Abstract
Numerous pre-clinical studies have demonstrated that non-contingent methamphetamine (METH) administration rapidly decreases both dopamine (DA) transporter (DAT) and vesicular monoamine-2 transporter (VMAT-2) function. Because of the importance of transporter function to the abuse and neurotoxic liabilities of METH, and previous research indicating that the effects of non-contingent METH treatment do not necessarily predict effects of contingent exposure, the present study examined the acute impact of METH self-administration on these transporters. Results revealed that five days of METH self-administration (4 h/session; 0.06 mg/infusion) decreased DAT and VMAT-2 activity, as assessed in synaptosomes and vesicles, respectively, prepared from striatal tissue 1 h after the final self-administration session. METH self-administration increased core body temperatures as well. Brain METH and amphetamine (AMPH) levels, assessed 1 h after the final self-administration session, were approximately twice greater in high-pressing rats compared to low-pressing rats despite similar changes in DAT function. In conclusion, the present manuscript is the first to describe transporter function and METH/AMPH levels after self-administration in rodents. These data provide a foundation to investigate complex questions including how the response of dopaminergic systems to METH self-administration contributes to contingent-related processes such as dependence.
Keywords: Dopamine Transporter, Vesicular Monoamine Transporter-2, Methamphetamine
Introduction
Methamphetamine (METH) is a dopamine (DA)-releasing agent that causes feelings of alertness, energy, and/or euphoria that, in turn, contribute to its abuse liability (Kish, 2008). Its effects are temporally associated with that of [11C]d-METH uptake within the striatum, suggesting a contribution of the latter to its reinforcing potential (Fowler et al., 2008; Newton et al., 2006). Chronic METH abuse is associated with neurochemical, psychiatric, and cognitive impairments that persist long after the short-term effects of the drug have been subsided (McCann et al., 1998, 2008; Sekine et al., 2001; Volkow et al., 2001). Decreases in striatal DA transporter (DAT) availability associated with psychiatric, memory and motor deficits have also been reported in METH abusers (McCann et al., 2008; Sekine et al., 2001; Volkow et al., 2001). Thus, an understanding of both the short- and long-term effects of METH on striatal dopaminergic markers is important.
Preclinical models demonstrate that experimenter-administered non-contingent METH treatment rapidly decreases striatal DAT and vesicular monoamine transporter-2 (VMAT-2) function and/or immunoreactivity. For example, a single METH injection rapidly, reversibly and dose-dependently decreases both striatal DAT and VMAT-2 function (Brown et al., 2002; Fleckenstein et al., 1997; Metzger et al., 2000; Sandoval et al., 2001). Multiple METH injections, given in a “binge”-like pattern (defined as 4 – 6 experimenter-administered injections, 7.5 – 15 mg/kg/injection, 2 – 6-h intervals), also rapidly decrease these transporters’ functions, although the magnitude and duration of these decreases are larger than after a single injection (Brown et al., 2000, 2002; Eyerman et al., 2007; Hadlock et al., 2009; Kokoshka et al., 2000; McFadden et al., 2011; Chu et al., 2010). Studies of METH-induced alterations in DAT and VMAT-2 function are important as these transporters are critical regulators of DA distribution, and thus contribute to both the abuse liability and the long-term deficits caused by the stimulant (for review, see Fleckenstein et al., 2007, 2009; Sulzer et al., 2005).
Because of its contingent nature, METH self-administration by rodents purportedly mimics aspects of human METH abuse (Reichel et al., 2011). Previous studies utilizing this model (Krasnova et al., 2010; Schwendt et al., 2009; Shepard et al., 2006; Stefanski et al., 1999, 2002) have focused on effects existing 1 – 30 d after the final METH exposure. While these studies provide important insight into long-term effects, little is known about the acute (defined herein as 1 h after the final session) effects of METH self-administration on dopaminergic markers such as striatal DAT and VMAT-2. Importantly, previous research demonstrates that the effects of non-contingent METH treatment do not necessarily predict effects of contingent exposure (Brennan et al., 2010; Frankel et al., 2011), and thus study of the impact of METH self-administration (vs. relying upon current preclinical literature involving investigator-administered METH) is of importance. Further, there is very little information concerning two factors that purportedly impact DAT and VMAT-2: brain levels of METH (and its metabolite, amphetamine (AMPH)), and METH-induced alterations in core body temperature. Thus, the purpose of this study was to investigate these issues in self-administering rats.
Methods
Male Sprague-Dawley rats (275–300 g; Charles River Laboratories, Raleigh, NC) were maintained under a 14:10 h light/dark cycle. Following surgery, each rat was individually housed, and water was available ad libitum. During food training, food was restricted, although no rat dropped below 90% of its initial body weight. The University of Utah Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, approved all experiments.
Food training (4 d; 14 h/d) and self-administration occurred in an operant chamber (Coulbourn Instruments, Whitehall, PA) as described previously (Hadlock et al., 2011). Catheters were constructed as described previously (Frankel et al.; 2008), and implanted in the jugular vein as described by Hadlock et al., (2011). Rats underwent 1 or 5 d of self-administration (4 h/d) during the light cycle in a room maintained at 28 ± 1°C (see Frankel et al., 2011). (±)METH hydrochloride (0.06 mg/10 µl; Research Triangle Institute; Research Triangle Park, NC) was dissolved in 0.9% sterile saline. For each active lever press, an infusion pump, connected to a liquid swivel suspended outside the operant chamber, delivered 10 µl of METH or saline per infusion (FR1) over a 5-s duration through a polyethylene tubing located within a spring leash tethered to the rat. During this period, both levers were retracted. Following the infusion, the levers remained retracted for an additional 20 s. The active lever was counterbalanced within each group. Pressing the inactive lever resulted in no programmed consequences although it was recorded. Rectal temperatures were measured using a digital thermometer approximately 30 min after each session (Physiotemp Instruments, Clifton, NJ).
Following decapitation, the striata were removed, and striatal plasmalemmal and vesicular [3H]DA uptake assays were conducted as described previously (Brown et al., 2001; Chu et al., 2008; Hadlock et al., 2009). Protein concentrations were determined according to Bradford (1976). METH and AMPH levels were determined as described previously (Truong et al., 2005). Whole brains (minus the striatum, hippocampus and frontal cortex that were retained for other assessments) were analyzed, and concentrations were determined using a ThemoQuest Quantum liquid chromatography tendem mass spectrometer (Thermo Electron Corporation, Walham, MA). The lower limit of quantitation was 0.5 ng/ml.
Statistical analyses among groups were conducted using one-way analysis of variance (ANOVA) followed by Newman-Keuls posthoc analyses, Pearson’s correlation, or a t-test (GraphPad Prism 5.01 (La Jolla, CA, USA)). Differences among groups were considered significant if the probability of error was less than 5%. Data represent means ± standard error of the mean of 3 – 10 animals/group.
Results
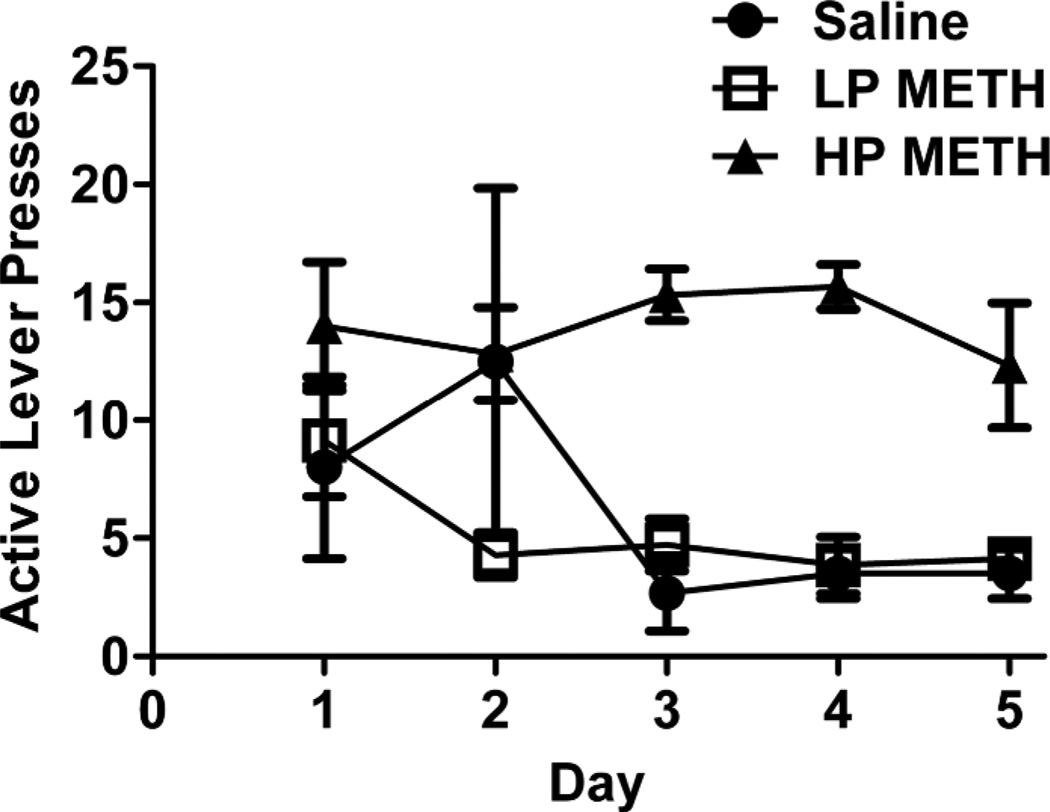
In the first study, rats were allowed to self-administer METH for 5 d, and sorted according to lever-pressing behavior, largely as described by Brennan et al. (2010; Figure 1). Specifically, animals that averaged 10 active lever presses/d and pressed the active lever on average at least 2-fold more than the inactive lever were considered high pressers (HP). Rats that did not meet these criteria were classified as low pressers (LP). Although not formally characterized, HP animals exhibited greater activity at the end of the sessions compared to LP animals.
Figure 1.
METH self-administration patterns vary among rats. Rats were sorted based on active lever pressing behavior. Rats self-administered METH (0.06 mg/infusion) or saline (10 µL) for 5 d (4 h/d). See Results for description of LP and HP rats.
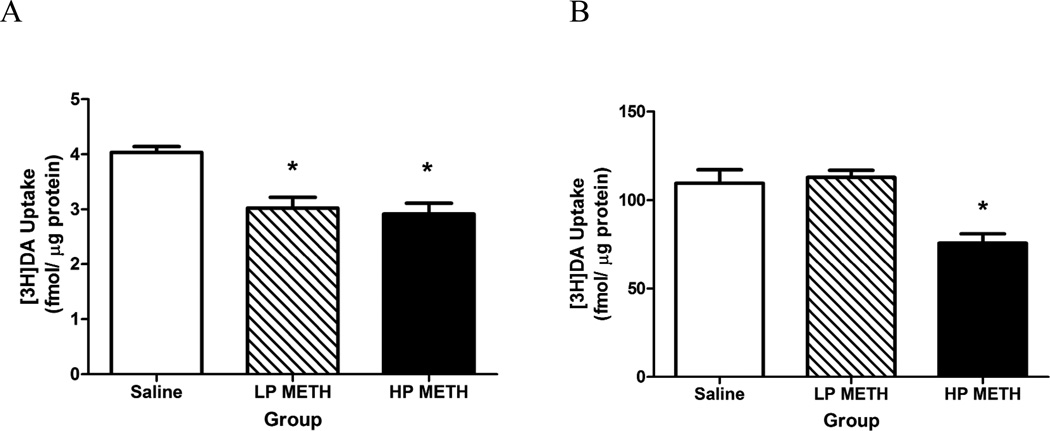
Results presented in Figure 2A revealed that 5 d of METH self-administration (0.06 mg/infusion, a dose based upon Frankel et al., 2011) decreased DAT function compared to saline self-administering rats in both HP and LP, as assessed in synaptosomes prepared from the striatum of rats sacrificed 1 h after the final session (F(2,9)=9.09, p< 0.05; Figure 2A). In this experiment, HP and LP rats self-administered a total of 4.40 ± 0.40 and 2.06 ± 0.31 mg METH, respectively over the 5 d course of exposure. This resulted in brain METH levels of 0.67 ± 0.11 and 0.35 ± 0.04 ng/mg wet weight, respectively (t(10)=3.25, p<0.05), and AMPH levels of 0.22 ± 0.05 and 0.06 ± 0.01 ng/mg, respectively (t(10)=3.93, p < 0.05). The reductions in DAT function were similar in magnitude, even though the HP rats administered nearly twice as much METH compared to LP rats (t(11)=5.69, p < 0.01). Similarly, the total amount of METH self-administered was not significantly correlated with DAT function (r=−0.06, ns). METH self-administration increased core body temperatures in both groups compared to saline-administering rats (37.48 ± 0.20, 38.17 ± 0.14 and 38.19 ± 0.13°C for saline, LP and HP rats, respectively; F(2,16)=37.80, p<0.05).
Figure 2.
METH self-administration decreased DAT (A) and VMAT-2 (B) function. Rats self-administered METH (0.06 mg/infusion) or saline (10 µL) for 5 d (4 h/d), and were sacrificed 1 h after the final session. Columns represent the mean (+SEM). *p < 0.05 vs. saline.
Of note, a separate group of rats was allowed to self-administer METH for one 4-h session. These rats lever-pressed for a total of 0.46 ± 0.06 mg METH. This treatment reduced DAT function, as assessed 1-h after the single session (Saline: 2.97 ± 0.33 fmol/µg protein, METH: 1.79 ± 0.16 fmol/µg protein; t(11)=3.49, p < 0.05), but was not significantly correlated with the total METH received (r=−0.54, p=0.11).
Results presented in Figure 2B demonstrate that after 5 d of METH self-administration, only the HP rats significantly decreased VMAT-2 function compared to saline self-administering rats, as assessed in striatal vesicles prepared from rats sacrificed 1 h after the final session (F(2,16)=12.73, p<0.01). In this experiment, HP and LP rats self-administered a total of 4.51 ± 0.23 and 2.08 ± 0.34 mg METH, respectively, over the entire 5-d course of treatment. The total amount of METH self-administered was significantly negatively correlated with VMAT-2 function (r=−0.78, p<0.01). METH self-administration increased core body temperatures, with the HP rats having greater hyperthermia than the LP rats (37.08±0.14, 37.73±0.11 and 38.47±0.06°C for saline, LP and HP rats, respectively F(2,16)=37.80, p<0.05). Because the decrease in VMAT-2 function only occurred in HP rats, effects of METH self-administration were not assessed after a single session.
Discussion
The present study is the first report of the acute (vs. long-term) effects of METH self-administration on DAT and VMAT-2. Study of the impact on transporter function is of importance, as the former can contribute to the persistent dopaminergic deficits (e.g., lasting weeks to months; e.g., Chu et al., 2008) caused by METH. Further, these transporters are critical regulators of extracellular DA levels and thus its abuse liability. Although there are several preclinical studies of the impact of non-contingent METH treatment, study of effects after self-administration is of particular significance since studies indicate that the effects of non-contingent METH treatment do not necessarily predict effects of contingent exposure (Brennan et al., 2010; Frankel et al., 2011). For example, Brennan et al. demonstrated that experimenter-administered ‘binge’ exposure to METH lead to decreases in DA content within the striatum both 24 h and 7 d after the exposure whereas 20 d of METH self-administration lead to decreased DA content at 24 h but not 7 d after the last self-administration session. Thus, the present study examined the acute impact of METH self-administration on DAT and VMAT-2.
Results revealed decreases in DAT function that occurred regardless of pressing behavior (i.e., low or high), as assessed ex vivo 1 h after the final METH session. Of note,Schwendt et al. (2009) reported a decrease in DAT immunoreactivity when assessed approximately 10 d after the last self-administration session. Further, emerging data from our laboratory demonstrate decreased DAT function 7 and 30 d after METH self-administration in HP animals only and attenuated the effects of a subsequent “binge” of METH (McFadden et al., In Press). These data permit speculation that the acute changes reported in the present study may be distinct from, and yet contribute to, these long-term changes. Such a phenomenon has been postulated after non-contingent “binge” METH treatment. In particular, changes occurring 1 h after this treatment (which may reflect transporter modification and/or internalization) likely contribute to the persistent dopaminergic deficits observed 7 d after treatment (which possibly reflect a loss of dopaminergic nerve terminals (Fleckenstein et al, 2009; Sulzer et al., 2005; Yamamoto et al.,2010).
Greater pressing behavior was required to alter VMAT-2 function, as reductions were only displayed in the HP rats. Noteworthy, neither these decreases nor the decrease in DAT function were likely due to the presence of METH during the assay resulting from its infusion during the final session since previous studies demonstrated that the preparation of synaptosomal and vesicular fractions washes the drug from the samples prior to assay (e.g., Fleckenstein et al., 1997). Of note, slight differences in hyperthermia were seen between the low-pressing groups in the DAT and VMAT-2 studies, (perhaps due to the proximity of the pressing behavior to the time the temperature was taken), may have attributed to these pressing-dependent temperature differences. Similar hyperthermia in HP and LP rats may have contributed to similar decreases in DAT function, whereas differences in hyperthermia in HP and LP rats may have contributed to differences in VMAT-2 function. Alternatively, it is established that D1 and D2 receptor activation contributes to the rapid effects of METH on DAT (Metzger et al., 2000) whereas only D2 (but not D1) receptors contribute to effects on VMAT-2 (Chu et al., 2010). Although the mechanism underlying the differential effect on DAT and VMAT-2 is not known, differential activation of these receptors or hyperthermia may underlie the dissimilar effects of self-administration on these transporters.
The effects of METH self-administration on DAT activity share some, but not all, features of effects of non-contingent experimenter-administered METH exposure. In particular, and as after a single METH self-administration session, a single METH injection (5 – 15 mg/kg, s.c.) rapidly decreases DAT function in a dose-dependent manner, with a magnitude of decrease comparable to that observed after METH self-administration (Fleckenstein et al., 1997). This effect is reversed 24 h later (Metzger et al., 1997). However, and in contrast to experimenter-administered METH exposure, these effects did not appear to be dose-dependent as low- and high-pressing rats displayed a nearly 2-fold difference in brain METH levels, and yet similar decreases in the magnitude of DAT function.
The route of administration may have played an important role in these differences. Although brain METH concentrations in the current study were lower than those 1 h following a single 15 mg/kg (s.c.; approximately 8 ng/mg) injection of METH or the METH-like substance AMPH (5 mg/kg, s.c.; approximately 1.5 µM), and postmortem brain levels in human METH abusers (1.11–56.52 ng/mg), intravenous administration may have allowed for a greater proportion of the drug administered to enter into striatal regions of the brain (Clausing et al., 1999; Fleckenstein et al., 1997; Wilson et al., 1996). Further, it can be speculated that the animals’ ability to self-administer the drug at any time point during the 4-h session may have resulted in more constant levels of the drug in the brain over a more prolonged time period than would occur if the drug was given in a single injection.
Several mechanisms likely contribute to the impact of METH on DAT including phosphorylation, internalization, oxidation and/or nitrosylation (Cervinski et al., 2005; Eyerman et al., 2007; Kokoshka et al., 1998; Pogun et al., 1994; Robertson et al., 2009). Increases in core body temperatures such as those facilitated by METH can exacerbate at least some of these (i.e., the formation of reactive oxygen and nitrogen species), and thus are likely contributors to the impact of METH self-administration on these transporters (Bowyer, 1995; Krasnova & Cadet, 2009; Yamamoto et al., 2010).
As with the DAT, the decreases in VMAT-2 function after METH self-administration in HP rats resemble effects of a single, non-contingent METH exposure. In particular, a previous study found that a single injection of METH (15 mg/kg, s.c.) decreases cytosolic VMAT-2 function as assessed in purified striatal vesicles prepared 1 h after treatment, but lower METH doses do not alter VMAT-2 function (Brown et al., 2001). These data are reminiscent of the present finding that only HP rats displayed reductions in VMAT-2 function as assessed 1 h after the last session. The importance of this decrease may involve the possibility that acute decreases in VMAT-2 function may contribute to persistent deficits caused by METH self-administration as has been reported by others and us (as has been suggested after “binge” METH treatment (Fleckenstein et al., 2009; Sulzer et al., 2005). Of note, we cannot preclude the possibility that the small increase in hyperthermia in the HP vs. LP group may have contributed to this decrease, as some (McFadden et al., 2011), but not all (Johnson-Davis et al., 2004) previous experimenter-administered METH studies have found that hyperthermia may contribute to changes in VMAT-2. The mechanism underlying the decrease in VMAT-2 function, whether following contingent or non-contingent administration, remains unknown, although it may involve a structural alteration in the VMAT-2 protein, per se, or a disruption of the pH gradient required for DA sequestration (for review, see Fleckenstein et al, 2009; Sulzer et al., 2005).
In conclusion, the present manuscript is the first to report acute alterations in DAT and VMAT-2 function. Further, it is the first to describe METH and AMPH levels after such exposure. These findings suggest the acute effects of the self-administration of METH resemble some, but not all of the effects seen after experimenter-administered METH, and provide groundwork to investigate complex questions including how the response of dopaminergic systems to METH self-administration contributes to contingent-related processes such as dependence.
Acknowledgements
This research was supported by NIH DA09407, 019447, 013367, 00869, 11389, 04222, and 00378.
References
- Bowyer JF. The role of hyperthermia in amphetamine's interactions with NMDA receptors, nitric oxide, and age to produce neurotoxicity. Ann N Y Acad Sci. 1995;765:309–310. doi: 10.1111/j.1749-6632.1995.tb16594.x. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74(5):2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther. 2001;296(3):762–767. [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302(2):497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, Vaughan RA. Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem. 2005;280(49):40442–40449. doi: 10.1074/jbc.M501969200. [DOI] [PubMed] [Google Scholar]
- Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, Hanson GR, Fleckenstein AE. Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol. 2008;590(1–3):105–110. doi: 10.1016/j.ejphar.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PW, Hadlock GC, Vieira-Brock P, Stout K, Hanson GR, Fleckenstein AE. Methamphetamine alters vesicular monoamine transporter-2 function and potassium-stimulated dopamine release. J Neurochem. 2010;115(2):325–332. doi: 10.1111/j.1471-4159.2010.06922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing P, Bowyer JF. Time course of brain temperature and caudate/putamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of d-amphetamine. Ann N Y Acad Sci. 1999;890:495–504. doi: 10.1111/j.1749-6632.1999.tb08031.x. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103(3):1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282(2):834–838. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(Suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Wong C, Ma Y, Kriplani A, Pradhan K, Schlyer D, Jayne M, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog K. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43(4):756–763. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther. 2011;336(3):809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Baucum AJ, 2nd, King JL, Horner KA, Cook GA, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. Mechanisms underlying methamphetamine-induced dopamine transporter complex formation. J Pharmacol Exp Ther. 2009;329(1):169–174. doi: 10.1124/jpet.108.145631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.184119. Epub 2011 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG. Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 2004;309:578–586. doi: 10.1124/jpet.103.062695. [DOI] [PubMed] [Google Scholar]
- Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178(13):1679–1682. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75(5):2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998;361(2–3):269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5(1):e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock G, Allen SA, Vieira-Brock P, Stout KA, Ellis JD, Hoonakker A, Andrenyak DM, Nielsen SM, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. Journal of Pharmacology and Experimental Therapeutics. doi: 10.1124/jpet.111.188433. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hoonakker AJ, Vieira-Brock PL, Stout KA, Sawada NM, Ellis JD, Allen SC, Walters ET, Nielsen SM, Gibb JW, Alburges ME, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine treatment during development attenuates the dopaminergic deficits caused by subsequent high-dose methamphetamine administration. Synapse. 2011;65(8):771–777. doi: 10.1002/syn.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther. 2000;295(3):1077–1085. [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Pogun S, Baumann MH, Kuhar MJ. Nitric oxide inhibits [3H]dopamine uptake. Brain Res. 1994;641(1):83–91. doi: 10.1016/0006-8993(94)91818-x. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36(4):782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol. 2009;39(2):73–80. doi: 10.1007/s12035-009-8053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci. 2001;21(4):1413–1419. doi: 10.1523/JNEUROSCI.21-04-01413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331(2):555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185(4):505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371(2–3):123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Lee SH, Yasar S, Cadet JL, Goldberg SR. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol. 2002;439(1–3):59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Fleckenstein AE. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. J Pharmacol Exp Ther. 2005;314(3):1087–1092. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]