Abstract
Human and animal research indicates that females may have a higher biological propensity for cocaine abuse than do males. Furthermore, reproductive status modulates the subjective effects of cocaine in women and self-administration rates in rats. Despite the attention that has been given to the modulation of appetitive responses by reproductive status and the well-known mixed positive and negative subjective effects of cocaine, it is unknown if similar effects are observed on aversive responses to cocaine. The present study examines the impact of sex and estrous cycle on approach-avoidance behavior for cocaine as measured in the runway self-administration model. Male and freely cycling female Sprague Dawley rats were trained to traverse a straight alley for single daily injections of 1.0 mg/kg intravenous cocaine over 21 trials. Relative to males, females had significantly longer start latencies but significantly faster approach and shorter run times during the first week of training. Further, estrus females displayed significantly fewer approach-avoidance retreats across all sessions relative to non-estrus females. These results suggest that females initially exhibit greater motivation for cocaine (faster approach) than do males and that the drug’s anxiogenic properties have a reduced impact on the motivation to seek cocaine (fewer retreats) in females during the estrus phase relative to other reproductive phases. These findings indicate that both sex and reproductive status contribute to the motivation for cocaine and that sex differences in addiction vulnerability may be attributable in part to differences in the motivational impact of both the appetitive and aversive properties of cocaine.
Keywords: cocaine, sex differences, self-administration, runway, conflict, drug reinforcement
Introduction
Despite its high dependence liability, cocaine has mixed appetitive and aversive properties whose relative impact may differ across individuals and thereby differentially impact addiction vulnerability. Sex differences in the profile of cocaine dependence have indicated that women, relative to men, transition faster from first use to entering treatment (Westermeyer, Kopka & Nugent 1997), report shorter cocaine-free periods (Kosten et al. 1993) and experience greater cocaine craving in response to psychological and physical stressors (Back et al. 2005). Sex differences in the responses to cocaine have also been described in animals. Relative to males, female rats display greater propensity to acquire cocaine self-administration (Lynch & Carroll 1999), increased self-administered intake of cocaine both during extended sessions (Roth & Carroll 2004) and under progressive ratio schedules (Lynch & Taylor 2004), and stronger cocaine-induced conditioned place preferences (Zakharova, Wade & Izenwasser 2009). Among females, the subjective and behavioral effects of cocaine vary across the reproductive cycle. For example, women report higher cocaine-induced pleasure during the follicular phase than during the luteal phase (Evans, Haney & Foltin 2002), and female rats display greater cocaine seeking during estrus compared to other cycle phases (Kippin et al. 2005; Feltenstein & See 2007; Kerstetter et al. 2008). Furthermore, female rats in estrus earn significantly more cocaine infusions under a progressive ratio schedule of cocaine reinforcement than during other cycle phases (Roberts, Bennett & Vickers 1989; Lynch 2008). Thus, estrus appears to be a specific ‘at risk’ phase of the female reproductive cycle with respect to cocaine taking and seeking. What remains unclear is whether or not this increased cocaine-seeking is due to perceived changes in the appetitive properties of cocaine, the aversive/anxiogenic properties of cocaine or both.
It has been well documented that, in addition to its well-known positive or rewarding actions, cocaine has potent aversive anxiogenic-like properties (e.g. Ettenberg & Geist 1991; DeVries & Pert 1998; Basso et al. 1999; Paine, Jackman & Olmstead 2002). Consistent with Solomon & Corbit’s (1974) ‘Opponent Process Theory’ of motivation, self-administered cocaine simultaneously produces appetitive and anxiogenic effects, specifically the initial positive experience is shortly followed by a ‘crash’ that is associated with agitation, anxiety and cravings (see review by Ettenberg 2004). For example, male rats will exhibit place preferences for environments paired with the immediate effects of cocaine and aversions for places paired with effect present 15 minutes after an intravenous (i.v.) cocaine injection (Ettenberg et al. 1999; Ettenberg & Bernardi 2007). In a runway model of drug self-administration, cocaine reinforcement elicits a behavioral profile in which male rats display a fast approach towards the goal-box but gradually develop a stop-and-retreat behavior reflective of a growing ambivalence about entering the goal-box (Ettenberg & Geist 1991). These ‘retreat’ behaviors are identical to those observed during approach of goal-box associated with both a positive reinforcer (food) and a punishment (footshock) (Geist & Ettenberg 1997), and appear to reflect approach/avoidance conflict about goal-box entry (Rogerio & Takahashi 1992; Yang et al. 1992; Simon, Dupris & Costentin 1994; Ettenberg et al. 1999). To date, no studies have assessed the potential mixed properties of cocaine in females, including assessment of estrous cycle modulation of this behavior. Thus, the present study was devised to assess whether males and females might differ in their response to cocaine in the runway model of self-administration and to assess the contribution of the estrous cycle to the expression of cocaine-induced approach/avoidance behavior.
Methods and Materials
Subjects
Male (n = 15, weighing between 275 and 300 g) and female (n = 28, weighing between 225 and 250 g) Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA) and served as subjects. Rats were individually housed in plastic cages within a temperature-controlled (23°C) vivarium maintained under a 12-hour light–dark cycle (lights off at 1900). Rats had continuous access to food (Purina Rat Chow) and water throughout the duration of the study. All animal handling and experimental procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California-Santa Barbara’s Institutional Animal Care and Use Committee.
Surgery
Rats were acclimated to human handling and vivarium conditions for 5 days prior to i.v. catheterization. Rats were anesthetized by inhalation of isoflourane gas (5% for induction; 2.5% for maintenance) and a jugular catheter was implanted. Chronic indwelling catheters were constructed using a bent steel cannula with a screw-type connector (Plastics One, Roanoke, VA, USA), silastic tubing (10 cm, i.d. 0.64 mm, o.d. 1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (Atrium Medical, Hudson, NH, USA) and cranioplastic cement. The end of the catheter was inserted into the right jugular vein and secured to surrounding tissue with suture. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. Following surgery, animals received the antibiotic, ticarcillin disodium/clavulanate potassium (Timentin; 50 mg/kg, i.v.) and 0.1 ml of heparin (1000 IU/0.1 ml prepared in 0.9% physiological saline, IV) as a prophylactic measure against microbial infection and to ensure catheter patency.
All rats were allowed a minimum of one week to recover from surgery before the onset of operant runway training. During this time, the catheters were flushed once daily with 0.1 ml of Timentin antibiotic (20 mg/kg, i.v.), followed by 0.1 ml of heparinized saline (1000 IU/0.1 ml, i.v.) to maintain catheter patency. Prior to the start of the experiment, catheter patency was confirmed in all rats by observing the behavioral impact of an IV injection of the fast-acting barbiturate, Brevital (2 mg/kg, 1.0 mg/0.1 ml water). Rats that were unresponsive to the Brevital (did not exhibit sedation) were re-implanted with a new catheter using the left jugular vein and given additional days for recovery.
Drugs
Cocaine (National Institute on Drug Abuse, Research Triangle Park, NC, USA) was dissolved in 0.9% physiological saline and delivered in a volume of 0.1 ml over a period of 4.3 sec via a 10-ml syringe (1.0 mg/kg/injection, i.v.) seated in a motorized syringe pump (Razel Scientific Instruments, St Albans, Vermont, USA). The dose of cocaine for this project was selected based upon prior studies that indicate it reliably produces approach-avoidance responding in the runway (e.g. Ettenberg & Geist 1991; Guzman & Ettenberg 2004; Ettenberg & Bernardi 2006).
Runway apparatus
All trials for this experiment utilized two identical wooden straight-arm alleys (160 cm in length × 12 cm wide × 44 cm high). A start box and goal box (each 23 cm × 20 cm × 44 cm) were attached to opposite ends of each runway. The floor of each apparatus consisted of 3 cm steel rods, laid in parallel 1.2 cm apart perpendicular to the runway walls. Aligned along the length of the runway were 13 pairs of infrared photodetector-emitter pairs spaced approximately 16 cm apart. The 1st pair was located within the start box and the 13th pair inside the goal box. The output from these sensors was fed to a desktop computer via a custom Any-Maze AMi interface (Stoetling Co, Wood Dale, IL, USA) that permitted the precise location of the animal in the alley to be recorded in real time throughout each trial.
Suspended above each apparatus were two long magnetic rails (spaced 3 cm apart) that ran in parallel along the entire length of the runway. Positioned between the rails was a liquid swivel (375-22PS, Instech Laboratories Inc., Plymouth Meeting, PA, USA) that connected the guide cannula on the rats’ back to a 10 ml drug-filled syringe, via polyethyne-20 tubing. Around the midsection of the swivel, a flat plastic collar was secured to prevent it from falling through the space between the magnetic rails. A pot magnet was attached to the underside of the collar with the polarity arranged to repel the swivel system from the magnetic rails. The magnetic repulsion between the swivel assembly and the rails permitted the swivel to float slightly above the tracks thereby providing a low-friction and low-resistance mechanism that allowed the rat to move freely throughout the alley, pulling the swivel assembly along behind and above the animal as it moved (for a more complete description of the runway apparatus, see Geist & Ettenberg 1990).
Estrous cycle monitoring
Estrous phase determination was based on vaginal smears collected daily before each runway trial, as described previously (Feder 1981; Becker et al. 2005; Kerstetter et al. 2008). Vaginal lumen samples were collected using a gentle sweeping motion with a sterile, saline-dipped, cotton-tipped applicator and smeared onto a glass slide. Smears were stained with Giemsa Stain (Sigma-Aldrich, St. Louis, MO, USA) and cell morphology was assessed under a light microscope set at 10× magnification. The metestrus (also known as diestrus I) phase was defined as the presence of approximately equal proportions of nucleated epithelial cells, non-nucleated cornified epithelial cells, and leukocytes. The diestrus (also known as diestrus II) phase was defined as a minimum amount of cells, including leukocytes and occasional epithelia. The proestrus phase was defined by the presence of more than 75% nucleated epithelial cells. The estrus phase (i.e. vaginal estrus as opposed to behavioral estrus) was defined as the presence of more than 75% nonnucleated cornified epithelial cells.
Runway procedure
Rats were habituated to the apparatus during a single 10-minute trial before the start of drug self-administration training. Rats were not permitted access into the goal box, but could travel throughout the rest of the runway and start box. Then, to begin each trial, the animal was connected to the drug delivery system and placed in the start box. After 5 seconds, the start door opened to allow the animal full access to the alley. Upon entry into the goal box (i.e. interruption of the infrared photobeams in the goal box) the goal door was closed and the syringe pump activated to deliver an i.v. injection of cocaine. Rats were left in the goal box for 5 minutes post-injection and then returned to their home cages. Testing continued daily for 21 single trial/day sessions.
Runway dependent variables
Four dependent measures were collected for every animal on every trial: start latency (time to leave the start box after the start door was opened), approach latency (on the very first approach to the goal each day, the time to reach the threshold of the goal-box independent of whether or not the animal entered or stopped); run time (time to traverse the runway and enter the goal box), and retreat frequency (number of times an animal stopped its forward progress toward the goal box and retreated back at least 32 cm toward the start box).
Data analysis
Sex differences may be present without estrous cycle effects or can be obscured by opposing estrous cycle effects; thus, the contribution of sex and cycle status were analyzed separately. In order to assess the effect of estrous, females were determined to be in Dietrus (I or II), Proestrus or Estrus, and the runway behaviors of females in the same phase were averaged across each week (seven trials). Females typically complete at least one full cycle within 7 days, so usually the same rat will be in all conditions within each week (except as noted in further discussion) and, typically, each datum for an individual rat for each cycle stage was dervied by averaging across multiple daily sessions.
Analysis of variance (ANOVA) was used to assess the statistical reliability of differences within each dependent measure. Mixed factor (Group × Time) ANOVA was used to analyze start latency, run time, retreats and approach with either sex/estrous status serving as the between-subjects variables and ‘week’ serving as the repeated measure variable as appropriate. Simple main effects analyses were conducted after significant interactions; post hoc t-tests with Bonferroni adjusted alpha levels were implemented to further examine significant effects of week, and post hoc Tukey tests were run for significant main effects of estrous. With the exception of the Bonferroni post hocs on the repeated measures factor, the alpha level for statistical significance was 0.05.
Results
Sex differences
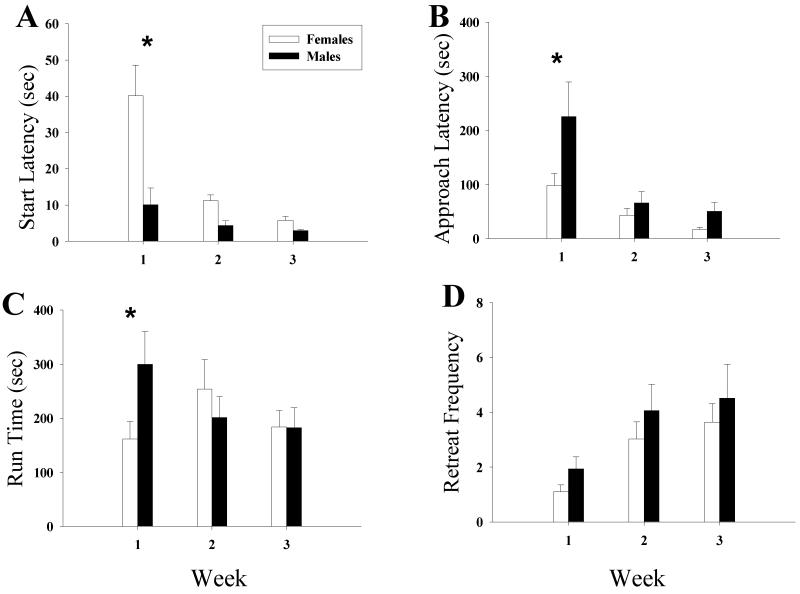
Relative to males, females initially took longer to enter the runway from the start box (see Fig. 1a). A 2 × 3 (sex × week) ANOVA of start latency revealed a significant sex-week interaction (F2,82 = 4.87, P < 0.05) and simple main effects analysis revealed a significant difference during week1 (F1,82 = 20.00, P < 0.05) with males leaving the start box significantly faster than females, but no significant differences during weeks 2 or 3. Additionally, there was a main effect of sex (F1,41 = 8.00, P < 0.05), with males leaving the start box significantly faster than females when averaged across all weeks, and a significant effect of week (F2,82 =, P < 0.05) stemming from the reduction in start latencies (i.e. faster responding) as testing progressed. Post hoc Bonferroni-corrected comparisons revealed significantly shorter start latencies during weeks 2 and 3 versus week 1, and during week 2 versus week 3 (Ps < 0.017).
Figure 1.
Effect of Sex on Runway Behavior across weeks. (a) Start latency, males left the start box significantly quicker than females during week 1. (b) Time to approach, rats became faster to approach the goal box over trials, females approached the goal box significantly faster than males during week 1. (c) Run time, females entered the goal box significantly faster than males during week 1. (d) Retreats, retreats from the goal box significantly increased over trials. *Indicates a significant difference between males and females for that week, † indicates a main effect of sex, γ indicates a main effect of week (Ps < 0.05)
Once subjects left the start box, the first approach of the goal box (see Fig. 1b) was completed more quickly by females than by males. A 2 × 3 (sex × week) ANOVA of the approach scores revealed a significant interaction of sex and week (F2,82 = 3.23, P < 0.05) and simple main effects analysis revealed a significant difference during week 1 (F1,82 = 15.79, P < 0.05) with females approaching the goal box significantly faster than males, but not during weeks 2 or 3.
Additionally, a significant main effect of sex was detected (F1,41 = 6.51, P < 0.05), with females on average approaching the goal box significantly faster than males across all 3 weeks, as well as a significant main effect of week (F2,82 = 18.56, P < 0.05), and post hoc Bonferroni-corrected comparisons revealed significantly shorter approach times across both sexes during weeks 2 and 3 relative to week 1 (Ps < 0.017).
Females also entered the goal box more rapidly than did males during week 1 but not later in the experiment (see Fig. 1c). A 2 × 3 (sex × week) ANOVA of run time revealed a significant interaction between sex and week (F2,82 = 4.27, P < 0.05), and simple main effects analysis identified a significant difference during week 1 (F1,82 = 8.32, P < 0.05) with females entering the goal box significantly faster than males, but not during weeks 2 or 3. There were no significant main effects for sex or week.
Males and females exhibited comparable development of approach-avoidance behavior with repeated testing (see Fig. 1d). A 2 × 3 (sex × week) ANOVA of retreats revealed a significant effect of week (F2,82 = 15.69, P < 0.05) and post hoc Bonferroni-corrected comparisons confirmed that significantly more retreats were made during weeks 2 and 3 compared to week 1 (Ps < 0.017). However, there was no significant main effect for sex or interaction between sex and week.
Effects of estrous
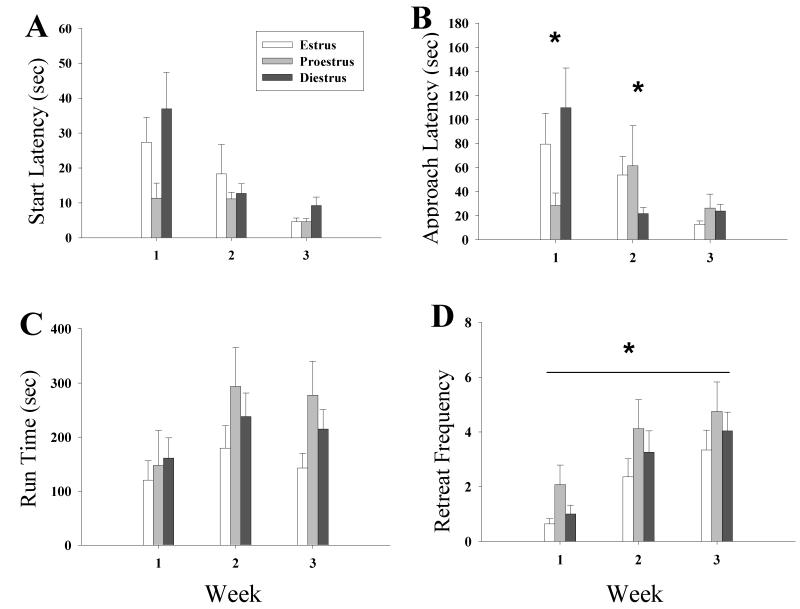
Females, regardless of cycle status, exhibited a progressive decline in the time to leave the start box with repeated cocaine reinforcement (see Fig. 2a). A 3 × 3 (cycle phase × week) ANOVA of start latencies revealed a significant effect of week (F2,128 = 4.67, P < 0.05) and post hoc Bonferroni-corrected comparisons revealed that females left the start box significantly faster during weeks 2 and 3 versus week 1 (Ps < 0.017). There was no significant main effect of estrous cycle, and no significant interaction between cycle and week.
Figure 2.
Effect of estrous cycle on runway behavior across weeks for estrus, proestrus and diestrus females. Data for each cycle phase was collapsed across rats for each week. (a) Start latency, females left the start box significantly faster over time. (b) Approach, females approached the goal box significantly faster over trials and estrous had a significant impact on approach time during weeks 1 and 2. (c) Runtime, females took longer to enter the goal box over trials. (d) Retreats, retreats from the goal box increased over trials, proestrus females retreated significantly more than estrus females. *Indicates a significant effect of the estrous cycle for that week, δ indicates a main effect of estrous, γ indicates a main effect of week (Ps < 0.05). Estrus and Diestrus n = 28 for all weeks. Proestrus n = 14 (week-1), N = 15 (week-2) N = 19 (week-3)
Initially, females in proestrus exhibited the fastest approach to the goal area during week 1 of reinforcement whereas females in diestrus exhibited the fastest approach during the second week of reinforcement; all females displayed equivalent rapid approach during the final week of testing (see Fig. 2b). A 3 × 3 (cycle phase × week) ANOVA of the approach latency data revealed a significant interaction between cycle phase and week (F2,126 = 2.69, P < 0.05) and simple main effects analyses revealed a significant differences in cycle phase during week 1 (F2,126 = 3.74, P < 0.05) and week 2 (F2,126 = 4.58, P < 0.05). Post hoc HSD Tukey tests on the cycle phases revealed that during week 1, proestrus females approached the goal box significantly faster than diestrus females (P < 0.05), and during week 2, diestus females approached the goal box significantly faster than estrus and proestrus females (P < 0.05). In addition, a significant main effect of week was detected (F2,126 = 3.88, P < 0.05) and post hoc Bonferroni-corrected comparisons determined that females approached the goal box significantly faster during weeks 2 and 3 relative to week 1 (Ps < 0.017). There was no significant main effect of estrous.
Females, regardless of cycle status, exhibited a progressive increase in the time to enter the goal box with repeated cocaine reinforcement (see Fig. 2c). A 3 × 3 (cycle phase × week) ANOVA on the run time data revealed a significant effect of week (F2,108 = 7.71, P < 0.05) and post hoc Bonferroni-corrected comparisons demonstrated that females entered the goal box significantly faster on week 1 versus week 3 (P < 0.05) with no other significant differences between weeks. There was no significant interaction between estrous cycle and week nor a significant main effect of estrous cycle.
All females exhibited an increase in approach-avoidance retreats with repeated testing with those in estrus exhibiting the fewest retreats (see Fig. 2d). A 3 × 3 (cycle phase × week) ANOVA of retreat frequency identified a main effect of estrous (F2,63 = 3.54, P < 0.05) and Tukey HSD post hoc tests revealed that estrus females displayed significantly fewer retreats than proestrus females (P < 0.05) and estrus females also tended to display fewer retreats than diestrus females although this difference failed to reach statistical significance. Further, there was a significant effect of week (F2,126 = 21.07, P < 0.05) and post hoc Bonferroni-corrected comparisions revealed significantly more retreats during weeks 2 and 3 versus week 1 (Ps < 0.017). There was no significant interaction between cycle phase and week.
Discussion
The present study examined the effects of sex and estrous cycle on the runway behavior of animals traversing a straight alley for i.v. cocaine delivered upon goal-box entry. In this model, the time it takes subjects to leave the start box and run the alley, as well as the presence or absence of retreat behaviors (ambivalence about entering the goal-box) provide indices about the rats’ motivation to seek the reinforcer that is available in the goal-box. Over the course of the experiment, rats left the start box and approached the goal box more quickly as testing progressed, indicating an increase in motivation to receive cocaine reinforcement. However, retreat frequency also increased over the course of the experiment, indicating that the subjects likely had mixed positive and negative associations with the goal-box, presumably stemming from cocaine’s mixed rewarding and anxiogenic properties (e.g. see review by Ettenberg 2004). New to the current study was the observation that runway behavior was modulated by both the sex of the animal and, in females, their estrous cycle status. Specifically, females exhibit faster running with equivalent avoidance (retreats) as males, and, moreover, females in estrus exhibit less approach-avoidance retreats than those in other reproductive stages.
Despite a lack of sex differences in retreat behavior, initial sex differences were detected for start latency as well as approach latency and run times. Across a variety of reinforcers, the time to traverse the alley of a runway is believed to provide an index of the motivation for the reinforcer (for a review, see Ettenberg 2009). Accordingly, the findings that females exhibited faster approach and entry into the goal area indicate that they have higher motivation for cocaine relative to males. This is consistent with evidence from cocaine conditioned place preference studies where female rats develop a preference for a cocaine-paired environment more quickly and to lower doses of cocaine than male rats (Russo et al. 2003; Zakharova et al. 2009), and cocaine self-administration studies where female rats acquire consistent lever press behavior for cocaine reinforcement more quickly than male rats (Lynch & Carroll 1999; Lynch 2008), make significantly more lever presses for cocaine than male rats during training and extinction (Fuchs et al. 2005; Kippin et al. 2005; Kerstetter et al. 2008; Kosten & Zhang 2008) and are willing to work harder for cocaine under progressive ratio schedules (Roberts et al. 1989; Lynch 2008). In contrast with these findings, males left the starting box significantly faster than females during week 1, suggesting that the males are less hesitant to enter the alley relative to females. This difference is unlikely to be due to higher inherent levels of anxiety in females because several reports suggest that female rats display less anxiety than males in the elevated plus maze (Johnston & File 1991; Imhof et al. 1993; Zimmerberg & Farley 1993), and female rats spent more time on the ledges of an open arena than males (Alstott & Timberlake 2009). One explanation for this finding may be that females are exhibiting an altered behavioral response to experimenter handling and placement into the apparatus (i.e. handling-induced behavioral inhibition lasting beyond the 5-second pre-trial habituation period). Alternatively, the contrasting effects of sex on start latency versus approach latency and run times may indicate that females exhibit approach-avoidance behavior at an earlier time within each trial than do males. Thus, the relation of initial sex differences in start latency to the motivation for cocaine remains unclear. Reproductive cycle and ovarian hormone fluctuations modulate female cocaine-related behavior across a variety of measures (Lynch, Arizzi & Carroll 2000; Hu & Becker 2003; Feltenstein & See 2007; Russo et al. 2008; Feltenstein et al. 2009). Specifically, females in the estrus phase will lever press more for cocaine than females in other reproductive phases during cocaine self-administration, extinction training, as well as reinstatement of lever responding produced by cocaine-priming injection (Kippin et al. 2005; Feltenstein & See 2007; Kerstetter et al. 2008). In a similar fashion, estrous cycle had a significant impact on both the initial time to approach the goal box and on retreat frequency. Females in proestrus displayed the fastest approach of the goal area during week 1 and females in diestrus exhibited the fastest approach during week 2.
This indicates that estrous status may not have a stable impact on the motivation for cocaine as measured by time to traverse the runway but, rather, this impact varies with the history of cocaine reinforcement. In contrast, the impact of the estrous cycle on approach-avoidance behavior (as measured by retreats) is stable with estrus females exhibiting fewer retreats than females in other phases. Given that retreat behaviors can be used as a measure of conflict anxiety that presumably reflects the anticipation of the mixed positive and negative properties of cocaine (Geist & Ettenberg 1997), reduced retreats could be due to reduced anticipation of the negative properties of cocaine, increased anticipation of the positive properties of cocaine, or both. The present findings that females in estrus exhibit reduced retreats in the absence of faster initial approach indicates that the differences in runway behavior of these females is largely determined by lower anticipation of the adverse effects while exhibiting equivalent (or slightly diminished) anticipation of the positive effects of cocaine. Given that the females were tested repeatedly across the estrous cycle, it appears that the impact of estrous status at the time of testing is unlikely due to differential experience with the appetitive and aversive properties of cocaine. Conversely, it is unclear if the reduced anticipation of cocaine’s aversive effects reflects differences in the actual magnitude of these effects across the estrous cycle (i.e. whether or not estrus females retreat less because they will experience a milder aversive response following the cocaine infusion). One potential method to examine this issue is the employment of place conditioning to detect the delayed aversive properties of cocaine (Ettenberg et al. 1999; Ettenberg & Bernardi 2007). Thus, estrous cycle differences in cocaine seeking in the runway appear to be mediated by a reduced avoidance of cocaine’s adverse effects and these effects may contribute to sex and reproductive cycle differences in other cocaine-related behaviors such as the rate of operant responding to receive repeated cocaine reinforcement.
Although sex differences in the anxiogenic effects of cocaine were unreliable, the finding that female rats in estrus exhibit lower avoidance behavior of cocaine reinforcement suggests that cocaine dependent women may be more likely to disregard the negative consequences of cocaine use during their early luteal phase of the menstrual cycle (most closely relates to estrus phase in rats). This is supported by evidence from Ambrose-Lanci et al. (2009), which indicates that cocaine dependent women in their early luteal phase of their menstrual cycle report the lower levels of anxiety relative to other phases, however it is unclear if the decreased anxiety impacted drug dependency. Therefore, further examination of the impact of sex and ovarian hormones on the aversive properties of cocaine is critical to elucidate the role of these variables in cocaine seeking.
Acknowledgements
This research was supported with funds from a National Alliance for Research on Schizophrenia and Affective Disorders Young Investigator Award to T.E.K and by the National Institute of Drug Abuse grants DA027525 and DA027115 awarded to T.E.K and DA05041 to A.E.
Footnotes
Authors Contribution
All authors were responsible for the study concept and design and participated in data analysis and interpretation of findings. KK and ZS collected animal data. KK and TK drafted the manuscript and all authors provided critical revision of the manuscript, critically reviewed content and approved the final version for publication.
References
- Alstott J, Timberlake W. Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav Brain Res. 2009;196:214–219. doi: 10.1016/j.bbr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Ambrose-Lanci LM, Sterling RC, Weinstein SP, Van Bockstaele EJ. The influence of intake urinalysis, psychopathology measures, and menstrual cycle phase on treatment compliance. Am J Addict. 2009;18:167–172. doi: 10.1080/10550490902772710. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology. 1998;137:333–340. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The opponent-process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;8:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behavior. 2006;85:393–399. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feder HH. Estrous cyclicity in mammals. In: Adler NT, editor. Neuroendocrinology of reproduction: physiology and behavior. Plenum Press; New York: 1981. pp. 279–348. [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in a runway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. Concurrent positive and negative goalbox events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol Biochem Behav. 1997;57:145–150. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- Guzman D, Ettenberg A. Heroin attenuates the negative consequences of cocaine in a runway model of self-administration. Pharmacol Biochem Behav. 2004;79:317–324. doi: 10.1016/j.pbb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY. Sex differences in non-reinforced responding for cocaine. Am J Drug Alcohol Abuse. 2008;34:473–88. doi: 10.1080/00952990802082206. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–523. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quiñones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupris J, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behavioral Brain Research. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychology Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Kopka S, Nugent S. Course and severity of substance abuse among patients with comorbid major depression. Am J Addict. 1997;6:284–92. [PubMed] [Google Scholar]
- Yang XM, Gormon AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Farley MJ. Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiol Behav. 1993;54:1119–1124. doi: 10.1016/0031-9384(93)90335-d. [DOI] [PubMed] [Google Scholar]