Abstract
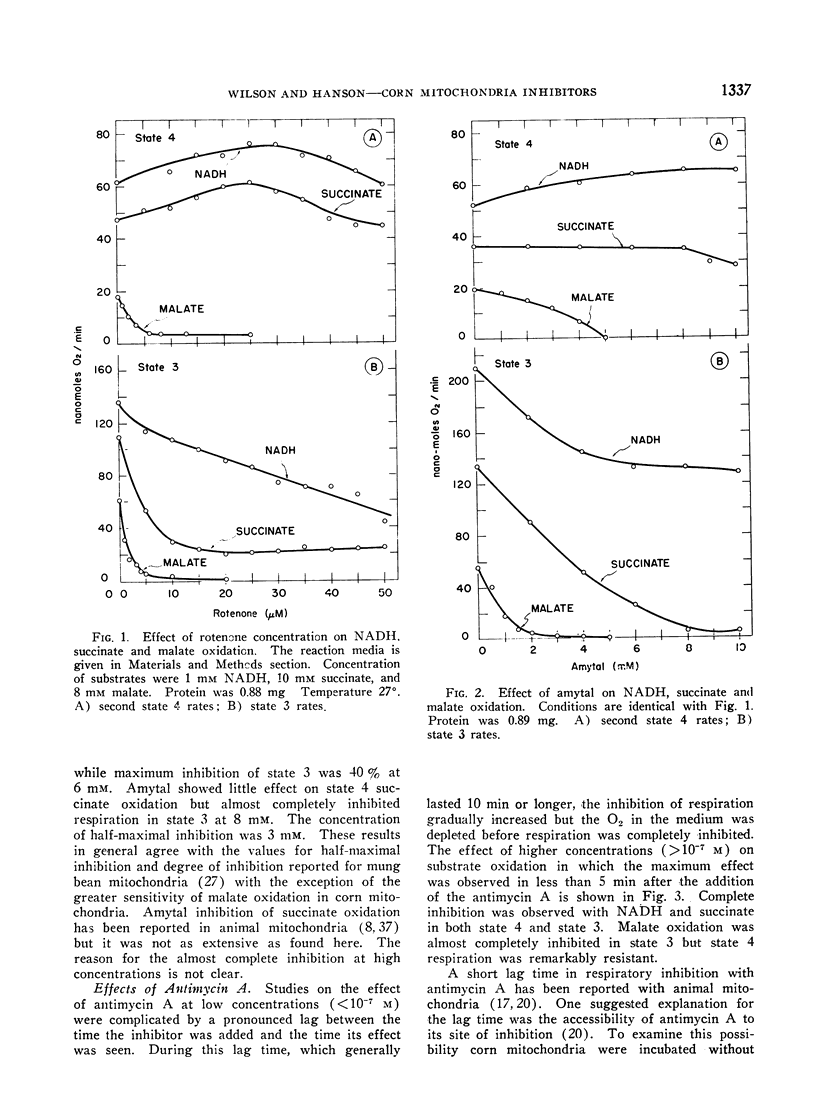
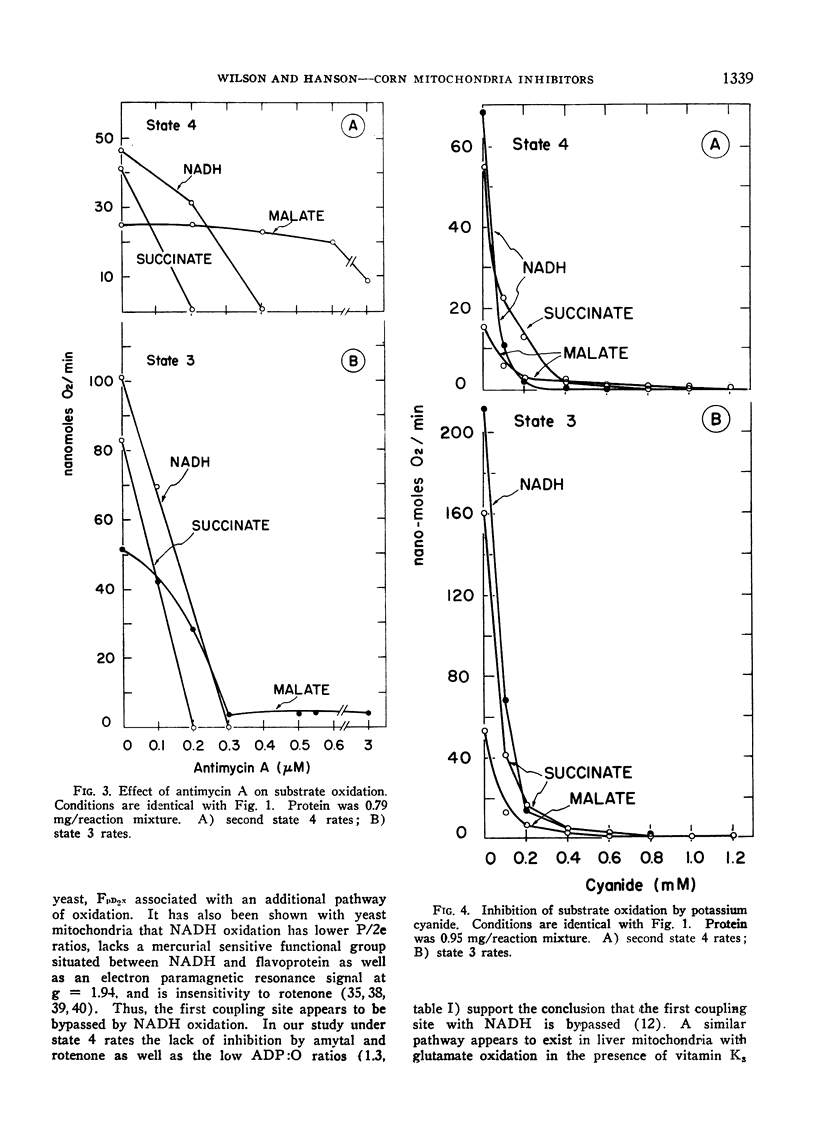
The effect of a series of respiratory inhibitors on the oxidation of NADH in state 4 and state 3 conditions was studied with corn shoot mitochondria. Comparisons were made using malate and succinate as substrates. The inhibitors, rotenone, amytal, antimycin A and cyanide, inhibited oxidation of NADH in state 3 but rotenone and amytal did not inhibit oxidation in state 4. The inhibition by antimycin A was partially overcome by the presence of cytochrome c. The results indicate the presence of alternative pathways available for NADH oxidation depending on the metabolic condition of the mitochondria. Under state 4 conditions, NADH oxidation bypasses the amytal and rotenone sensitive sites but under state 3 conditions a component of the NADH respiration appears to be oxidized by an internal pathway which is sensitive to these inhibitors. Still a third pathway for NADH oxidation is dependent on the addition of cytochrome c and is insensitive to antimycin A. Succinate oxidation was sensitive to cyanide and antimycin A under both state 4 and state 3 conditions as well as amytal and rotenone under state 3 conditions but was not inhibited by amytal and rotenone under state 4 conditions. Malate oxidation was inhibited by cyanide, rotenone and amytal under both state 4 and state 3 conditions. Antimycin A inhibited state 3 but did not appreciably alter state 4 rates of malate oxidation. With all substrates tested inhibition by antimycin A was greatly facilitated by preswelling the mitochondria for 10 min. This was interpreted to indicate that swelling increases the accessibility of antimycin A to the site of inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVI-DOR Y., TRAUB A., MAGER J. Inhibition studies on sarcosomal DPNH oxidase. Biochim Biophys Acta. 1958 Oct;30(1):164–168. doi: 10.1016/0006-3002(58)90253-1. [DOI] [PubMed] [Google Scholar]
- BIRT L. M., BARTLEY W. The distribution and metabolism of intra- and extra- mitochondrial pyridine nucleotides in suspensions of liver mitochondria. Biochem J. 1960 May;75:303–315. doi: 10.1042/bj0750303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M. Cytochrome Components & Electron Transfer in Sweet Potato Mitochondria. Plant Physiol. 1962 Jan;37(1):90–97. doi: 10.1104/pp.37.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., THORELL B. Localization and kinetics of reduced pyridine nucleotide in living cells by microfluorometry. J Biol Chem. 1959 Nov;234:3044–3050. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CONOVER T. E., ERNSTER L. DT diaphorase. II. Relation to respiratory chain of intact mitochondira. Biochim Biophys Acta. 1962 Apr 9;58:189–200. doi: 10.1016/0006-3002(62)90998-8. [DOI] [PubMed] [Google Scholar]
- Chance B., Ernster L., Garland P. B., Lee C. P., Light P. A., Ohnishi T., Ragan C. I., Wong D. Flavoproteins of the mitochondrial respiratory chain. Proc Natl Acad Sci U S A. 1967 May;57(5):1498–1505. doi: 10.1073/pnas.57.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W. P. Oxidation of Externally Added NADH by Isolated Corn Root Mitochondria. Plant Physiol. 1964 Jul;39(4):699–703. doi: 10.1104/pp.39.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESHPANDE P. D., HICKMAN D. D., VON KORFF R. W. Morphology of isolated rabbit heart muscle mitochondria and the oxidation of extramitochondrial reduced diphosphopyridine nucleotide. J Biophys Biochem Cytol. 1961 Oct;11:77–93. doi: 10.1083/jcb.11.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVLIN T. M., BEDELL B. H. Effect of acetoacetate on the oxidation of reduced diphosphopyridine nucleotide by intact rat liver mitochondria. J Biol Chem. 1960 Jul;235:2134–2139. [PubMed] [Google Scholar]
- ERNSTER L., JALLING O., LOW H., LINDBERG O. Alternative pathways of mitochondrial DPNH oxidation, studied with amytal. Exp Cell Res. 1955;(Suppl 3):124–132. [PubMed] [Google Scholar]
- ERNSTER L., LEE C. P. BIOLOGICAL OXIDOREDUCTIONS. Annu Rev Biochem. 1964;33:729–790. doi: 10.1146/annurev.bi.33.070164.003501. [DOI] [PubMed] [Google Scholar]
- ERNSTER L. Organization of mitochondrial DPN-linked systems. II. Regulation of alternative electron transfer pathways. Exp Cell Res. 1956 Jun;10(3):721–732. doi: 10.1016/0014-4827(56)90049-0. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. Observations on the antimycin A inhibition of biological oxidations. I. Stoichiometry and pH effects. Biochim Biophys Acta. 1962 Jul 2;60:236–248. doi: 10.1016/0006-3002(62)90399-2. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. E., CONN E. E. The oxidation of reduced diphosphopyridine nucleotide by lupine mitochondria. Arch Biochem Biophys. 1956 Jan;60(1):226–243. doi: 10.1016/0003-9861(56)90413-1. [DOI] [PubMed] [Google Scholar]
- Hackett D. P. Effects of salts on DPNH oxidase activity & structure of sweet potato mitochondria. Plant Physiol. 1961 Jul;36(4):445–452. doi: 10.1104/pp.36.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenefick D. G., Hanson J. B. Contracted state as an energy source for ca binding and ca + inorganic phosphate accumulation by corn mitochondria. Plant Physiol. 1966 Dec;41(10):1601–1609. doi: 10.1104/pp.41.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L. Oxidative phosphorylation. Harvey Lect. 1953;49:176–215. [PubMed] [Google Scholar]
- LEHNINGER A. L. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J Biol Chem. 1951 May;190(1):345–359. [PubMed] [Google Scholar]
- LINDAHL P. E., OBERG K. E. The effect of rotenone on respiration and its point of attack. Exp Cell Res. 1961 Mar;23:228–237. doi: 10.1016/0014-4827(61)90033-7. [DOI] [PubMed] [Google Scholar]
- LINNANE A. W., ZIEGLER D. M. Studies on the mechanism of oxidative phosphorylation. V. The phosphorylating properties of the electron transport particle. Biochim Biophys Acta. 1958 Sep;29(3):630–638. doi: 10.1016/0006-3002(58)90021-0. [DOI] [PubMed] [Google Scholar]
- MALEY G. F. Phosphorylations associated with the oxidation of external reduced diphosphopyridine nucleotide by rat liver mitochondria. J Biol Chem. 1957 Feb;224(2):1029–1038. [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. An inhibition by amytal of succinate oxidation in tightly coupled mitochondria. Biochem Biophys Res Commun. 1962 Jun 19;8:92–96. doi: 10.1016/0006-291x(62)90242-5. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Secchi A. G., Quagliariello E. Utilization of respiratory substrates in calf-retina mitochondria. Biochim Biophys Acta. 1967 Mar 8;131(2):288–294. doi: 10.1016/0005-2728(67)90142-9. [DOI] [PubMed] [Google Scholar]
- Schatz G., Racker E. Stable phosphorylating submitochondrial particles from baker's yeast. Biochem Biophys Res Commun. 1966 Mar 8;22(5):579–584. doi: 10.1016/0006-291x(66)90314-7. [DOI] [PubMed] [Google Scholar]
- Schatz G., Racker E., Tyler D. D., Gonze J., Estabrook R. W. Studies of the DPNH-cytochrome b segment of the respiratory chain of baker's yeast. Biochem Biophys Res Commun. 1966 Mar 8;22(5):585–590. doi: 10.1016/0006-291x(66)90315-9. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner C. D., Hanson J. B. Swelling and contraction of corn mitochondria. Plant Physiol. 1966 Feb;41(2):255–266. doi: 10.1104/pp.41.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. II. Morphology and oxidative phosphorylation capacity of mitochondria and derived particles from baker's yeast. J Biophys Biochem Cytol. 1961 Mar;9:701–710. doi: 10.1083/jcb.9.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B., Mollenhauer H. H. Active swelling and acetate uptake in corn mitochondria. Biochemistry. 1969 Mar;8(3):1203–1213. doi: 10.1021/bi00831a055. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]



