Abstract
Preclinical models suggest that repeated high-dose methamphetamine (METH) exposures, administered in a “binge-like” pattern, acutely decrease norepinephrine (NE), and acutely and persistently decrease serotonin (5-hydroxytryptamine; 5HT) content in the frontal cortex. However, the impact of METH self-administration on this region is unknown. Because of the importance of the monoaminergic neurons in the frontal cortex to a variety of cognitive and addictive processes, effects of METH self-administration on cortical NE and 5HT content were assessed. Results revealed several novel findings. First, METH self-administration decreased cortical NE content as assessed 24 h after last exposure. Consistent with previous preclinical reports after a binge METH regimen, this decrease was reversed 8 d after the final METH exposure. Second, and in contrast to our previous reports involving the hippocampus or striatum, METH self-administration caused persistent decreases in 5HT content as assessed 8 d after the final METH exposure. Of note, the magnitude of this decrease (~20%) was less than that observed typically after a binge METH treatment. Third, prior METH self-administration attenuated METH-induced serotonergic deficits as assessed 7 d, but not 1 h, following a neurotoxic METH regimen. No protection was observed when the binge exposure occurred 15 d after the last self-administration session. Taken together, these data demonstrate important and selective alterations in cortical serotonergic neuronal function subsequent to METH self-administration. These data provide a foundation to investigate complex questions involving “resistance” to the persistent deficits caused by neurotoxic METH exposure, and frontal cortical function.
Keywords: Methamphetamine, Self-administration, Cortex, Serotonin, Norepinephrine
Introduction
Methamphetamine (METH) users often display abnormalities in the frontal cortical structure and function that may contribute to impairments in executive functions and addiction (for review see Goldstein et al., 2011). For example, reduced activations of areas of the frontal cortex have been reported in abstinent METH users during a Stroop task (Nestor et al., 2011). Further, decreases in serotonin (5-hydroxytryptamine; 5HT) transporter (SERT) densities in the orbitofrontal, temporal, and anterior cingulate cortices as measured by [11C](+)McN-5652 binding have been associated with increases in aggression ratings in abstinent METH users (Sekine et al., 2006). Lastly, postmortem studies have shown decreases in 5HT content in Brodmann areas 11 and 12 in human METH users (Wilson et al., 1996). These findings suggest that serotonergic neuronal changes within the frontal cortex in METH users may contribute to cognitive and psychiatric symptoms.
Norepinephrine (NE) in the prefrontal cortex also plays an important role in cognition such as working memory (Arnsten, 2011). In contrast to 5HT, no changes in NE content were found in METH users when assessed postmortem (Wilson et al., 1996).
Preclinical studies also indicate that METH administration, given according to a regimen that mimics some aspects of human “binging,” rapidly decreased 5HT content, and tryptophan hydroxylase activity as assessed 1–2 h after METH exposure (Fleckenstein et al., 1997; Graham et al., 2008; Kokoshka et al., 2000). Further, such treatment decreased 5HT content and SERT immunoreactivity or [125I]RTI-55 binding in the frontal cortex as assessed at least 7 d following METH exposure (Brennan et a l., 2010; Johnson-Davis et al., 2003; Preston et al., 1985; O’Dell et al., 2012; Reichel et al., 2012; Son et al., 2013; but see also Kosheleff, Grimes, et al., 2012 and Kosheleff, Rodriguez, et al., 2012) with decreases in 5HT content persisting for 139 d after METH exposure (Friedman et al., 1998). In contrast, preclinical studies indicate that METH exposure causes acute but not persistent changes in NE content in the frontal cortex (Graham et al., 2008; Krasnova et al., 2010; Preston et al.,1985).
Recent studies have focused on the impact of METH self-administration and have included studies wherein the impact of prior self-administration on subsequent METH exposure has been investigated. For example, 7 d of METH self-administration followed by a binge exposure of METH 24 h after the start of the last self-administration session lead to: 1) attenuated hyperthermia during the binge exposure of METH; 2) no alterations in brain METH concentration 1 h after binge exposure; and 3) attenuated persistent SERT deficits within the hippocampus compared to saline self-administration and METH challenged rats. The present study extends these findings by investigating the impact of METH self-administration alone or prior to binge METH exposure on 5HT and NE content in another brain region, the cortex, which is thought to be affected by drug abuse (Goldstein et al., 2011). These findings may have important implications for understanding tolerance to binge METH exposure. Further, findings of the current study may help elucidate the changes underlying the compulsive drug taking and disadvantageous behaviors associated with addiction.
Methods
Male Sprague-Dawley rats (275–300 g; Charles River Laboratories, Portage, MI) were housed four rats/cage. Following surgery, each rat was individually housed. Water was available in their home cage ad libitum. During food training, rats were food restricted such that no rat dropped below 90% of their starting body weight. Rats were maintained under the same 14:10 h light/dark cycle in the animal facility and in the operant chambers. Animals were sacrificed by decapitation. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Core body temperatures and other data from these experiments have been reported previously (McFadden et al., 2012b) as noted below.
Prior to self-administration, rats were food trained and received a catheter surgery as described in McFadden et al. (2012b). Because others and we have reported that food training predicts an animal’s ability to acquire drug self-administration, all rats used were required to successfully acquire lever-pressing behavior for food (Frankel et al. 2011; Fuchs et al., 2005; Hanson et al. 2012; McFadden et al., 2012a; See et al., 2007). Immediately following surgery and daily thereafter, catheters were infused with 0.1 ml of cefazolin followed by 0.05 ml of heparinized saline and heparinized glycerol. Catheter patency was checked by infusing 0.03 ml (20 mg/ml) of xylazine. Rats underwent 7 d of self-administration (8 h/session; FR1; 0.12 mg/infusion racemic-METH [expressed in free-base form] or saline) during the light cycle in a room maintained at 29±1°C to promote lever pressing (Cornish et al., 2008). For each active lever press, an infusion pump delivered 10 ul of METH or saline over a 5-s duration (Coulbourn Instruments, Whitehall, PA USA). During this period both levers were retracted. Following the infusion, the levers remained retracted for an additional 20 s. Pressing the inactive lever was recorded but resulted in no programmed consequences. METH self-administering rats were only included if they: 1) pressed an average of more than 10 active lever presses per d; and 2) the ratio of active/inactive lever presses was ≥ 2:1. These criteria were largely based upon those of Brennan et al., 2010. An escalation in daily METH intake occurred in all METH self-administration experiments similar to that described in McFadden et al. (2012a; see Figures 4A and 6A therein). Rectal temperatures were measured using a digital thermometer (Physitemp Instruments, Clifton, NJ) as previously described (Danaceau et al., 2007; Doyle et al., 2010; Kelly et al., 2012; Kokoshka et al., 2000; Kosheleff et al., 2012; McFadden et al., 2012a,b; Son et al., 2013) and were recorded after a stable reading for at least 5 s. Animals were sacrificed 24 h or 8 d after the start of the last self-administration session or received a binge of METH or saline.
Twenty-four h or 15 d after the last self-administration session, rats were challenged with 4 injections of METH (7.5 mg/kg/injection freebase) or saline (1 ml/kg/injection) at 2-h intervals. Prior to the first injection, all rats were housed in a 22–23°C room. Following recording of basal temperatures, METH self-administering rats challenged with METH (METH/METH) were maintained in a warm environment (25°C) to permit hyperthermia (see McFadden et al., 2012b for greater details of these methods). Saline self-administering rats challenged with METH (Saline/METH) were maintained in a 22–23°C room. Animals were sacrificed 1 h or 7 d after the binge exposure. Rectal temperatures were measured using a digital thermometer (Physiotemp Instruments, Clifton, NJ) approximately 30 and 90 min after each injection as previously described (McFadden et al., 2012a,b).
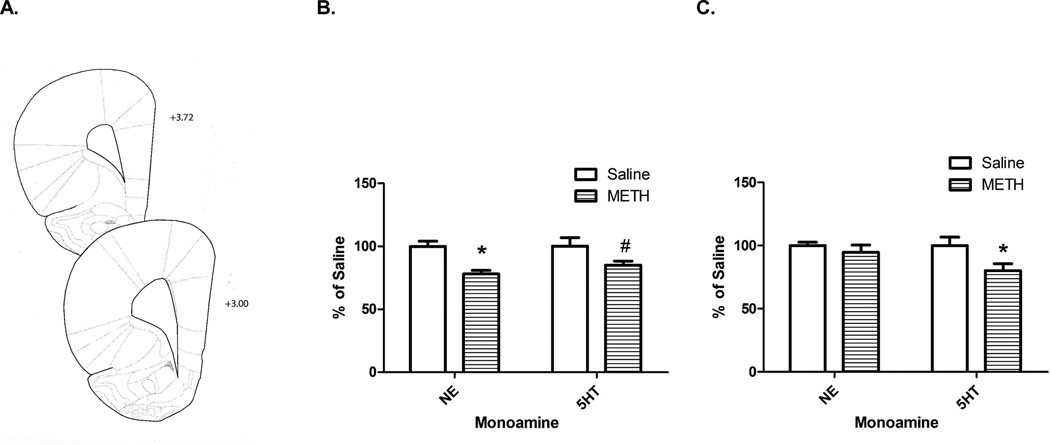
The whole brain was frozen on dry ice following striatum and hippocampus removal. A coronal slice (spanning +3.72 to +3.00 mm anterior to bregma including the motor, insular, and pyriform cortex; Paxinos et al., 2007; Figure 1A) of the left side of the brain was dissected from the frozen brains (approximately 10 mg wet weight) similar to that described in Kelly et al., 2012. This coronal slice was stored at −80°C until it was sonicated for 3–5 s in 0.5 ml tissue buffer (0.1 M phosphate/citrate buffer, pH=2.5, containing 10% methanol). Fifty µl were injected onto a partisphere C-18 reverse-phase analytical column (5-µm spheres; 250 X 4.6 mm; Whatman, Clifton, NJ, USA). Mobile phase consisting of 0.05 M sodium phosphate, 0.03 M citrate buffer, 0.1 M EDTA, 0.03% sodium octylsulfate, and 20% methanol (pH=2.85, flow rate=0.75 ml/min) was used. NE and 5HT were detected using an ampherometric electrochemical detector with the working electrode potential set at +0.70 V relative to an Ag+/AgCl reference electrode. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories Inc., Hercules, CA).
Figure 1.
METH self-administration decreased monoamine content in the cortex (histology represented in Panel A). Rats self-administered METH (0.12 mg/infusion) or saline (10 µl/infusion) for 7 d (8 h/d). Rats were sacrificed 24 h (Panel B; Saline NE: 7.58±0.31 pg/µg protein; Saline 5HT: 7.22±0.50 pg/µg protein) or 8 d (Panel C; Saline NE: 10.16±0.27 pg/µg protein; Saline 5HT: 8.72±0.59 pg/µg protein) after the start of the last self-administration session and cortex monoamines were assessed. *p<0.05 METH vs. Saline; #p<0.10 METH vs. Saline
Statistical analysis was conducted in GraphPad Prism (La Jolla, CA). Statistical analyses among groups were conducted using a t-test, analysis of variance (ANOVA) or repeated measures ANOVA followed by Newman-Keuls posthoc analyses. The data represent means ± standard error of the mean (S.E.M.) of 6–9 rats/group.
Results
METH self-administration led to reductions in cortical NE (t(12)=4.38, p<0.05) and a trend towards reductions in 5HT content (t(13)=1.85, p=0.09; Figure 1B) 24 h after the start of the last session. As reported previously (McFadden et al., 2012b), rats administered a total of 22.33±1.82 mg METH and had elevated body temperatures following self-administration (Saline: 37.39±0.10°C; METH: 38.51±0.12°C; t(13)=6.76, p<0.05). When assessed 8 d later, METH self-administration reduced 5HT content in the cortex (t(12)=2.28, p<0.05), and resulted in no change in NE (t(12)=0.85, ns; Figure 1C). As previously reported (McFadden et al., 2012b), rats self-administered a total of 26.33±1.49 mg METH and had significantly elevated average temperature following self-administration (Saline: 38.0±0.1°C; METH: 38.5±0.1°C; t(12) = 4.32, p < 0.05).
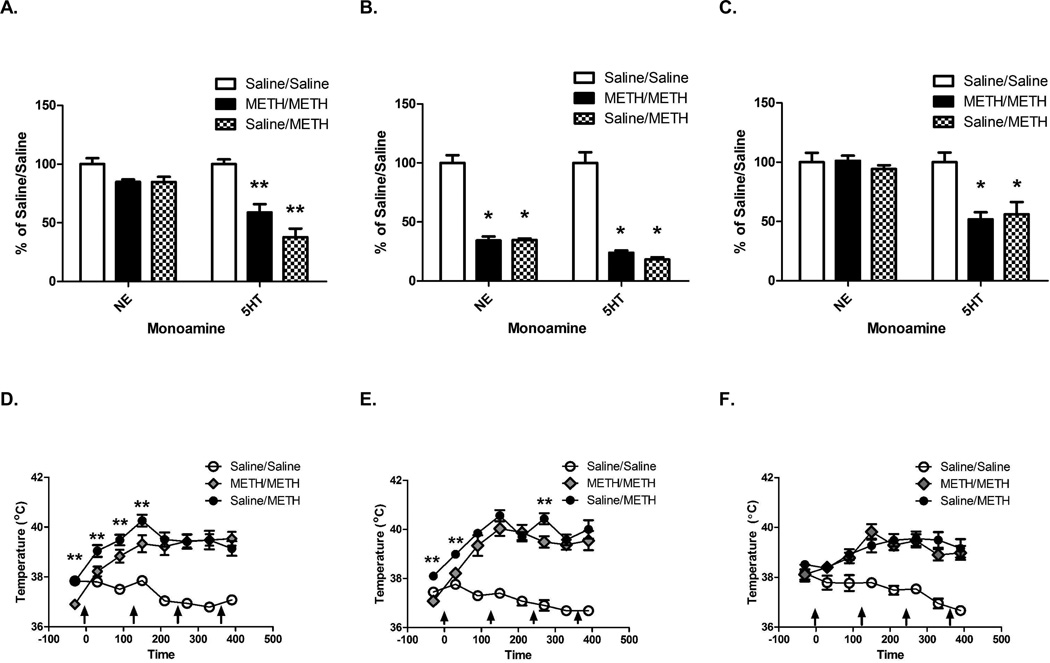
Prior METH self-administration reduced cortical 5HT deficits induced by binge METH exposure received 1 d after self-administration as assessed 7 d after the binge exposure (F(2,16)=29.84, p<0.05; Figure 2A). Although there was an overall significant effect, posthoc analysis revealed no significant differences among groups in NE content within the cortex (F(2,16)=4.39, p=0.03; Newman-Keuls Multiple Comparison Test: Saline/Saline vs. Saline/METH: q=3.435, ns; Figure 2A). As reported previously, animals self-administered a total of 21.68±1.86 mg METH (McFadden et al., 2012b). Prior METH self-administration attenuated the increase in core body temperatures caused by the subsequent binge METH treatment during the first 2.5 h as well as the basal temperature (F(2,16)=103.3, p<0.05; see Figure 2D).
Figure 2.
Prior METH self-administration reduced the persistent but not acute serotonergic deficits in the cortex induced by a binge exposure to METH 1 d after the start of the last self-administration session. No protection was afforded by prior self-administration when the binge exposure occurred 15 d after the last self-administration session. Rats self-administered METH (0.12 mg/infusion) or saline (10 µl/infusion) for 7 d (8 h/d), received a binge exposure to METH 1 d after the start of the last self-administration session, and were sacrificed 7 d (Panel A and D; Panel A- Saline/Saline NE: 9.22±0.47 pg/µg protein; Saline/Saline 5HT: 8.11±0.33 pg/µg protein) or 1 h (Panel B and E; Panel B-Saline/Saline NE: 8.40±0.55 pg/µg protein; Saline/Saline 5HT: 8.73±0.79 pg/µg protein) after the last injection. Rats in Panels C and F self-administered as described above, but received the binge exposure to METH 15 d after the last self-administration session and were sacrificed 7 d later (Panel C- Saline/Saline NE: 8.29±0.66 pg/µg protein; Saline/Saline 5HT: 5.97±0.49 pg/µg protein). *p<0.05 compared to Saline/Saline; **p<0.05 compared to all other groups. Panels D, E, and F are from McFadden et al., 2012B with permission.
The acute (1-h) decreases in 5HT and NE content following the binge METH exposure were similar between the Saline/METH and METH/METH groups when administered 1 d following self-administration (5HT: F(2,20)=66.03; p<0.05; NE: F(2,20)=75.45, p<0.05; Figure 2B). Rats self-administered a total of 21.61±1.23 mg METH (McFadden et al., 2012b). The METH/METH group had attenuated the hyperthermia (F(14,154)=12.09, p<0.05; Figure 2E), 30 min following the first and third injection of METH and decreased basal temperatures.
Prior self-administration of METH did not protect against cortical 5HT deficits (F(2,20)=8.95, p<0.05; Figure 2C) when the binge of METH was administered 15 d after self-administration as assessed 7 d after binge exposure. No changes were found in NE content (F(2,20)=0.59, ns; Figure 2C). As reported previously, rats administered a total of 20.57±0.83 mg of METH during self-administration (McFadden et al., 2012b). Similar hyperthermia during the binge of METH was achieved between the Saline/METH and METH/METH groups (F(2,20)=6.02, p<0.05; Figure 2F).
Discussion
The current study is among the first to investigate the impact of METH self-administration on the frontal cortex. In particular, results revealed that METH self-administration reduced NE content 1 d after the start of the last session, although this effect recovered by 8 d following self-administration. These data confirm previous findings that noradrenergic neurons are resistant to persistent deficits caused by METH (Krasnova et al., 2010; Preston et al., 1985). Further, and in contrast to the hippocampus or striatum, a second novel finding of this study is METH self-administration resulted in persistent cortical 5HT content depletions that were smaller in magnitude than those following a binge exposure. Third, prior METH self-administration attenuated METH induced depletions in 5HT content 7 d, but not 1 h, following the binge exposure when administered 1 d after self-administration
As noted above, reductions in 5HT content were observed 8 d following METH self-administration in the frontal cortex. These deficits are consistent with clinical findings (Wilson et al., 1996). These decreases in 5HT content may be of significance to the cognitive deficits or psychiatric symptoms found in METH users (Robbins et al., 2007). For example, decreased cortical SERT densities as measured by positron emission tomography in abstinent METH abusers were associated with increased aggression (Sekine et al., 2006). Aggression is a psychiatric symptom of antisocial personality and conduct disorder which often comorbidly occur with METH or substance abuse (Glasner-Edwards et al., 2010; Yen et al., 2006). Further, 5HT is an important regulator of neuronal excitability in the frontal cortex (Andrade, 2011). Abstinent human METH abusers often display decreased frontal cortex activation and a greater number of errors during a Stroop task (Nestor et al., 2011). This task is thought to reflect impairments in executive functioning and is impaired in people with attention deficit-hyperactivity disorder (Lansbergen et al., 2007). Of note, deficits in attentional set-shifting were observed in rats following self-administration and these changes were related to changes in higher basal firing frequency and a greater proportion of burst-firing cells within the frontal cortex (Parsegian et al., 2011). It is speculated that decreases in serotonergic function in the cortex may contribute to these cognitive deficits and drug addiction by impairing decision making and contributing to relapse.
METH self-administration prior to the binge of METH did not protect against the acute effects of METH on cortical NE and 5HT content. Similarly, escalating-dose pretreatments of METH did not protect against binge METH-induced NE and 5HT depletions in the cortex as assessed 2 h after binge METH exposure (Graham et al., 2008). These findings suggest that the ability of a high-dose METH treatment to reduce NE and 5HT content and/or to alter the function NE and 5HT transporters are similar regardless of previous METH exposure.
In contrast to the acute effects, prior METH self-administration attenuated the persistent deficits induced by binge exposure to METH when given 1 d later. Animals that self-administered METH prior to the binge METH exposure had attenuated serotonergic deficits 7 d after the binge exposure. This is consistent with escalating-dose pretreatments in that prior exposure to METH decreased binge-induced serotonergic deficits as assessed 7 d later (Johnson-Davis et al., 2003). It can be speculated that the attenuated binge METH-induced persistent deficits afforded by prior METH exposure may explain why larger deficits are not observed in human METH users (Wilson et al., 1996). However, in both the escalating-dose pretreatment and the current study, protection against the persistent serotonergic deficits in the cortex induced by binge METH exposure were lost when the binge exposure occurred 14–15 d after the last METH pretreatment (Danaceau et al., 2007). These findings suggest METH exposure after prolonged abstinence may be a particularly vulnerable time for serotonergic deficits in the frontal cortex.
Attenuations in hyperthermia may be one contributing factor to the protection against persistent serotonergic deficits. The METH/METH group had attenuated temperatures during the binge exposures as well as basal temperatures compared to the Saline/METH group. Previous research has suggested that METH-induced hyperthermia can contribute to depletions in 5HT content, reduced tryptophan hydroxylase activity, and the formation of oxygen radicals (Bowyer et al., 2008; Fleckenstein et al., 1997; Yamamoto et al., 2010). METH-induced hyperthermia can contribute to the breakdown of the blood-brain barrier (Bowyer et al., 2006; Bowyer et al., 2008) which is particularly prevalent in the cortex (Kiyatkin and Sharma, 2009). This permeability of the blood-brain barrier is hypothesized to contribute to a variety of other events including the activation of glial cells and the increase in brain water content which are thought to contribute to the persistent neurotoxic effects of METH (Kiyatkin and Sharma, 2009). Further, amphetamine administrations the also effects the meninges, arachnoid and pia membranes, and associated vasculature to the forebrain by increasing mRNA expression of endoplasmic reticulum stress response related genes, vascular tone regulating genes, inflammatory response related genes and altered genes related to angiognesis (Thomas et al., 2009; Thomas et al., 2010). In rats, hyperthermia promotes the formation of oxygen radicals and reduces tryptophan hydroxylase activity (Fleckenstein et al., 1997; Stone et al., 1989). The increased hyperthermia observed in the rats that self-administered saline prior to the binge exposure of METH in the present study may have contributed to an increase in the blood-brain barrier permeability, edema, glial activation, oxygen radical formation, a disruption the vasculature to the frontal cortex, and a decrease in tryptophan hydroxylase activity compared to the animals with previous METH exposure during self-administration and may have resulted in greater 5HT depletions within the cortex 7 d after the binge METH exposure.
Of note, other factors such as alterations in corticosteroids may also contribute to these findings (for reviews see Yamamoto et al., 2010). Extended access to METH self-administration reduced plasma corticosterone levels (Mandyam et al., 2008). Increased corticosterone, administered through the drinking water, increases proinflammatory cytokines and chemokines mRNA in the frontal cortex which can be associated with neuronal damage (Kelly et al., 2012). Further, chronic stress increases METH-induced plasma corticosterone levels, hyperthermia and serotonergic toxicity in the hippocampus (Doyle et al., 2010). Prior METH self-administration may attenuate METH-induced corticosterone levels which may have reduced hyperthermia and 5HT depletions in the cortex. It is unknown how long the reduction in corticosterone levels occurs following METH self-administration, so it is unknown if a recovery in corticosterone levels may have resulted in the different findings when the binge was administered 1 d versus 15 d after self-administration. Future studies may investigate the role of corticosterone in tolerance induced by METH self-administration.
Overall, the present data demonstrate that METH self-administration leads to persistent reductions in 5HT content within the cortex. Further, METH self-administration 1 d prior to binge METH exposure attenuates persistent, but not acute, 5HT depletions within the cortex. However, this reduction of persistent binge-induced deficits did not occur following 15 d of abstinence from METH self-administration. Future studies will investigate the mechanisms underlying these changes and the behavioral consequences of these changes in 5HT content within the cortex. Overall, these findings are of importance for future studies investigating the relationship between monoamines in the frontal cortex, tolerance, and drug abuse.
Acknowledgements
Funding for this study was provided by NIH grants: DA033097, 011389, 019447, 000378, 013367, 031883
References
- Andrade R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology. 2011;61(3):382–386. doi: 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry. 2011;69(12):e89–e99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60:521–532. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Robinson B, Ali S, Schmued LC. Neurotoxic-related changes in tyrosine hydroxylase, microglia, myelin, and the blood-brain barrier in the caudate-putamen from acute methamphetamine exposure. Synapse. 2008;62:193–204. doi: 10.1002/syn.20478. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Ladenheim B, Cai NS, McCoy MT, Atianjoh FE. Methamphetamine preconditioning: differential protective effects on monoaminergic systems in the rat brain. Neurotox Res. 2009;15:252–259. doi: 10.1007/s12640-009-9026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Clemens KJ, Thompson MR, Callaghan PD, Dawson B, McGregor IS. High ambient temperature increases intravenous methamphetamine self-administration on fixed and progressive ratio schedules in rats. J Psychopharmacol. 2008;22:100–110. doi: 10.1177/0269881107082286. [DOI] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Doyle JR, Yamamoto BK. Serotonin 2 receptor modulation of hyperthermia, corticosterone, and hippocampal serotonin depletions following serial exposure to chronic stress and methamphetamine. Psychoneuroendocrinology. 2010;35(4):629–633. doi: 10.1016/j.psyneuen.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Beyeler ML, Jackson JC, Wilkins DG, Gibb JW, Hanson GR. Methamphetamine-induced decrease in tryptophan hydroxylase activity: role of 5-hydroxytryptaminergic transporters. Eur J Pharmacol. 1997;324:179–186. doi: 10.1016/s0014-2999(97)00081-2. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR. Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. J Pharmacol Exp Ther. 1997;283:281–285. [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol. Exp. Ther. 2011;36:809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Castañeda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Fuchs R, Evans K, Ledford C, Parker M, Case J, Mehta R, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29:12–20. doi: 10.1111/j.1465-3362.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–269. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, Frankel PS. Response of limbic neurotensin systems to methamphetamine self-administration. Neurosci. 2012;203:99–107. doi: 10.1016/j.neuroscience.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Miller DB, Bowyer JF, O'Callaghan JP. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. J Neurochem. 2012;122:995–1009. doi: 10.1111/j.1471-4159.2012.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Sharma HS. Acute methamphetamine intoxication brain hyperthermia, blood-brain barrier, brain edema, and morphological cell abnormalities. Int. Rev. Neurobiol. 2009;88:65–100. doi: 10.1016/S0074-7742(09)88004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O'Dell SJ, Marshall JF, Izquierdo A. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology. 2012;219:411–420. doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosheleff AR, Rodriguez D, O'Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012;224:459–467. doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen MM, Kenemans JL, van Engeland H. Stroop interference and attention-deficit/hyperactivity disorder: a review and meta-analysis. Neuropsychology. 2007;21:251–262. doi: 10.1037/0894-4105.21.2.251. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–565. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Anderyak DM, Neilson SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012a;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hunt MM, Vieira-Brock PL, Muehle J, Nielsen S, Allen SC, Hanson GR, Fleckenstein AE. Prior methamphetamine self-administration attenuates serotonergic deficits induced by subsequent high-dose methamphetamine administrations. Drug Alcohol Depend. 2012b;126:87–94. doi: 10.1016/j.drugalcdep.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell SJ, Galvez BA, Ball AJ, Marshall JF. Running wheel exercise ameliorates methamphetamine-induced damage to dopamine and serotonin terminals. Synapse. 2012;66:71–80. doi: 10.1002/syn.20989. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London, UK: Elsevier, Inc.; 2007. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott J, Feltenstein M. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Son JH, Kuhn J, Keefe KA. Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology. 2012;67C:95–103. doi: 10.1016/j.neuropharm.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Johnson M, Hanson GR, Gibb JW. Acute inactivation of tryptophan hydroxylase by amphetamine analogs involves the oxidation of sulfhydryl sites. Eur J Pharmacol. 1989;172:93–97. doi: 10.1016/0922-4106(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Thomas M, George NI, Patterson TA, Bowyer JF. Amphetamine and environmentally induced hyperthermia differentially alter the expression of genes regulating vascular tone and angiogenesis in the meninges and associated vasculature. Synapse. 2009;63:881–894. doi: 10.1002/syn.20661. [DOI] [PubMed] [Google Scholar]
- Thomas M, George NI, Saini UT, Patterson TA, Hanig JP, Bowyer JF. Endoplasmic reticulum stress responses differ in meninges and associated vasculature, striatum, and parietal cortex after a neurotoxic amphetamine exposure. Synapse. 2010;64:579–593. doi: 10.1002/syn.20763. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Chong MY. Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr Psychiatry. 2006;47:215–220. doi: 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]