroke captured from the middle of the imaging field and then finding the local maxima of the correlation operation. Qualitatively similar phenomena were seen in all experiments (n = 6). Results are representative examples of the observed phenomena.
RESULTS
Battery Application
Battery application during diastole: make excitation production.
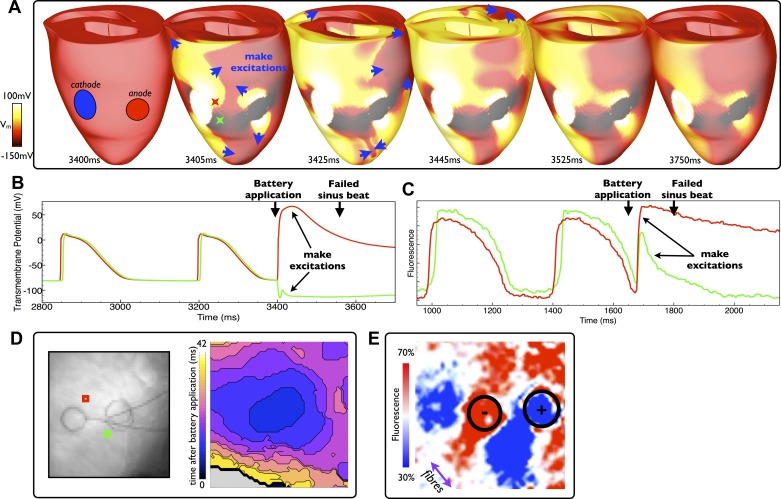
For battery application some time (>150 ms) after the last sinus beat, the ventricular tissue is excitable and both anodal and cathodal make excitations are elicited on the epicardial surface in both simulation and experiment, rapidly spreading across the ventricles, as shown in Fig. 2, A and D, respectively. As these make excitations propagate while the battery is still being applied, the make excitation wavefronts propagate through (and interact with) the static virtual electrode patterns established in the vicinity of the battery electrodes evident in the simulation images shown in Fig. 2A. A single ectopic beat results, but there is no reentry, as the wavefronts rapidly collide with one another due to their unhindered propagation pathways throughout the rest of the ventricles distal to the electrodes.
Fig. 2.
Battery application during diastole. A: epicardial distribution of Vm in the model at different times after battery application (at 3,400 ms) with make excitation wavefront propagation indicated by blue arrows. D: the experimental activation map (right) along with electrode locations (left) of excitation patterns after a similar battery application episode (at 1,680 ms). Stimulus durations were both of relatively long duration: 700 and 500 ms in the model and experiment, respectively. B and C: individual temporal model epicardial Vm (B) and fluorescent signal traces (C) from locations shown by the colored Xs in B (3,405 ms) and squares in D. Make excitation induction is seen immediately after battery application in both the model and experiment, which subsequently excites the rest of the ventricles and causes the next sinus beat to be blocked. After recovery of the tissue from this initation excitation, a static virtual electrode pattern is seen around the ventricles in the model (A, 3,750 ms) and experiment (E). The color bar scale in E is relative to fluorescent intensity of the action potential amplitude recorded during sinus pacing. The approximate fiber orientation is shown by the purple arrow.
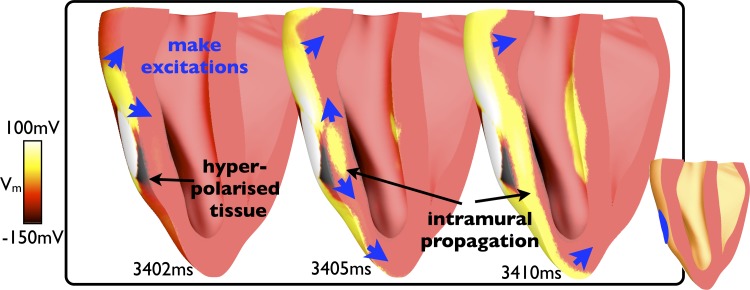
The individual respective Vm and fluorescent signal traces shown in Fig. 2, B and C, highlight the depolarization of tissue due to make excitation production in surface regions positively (virtual cathode) and negatively (virtual anode) affected by the shock (red and green traces, respectively). Noticeably, a much stronger depolarization was observed in the experimental preparation in the region negatively affected by the shock (Fig. 2C, green trace) due to intramural differences in polarization levels picked up in the fluorescent optical signal. This effect is shown in Fig. 3, which demonstrates that although the epicardial surface of the model is strongly hyperpolarized in this region, substantial make excitation wavefronts propagate intramurally through tissue that has been left excitable. Due to the well-acknowledged effect of photon scattering in the optical mapping technique, the fluorescent signal is collected from a widely distributed three-dimensional volume beneath the epicardial surface (5). Consequently, information regarding the propagation of intramural wavefronts, which pass within this scattering volume of tissue, is picked up in the optical signal, increasing the strength of the apparent depolarization recorded from this pixel in the optical trace. The phenomenon also explains the appearance of more uniform activation in the experimental maps shown in Fig. 2D.
Fig. 3.
Intramural polarization levels and wavefront propagation in battery application during diastole (shown in Fig. 2). Images show Vm levels within the three-dimensional model where a clipping plane (shown in the middle) has been used to expose the intramural tissue state beneath the cathode at different instances in time after battery application (at 3,400 ms).
Approximately 100 ms after the battery application, a sinus beat is initiated in both the model and experiment, which subsequently fails to capture as the ventricles are still refractory at this time. As the tissue recovers, a static virtual electrode pattern is established by the battery in the model (Fig. 2A, 3,750 ms). A similar static virtual electrode pattern is also evident in the experiments (Fig. 2E). Both patterns resemble the characteristic “dog bone” distribution of surface polarization under unipolar stimulation (28, 29) with regions of depolarization and hyperpolarization in the vicinity of the anode and cathode. However, due to the close proximity of the electodes, combined with the irregular ventricular geometry, a complex distribution of tissue polarization levels across the LV results. Furthermore, the well-documented effects of photon scattering (5) are also responsible for the virtual electrode pattern in the vicinity of the battery electrodes being less well defined in the experimental image shown in Fig. 2E than in the Vm simulation images shown in Fig. 2A. As shown in Fig. 3, although the epicardial surface is strongly polarized by the battery, intramural tissue directly beneath it is of intermediate polarization. Thus, the three-dimensional spatial averaging effects of the fluorescent photon scattering attenuate the strength of the apparent surface fluorescent signals, as previously described, leading to the virtual electrode pattern being significantly less distinct in the experimental image.
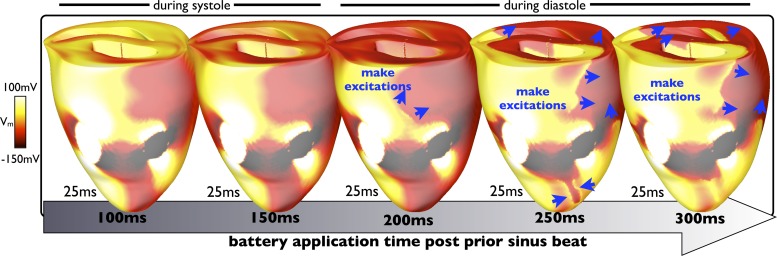
Analysis of simulation data shown in Fig. 4 demonstrates how the progression of make excitations varies after battery application when applied at different time instances (100–300 ms) after the previous sinus beat, all showing the tissue state 25 ms after battery application. When the battery is applied to relatively refractory tissue (100–150 ms), no make excitations are elicited. However, for application during diastole (200–300 ms), make excitations are elicited by the battery. The initial propagation speed of these excitations can then be seen to depend strongly on the recovered excitability of the surrounding tissue. When fully recovered (300 ms), more make excitations are elicited, which progress much further around the ventricles during the first 25 ms of battery application than when the tissue has only just regained excitability (200 ms). For example, when the battery is applied 300 ms after the sinus beat, the corresponding make excitations have propagated right around the ventricles within the first 25 ms and are beginning to depolarize the right ventricle, which is not the case when the battery is applied 200 ms after the sinus beat, in which case the wavefronts have progressed significantly less far.
Fig. 4.
Progression of make excitations after battery application in the model. Epicardial distributions of Vm at 25 ms after battery application, applied at varying instances in time (100–300 ms) after a sinus beat, are shown. As the battery is applied a longer time after the prior sinus beat (to progressively more recovered tissue), make excitations propagate faster, reaching further around the ventricles after 25 ms.
Battery application during systole: deexcitation.
For battery application soon after a sinus beat (<150 ms), surrounding refractory tissue prevents make excitation production in the model and experiment. However, in both cases, the tissue in the region negatively affected by the battery is rapidly deexcited from the plateau potential, whereas the region positively affected by the battery experiences prolonged depolarization. Continual application of the battery results in similar static virtual electrode patterns being established on the epicardial surface in tissue proximal to the electrodes. A few hundred milliseconds after battery application, a sinus beat is initiated, which is successfully captured due to tissue distal to the electrodes having regained excitability. The resulting wavefronts propagate around, and interact with, the virtual electrode pattern established by the battery, in both cases causing brief depolarization of the tissue close to the electrodes.
Reentry initiation.
Self-sustained reentrant activity could not be induced in the model or in any of the experimental cases during the process of battery application, neither during systole nor diastole. A brief interaction of sinus beat wavefronts with the static virtual electrode pattern established by the battery also did not induce reentry. A steady state thus took hold, periodically disturbed by sinus beats.
Battery Release
Battery release during diastole: break excitation production.
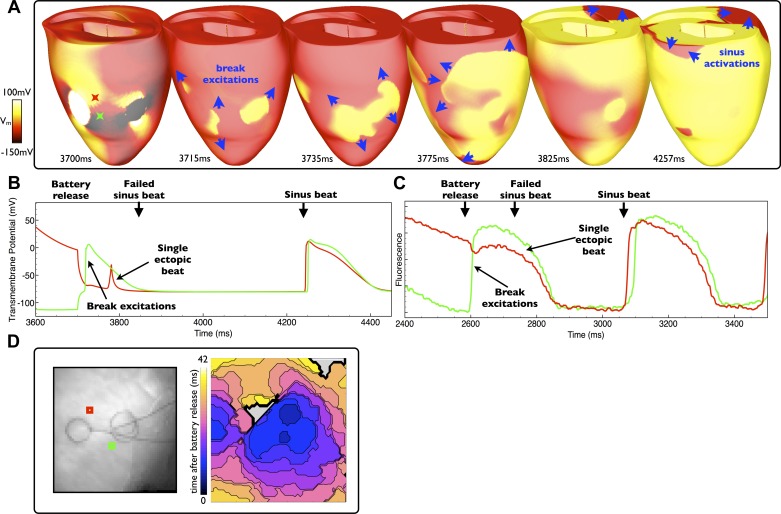
For battery release during diastole, tissue distal to the electrodes has fully regained excitability with only tissue in the vicinity of the electrodes strongly polarized due to the action of the battery electrodes before removal. Upon cessation of the stimulus, the virtual anodes and virtual cathodes induced by the battery electrodes initiate both anodal and cathodal break excitation wavefronts (29). Similar to make excitation production upon application, the break excitations induced upon battery release during diastole result in a single ectopic beat, as shown in Fig. 5, A and D, for the model and experiment, respectively, and in the corresponding individual model Vm and experimental fluorescent signal traces, shown in Fig. 5, B and C, from regions positively/negatively (red/green traces) affected by the battery. In both the simulation and experiment, propagation is initially highly nonuniform as the break excitations interact with neighboring refractory tissue due to the virtual electrode pattern established by the battery. Contrary to the case of make excitation propagation, however, the virtual electrode pattern begins to disappear upon stimulus termination. Upon release, as ventricular tissue distal to the electrodes has fully regained excitability, the induced break excitation wavefronts (blue arrows in Fig. 5A) rapidly sweep across the rest of the ventricles unhindered, colliding with each other and self-terminating. Activation of the rest of the ventricles by the break excitation wavefronts causes a single ectopic beat, leaving the tissue refractory when the next sinus beat occurs (some 150 ms after release), which consequently fails to capture. Once the tissue excited by the break excitation has recovered, however, the next sinus beat is able to capture as normal, as demonstrated by the individual traces from both the simulation and experiment shown in Fig. 5, B and C, respectively.
Fig. 5.
Battery release during diastole. A: epicardial distribution of Vm in the model at different times after the battery release (at 3,700 ms) with induced break excitation wavefront propagation indicated by blue arrows. D: the experimental activation map (right) along with electrode locations (left) of excitation patterns after a similar battery release (at 2,600 ms). B and C: individual temporal model epicardial Vm (B) and fluorescent signal traces (C) from locations shown by the colored Xs in B (3,700 ms) and squares in D. Battery release induces break excitations that activate the entire ventricles, resulting in a single ectopic beat (but no reentry) in both the simulation and experiment. After the ectopic, sinus rhythm returns in both cases.
Battery release during systole.
For longer battery application durations, the sinus beat may capture while the battery is still being applied (as described in Battery Application During Systole: Deexcitation). In these circumstances, when the battery is subsequently released soon after such sinus beat capture (∼100 ms after), much of the tissue away from the electrodes (and intramurally) is refractory. Thus, upon release, break excitations can only propagate into the hyperpolarized tissue immediately in the vicinity of the electrodes, upon which they rapidly interact with unexcitable tissue and terminate.
Conditions for Reentry Induction During Short Battery Applications
Interaction of make and break excitations.
Extensive investigations using the computational model allowed us to uncover the conditions under which reentry was most likely induced within rabbit ventricles by the battery. Such conditions involved the relatively short (<150 ms) battery applications in cases where the battery was initially applied to the tissue during diastole, thus successfully inducing make excitations that propagated across the ventricles. Releasing the battery a relatively short time after make excitation initiation allowed the resulting break excitations to initially propagate, but, importantly, allowed them to also interact with the previous make excitation wavefronts themselves, in addition to the heterogeneous distribution of refractory tissue that the make excitations leave in their wake. Such an interaction results in the slowing of the break excitation wavefronts and nonuniform conduction block as they encounter refractory tissue, restricting their pathways, providing the necessary substrate to encourage reentry. Of the four different short battery application durations simulated (50, 75, 100, and 150 ms), those applied to diastolic tissue (states 200, 250, and 300 ms postsinus beat), successfully induced initial reentrant circuits in 9 of 12 cases (75%), whereas longer application durations failed to induce. In the experiments, 12 of 16 (75%) of short-duration shocks (125 ms) applied to diastolic tissue (225–325 ms postsinus beat) successfully induced reentry.
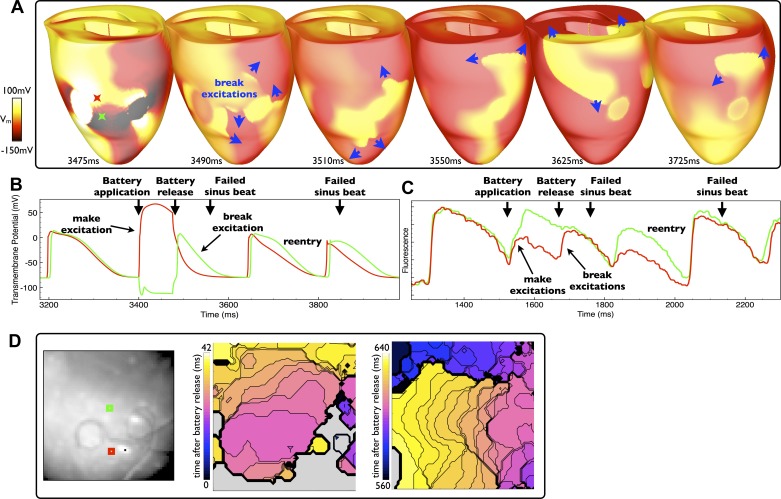
An example of reentry induction via a short battery application is shown in Fig. 6 for the simulation and experiment. Figure 6, A and D, shows the induction of break excitation wavefronts upon battery release, their subsequent convoluted pathways, and the formation of self-sustained reentry. In both cases, the battery was applied during diastole and was applied for a relatively short duration. Therefore, not only were make excitations elicited upon battery application, but also the subsequent break excitations elicited upon battery release successfully interacted with the refractory tails of the prior make excitations, causing the break excitations to slow and reenter. The slowing of initial break excitations and their nonuniform propagation patterns is evident in Fig. 6A (3,490–3,550 ms) as well as Fig. 6D (first experimental activation map). Most evident in the simulation images is that both cathodal and anodal break excitation wavefronts are initiated that begin by propagating into the virtual anode regions associated with each individual electrode. As the surrounding tissue is still partially refractory, critical points are established as the break excitations begin to propagate. As the tissue regains its excitability, the break excitation wavefronts pivot around these critical points and start to reenter (28). Furthermore, the individual Vm and fluorescent signal traces shown in Fig. 6, B and C, respectively, also show blockage of sinus beats in both the simulation and experiment, both immediately after battery release (when the initial break excitations are active) as well as later during self-sustained reentry.
Fig. 6.
Reentry induction due to the interaction of make and break excitations. A: epicardial distribution of Vm in the model after a relatively short application (duration: 75 ms), applied during diastole, with induced break excitation wavefront propagation indicated by blue arrows. D: two experimental activation maps (right) along with electrode locations (left) of excitation patterns after a similar episode of battery release (application duration: 146 ms). B and C: individual temporal model epicardial Vm (B) and fluorescent signal traces (C) from the locations shown by colored Xs in B (3,475 ms) and squares in D. In both the model and experiment, the battery is released sufficiently soon after application such that the induced break excitations (upon release) interact with the refractory tails caused by the prior make excitations, facilitating reentry.
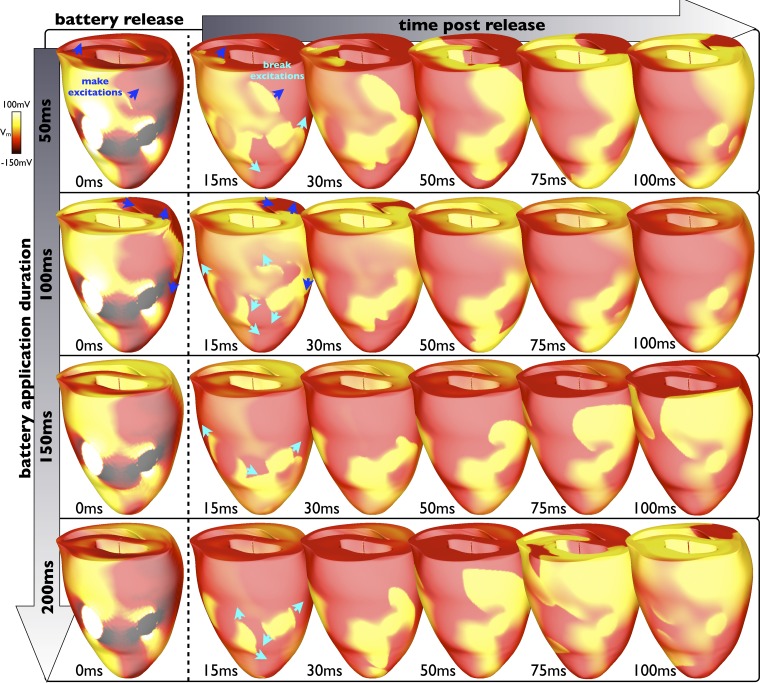
The specific duration of battery application (when applied during diastole such that make excitations are successfully induced) is very important as it governs the time between make and break excitation production. Figure 7 shows this interaction, showing the progressive propagation of make excitation wavefronts during battery application (images down the first column), before its release for different application durations. Shown along each row are the subsequent interactions of the resulting break excitations with these make excitations for different battery release times. The battery was applied at the same time (a point in diastole) in each row. Each row therefore shows the evolution of activity when that battery is applied for different durations. For short battery applications (50–150 ms), make excitations (dark blue arrows) are still active within the ventricles when the break excitations begin to propagate (turquoise arrows), slowing their initial propagation and restricting their paths. The different stage of make excitation wavefront progression at the point of battery release in these cases is thus seen to govern the initial propagation speed and pathways taken by the break excitations, evident by comparing the evolution of break excitation wavefronts between the 15- and 50-ms images along each row (for battery application durations of 50, 100, and 150ms). The specific stage of make excitation wavefront propagation at the time of release therefore dictates the heterogeneous substrate that causes reentry. Again, establishment of critical points can be seen here for all cases as the break excitations begin by propagating into the virtual anodes associated with the electrodes upon cessation of the battery stimulus, which encourages the waves to initially reenter.
Fig. 7.
Interaction of make and break excitations. Epicardial Vm distributions show the evolution of break excitation wavefronts at different time instances in the first 100 ms (columns) after battery release for different total battery application durations from 50 to 200 ms (rows) when initially applied 200 ms after the previous sinus beat (during diastole). Make excitations are shown by blue arrows; break excitations are shown by turquoise arrows. As the battery is applied for longer (going down the first column), the make excitations induced upon its application have advanced further and the ventricles are progressively more recovered when the battery is released, allowing the subsequent induced break excitations to propagate more freely, thus encountering less refractory tissue.
Interaction of break excitations with prior refractory tissue.
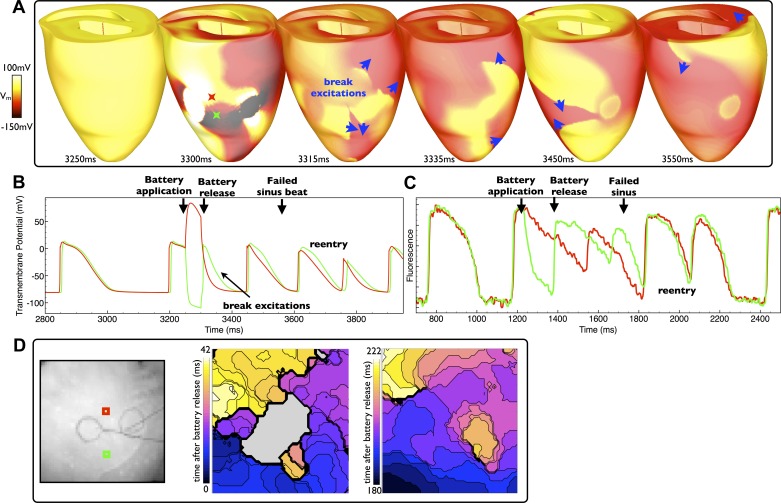
For battery application during systole, no make excitations are induced for the break excitations to interact with. However, upon battery release, break excitations may still be elicited, propagating initially into the hyperpolarized regions of tissue proximal to the electrodes deexcited by the application of the battery. An example of such an effect is shown in Fig. 8 in both the model and experiment. Figure 8A shows that the ventricles are still refractory upon battery application (3250 ms), which was applied just 100 ms after a previous sinus beat. Upon release (just 50 ms after the application, at 3,300 ms), break excitations (blue arrows) initially progress into the regions deexcitated and hyperpolarized by the action of battery (virtual anodes). Due to the fact that the battery was applied during systole, tissue distal from the electrodes, unaffected by the virtual electrodes established in the vicinity of the battery, remains relatively refractory, thus slowing the propagation of the break excitations as they attempt to capture the rest of the myocardium (3,335- and 3375-ms images). Such slowing of the progression of break excitation wavefronts provides sufficient time for both the refractory tissue depolarized by the action of the battery in addition to tissue distal to the electrodes activated by the prior sinus beat to recover, allowing the break excitation wavefronts to propagate into these regions before reentering. Figure 8B shows the corresponding individual Vm, as before, demonstrating the production of break excitations only into the region negatively affected by the battery (green trace). Such a mechanism of reentry induction was more likely for short-duration shocks applied during systole. For example, of the four different short battery application durations simulated (50, 75, 100, and 150ms), those applied to systolic tissue (states 0, 50, and 100 ms postsinus beat) successfully induced initial reentrant circuits in 7 of 12 cases (58%), of which 5 cases were for the shorter shocks (50 and 75ms) and 2 cases for the relatively longer shocks (100 and 150 ms). In this case, longer battery applications interacted with the next sinus activation, and thus reentry induction was governed by the mechanism discussed below in Conditions for Reentry Induction During Longer Battery Application. In the experiments, 7 of 17 (41%) of short-duration shocks (125 ms) applied to systolic tissue (<150 ms postsinus beat) successfully induced reentry. A similar example of the induction of a number of reentrant cycles in the experimental model is shown in Fig. 8D for battery application during systole (50 ms after sinus beat). Activation maps highlight the initial complex propagation patterns of break excitations followed by the later establishment of a reetrant circuit. Similar reentrant behavior to that witnessed in the model is also evident in the fluorescent signal trace shown in Fig. 8C, which also shows the production of break excitations only into the virtual anode region.
Fig. 8.
Reentry induction after battery application during sysole. A: epicardial distribution of Vm in the model after a relatively short application (duration: 50 ms), applied during systole, with induced break excitation wavefront propagation indicated by blue arrows. D: two experimental activation maps (right) along with electrode locations (left) of excitation patterns after a similar episode of battery release (application duration: 130 ms). B and C: individual temporal model epicardial Vm (B) and fluorescent signal traces (C) from the locations shown by colored Xs in B (3,300 ms) and squares in D. As the battery is applied to refractory tissue, no make excitations are produced. However, both the model and experiment show rapid deexcitation of tissue negatively affected by the shock (virtual anode) in additon to depolarization of the tissue positively affected by the shock (virtual cathode). The relatively short battery application time means that break excitations elicited upon battery release interact with tissue still recovering from the prior sinus beat, providing the substrate for reentry.
Conditions for Reentry Induction During Longer Battery Application
Antiarrhythmogenic conditions.
For battery release a long time (200 ms) after application to diastolic tissue, all make excitation wavefronts have died away and the heterogeneous refractory tissue that they established in tissue distal from the ventricles has also recovered (3,750-ms image shown in Fig. 2A). Upon release, the break excitations are then free to sweep around the ventricles in a faster-moving, larger wavefront whose path is not restricted by heterogeneous refractory tissue, rapidly exciting the rest of the ventricles and self-terminating (as shown in Figs. 6 and 7).
For longer battery applications applied to systolic tissue, the tissue left refractory by the prior sinus beat regains excitability during the battery application period, leaving the rest of the ventricles relatively homogeneously excitable. In this case, battery release induces break excitations that again simply sweep across the ventricles unrestricted, inducing only a single ectopic, but no reentry.
Interaction of break excitations with sinus beat.
For longer battery application durations, although any make excitations have died away, the induction of short-lived cycles of reentry was still possible through the interaction of the break excitations with sinus activations. However, the majority of release timings during sinus rhythm resulted in early termination of reentry.
In such a scenario that a sinus beat captures during battery application, if the battery is then released soon after this sinus activation, the majority of the tissue is still refractory. The break excitations upon battery release then either fail to propagate or rapidly annihilate with the sinus activation wavefronts. For battery removal just before a sinus beat, break excitations rapidly render the majority of the ventricular tissue refractory and, in addition to anterograde propagation up the PS, means that the next sinus beat fails to capture (shown in Fig. 5, B and C).
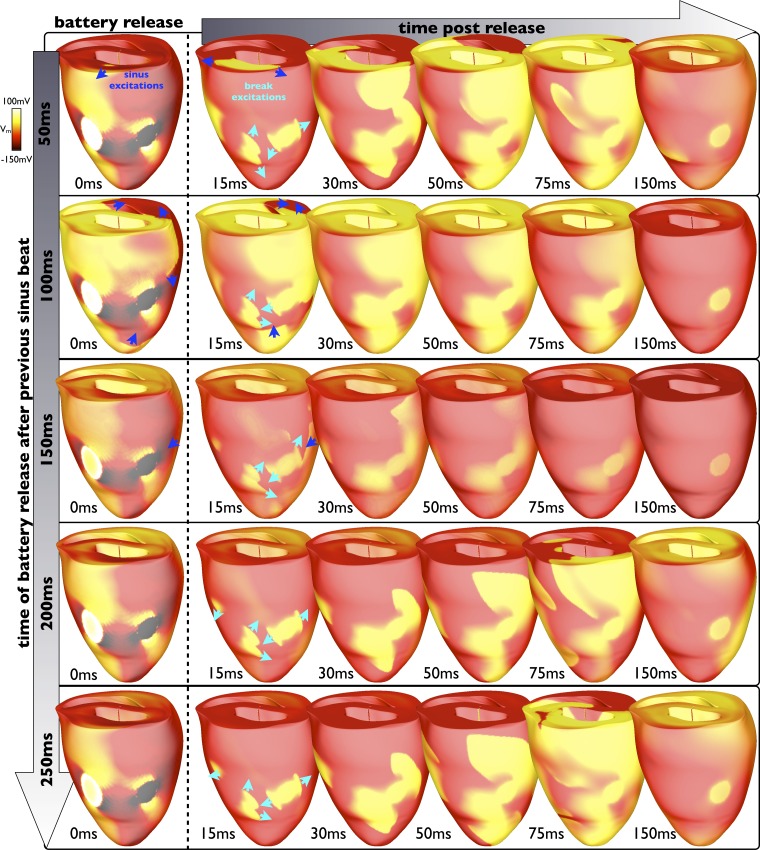
However, if the battery is released a longer time (>150 ms) after the previous sinus beat (although not too close to the next sinus beat), much of the ventricular tissue distal to the electrodes has regained excitability, allowing break excitations to propagate more freely. Initial reentrant cycles are established, but they tend to terminate quickly. An example of such a mechanism is shown in Fig. 9. Figure 9 shows different examples of the evolution of break excitations (along the rows) when the battery is released at different times after the previous sinus beat (images down the first column). As can be seen, when the battery is released after 50, 100, or 150 ms after the previous sinus beat, although break excitations are successfully initiated (turquoise arrows), their propagation is hindered via an interaction with sinus activation wavefronts (blue arrows) and their corresponding refractory tails, leading to the rapid termination of activity. However, when the battery is released 200 or 250 ms after the previous sinus beat, the sinus activations have passed, and the tissue has recovered sufficiently to allow the break excitation wavefronts to propagate more readily. Initial cycles of reentry are induced (as shown in the 75-ms images of rows corresponding to 200 and 250 ms after battery release after the previous sinus beat) as the activations are still slowed somewhat by the tissue recovering from the sinus beat, although sustained reentry is not induced.
Fig. 9.
Interaction of break excitations with sinus activations. Epicardial Vm distributions show the evolution of break excitation wavefronts at different time instances in the first 150 ms (columns) after battery release at different times between 50 and 250 ms after the previous PS sinus beat (having occurred during battery application). The battery was initially applied 150 ms after a sinus beat; thus, total application durations ranged from 250 to 450 ms. If the battery is released too soon after the previous sinus beat (<150 ms), the induced break excitations have little excitable tissue to propagate into and thus rapidly terminate. If released later after the previous sinus beat (>150 ms), the distal tissue has recovered sufficiently to allow initial break excitation propagation, which is slowed slightly, facilitating reentry.
DISCUSSION
Using advanced computational modeling combined with high-resolution optical mapping measurements, we have uncovered the hitherto unknown conditions under which DC from a battery interacts with myocardial tissue to induce reentry within rabbit ventricles. We have further presented our findings regarding the highly complex interaction and dependence of these mechanisms upon battery application timing relative to sinus activation and battery application duration.
Summary of Arrhythmogenic Mechanisms
In the rabbit, although self-sustained VF was not induced at all in the model or in the experiment with battery application, relatively sustained cycles of reentry were inducible. In all cases of arrhythmia induction, reentry was initiated due to the interaction of break excitations (produced by both anodal and cathodal electrodes upon battery release) with:
1. Refractory tissue due to prior make excitations (elicited upon battery application), requiring relatively short battery applications applied to diastolic tissue;
2. Refractory tissue due to prior sinus beats occurring either before the battery was applied (requiring relatively short battery applications to systolic tissue) or occurring during the battery application (requiring relatively longer application durations).
Reentry was induced in these circumstances as the break excitation wavefronts were slowed and forced to take convoluted pathways upon interaction with the heterogeneous distributions of refractory tissue. Such refractory tissue was located both proximal to the battery (due to the direct effect of the virtual electrodes induced by the battery itself) and distal to the battery (due to the complex propagation patterns of the prior make excitations and sinus beats). The identification and dissection of these arrhythmogenic mechanisms that are highly specific to the application of DC via a 9-V battery to the ventricles represent novel and intriguing findings uncovered in this study.
It should also be noted that central to the initiation of reentry in all the cases studied here is the initial establishment of critical points at the border of virtual anode and virtual cathode regions associated with each electrode. At the end of the stimulus, anodal and cathodal break excitation wavefronts initially propagate into the virtual anode regions of tissue forming critical points with the neighboring refractory tissue, which was depolarized by the stimulus (28). These critical points provide the substrate around which the break excitations begin to reenter. Due to the close proximity of the battery electrodes, combined with the heterogeneous distribution of refractory tissue (from prior make excitations or sinus beats), many of these initial reentrant waves rapidly terminate either through interaction with oneanother or with inexcitable tissue. Those that survive, however, provide the foundation for sustained reentrant activity.
Other mechanisms do not appear to be necessary for reentry induction. The battery produces a single make excitation (if applied to diastolic tissue) and a single break excitation when released, so repetitive discharges do not occur. The virtual electrode pattern is essentially static, developing over a few milliseconds, and is large enough that it provides an obstacle with which sinus wavefronts may propagate around. Ionic gradients are not necessary as qualitatively no mechanistic differences were seen using an electrophysiologically homogeneous model (data not shown). The PS was required for delivering the sinus pulse that could interact with the battery excitations in some cases to produce reentry, but it was not a special component of the ensuing reentrant circuit. Mechanical aspects were not considered, namely, stretch-activated channels. These may be opened near the electrode near the boundary of the tissue persistently depolarized by the battery. However, due to the static nature of the response and the fact that an action potential is generated regardless, and more importantly, that arrhythmias can reliably be induced in motion arrested hearts with a 9-V battery, it would not appear that such mechanical effects would alter the outcomes of this study significantly.
Shorter battery applications.
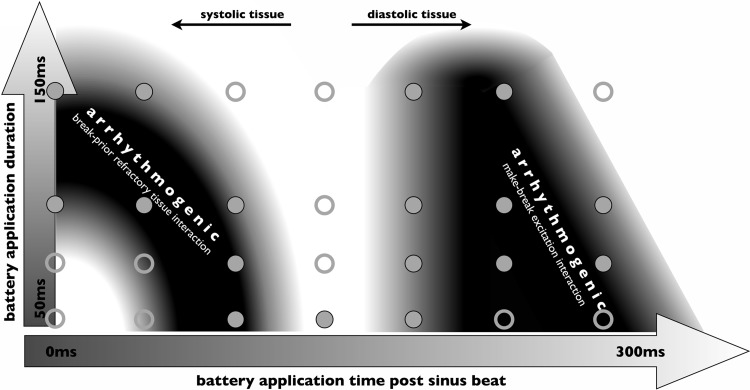
The arrhythmogenic and antiarrhythmogenic mechanisms described in this study after short battery application durations are summarized in Fig. 10. Figure 10 shows an intentionally schematic representation of the qualitative results uncovered in this study from both the model and experiment demonstrating how vulnerability to reentry induction depends strongly on both the timing of the application relative to the prior sinus beat and the duration of the application itself. Reentry can be induced when the battery is applied during systole in the absence of make excitations (which are not induced upon battery application, as tissue is refractory). Here, relatively longer applications are required when the initial application occurs very soon after the sinus beat, as this allows more time for the refractory tissue resulting from the sinus activations to recover, allowing break excitations to propagate in the rest of the ventricles upon release. However, the application must still be sufficiently short such that the break excitations are slowed by tissue that has not fully regained excitability after the sinus beat.
Fig. 10.
Schematic summary of vulnerability to reentry induction after short battery application durations for simulations. The dependence of different arrhythmogenic mechanisms to battery application timing after the previous sinus beat and for different application durations is highlighted. Solid circles demonstrate successful induction in simulations; open circles demonstrate failed reentry attempts.
Reentry is also likely induced due to the interaction between make and break excitations after battery application during diastole. In this case, the break excitation wavefronts interact with the repolarization tail of the prior wave that has been induced by the make excitation. When applied a longer time after the previous sinus beat, the make excitations have propagated more rapidly through the ventricles as the rest of the tissue has regained more excitability, meaning that battery application duration needs to be shorter to ensure a sufficient interaction between make and break excitations.
Longer battery applications.
For longer battery application durations, all tissue refractory from the prior sinus beat and that tissue refractory from the potential make excitations (if applied to diastolic tissue) is recovered. Break excitations will thus propagate relatively uniformly and unhindered upon release, again leading to a single ectopic, but no reentry, unless they interact with additional sinus beats occurring during battery application. However, often the break excitations either directly interact with sinus wavefronts (if released just after sinus capture) and mutually annihilate or else get blocked by relatively homogeneously refractory tissue established by the sinus wavefronts captured during application. Nonetheless, such interactions were frequently noted to be sufficiently complex to result in one to two cycles of reentry, although they were generally not sufficiently complex to induce fully self-sustained reentry here in the rabbit. The interaction of break excitations with prior make excitations is more arrhythmogenic than with prior sinus activation wavefronts due to the nonuniformity of the make excitation propagation pathways, relative to the more spatially uniform nature of sinus activation patterns, even in the presence of the virtual electrode pattern induced by the battery.
Relation to Other Species
The focus of this study was healthy rabbit ventricles, which are widely recognized to be difficult to induce sustained VF in (3, 12, 14). The lack of VF induction in our simulations and experiments likely explains the lack of evidence in the literature for the use of a 9-V battery for such purposes in the rabbit, as opposed to its widespread use in species such as the pig (8, 9, 17, 22) and dog (11, 24), which are known to be easier in which to induce more complex arrhythmias (25) due to their relatively larger effective electrical size (19). Therefore, the larger effective size of other species may prevent the rapid self-termination of initial reentrant cycles induced by the battery, which may then likely degenerate into more complex, self-sustained fibrillatory-like activity. We believe that the fundamental interaction of the battery with the myocardial tissue to produce make/break excitations and their subsequent interactions with one another and with sinus activations highlighted here will hold and be equally as relevant across different species, as such phenomena are governed primarily by the bidomain nature of cardiac tissue (28, 29).
Application to Practical Use Within the Experimental Laboratory
The practical use of a 9-V battery for VF induction is often conducted in a less controlled environment than that represented in the simulations and experimental setups presented in this study. Creating a constant, steady contact of the battery electrodes with the epicardial surface is often difficult due to movement of the heart, which is particularly problematic in a Langendorff setup and in the absence of mechanical uncouplers. In reality, this will result in slight movements of the battery electrodes, periodically causing them to lose contact with the surface. Consequently, this will result in the production of additional make/break excitations each time contact is lost and reestablished. Despite this significantly more complex, and less predicable, scenario, we believe that the fundamental mechanisms of make/break excitation interaction uncovered in this study will be the driving force leading to reentry initiation.
Furthermore, in the practical use of the battery, it is often necessary to slightly “jiggle” the battery to induce sustained arrhythmia in human Langendorf preparations (personal observations, K. Nanthakumar). We have performed test simulations of this action (data not shown) and seen how such a jiggling of the battery back and forth produces a similar induction of make/break excitations upon each individual movement. Despite the witnessed complex interaction between make and break excitations after each successive movement, the propagation dynamics of the final set of break excitations still depends strongly on the duration of the final application and timing of release. Successive back-and-forth movements may also induce complex activations during the application, which, in more arrhythmogenic species, may indeed be sufficient to increase the likelihood of sustained reentry induction either through make-break excitation interactions or interactions with sinus activations.
Study Limitations
Due to restrictions in mesh resolution of our unstructred finite-element mesh, we modeled the battery electrodes in the simulations as solid “disks” on the epicardial surface, whereas, experimentally (and using a real 9-V battery), these electrodes are rings of the same diameter but of ∼1-mm width. We have performed test simulations using higher-resolution slab meshes that have demonstrated the very close similarity in Vm distribution patterns around the electrodes during battery application when represented by either rings or disks. These similarities are due to the fact that the area within the disk is effectively caged, so its activity has little effect, in addition to the established edge effects of disk electrodes (20) from where current mostly flows.
Furthermore, our model does not include a representation of electrode polarization impedance at the tissue/metal interface (21), which may slightly affect current flow from the electrodes during battery application/removal. However, as has been shown experimentally (18), the effects of electrode polarization impedance are most relevant for weaker shocks, below the normal anode make threshold, where this effect can then cause artifactual anode break excitations. In our case, the stimulus strength was always sufficiently strong to cause anode make and break excitations to diastolic tissue (without including electrode polarization impedance), and, thus, this small additional effect would not be expected to significantly affect our results. This issue is further supported by the close match between the make/break excitation phenomena witnessed here between the simulation and experiment.
Conclusions
Through close agreement of model simulations and experimental data, we demonstrate the mechanism of reentry induction by DC application through a 9-V battery as involving the interaction of break excitations upon battery release with refractory tissue from prior make excitations elicited upon battery application or prior sinus beats. Reentry induction was most likely during shorter battery applications (<150 ms) and interactions with prior make excitations. Although not witnessed here in healthy rabbit ventricles, degeneration into sustained VF is likely in other more arrhythmogenic species.
GRANTS
M. J. Bishop acknowledges support from a Sir Henry Wellcome Postdoctoral Fellowship and the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and Engineering and Physical Sciences Research Council (Grant WT 088641/Z/09/Z). R. A. B. Burton is an EP Abraham Cephalosporin Junior Research Fellow, Linacre College (Oxford, UK), and acknowledges support from the British Heart Foundation. G. Plank acknowledges Austrian Science Fund Grant F3210-N18 and National Heart, Lung, and Blood Institute Grant RO1-HL-10119601. G. Bub acknowledges support from the British Heart Foundation Centre of Research Excellence (RE/08/004). E. J. Vigmond is supported by the Government of France through the Agence National de la Recherche programme “Investissements d'Avenir” with Grant ANR-10-IAHU-04.
DISCLAIMER
The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.B. and E.J.V. conception and design of research; M.J.B., R.-A.B.B., M.K., and G.B. performed experiments; M.J.B., G.B., and E.J.V. analyzed data; M.J.B., K.N., G.P., G.B., and E.J.V. interpreted results of experiments; M.J.B. and G.B. prepared figures; M.J.B., G.B., and E.J.V. drafted manuscript; M.J.B., R.-A.B.B., K.N., G.P., G.B., and E.J.V. edited and revised manuscript; M.J.B., K.N., G.P., G.B., and E.J.V. approved final version of manuscript.
ACKNOWLEDGMENTS
M. J. Bishop acknowledges the support of the National Institute for Health Research Biomedical Research Centre at Guy's and St Thomas' National Health Service Foundation Trust and King's College London.
REFERENCES
- 1.Ashihara T, Trayanova NA. Asymmetry in membrane responses to electric shocks: insights from bidomain simulations. Biophys J 87: 2271–2282, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslanidi O, Sleiman R, Boyett M, Hancox J, Zhang H. Ionic mechanisms for electrical heterogeneity between rabbit Purkinje fiber and ventricular cells. Biophys J 98: 2420–2431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop MJ, Plank G. The role of fine-scale anatomical structure in the dynamics of reentry in computational models of the rabbit ventricles. J Physiol 590: 4515–4535, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop MJ, Vigmond EJ, Plank G. The functional role of electrophysiological heterogeneity in the rabbit ventricle during rapid pacing and arrhythmias. Am J Physiol Heart Circ Physiol 304: H1240–H1252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop MJ, Bub G, Garny A, Gavaghan DJ, Rodriguez B. An investigation into the role of the optical detection set-up in the recording of cardiac optical mapping signals: a Monte Carlo simulation study. Physica D 238: 1008–1018, 2009 [Google Scholar]
- 6.Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol 255: 335–346, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deo M, Boyle P, Plank G, Vigmond EJ. Arrhythmogenic mechanisms of the Purkinje system during electric shocks: a modeling study. Heart Rhythm 6: 1782–1789, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euler DE, Whitman TA, Roberts PR, Kallok MJ. Low voltage direct current delivered through unipolar transvenous leads: an alternate method for the induction of ventricular fibrillation. Pacing Clin Electrophysiol 22: 908–914, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Cheng KA, Dosdall DJ, Smith WM, Ideker RE. Role of maximum rate of depolarization in predicting action potential duration during ventricular fibrillation. Am J Physiol Heart Circ Physiol 293: H2530–H2536, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser S, Weirich J, Antoni H. Influence of direct current on the electrical activity of the heart and on its susceptibility to ventricular fibrillation. Bas Res Cardiol 77: 237–249, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Kong W, Ideker RE, Fast VG. Transmural optical measurements of Vm dynamics during long-duration ventricular fibrillation in canine hearts. Heart Rhythm 6: 796–802, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou Q, Li W, Efimov IR. The role of dynamic instability and wavelength in arrhythmia maintenance as revealed by panoramic imaging with blebbistatin vs. 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol 302: H262–H269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan A, Shiferaw Y, Sato D, Baher A, Olcese R, Xie LH, Yang MJ, Chen PS, Restrepo JG, Karma A, Garfinkel A, Qu Z, Weiss JN. A rabbit ventricular action potential model replicating cardiac dynamics at rapid heart rates. Biophys J 94: 392–410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manoach M, Netz H, Erez M, Weinstock M. Ventricular self-defibrillation in mammals: age and drug dependence. Age Ageing 9: 112–116, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Moreno J, Zaitsev A, Warren M, Berenfield O, Kalifa J, Lucca E, Mironov S, Guha P, Jalife J. Effect of remodelling, stretch and ischaemia on ventricular fibrillation frequency and dynamics in a heart failure model. Cardiovasc Res 65: 158–166, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Nair K, Umapathy K, Farid T, Masse S, Mueller E, Sivanandan RV, Poku K, Rao V, Nair V, Butany J, Ideker RE. Intramural activation during early human ventricular fibrillation. Circ Arrhyth Electrophysiol 4: 692–703, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Nanthakumar K, Huang J, Rogers JM, Johnson PL, Newton JC, Walcott GP, Justice RK, Rollins DL. Regional differences in ventricular fibrillation in the open-chest porcine left ventricle. Circ Res 91: 733–740, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Nikolski V, Sambelashvilli A, Efimov IR. Anode-break excitation during end-diastolic stimulation is explained by half-cell double layer discharge. IEEE Trans Biomed Eng 49: 1217–1220, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Panfilov AV. Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts? Heart Rhythm 3: 862–864, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Patel SG, Roth BJ. How electrode size affects the electric potential distribution in cardiac tissue. IEEE Trans Biomed Eng 47: 1284–1287, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Patel SG, Roth BJ. How epicardial electrodes influence the transmembrane potential during a shock. Ann Biomed Eng 29: 1028–1031, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Robertson PG, Huang J, Chen KA, Chen X, Dosdall DJ, Tabereaux PB, Smith WM, Ideker RE. Increased cycle length during long-duration ventricular fibrillation is caused by decreased upstroke velocity as well as prolonged refractoriness. Heart Rhythm 6: 378–384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma AD, Fain E, O'Neill PG, Skadsen A, Damie R, Baker J, Chauhan V, Mazuz M, Ross T, Zhang Z. Shock on T versus direct current voltage for induction of ventricular fibrillation. Pacing Clin Electrophysiol 27: 89–94, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Tabereaux PB, Dosdall DJ, Ideker RE. Mechanisms of VF maintenance: wandering wavelets, mother rotors, or foci. Heart Rhythm 6: 405–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ten Tusscher KH, Mourad A, Nash MP, Clayton RH, Bradley CP, Paterson DJ, Hren R, Hayward M, Panfilov AV, Taggart P. Organization of ventricular fibrillation in the human heart: experiments and models. Exp Physiol 94: 553–562, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Vigmond EJ, Hughes M, Plank G, Leon L. Computational tools for modeling electrical activity in cardiac tissue. J Electrocardiol 36: 69–74, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Weismuller P, Richter P, Binner L. Direct current application: easy induction of ventricular fibrillation for the determination of the defibrillation threshold in patients with implantable cardioverter defibrillators. Pacing Clin Electrophysiol 15: 1137–1143, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Wikswo JP, Roth BJ. Virtual electrode theory of pacing. In: Cardiac Bioelectric Therapy Mechanisms and Practical Implications, edited by Efimov IR, Kroll MW, Tchou PJ. New York: Springer, 2009, p. 283–330 [Google Scholar]
- 29.Wikswo JP, Lin SF, Abbas RA. Virtual electrodes in cardiac tissue: a common mechanisms of anodal and cathodal stimulation. Biophy J 69: 2195–2210, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]