Abstract
We report on a multifunctional nucleic acid, termed AptamiR, composed of an aptamer domain and an antimiR domain. This composition mediates cell specific delivery of antimiR molecules for silencing of endogenous micro RNA. The introduced multifunctional molecule preserves cell targeting, anti-proliferative and antimiR function in one 37-nucleotide nucleic acid molecule. It inhibits cancer cell growth and induces gene expression that is pathologically damped by an oncomir. These findings will have a strong impact on future developments regarding aptamer- and antimiR-related applications for tumor targeting and treatment.
Introduction
Nucleic acid drugs that interfere with biological function are recognized as molecules with emerging therapeutic value. Antisense oligonucleotides that bind to messenger RNA (mRNA) molecules and, thus, either interfere with ribosome scanning or the induction of mRNA hydrolysis by recruiting RNAse H represent the first generation of potential nucleic acid-based drugs and tools for target validation (Malik et al, 2011). The discovery of RNA interference and the related endogenous micro RNA processing pathway has further surged the oligonucleotide therapeutic field (Kanasty et al., 2012). Endogenous micro RNA biosynthesis culminates in the generation of short non-coding RNA, designated as miR (micro RNA) molecules, which recognize RNA elements within the 5′- or 3′-untranslated regions (UTRs) of mRNA (Lim et al., 2003). Thereby, miR molecules regulate gene expression by means of induced mRNA hydrolysis or inhibition of translation initiation. Although micro RNA function is critical for diverse cellular activities, such as the regulation of cellular differentiation, apoptosis and proliferation, there are pathologies in which impaired activity of individual miR molecules is maladaptive (Tong et al., 2008). A well-studied micro RNA is miR-21 and its pathogenic role in tumorigenesis (Krichevsky et al., 2009). For example, in breast cancer cells the level of miR-21 is higher compared to normal breast tissues (Lou et al., 2011; Yan et al., 2011). In analogy to proteins overexpressed in tumor tissues and, thus, termed as oncogenes, upregulated micro RNA molecules associated with tumors are referred to as oncomirs (CHO, 2007). Consistent with this observations is the finding that transfection of miR-21 antimiRs into breast cancer cells reduces cell proliferation (Yan et al., 2011). AntimiRs, also termed antagomirs, are chemically modified oligonucleotides allowing specific interaction with individual micro RNA molecules. Upon base pairing, they prevent the interaction of miR molecules with their corresponding target region embedded in mRNA molecules and, thus, the associated down regulation of gene expression. Besides short interfering RNA (siRNA) molecules antimiRs represent novel synthetic nucleic acid-based drugs with enormous potential and in vivo activity has been shown in seminal proof-of-concept studies (Elmen et al., 2008).
Locked nucleic acid (LNA)-based antimiRs have proved highly efficient in targeting miR molecules both in cell cultures and in vivo. One example is SPC3649, a 15-mer antimiR composed of alternating DNA and LNA nucleotides, which has been shown to inhibit the function of miR-122 (Elmen et al., 2008) which among other functions is a crucial host factor for hepatitis C virus. SPC3649 has since been demonstrated to reduce the hepatitis C virus titer in infected chimpanzees (Lanford et al., 2010) and is currently in phase 2 clinical trials in humans (www.santaris.com). Recently, so-called seed-targeting tiny LNA antimiRs have been developed. They are 8-mer all-LNA phosphorothioate (PTO) oligonucleotides designed to be complementary to the 5’ end (seed region) of miR molecules, and they have been shown to efficiently inhibit the function of a number of miR molecules, including miR-21 (Obad et al., 2011).
However, due to the highly polar nature of nucleic acids their cellular bioavailability is poor compared to classical low molecular weight drugs. Circumvention of this obstacle is considered to be one of the most important success factors for the continued advancement of nucleic acid therapeutics for tackling key elements inside cells of pathogenic tissue (Kanasty et al., 2012). Various strategies were developed to enhance bioavailability of nucleic acid-based drugs, the most convenient of which use hydrophobic groups, such as lipids and cholesterol, or nanoparticles to trigger transmembrane passage of nucleic acids.
Aptamers represent a further class of short nucleic acids that interact with target molecules due to defined three-dimensional shapes (MAYER, 2009). They are mainly isolated from synthetic combinatorial nucleic acid libraries by an in vitro selection process, also termed SELEX (systematic evolution of ligands by exponential enrichment). Aptamers have been isolated that target various molecules, including small molecules, peptides, and proteins. The application of aptamers as therapeutics is an important field, since frequently they not only interact with related target molecules, but also inhibit target's associated biological functions. Besides their potential therapeutic use, the application of aptamers as molecular vehicle recently has gained emerging interest (Zhou et al., 2011). Aptamers can be generated to selectively target tumor cells (Mayer et al., 2010; Sefah et al., 2010). Upon tumor cell recognition some of these aptamers have been shown to trigger or hijack internalization processes and, thus, maintain delivery of attached cargo molecules (Reyes-Reyes et al., 2010). This has been exploited to enable cellular delivery of chemotherapeutics, toxins, and siRNA molecules (Chu et al., 2006; Farokhzad et al., 2006; McNamara et al., 2006). However, the delivery of antimiRs into tumor cells employing targeting aptamers still remains elusive. Here we fill this gap and report on the use of an aptamer, named AS1411, whose uptake by tumor cells is mediated by a processes referred to as macropinocytosis (Reyes-Reyes et al., 2010). AS1411 is a G-nucleotide-rich aptamer that inhibits proliferation of tumor cells (Bates et al., 2009). AS1411 destabilizes BCL2 (B-cell lymphoma 2) mRNA and modulates arginine methyltransferase 5-nucleolin complexes (Teng et al., 2007; Soundararajan et al., 2008). Due to these characteristics, AS1411 was considered as regimen for patients suffering from acute myeloid leukaemia (Mongelard et al., 2010). We have synthesized chimeric molecules, termed AptamiRs (a word-chimera derived from Aptamer and antimiR), and demonstrated that these molecules enable the intracellular delivery of covalently bound tiny-LNA-antimiR sequences targeting the oncomir miR-21. Upon uptake, the antimiR moiety inhibits endogenous micro RNA function, whereas the AS1411 domain of the molecule still retains cell-recognition and anti-proliferative activity. Since nucleolin and miR-21 are overexpressed in many tumor cells the AptamiRs described in this study are considered as broad-spectrum tumor targeting reagents.
Material and Methods
Cell culture
The tumor cell lines A549 (human non–small-cell lung cancer), MCF-7 (human breast cancer), MDA-MB-231 (human breast cancer) and PC-9 (human non–small-cell lung cancer) were obtained from the American Type Culture Collection (Manassas) or DSMZ (Heidelberg), respectively. Cells were grown in a standard incubator at 37°C, 5% CO2 in RPMI or MEM (Gibco) supplemented with 10% fetal bovine serum (Sigma, instant fetal bovine serum).
miR21-EGFP plasmid transfection
Cells were plated with a density of 4×104 cells per well. After 18 hours, cells were transfected using 800 ng plasmid and Lipofectamin 2000 according to the manufacturers protocol. Fluorescence of cells was analyzed 24 hours after transfection using flow cytometry. Target sequence of miR-21 embedded in the reporter EGFP-plasmid: 5′-GGCCGCTCAACATCAGTCTGATAAGCTACTAATCAACATCAGTCTGATAAGCTACTAATCAACATCAGTCTGATAAGCTACTAATCAACATCAGTCTGATAAGCTAGC-3′
Transfection of antimiR21-LNA
For transfection cancer cells were seeded in low density (1×103) in a 96-well plate. After complete adhesion, cells were transfected with antimiR-LNA or control antimiR-LNAs using Lipofectamin 2000 (0.25 μL per well). Viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay 72 hours post transfection.
Generation of miR21-EGFP expressing MCF-7 cell line
MCF-7 cells were transfected with miR-21-EGFP plasmid using 4 μg plasmid DNA and Lipofectamin 2000. After 2 weeks of selection in the presence of G418 (1500μg/mL) cells were transfected with antimiR-21 (100 nM) and single EGFP-positive cells were isolated in 96-well plates using a fluorescent-activated cell sorter device (FACS; Aria).
MTT assays
Cells were plated at low density (1×103 to 3×103 cells per well) in 96-well plates in the appropriate serum-supplemented medium. After 18 hours, oligonucleotides [or phosphate buffered saline (PBS) as control] were added to the culture medium, resulting in a final concentration as indicated. The culture medium was not changed throughout the duration of the experiment. Viable cells were assayed using MTT assay on day 4 after seeding. Therefore, 15 μL of MTT dye solution (5 mg/mL MTT in PBS) per well was added and incubated for 4 hours at 37°C. Following incubation, cells were lysed using 100 μL of MTT-lysis buffer pH 4.7 (250 mL DMF, 250 mL ddH20, 100 g SDS, 10 mL acetic acid). Absorption at λ=570 nm was measured using a Varioscan plate reader (Thermo). All experiments were performed in triplicate.
Interaction of oligonucleotides with cancer cell lines
Cells (4×104) were plated into 24-well plates and incubated for 18 hours. Subsequently, cells were incubated for 3 hours at 37°C with fluorescein-labeled oligonucleotides or control molecules at the indicated concentrations. After incubation, cells were washed three times with PBS, trypsinized, and centrifuged (200 g, 5 minutes). Cells were suspended in PBS and measured by flow cytometry (FACSCanto 2). The data was analysed using FlowJo 7.6.1. All experiments were performed in duplicate.
Reporter gene assays with stable cell line
Thirty thousand cells were plated into 24-well plates for 18 hours before treatment with the respective oligonucleotide. Therefore, the oligonucleotides were diluted in OptiMEM and directly added to the culture media. After 72–96 hours of incubation at 37°C, expression of EGFP was analyzed using fluorescence microscopy and flow cytometry (FACSCanto 2). All experiments were performed in triplicate.
Oligonucleotide synthesis
Oligonucleotides were synthesized in 1.0 μmol scale using standard phosphoramidite chemistry on an automated nucleic acid synthesizer. LNA phosphoramidites were purchased from Exiqon (www.exiqon.com). The synthesis conditions used were as follows: trichloroacetic acid in CH2Cl2 (3:97) as detritylation reagent; 0.25 M 4,5-dicyanoimidazole in CH3CN as activator; acetic anhydride in tetrahydrofuran (THF) (9:91, v/v) as cap A solution; N-methylimidazole in THF (1:9, v/v) as cap B solution. As an oxidizing solution, 0.02 M iodine in H2O/pyridine/THF was used for phosphate linkages whereas a thiolation solution 0.02 M PADS (phenylacetyl disulfide) in 3-picoline/acetonitrile (1:1, v/v) was used for phosphorothioate linkages. Coupling times used were 12 minutes for incorporation of both LNA and DNA monomers. The stepwise coupling yields (∼99% per step) were determined from the absorbance of the dimethoxytrityl cations (DMT+) released after each coupling step. Cleavage from the support was carried out using 32% aqueous ammonia solution (12 hours at 55°C). All oligonucleotides were purified by reversed-phase high-performance liquid chromatography (RP-HPLC) using a Waters 600 system equipped with an XBridge OST C18 (2.5 μm, 19×100 mm) column and an XBridge Prep C18 (5 μm, 10×10 mm) precolumn. After removal of the DMT group, oligonucleotides were characterized (purity>90%) by ion-exchange HPLC on a Dionex system HPLC (VWR International) and their composition verified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry on a Microflex Maldi (Bruker instruments).
Results
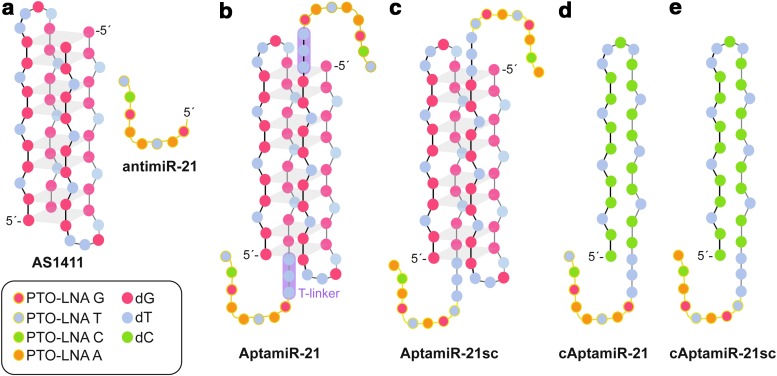
We designed and synthesized chimeric molecules built from the nucleolin-binding aptamer AS1411 and a phosphorothioate-LNA antimiR-21 domain, targeting the oncomir miR-21 (Fig. 1a, Table 1). We extended AS1411 by three thymidine residues as spacer at the 3′ end followed by the antimiR-21 moiety. This arrangement of oligonucleotide entities was termed AptamiR-21 (Fig. 1b). We also synthesized AptamiR-variants that consist of a nonfunctional LNA moiety, named AptamiR-21sc (Fig. 1c), or bear a non-tumor targeting control sequence in conjunction with the LNA portion (cAptamiR-21) or the non-functional LNA moiety (cAptamiR-21sc Fig. 1d, e). These molecules were used for control experiments throughout the study. To choose a nonfunctional LNA moiety, we initially performed cell viability assays with three different nontargeting LNA molecules (Table 1). Therefore, we transfected various tumor cell lines with either the antimir-21 or the different control sequences (antimiR-21sc_1, antimiR-21sc_2, and antimiR-21sc_3). Subsequently, cell viability was measured and the results of these experiments revealed that antimiR-21, as expected, inhibits proliferation of MCF-7 (human breast cancer) cells (Supplementary Fig. S1a; Supplementary Data are available online at www.liebertpub.com/nat). However, the control sequence antimiR-21sc_1 had also a strong impact on the proliferation of MCF-7 cells. The two other control sequences showed almost no effect, whereas the antimiR-21sc_2 revealed comparable results throughout all investigated cell lines. Thus, we choose this PTO-LNA sequence to be included into control AptamiRs and to be used in subsequent studies. Noteworthy sole addition of the antimiR-21 and antimir-21sc_1 to the cell culture did not lead to inhibition of cell proliferation (Supplementary Figure 1b). These data underline that the choice of suitable control LNA-oligonucleotides is a critical step in evaluating LNA molecules and their impact on the proliferation of tumor cells.
FIG. 1.
AptamiRs. AptamiRs were generated by extending the aptamer AS1411 at the 3′ end with three thymidine nucleotides followed by a phosphorothiolated (PTO) antimiR locked nucleic acid (LNA) oligonucleotide sequence targeting the micro RNA miR-21 (a and b). As control molecules AptamiR variants with either a nonfunctional LNA moiety (c, e) or nontargeting control sequence (d, e) were synthesized.
Table 1.
Sequences of AptamiR Constructs and Control Oligonucleotides Used in This Study
| Oligonucleotide | Sequence (5′–3′)* |
|---|---|
| AS1411 | GGTGGTGGTGGTTGTGGTGGTGGTGG |
| c1 AS1411 | CCTCCTCCTCCTTCTCCTCCTCCTCC |
| AptamiR-21 | GGTGGTGGTGGTTGTGGTGGTGGTGGTTTGATAAGCT |
| AptamiR-21sc+ | GGTGGTGGTGGTTGTGGTGGTGGTGGTTTTCATACTA |
| cAptamiR-21 | CCTCCTCCTCCTTCTCCTCCTCCTCCTTTGATAAGCT |
| cAptamiR-21sc+ | CCTCCTCCTCCTTCTCCTCCTCCTCCTTTTCATACTA |
| antimiR-21 | GATAAGCT |
| antimiR-21sc_1 | TGATGACA |
| antimiR-21sc_2 | TCATACTA |
| antimiR-21sc_3 | TCTACAAT |
| antagomir | CAACATCAGTCTGATAAGCTA# |
| miR-21 | UAGCUUAUCAGACUGAUGUUGA |
Bold indicates phosphorothioate locked nucleic acid (PTO-LNA); underscore, T-Linker phosphodiester linkage; italic, miR-21 seed sequence.
AptamiR-21sc and cAptamiR-21sc are composed of AS1411, T-linker, and the antimiR-21sc_2.
all nucleotides 2′ O-Methyl (2′-OMe).
Per definition, we would expect AptamiR molecules to reveal certain properties that are associated with the individual sub-domains they are built from. Thus, AptamiR-21 (Fig. 1b) should interact with nucleolin-expressing tumor cells and inhibit tumor cell proliferation. Both functions associated with the AS1411 aptamer domain. Furthermore, the antimiR-21-domain should prevent the interaction of miR-21 with its target sites on mRNA molecules and contribute to cell growth inhibition, since miR-21 blockade has been reported to influence proliferation and migration of tumor cells (Yan et al., 2011).
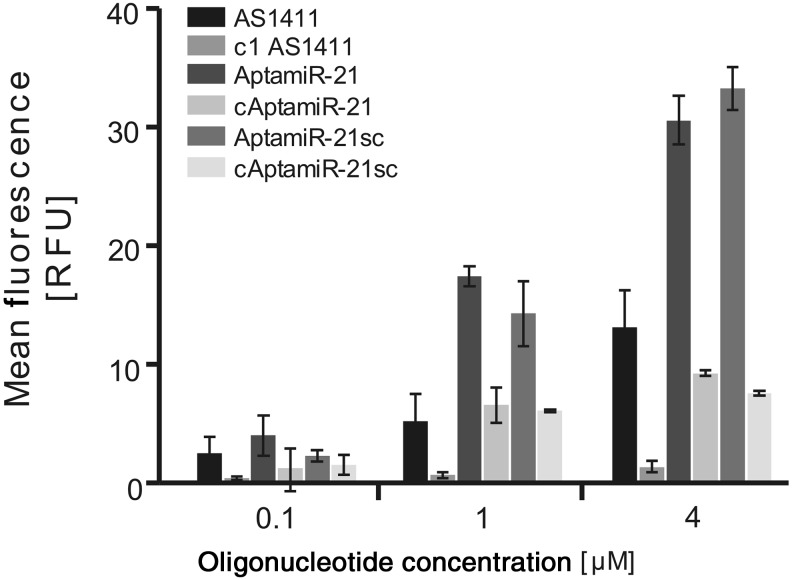
Having synthesized the AptamiR molecules, we first investigated whether they retain interaction properties with breast cancer cells, mediated by the inherent AS1411 domain. Therefore, we incubated increasing concentrations of fluorescently labelled AptamiR variants with MCF-7 cells and monitored binding by flow cytometry. All oligonucleotides and chimeras that possess an AS1411 moiety showed concentration dependent interaction with MCF-7 cells, whereas the control molecules showed comparable low signals (Fig. 2). At concentrations of 1 μM and 4 μM, the signals obtained from AptamiRs were even 2- to 3-fold higher than those from AS1411, indicating that the LNA moieties have an impact on the AptamiR's cell recognition properties. Most likely, the LNA domains stabilize an active conformation of the AS1411 domain and contribute to binding due to their phosphorothioate nature. Noteworthy, LNA-antimiRs associate with MCF-7 cells but only at high concentrations and in a non-selective manner (Supplementary Fig. S2). This explains why AptamiR molecules with nonfunctional aptamer domain reveal a higher background signal regarding MCF-7 cells compared to molecules that lack a PTO-LNA moiety (c1 AS1411). But clearly, stronger interaction with cancer cells was obvious from those AptamiRs that bear an intact aptamer domain. This was also revealed from studies using further cancer cell lines that show similar interaction properties of the AptamiRs as those reported previously for AS1411 alone (Supplementary Fig. S3) (Bates et al., 2009). These data indicate that within the AptamiR molecules the cell recognition properties of AS1411 are preserved.
FIG. 2.
Interaction of AptamiRs with breast cancer cell line MCF-7. Interaction of AptamiR molecules with cancer cells was studied using fluorescein-labeled variants of the indicated molecules. Cells were incubated with the aptamer AS1411, AptamiR-21 molecules, or the indicated control oligonucleotides. After washing, fluorescence was detected by flow cytometry. Shown are mean fluorescence values.
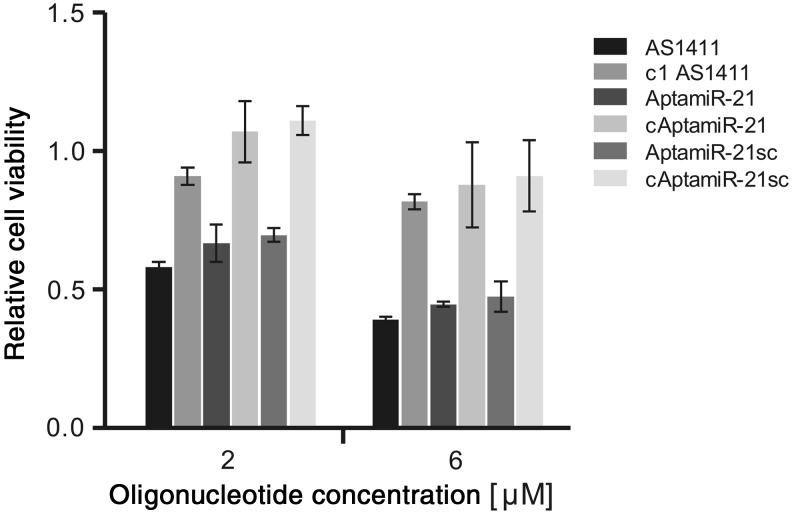
Next we measured the impact of AptamiRs on tumor cell proliferation. AS1411 has been reported to inhibit cell proliferation, which was confirmed by our studies (Fig. 3). Importantly, the AptamiRs with an intact AS1411 domain (AptamiR-21 and AptamiR-21sc) were also found to inhibit cell proliferation in a concentration-dependent manner (Fig. 3). In contrast, AptamiRs with a nonfunctional aptamer domain (cAptamiR-21 and cAptamiR-21sc) had no effect on tumor cell proliferation (Fig. 3). These data underline that AptamiR-21 not only has preserved interaction but also functional properties of the aptamer domain. The observed effect on cell proliferation of AptamiR-21 was as strong as the effect determined by employing AS1411. This is surprising since AptamiRs revealed a stronger interaction potential with MCF-7 cells compared to AS1411 and, thus, a greater impact on cell proliferation might be expectable. Additionally, AptamiRs bear an antimiR-21 moiety, which has anti-proliferative activity per se (Supplementary Fig. 1). However, we detected any additive activity regarding inhibition of cell proliferation. This indicates that stronger binding of AptamiR molecules compared to AS1411 not necessarily converges into a greater impact on cell viability. Furthermore, AS1411-mediated cellular provision of the antimiR-21 moiety might be limited, thus, preventing the event of synergistic effects.
FIG. 3.
Impact of AptamiRs on MCF-7 cell proliferation. Proliferation assays were performed to analyze the AptamiR molecules impact on cell viability. Therefore, cells were grown for 72 hours in the presence of AS1411, AptamiRs, or control oligonucleotides at the indicated concentrations. Data were normalized to the growth of untreated cells.
We next investigated whether the function of the antimiR-21-domain is also preserved in the AptamiRs or whether the presence of an aptamer domain has an impact on antimiR-function. We therefore generated a MCF-7 cell line that expresses enhanced green fluorescent protein (EGFP) under the control of miR-21. We therefore cloned the miR-21 target site into the 3′-UTR of the mRNA of an EGFP encoding plasmid. This plasmid was used to generate the recombinant EGFP-expressing MCF-7 cell line, termed miR-21 MCF-7, by G418 selection. Since MCF-7 cells have a high endogenous miR-21 level, expression of EGFP in the new cell line is prevented (Supplementary Fig. S4a). However, the transfection of antimiR-21 into these cells specifically induced EGFP expression, indicating that the generated cell line is suitable to investigate the inhibition of the interaction of endogenous miR-21 with its target sequence (Supplementary Fig. S4a). Noteworthy, transfection of AptamiRs with an intact antimiR-21 moiety also induced EGFP expression (Supplementary Fig. S4a). However, transfecting increasing concentrations of AptamiR-21 led to a decrease of EGFP expression rather than to an increase (Supplementary Fig. S4a). This might be mainly attributed to the presence of the anti-proliferative AS1411 domain (Supplementary Fig. S4c, d). Beyond that, the interaction and anti-proliferative properties of the AptamiR molecules regarding the generated cell line miR-21 MCF-7 were shown to be very similar compared to the non-modified cell line MCF-7 (Supplementary Fig. S5).
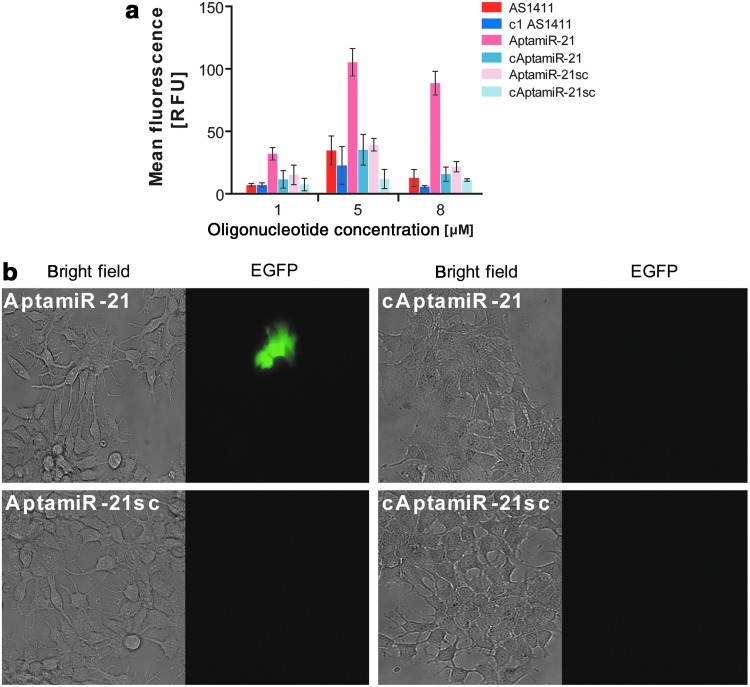
Next we investigated whether the sole addition of AptamiR-21 to the growth medium of the generated miR-21 MCF-7 cells also induces EGFP expression. Figure 4a clearly shows that AptamiR-21 addition induces EGFP expression in a concentration dependent manner. This result reflects both the cellular uptake of AptamiR-21 and the blocking of the association of endogenous miR-21 with its target sequence embedded in the EGFP-mRNA (Fig. 4a). Notably, variants with a scrambled antimiR-21 domain (AptamiR-21sc) or an inactive aptamer moiety (cAptamiR-21) did not induce reporter gene expression (Fig. 4a). The addition of AS1411 to the cells did not affect EGFP expression (Supplementary Fig. S6), whereas addition of antimiR-21 at high concentrations induced EGFP expression in the generated mir-21 MCF-7 cell line. This most likely reflects a nontargeted uptake of short oligonucleotides by a mechanism referred to as gymnosis (Supplementary Fig. S6) (Stein et al., 2010).
FIG. 4.
AptamiR molecules interfere with endogenous miR21 function. Aptamer-triggered import of antimiR-21 into breast cancer cells was investigated employing a recombinant MCF-7 cell line that contains a miR-21-targeted site in the 3′-untranslated region of enhanced green fluorescent protein (EGFP). Oligonucleotides were directly added to the culture media at the indicated concentrations (a) or at 5μM in (b) and induced EGFP-fluorescence was analyzed 72 hours after incubation by flow cytometry (a) and fluorescence microscopy (b).
Induction of EGFP expression is also visible from studies using fluorescence microscopy (Fig. 4b). These data indicate that the aptamer delivered the adjacent antimiR-21 domain into breast cancer cells. Furthermore, the antimiR-21 moiety remains functional and prevents the interaction of endogenous miR-21 with its mRNA target site. However, the efficiency of AS1411-mediated antimiR delivery by means of induction of EGFP expression is limited (Supplementary Fig. S7). This could be due to restricted availability of the antimiR sequence upon AS1411-guided uptake, which presumes an endosome escape for most reaching the actual target micro RNA. Additionally, antiproliferative activity of AS1411 was also shown to impair the expression of EGFP and, thus, to lower the visible effect of antimiR molecules (Supplementary Fig. 4b, c).
Here we report the delivery of antimiR molecules into cells by a targeting aptamer domain, to the best of our knowledge, for the first time. The results shown complement efforts employing aptamer-based targeting of drugs or potential drug candidates. We termed the arrangement of an aptamer and antimiR molecule “AptamiR.” Furthermore, the AptamiR-21 molecule represents the first example of a trifunctional nucleic acid—combining cell targeting, antiproliferative, and antimiR functions in one nucleic acid molecule, being as short as 37 nucleotides. These findings may have a strong impact on future developments regarding antimiR delivery and their application as selective and effective drugs. Albeit the efficiency of the reported system is not optimal as of yet, it is likely to be improved using other existing aptamers as delivery domains or to select for ones that efficiently allow the cell-specific recognition and cellular uptake combined with early or late endosomal escape. Moreover, our study might open the door to constructing other multivalent nucleic acids with multifunctional properties.
Supplementary Material
Acknowledgments
The work of this study was in part made possible by grants from the Federal Ministry of Education and Research (0313942C) to GM. JW thanks the European Research Council for support under the European Union Program (FP7/2007–2013)/ERC Grant agreement No. 268776.
Author Disclosure Statement
JW is founder of RiboTask ApS, which owns Intellectual Properties on chemically modified variants of AS1411.
References
- BATES P.J., LABER D.A., MILLER D.M., THOMAS S.D., and TRENT J.O. (2009). Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 86,151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO W.C. (2007). OncomiRs: the discovery and progress of microRNAs in cancers. Mol. Cancer 6,60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T.C., MARKS J.W., 3rd, LAVERY L.A., FAULKNER S., ROSENBLUM M.G., ELLINGTON A.D., and LEVY M. (2006). Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 66,5989–5992 [DOI] [PubMed] [Google Scholar]
- ELMEN J., LINDOW M., SCHUTZ S., LAWRENCE M., PETRI A., OBAD S., LINDHOLM M., HEDTJARN M., HANSEN H.F., BERGER U., et al. (2008). LNA-mediated microRNA silencing in non-human primates. Nature 452,896–899 [DOI] [PubMed] [Google Scholar]
- FAROKHZAD O.C., CHENG J., TEPLY B.A., SHERIFI I., JON S., KANTOFF P.W., RICHIE J.P., and LANGER R. (2006). Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. U. S. A. 103,6315–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANASTY R.L., WHITEHEAD K.A., VEGAS A.J., and ANDERSON D.G. (2012). Action and Reaction: The Biological Response to siRNA and Its Delivery Vehicles. Mol. Ther. 20,513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRICHEVSKY A.M., and GABRIELY G. (2009). miR-21: a small multi-faceted RNA. J. Cell Mol. Med. 13,39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANFORD R.E., HILDEBRANDT-ERIKSEN E.S., PETRI A., PERSSON R., LINDOW M., MUNK M.E., KAUPPINEN S., and ORUM H. (2010). Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327,198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM L.P., GLASNER M.E., YEKTA S., BURGE C.B., and BARTEL D.P. (2003). Vertebrate microRNA genes. Science 299,1540. [DOI] [PubMed] [Google Scholar]
- LOU Y., CUI Z., WANG F., YANG X., and QIAN J. (2011). miR-21 down-regulation promotes apoptosis and inhibits invasion and migration abilities of OVCAR3 cells. Clin. Invest. Med. 34,E281. [DOI] [PubMed] [Google Scholar]
- MALIK R., and ROY I. (2011). Making sense of therapeutics using antisense technology. Expert Opin. Drug Discov. 6,507–526 [DOI] [PubMed] [Google Scholar]
- MAYER G. (2009). The chemical biology of aptamers. Angew Chem. Int. Ed. Engl. 48,2672–2689 [DOI] [PubMed] [Google Scholar]
- MAYER G., AHMED M.S., DOLF A., ENDL E., KNOLLE P.A., and FAMULOK M. (2010). Fluorescence-activated cell sorting for aptamer SELEX with cell mixtures. Nat. Protoc. 5,1993–2004 [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O., 2nd, ANDRECHEK E.R., WANG Y., VILES K.D., REMPEL R.E., GILBOA E., SULLENGER B.A., and GIANGRANDE P.H. (2006). Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24,1005–1015 [DOI] [PubMed] [Google Scholar]
- MONGELARD F., and BOUVET P. (2010). AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 12,107–114 [PubMed] [Google Scholar]
- OBAD S., DOS SANTOS C.O., PETRI A., HEIDENBLAD M., BROOM O., RUSE C., FU C., LINDOW M., STENVANG J., STRAARUP E.M., et al. (2011). Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 43,371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYES-REYES E.M., TENG Y., and BATES P.J. (2010). A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 70,8617–8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEFAH K., SHANGGUAN D., XIONG X., O'DONOGHUE M.B., and TAN W. (2010). Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 5,1169–1185 [DOI] [PubMed] [Google Scholar]
- SOUNDARARAJAN S., CHEN W., SPICER E.K., COURTENAY-LUCK N., and FERNANDES D.J. (2008). The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 68,2358–2365 [DOI] [PubMed] [Google Scholar]
- STEIN C.A., HANSEN J.B., LAI J., WU S., VOSKRESENSKIY A., HOG A., WORM J., HEDTJARN M., SOULEIMANIAN N., MILLER P., et al. (2010). Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 38,e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENG Y., GIRVAN A.C., CASSON L.K., PIERCE W.M., JR., QIAN M., THOMAS S.D., and BATES P.J. (2007). AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 67,10491–10500 [DOI] [PubMed] [Google Scholar]
- TONG A.W., and NEMUNAITIS J. (2008). Modulation of miRNA activity in human cancer: a new paradigm for cancer gene therapy? Cancer Gene Ther. 15,341–355 [DOI] [PubMed] [Google Scholar]
- YAN L.X., WU Q.N., ZHANG Y., LI Y.Y., LIAO D.Z., HOU J.H., FU J., ZENG M.S., YUN J.P., WU Q.L., et al. (2011). Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 13,R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU J., LI H., ZHANG J., PIOTR S., and ROSSI J. (2011). Development of cell-type specific anti-HIV gp120 aptamers for siRNA delivery. J. Vis. Exp. 52,2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.